Abstract
The Neisseria meningitidis outer membrane protein PorA from a Chilean strain was purified as a recombinant protein. PorA mixed with AbISCO induced bactericidal antibodies against N. meningitidis in mice. When PorA was fused to the Helicobacter pylori HpaA antigen gene, the specific response against H. pylori protein increased. Splenocytes from PorA-immunized mice were stimulated with PorA, and an increase in the secretion of IL-4 was observed compared with that of IFN-γ. Moreover, in an immunoglobulin sub-typing analysis, a substantially higher IgG1 level was found compared with IgG2a levels, suggesting a Th2-type immune response. This study revealed a peculiar behavior of the purified recombinant PorA protein per se in the absence of AbISCO as an adjuvant. Therefore, the resistance of PorA to proteolytic enzymes, such as those in the gastrointestinal tract, was analyzed, because this is an important feature for an oral protein adjuvant. Finally, we found that PorA fused to the H. pylori HpaA antigen, when expressed in Lactococcus lactis and administered orally, could enhance the antibody response against the HpaA antigen approximately 3 fold. These observations strongly suggest that PorA behaves as an effective oral adjuvant.
Introduction
Neisseria meningitidis, a Gram-negative bacterium, typically serves a commensal role as part of the normal nasopharyngeal flora. However, under certain circumstances, the bacterium can cause severe meningitis and septicemia. N. meningitidis is unique among meningitis-causing pathogens for its ability to cause large-scale epidemics.Citation1
Currently, 13 different N. meningitidis serogroups have been defined based on the immunoreactivity of their cell surface polysaccharides (CSP). Among them, groups A, B, C, W-135, Y and, recently, X2 are responsible for over 90% of severe meningitis and septicemia cases due to N. meningitidis.Citation1,3 However, the dynamics of meningococcal epidemiology is changing.Citation4
Several conjugated vaccines have been developed to control the severe consequences of infection.Citation5 The meningococcal group C conjugate (MenC) vaccine was developed by Chiron and Wyeth using N. meningitidis outer membrane vesicles and a genetically detoxified diphtheria toxoid (CMR197) as the adjuvant. Baxter Vaccines developed a similar vaccine using tetanus toxoid as the carrier.Citation6 In young infants, these vaccines appear to be safe and immunogenic by inducing long-term immune memory.Citation7 A tetravalent conjugate vaccine has been developed at Sanofi-PasteurCitation8 that incorporates polysaccharides from groups A, C, Y and W-135 covalently linked to diphtheria toxin (MCV4). In all cases, adjuvants were required. Finally, quadravalent meningococcal conjugated vaccines have been developed by Sanofi-Pasteur-Merck, Novartis and GlaxoSmuthKline.Citation9
N. meningitidis group B, responsible for approximately 50% of meningococcal disease cases worldwide, is the only serogroup whose infection cannot be prevented by a polysaccharide vaccine, because a similar polysaccharide is present in human tissues that increases the possibility of autoimmunity as a consequence of vaccination.Citation10 Although new serogroup B vaccines have been recently reported,Citation11,12 the development of safe serogroup B vaccines against N. meningitidis remains a challenge. Recently, Novartis developed a vaccine with wider coverage (4CMenB vaccine), based on 3 components and outer membrane vesicles (OMV) of the MenZB strain, and this vaccine has undergone clinical trials in ChileCitation13 and the UK.Citation14
Research on vaccines for serogroup B have primarily focused on outer-membrane vesicles (OMV) carrying either native or heterologous cell surface protein antigens. Native OMVs, usually prepared by detergent extraction from the bacteria, contain intact outer membrane proteins (OMP) and lipo-oligosaccharides (LOS).Citation15-17
More extensive studies on group B OMV vaccines have been performed in response to national outbreaks in Cuba, Norway, Brazil and New Zealand. These vaccines were found to be 47–83% effective,Citation12 however, they did not provide sufficient protection in children under 4 years old.Citation18 These vaccines showed lower efficacies in ChileCitation19 and Brazil.Citation20 Additionally, a vaccine based on a similar development strategy has been recently licensed and trialed in New Zealand, where an epidemic of meningococcal disease caused by a single serosubtype emerged.Citation21,22 This vaccine, however, provided limited protection for only specific meningococcus B strains.Citation23
The PorA protein is the major OMP present on the meningococcal surface, and it is expressed by almost all meningococcal strains. It has a molecular weight of approximately 42 kDa and functions as a cationic porin.Citation24 The PorA protein was identified as an immunodominant antigen that is a target of bactericidal antibodies.Citation25 Additionally, the bactericidal activity detected in sera from human subjects immunized with experimental OMV vaccines correlated with antibodies against PorA.Citation26 PorA porin has been proposed as a promising target for the development of new meningococcal vaccines.
However, PorA-based vaccines have a limited protection coverage, i.e., there is no universal PorA-based vaccine. Moreover, in most cases, vaccine protection is effective against the strain with the same typing as that used for immunization, as in the case of the tailor-made MeNZB vaccine.Citation22,23
Protective immunological cross reaction by PorA is rather modest. This is due to the PorA segments, which are involved in the induction of bactericidal immune response (VR1 and VR2 sequences) and correspond to the same variable regions utilized for typing the wide diversity of N. meningitidis strains. It is well known that a huge number of N. meningitidis strains have been typed using VR1 and VR2 sequences, which have been deposited at the Neisseria PorA typing data bank.Citation27 Therefore, as previously reported developing a PorA-based vaccine with a wide range of coverage will be a difficult task.
Recombinant technology has been used to produce an OMV hexavalent vaccine containing 6 PorA subtypes.Citation26 In addition, it was found that the immune response in mice caused by a mixture of OMV carrying 6 different PorA antigens per particle (HexaMix) was different in magnitude compared with that induced by a mixture of monovalent OMV (HexaMen) with the same 6 genotypes each contained on a single OMV particle. However, these responses were subtype-biased and showed the same serum bactericidal antibodies (SBA) titer profile.Citation28 Regarding these aspects, a discussion about different strategies for development of vaccines against serogroup B has been published.Citation29 These observations indicated difficulties in obtaining a PorA-based vaccine capable of inducing a bactericidal response against different VR1/VR2 genotypes with similar titer values for each serosubtype.
In addition, a design with 9 distinct PorA subtypes (NonaMen) has been obtained.Citation30,31 Multiple PorA serotype vaccines have been administered as a 3-dose series in infants; nevertheless, they showed a modest efficacy. However, after a fourth dose in toddlers, a satisfactory immune response could be induced.Citation32,33
Another disadvantage of designing vaccines using purified PorA is that PorA behaves as a better antigen in its natural environment, i.e., the outer membrane. For instance, it has been reported that 96% of LPS is removed from PorA after PorA purification, and antibodies raised by OMV were bactericidal, but those raised by purified PorA were not. However, this problem was resolved when PorA was incorporated into liposomesCitation34 Because of this, most of the recent efforts on N. meningitis vaccines have been focused on the use of OMVs.Citation35
Immune Stimulating Complexes (ISCOMs) had been developed as a different approach to deliver antigens to the immune system. They are artificial hollow particles with lipids and a natural detergent, which bind antigens on their surface and those within them.Citation36 ISCOM formulations require a simple mixture with a defined antigen that can induce bactericidal antibody as a response in animal models, to be tested as potential vaccines.Citation37
Using this approach, PorA has been purified from meningococcal strains and mixed with aluminum phosphate, QuilA and ISCOM as adjuvants.Citation38 This attractive strategy for developing potential new meningococcal serogroup B vaccines has been tested with recombinant outer membrane proteins, purified from other bacterial membrane components, particularly meningococcal LPS and capsule components. The recombinant PorA (rPorA) protein from a single strain of N. meningitidis serogroup B has been purified from E. coli cells and incorporated into liposome particles. When this type of vaccine was tested in a murine model, it induced a protective immune response.Citation34,39 Additionally, an extended approach has been tested, which is a liposome-based vaccine containing recombinant PorA from 4 serosubtypes, protecting against a broad range of meningococcal strains.Citation40
To improve the immune response of rPorA, AbISCO TM-100® (an ISCOM with the antigen adsorbed on the matrix surface, as described by Lövgren et al.Citation41) was selected as a novel adjuvant, particularly because of its immunomodulation properties after simple addition of purified protein antigens.Citation42
Presently, the availability of safe adjuvants approved by the FDA for human use is limited. Alum (AL(OH)3 gel), MF59 (nano emulsions of squaleno oil), MPL (a glycolipid), QS21 (obtained from the tree Quillaja saponaria), cholera toxin B subunit and VLP (viral self-assembled particles) have been used in human vaccines.Citation43 Precisely mutated adjuvants derived from cholera (CT) and diphtheria (DT) toxins produce serious side effects. The development of new adjuvants for production-efficient vaccines is highly desirable.
In this report, we describe the purification of the rPorA protein, whose gene was obtained from a Chilean bacterial strain, its formulation with AbISCO TM-100® and its oral immunization assay using BALB/c mice as an animal model for vaccination. We observed a protective immune response against N. meningitidis serogroup B, according to the international definition of protective responses. We used the N. meningitidis strain B:15:P1.3 as the source of PorA, because it has been the most prevalent and frequent serotype isolated in Chile over the last 20 years.Citation44
When PorA alone was used as an immunization control antigen delivered by oral route, we found that the bactericidal antibody titers were similar or even higher than those achieved with the AbISCO TM-100® mixtures. These adjuvant-like and strong immunizing properties of the PorA molecule and its enhanced ability to overcome protease digestion during gastrointestinal transit have not been previously described. Additionally, these features suggest that the intrinsic properties of this protein, which go beyond the strong immunogenic characteristics of an antigen, are optimal not only for developing a single vaccine component but also for a potential use as an adjuvant with apparently no toxicity, for formulating various vaccines.
Results
Cloning, expression and purification of the rPorA protein from a Chilean meningococcal strain
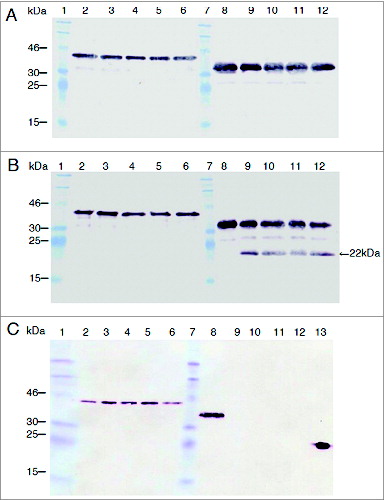
The porA gene was amplified by PCR using DNA from N. meningitidis B:15:P1.3 as the templateCitation45 and Pfu DNA polymerase (Stratagene). The strain has been annotated as B15: P1.7b,3, following the PorA VR type designation,Citation46 and the original PorA gene sequence has been depositedCitation45 at the GenBank as acc # L02929. This strain belongs to the clonal complex group ET-5.Citation47 The PCR product, as expected, was approximately 1.2 kb. This dA tailed fragment was cloned in pGEM-T/ DH5α cells and subcloned in the expression system pET21a/BL21 (DE3) (). With respect to the design, the clone lacked the initial 8 codons and the last 11 codons to avoid the assembly of PorA in the outer membrane; however, it included 21 bp of the pET21 polylinker region and 18 bp of additional nucleotides provided by the vector encoding a polyHis tail as the PorA C-terminus. The porA gene was sequenced and the corresponding sequence perfectly matched the one previously described.Citation45 The open reading frame was 1155 bp, close to the size of the wild-type sequence. These features promoted the PorA expression as inclusion bodies. Recombinant PorA (rPorA) was expressed in E. coli BL21 (DE3) cells after 2 hours of IPTG induction and was purified from inclusion bodies using a Ni-NTA-Sepharose gel column. Several 0.5 ml eluted fractions from the column were analyzed by SDS-PAGE () and Western blotting with anti-PorA rabbit antibodies (), revealing a single protein band of approximately 42 kDa. rPorA was additionally identified using a commercial (Novagen) monoclonal antibody against His-tag (data not shown). To eliminate E. coli LPS as a contaminant in the purified protein, a washing step with isopropanol before the final column elution was performed. Finally, an additional step for pyrogen removal (Detoxi-GelTM ) was included (see methods).
Figure 1. Cloning of the porA gene from N. meningitidis B15 P1.3 in the plasmid pET21a. A. PorA gene PCR amplification. The fragments were separated on a 1% agarose gel and stained with ethidium bromide. Lane 1 = λ/Hind III-digested molecular weight DNA standard. Lane 2 = PCR control reaction with a single PorA-1F primer. Lane 3 = control reaction with PorA-1R primer. Lane 4 = reaction with both primers and N. meningitidis chromosome as template. Lane 5 = λ/Bst II-digested DNA molecular weight standard (Gene Craft). B. Cloning of the amplified products by ligation to the pET21a plasmid. Lane 1 = λ/Bst II-digested DNA molecular weight standard. Lane 2 = pET21a-porA plasmid DNA without digestion. Lane 3 = pET21a-porA plasmid DNA under double digestion with Nde I and Hind III.
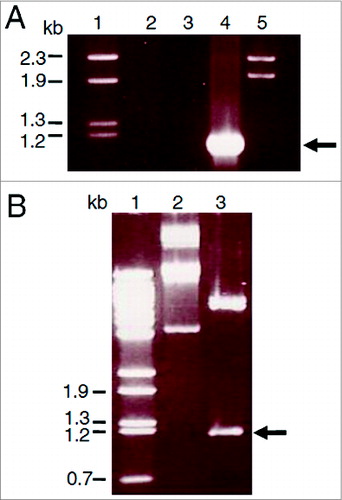
Figure 2. Purification of PorA recombinant protein from E. coli BL21 (DE3) culture using Ni-NTA column chromatography. A. Analysis of fractions by 10% SDS-PAGE. Lane 1 = Mark12TM unstained protein standard (Invitrogen). Lane 2 = total bacterial extract before purification. Lanes 3 to 10 = 2 ml fractions of purified rPorA eluted from a Ni-NTA column under denaturing conditions (8 M urea), as described in the methods section. B. Western Blot of the same purified rPorA fractions. Lane 1 = PageRulerTM Prestained Protein (Fermentas). Lane 2 = Total bacterial extract before purification. Lanes 3 to 10 = Fractions of purified rPorA.
Immunogenicity of the rPorA preparations in mice by oral route
For evaluating the specific IgG response against rPorA, suspensions containing 5 and 10 μg of the purified rPorA protein were mixed with 25 μg of AbISCOTM-100® adjuvant (expressed as micrograms of Quillaja saponins), following the instructions from the AbISCOTM manufacturer, and the response after the oral immunization of the mice was analyzed. Five groups of 8 mice each were formed: one group was immunized with 5 μg of rPorA mixed with AbISCO TM-100® as the adjuvant, another group was immunized with the same mixture but containing 10 μg of rPorA, 2 other groups were immunized with the corresponding amounts of the protein alone, and the last group was immunized with 25 μg of adjuvant (AbISCO TM-100®) alone. Pools of sera from each group were analyzed in triplicates.
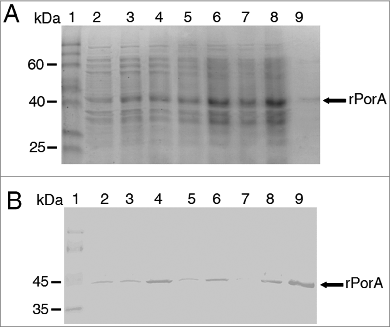
The mice immune response to the rPorA protein was initially analyzed by the IgG reactivity of the sera, using ELISA. Except for the control group immunized with the AbISCO TM-100® adjuvant alone, all experimental groups presented a significant increase in the level of IgG anti-PorA activity (, P < .05). The groups immunized using 10 μg of the rPorA protein with or without AbISCO TM-100® as adjuvant showed the highest serum titers compared with the corresponding groups immunized using 5 μg of the rPorA protein. This result demonstrated a PorA concentration-dependent response. We did not observe a significant difference (P < .05) between the formulations with or without AbISCO TM-100® as the adjuvant (). The integrity of the rPorA protein in these formulations before mice immunizations was verified by SDS-PAGE and Western blotting, and no degradation was observed (data not shown). An assay with the PorA protein administered by injection and aluminum as the adjuvant was not performed, because the purpose of the above experiment was to validate PorA as an adjuvant through the oral route. Different immunization routes induce different immunological responses,Citation48 rendering this comparative analysis more difficult to evaluate.
Figure 3. Anti-PorA antibodies responses to oral immunization in BALB/c mice using different rPorA formulations: PorA formulations rPorA 5 y 10 μg, AbISCO-PorA 5 y 10 μg. Immunization was evaluated in 8 groups of mice as described in the materials and methods section. The ELISA was performed as described in the materials and methods section.
Analysis of the predominant IgG subclass in mice immune sera raised against the rPorA protein
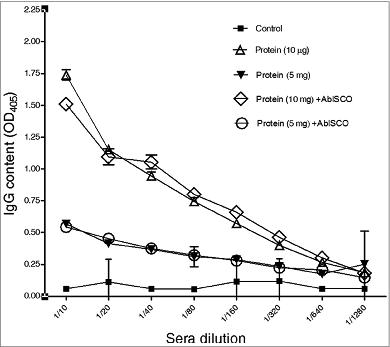
To evaluate whether the induced immune response of the mice against rPorA was class-specific IgG1 and/or IgG2a in the experimental groups, the Mouse Immunoglobulin Isotyping ELISA Kit assay was performed after being modified as described in the methods section. shows the distribution of IgG isotypes in all experimental groups. The IgG1 isotype () was significantly more predominant (P < 0.05) compared with IgG2a (). The formulation with 5 μg of PorA (with or without AbISCOTM -100®) induced the highest relative units of IgG1 compared with the formulation with 10 μg of rPorA. No significant differences (P < 0.05) were observed between the groups with or without AbISCOTM-100®.
Figure 4. Anti-PorA specific responses of IgG1 and IgG2a antibodies to immunization with different rPorA formulations. (A). Antibodies anti-IgG1 in different immunized group formulations: AbISCO-PorA 5 and 10 μg and rPorA 5 and 10 μg alone. The immunization was evaluated in 8 groups of mice, as described in the material and methods section. ELISA was performed as described in the materials and methods section. (B). Antibodies anti-IgG2a in different immunized group formulations with 5 and 10 μg of the recombinant protein AbISCO-PorA and 5 and 10 μg rPorA alone. ELISA was performed as described in the methods section. All values were expressed in relative units.
Secretion of IL-4 and IFN-γ cytokines in cultured splenocytes from immunized mice after in vitro rPorA pulse
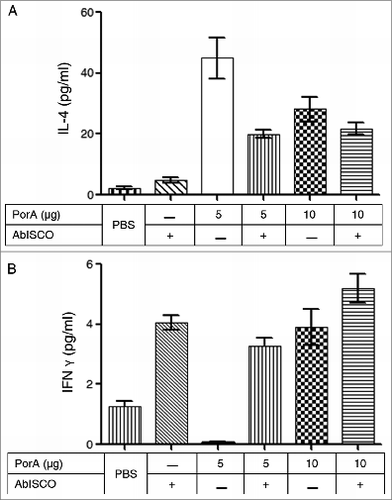
Cultured splenocytes were pulsed with 5 μg of the rPorA protein. IL-4 secretion was predominant over IFN-γ cytokine secretion in all experimental groups (). The secretions of IFN-γ in the same experimental groups was barely detectable. A significant increase of IL-4 secretion was observed in the experimental group immunized with 5 μg of the rPorA protein (, P < .05).
Figure 5. Secretion of interleukin from splenocytes in response to stimulation with rPorA in different experimental groups. (A). ELISA: IL-4 secretion in splenocytes stimulated with 5 μg of rPorA. (B). ELISA: IFN-γ secretion in splenocytes stimulated with 5 μg of rPorA. A significant increase in IL-4 secretion in the experimental group immunized with 5 μg of PorA protein was observed (P < .05).
Bactericidal activity of PorA-immunized mice sera
Detection of bactericidal activity in immunized sera strongly supports the notion that a relationship exists between the presence of complement-mediated bactericidal antibodies in the serum and protection against developing invasive meningococcal disease.Citation12 In general, a dilution titer ≥ 1:4 is accepted as a protective threshold titer in Europe, whereas a dilution titer ≥ 1:8 is used for the same criterion in the United States.
The ability of immunized mouse sera to promote in vitro complement-mediated killing of meningococci was tested via a standardized assay using our experimental groups. The bactericidal activity of the pools of murine sera from each group was determined in triplicates, against the same strain used as the source for the porA gene cloning. Human serum was used as an exogenous source of complement. We observed bactericidal activity for dilutions up to 1:8 dilution in all sera from each experimental group (). The experimental groups immunized with 5 μg of PorA showed the highest bactericidal activity (dilution serum = 1:16).
Table 2. Sequences of primers used in this work
Table 1. SBA titers of mice immunized with rPorA or rPorA + AbISCO*
Enhanced IgG antibody response against H. pylori HpaA antigen after the oral immunization of the mice with Lactococcus lactis expressing a PorA-HpaA hybrid construction
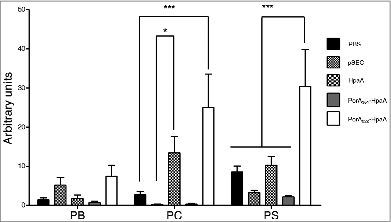
To demonstrate that PorA may actually perform as an adjuvant, 2 hybrid constructions obtained by the in frame ligation of different PorA gene segments to the gene encoding the H. pylori HpaA adhesin were used as oral immunization agents in the mice. One hybrid gene was formed by the segment encoding from Ser61 to Gly222 bound to the entire hpaA gene through its ATG start codon. Additionally, the other hybrid covering from Asp1 to Gly351 was bound to the complete hpaA gene. Each hybrid was separately inserted into the pSEC vector and transferred to L. lactis by electroporation. The expression of both hybrids was confirmed using SDS-PAGE and Western blotting. Five immunization groups of 7 mice each were formed: group 1 was treated with PBS, group 2 with L. lactis carrying only the pSEC vector, group 3 with L. lactis harboring the pSEC-HpaA plasmid, group 4 with L. lactis expressing the pSEC PorA351-HpaA hybrid, and group 5 with L. lactis expressing the PorA222-HpaA hybrid. Each L. lactis immunization dose was approximately 1 × 10Citation7 UFC per mice and each mouse received 6 doses during the immunization protocol. The mice immunized with the PorA222-HpaA hybrid induced 3 fold more anti-HpA antibodies compared with the mice immunized with the HpaA antigen alone (). In contrast, the hybrid PorA351-HpaA did not induce a comparable level of antibodies. In addition, the IgG immune response against HpaA was not enhanced in the L. lactis strain carrying only the pSEC empty plasmid, indicating that this increased antibody response was due to the presence of PorA222 in the fused fragment, which was acting as an adjuvant.
Figure 6. Humoral response of IgG anti-HpaA in mice after oral immunization with Lactococcus lactis strain expressing a PorA-HpaA hybrid driven by nisin-inducible promoter in the pSEC plasmid. Antibodies (as arbitrary units) were determined through ELISA using purified HpaA antigen bound to the plate wells. Five groups of 7 mice each were immunized with PBS, L. lactis carrying only the pSEC vector, L. lactis expressing H. pylori HpaA antigen, the PorA351-HpaA hybrid and the PorA222-HpaA hybrid. The antibodies were evaluated at different stages during the immunization protocol: PI = previous to primary immunization; PB = previous to boosting (week 3), PC = previous to challenge (week 6) and PS = previous to sacrifice (week 9). The PtdIns values were subtracted from the other samples. (*) P < 0.05 and (***) P < 0.001.
Altogether, these results demonstrated that rPorA and PorA covalently bound to the non-related HpaA antigen could perform as adjuvants when orally administered in mice, either as the purified recombinant protein or when expressed in L. lactis.
In vitro PorA resistance against gastrointestinal tract proteases, in comparison with PorB adjuvant
Because N. lactamica PorB has been defined as a potential adjuvantCitation49 and to compare the usefulness of PorA as an oral adjuvant, we submitted the PorA and PorB proteins to in vitro protease degradation for comparing their stability properties. The purified PorA and PorB recombinant proteins were incubated with pepsin, trypsin and chymotrypsin for 16 min at 1.2/1, 1/50 and 1/20 protease/substrate mass ratios, respectively. To evaluate the protease susceptibility of the antigens, the PorA and PorB protease-treated products were separated by SDS-PAGE, and the entire protein and fragments containing the C-terminus His-tag region were detected using Western blotting with a commercial anti-His6 antibody (). However, this method is unable to detect protease cuts located close to the N-terminus, because the release of a few amino acids will not alter the electrophoretic migration of the digested antigen. PorA stood undigested for at least 16 min when submitted to these 3 proteases under the assay conditions. In contrast, PorB incubated with trypsin gave a 22 kDa intermediate product detectable after a 4-min incubation, and after the incubation with chymotrypsin, no His-tag was detected, indicating early PorB degradation. However, PorB was not degraded by pepsin under the assay conditions, but incubations longer than 20 min gave 15 kDa and 22 kDa products (not shown). These results indicate that PorA, unlike PorB adjuvant, has the potential to be used directly as an oral adjuvant or as a hybrid protein fused in frame to the N-terminus of a particular antigen and expressed in L. lactis.
Figure 7. PorA resistance to protease digestion compared with that of PorB. In vitro incubations were performed with pepsin (A), trypsin (B) and chymotrypsin (C) in the final volume of 300 μl. Twenty-five microliter of rPorA at 0, 4, 8, 12 and 16 min (lanes 2 to 6) and PorB (same times, lanes 8 to 12) were collected and analyzed through SDS-PAGE and Western blotting using His-tagged PorA and PorB proteins. The protease/substrate ratio were 1.2/1 (pepsin), 1/20 (trypsin) and 1/50 (chymotrypsin). The active enzyme added was approximately 0.3 units per assay. The MW for rPorA and rPorB were 42 kDa and 38 kDa, respectively. A single 22 kDa trypsin degradation product was observed for PorB (panel B, lanes 9-12) but none for PorA. PorB was degraded early and completely by chymotrypsin (panel C, lanes 9-12). The relevant bands of Prestained protein standard (Fermentas) are indicated. A positive control reaction (3 ng of His-tagged VP2 protein from the Infectious Pancreatic Necrosis Virus) was included (panel C, lane 13).
Discussion
The development of an effective vaccine against meningococcus serogroup B continues to be a challenge for researchers, and the prevention of meningococcal infection around the world depends on the availability of an appropriate vaccine.Citation12,50
Until now, several proteins from the outer membrane of meningococcal B have been tested using different vaccine strategies. Among these proteins, PorA,Citation28,34,39,50-52 NspA,Citation53 Transferrin-binding proteins,Citation54,55 LP 2086,Citation56 GNA 1870,Citation15 GNA 2132Citation57 and NadACitation58 are the most studied.
PorA protein is a compelling potential antigen for the development of a meningococcal B vaccine; it has been described as a major target for bactericidal antibodies in patients suffering from invasive meningitis disease,Citation59 as well as in experimental OMV vaccines.Citation34,35,60
Nevertheless, the application of PorA-based vaccines has limitations. Due to the wide variability of PorA epitopes located at the VR1 and VR2 regions, a reduced spectrum of bactericidal antibody cross-reactivity can be expected for a particular PorA subtype. To obtain a wider coverage, vaccine strains carrying 6 (HexaMen) and 9 (NonaMen) different PorA serosubtypes have been designed.Citation26,31 Additionally, it has been estimated that NonaMen vaccine will probably cover 75% of the serogroup B strains present worldwide.Citation30 However, this will not be enough to protect from hundreds of PorA serosubtypes existing around the world.Citation27 However, a tailor-made vaccine designed to protect from a particular PorA subtype strain has been successfully used to control a particular epidemic outbreak in New Zealand.Citation22
In this study, the porA gene was cloned from a meningococcal B clinical isolate using PCR. The amplified product was ligated to the pET21a plasmid without the last 11 codons, to avoid the assembly of the protein in the outer membrane, and without the 8 initial codons of the signal peptide, favoring the PorA expression in E. coli cytoplasm as inclusion bodies. In addition, the normal translation stop codon of the gene was removed, and a His-tag coding region and a new stop codon were provided by the vector to facilitate protein purification. The rPorA protein was expressed in E. coli cells as inclusion bodies and purified under denaturing conditions (8 M urea), to obtain a soluble protein. Urea was removed by dialysis, and rPorA was purified and isolated as a trimeric structure after refolding, as described by others.Citation34,40 The conformation of the soluble rPorA as a trimer does not necessarily represent a correctly assembled structure as in the native protein, after insertion into the outer membrane of N. meningitidis. Native PorA folding is assisted by Omp85, a periplasmic assembly factor.Citation61 However, in E. coli cells during heterologous expression of OMPs, YaeT, a corresponding E. coli Omp85 counterpart is available at the periplasm,Citation62 but it may not be able to render the native folded structure for rPorA. It has been suggested that the role of YaeT in the assembly of Neisseria OMPs is not through lipid biogenesis, as debated for Omp85 in Neisseria, thus explaining the limited assembly function of the YaeT Omp85 homolog. In addition, this Omp85 interaction, which is required for PorA folding, presents species specificity, explaining the inefficient assembly of OMPs from Neisseria in E. coli. In this aspect, the in vivo assembly of the Neisserial porin rPorA into the E. coli outer membrane has been accomplished after adapting its signature sequence, demonstrating that the Omp85 assembly machinery recognizes OMPs by virtue of their C-terminal signature sequence.Citation63
We suggest that in vitro refolded rPorA monomer may have a different 3D structure but is still capable of adopting a trimeric structure and withstanding the traffic through the gastrointestinal system of mice. In this regard, it has been reported that in vitro folded trimeric PorA is resistant to 2% SDS and low pH conditions.Citation64 In addition, we tested its integrity after trypsin incubation, observing that rPorA is additionally resistant to 50 μg/ml of protease for 20 min. These findings suggest that rPorA may be able to resist passage along the gastrointestinal system after oral administration. We propose that refolded PorA may expose strongly immunogenic linear or conformational epitopes to the immune system, thus explaining its remarkable serological response after immunization without an adjuvant. However, this proposal demands further analysis.
We partially characterized the immune response and observed that all experimental mice groups presented a serological response of increased rPorA-specific IgG. Additionally, we analyzed the secretion of cytokines in splenocytes pulsed with rPorA and observed a predominant enhancement of IL-4 over IFN-γ in all experimental groups, indicating that oral immunization with PorA (with or without adjuvant) induces a cytokine pattern corresponding to a Th2-type response. Accordingly, we believe that a Th2 response may be the most effective in controlling and protecting against Neisserial infection. The in vitro measurement of IL-4 showed a cellular response against PorA by oral immunization, suggesting the induction of an effective mechanism against N. meningitidis. Isotype antibody identification corroborates the results of the in vitro assays, exhibiting the predominance of IgG1 over IgG2a in the immunized sera. Furthermore, the predominance of IgG1 indicates that a cellular response induced by PorA stimulates an isotype switch to IgG1 production, a common bactericidal isotype.Citation65 This finding is compelling because rPorA is capable of inducing a systemic Th2 response following oral immunization.
Residual E. coli LPS bound to rPorA might explain the adjuvant properties described here for rPorA. However, rPorA was expressed in E. coli as inclusion bodies accumulated at the cytoplasm, and subsequently, the protein was purified, solubilized and renatured after dialysis, with decreasing amounts of urea. Therefore, rPorA may have had little chance to bind E. coli LPS in vivo during protein transit to achieve outer membrane assembly. However, LPS could be captured as a contaminant after bacterial lysis required for rPorA purification. Nevertheless, the LPS level in our rPorA preparations was approximately 100 units of endotoxin/ml, as measured using the Limulus Amebocyte Lysate (LAL) kit (results not shown). Moreover, this amount of LPS in our rPorA preparations was unable to induce IL-4 secretion in the total splenocyte culture assay.
Our results contrast with those reported by Peeters et al.,Citation38 regarding the ability of PorA to induce bactericidal antibodies after the oral immunization of mice. They described that after testing various presentation forms of N. meningitidis PorA in mice, this protein (up to 10 μg per dose), using either AlPO4 or QuilA as adjuvant, was unable to induce bactericidal antibodies when used for subcutaneous immunization. They additionally reported that bactericidal antibodies were produced with either the OMV of N. meningitidis B strain or PorA-ISCOM formulations. Additionally, only PorA folded in its native conformation, inserted in OMV or liposomes, could induce bactericidal antibodies.Citation34 These results contrast with ours, indicating that purified rPorA administered via oral route is able to induce bactericidal antibodies. We propose that the route of administration, a well-known factor in vaccine efficacy, or the PorA itself (a different strain was used as the PorA source in the other reportCitation34) possibly accounts for the differences observed in these reports.
We did not include injected PorA as a control in our assays, as part of the comparative studies on PorA as an antigen administered by different routes. Using this control could have increased our understanding of the immune responses in these cases. The lack of this control does not allow comparison studies when using different immunization routes.
Finally, it was clear that when using the PorA222-HpaA hybrid expressed in L. lactis, administered by oral route, the PorA moiety of the hybrid behaved as an adjuvant, increasing the level of anti-HpaA antibodies 3 times. The bactericidal activity of the antibodies induced by the PorA-HpaA hybrid was not tested, because it has not been reported that HpaA antigen alone is capable of inducing mice antibodies with the ability to lyse H. pylori cells.
We did not test the ability of the PorA222 fragment to induce bactericidal antibodies, because PorA222 lacks the VR1 region, and VR1, as well as the VR2 regions are primarily involved in the induction of bactericidal antibodies for the strain B15:P1.7,16;Citation26 therefore, we anticipated that the ability to induce bactericidal antibodies against PorA may be reduced.
Altogether, the above results and the bactericidal activity detected in all experimental mice groups support the possibility that PorA may function as a strong adjuvant, particularly rPorA, and may be a promising antigen in vaccines against N. meningitidis B. It should be noted that the detection of antibodies with bactericidal activity is the gold standard technique for measuring protection against meningococcal B bacteria.Citation34,51,66 The titers of bactericidal activity were between 1:8 and 1:16, surpassing the accepted values for protective bactericidal activity titers in Europe (≥1:4) and in the US (≥1:8).Citation67
In summary, these data support the use of rPorA as an adjuvant and in the development of a novel meningococcal B oral vaccine. The next-generation vaccine NonaMen induced high SBA titers against all tested MenB strains, when administered subcutaneously in rabbits and mice, regardless of whether aluminum phosphate was used as an adjuvant,Citation31 suggesting that an adjuvant was not necessary.
Methods
Bacterial strains and growth conditions
Group B N. meningitidis, strain B:15:P1.3, obtained from the meningococcus reference laboratory of the Instituto de Salud Pública (ISP-Chile) as strain M-225/2003, was grown on proteose peptone agar at 37°C for 18 h in a humid atmosphere containing 5% (v/v) CO2. E. coli BL21 (DE3), used for expressing the recombinant PorA protein, was grown in Luria-Bertani (LB) medium with 100 μg/ml ampicillin. The plasmids were maintained in E. coli DH5α grown in the above LB medium, as well as on LB-agar plates with the same amount of ampicillin. Lactococcus lactis NZ9000 containing pSEC plasmid and its derivatives was grown for 16 hours in M17 medium at 37°C with 10% CO2, 0.5% glucose and 30 μg/ml chloramphenicol. For plasmid transfer, all bacteria were electroporated under standard conditions (0.2 cm electroporation cuvettes, with an electric field of 2000 volts/cm, solution resistance adjusted to 400 ohms and 25 μF capacitance), in a BioRad (Hercules, California, USA) Gene Pulser device.
Cloning of porA and porB genes in a plasmid expression system
The porA gene was obtained via PCR, using cell lysate from the strain N. meningitidis M-225/2003 as the DNA source, Pfu DNA polymerase and PorAF and PorAR as the primers. Both primers (sequences displayed in ) were designed using the PorA gene sequence described by Saunders et al.Citation45 PCR included the following steps: 94°C for 15 sec, 55°C for 30 sec and 68°C for 90 sec, over 30 cycles. The amplified porA gene included NdeI and HindIII restrictions sites at its 5′ and 3′ ends, respectively, as they were introduced into the primer sequences. The PCR product was dA-tailed with Taq polymerase and ligated into the pGEM-T vector and, after electroporation, replicated in E. coli DH5α. The amplicon was further identified by DNA sequencing. The porA gene was subcloned into the pET21 expression vector by ligation to the NdeI and HindIII vector sites and then transformed into E. coli DH5α for plasmid storage and into E. coli BL21 (DE3) for expression studies.
The porB gene was also amplified from the Chilean strain B:15:P1.3 (strain M225-2003, ISP-Chile) by PCR, using Pfu polymerase and PorBF and PorBR2 as primers (), and ligated into pGEMT-Easy, following dA-tailing at its 3′ends. Next, the NcoI-XhoI gene insert was released and ligated to pET21d for expression in E. coli BL21 (DE3). The forward primer included an NcoI site, and the PorB signal peptide and reverse primer contained an XhoI site in frame to the 3′ end of the porB gene to add the His-Tag provided by the vector. After 0.5 mM IPTG induction for 4 hours, the PorB expression was verified through SDS-PAGE using a 12% gel, followed by Western blotting with rabbit anti-His-Tag as the primary antibody (1:1000 dilution) and goat anti-rabbit HRP (1:1000 dilution) as the secondary antibody. The nitrocellulose membrane was analyzed using AP Conjugate Substrate kit (BioRad). DNA sequencing revealed 99% identity to the porB gene from the fully sequenced strain MC58.
Construction of PorA hybrid genes
Hybrids were designed with the PorA moiety at the N-terminus and HpaA carrying the C-terminus. The N-terminus of PorA222 and PorA351 sub-fragments for hybrid constructions were amplified using purified the previously obtained pET21a-PorA plasmid as the template (100 ng per assay). For PorA222 amplification, the primers used were PorAF222 and PorAR222. The primer sequences are presented in . The PCR reactions (50–100 μl final volume) were performed with GoTaq DNA polymerase, following standard assay conditions as described by the manufacturer. The thermocycling conditions were as follows: Initial denaturation step for 1 min at 94°C; 35 cycles with denaturation (1 min at 94°C), annealing (2 min at 57°C) and elongation (3 min at 72°C) steps; and a final elongation (10 min at 72°C). The amplicons were ligated to pGEMT-Easy, transferred by electroporation to the E. coli cells, released by NcoI and XhoI digestions and ligated to the NcoI/XhoI-digested pSEC-HpaA plasmid. The pSEC plasmids were transferred to L. lactis by electroporation. Both constructions lacked signal peptides but were in frame with the signal peptide provided by the pSEC plasmid for expression in L. lactis.
Expression and purification of recombinant PorA (rPorA) protein
PorA for the immunization studies was purified from a single bacterial colony of E. coli BL21 (DE3) containing the pET21a-porA plasmid. The colony was inoculated into LB-ampicillin medium and incubated overnight at 37°C with shaking (220 rpm). A fresh LB medium was inoculated using a 1:100 dilution of an overnight culture and incubated as described above, until an OD600 of 0.4–0.5 was reached. PorA expression was then induced using 0.5 mM IPTG (isopropyl-β-D-thiogalactoside) and by incubating further for 2 hours at 30°C with shaking at 220 rpm. The cells were harvested by centrifugation (10,000 rpm, Sorvall RC2B) and stored at −20°C.
The recombinant PorA contained an additional C-terminal His tag to allow purification through a Ni-NTA column. The protein was purified under denaturing conditions, according to the instructions of the manufacturer (Invitrogen, cat K850-01). Before eluting PorA, E. coli LPS-bound protein contaminations were eliminated by washing the column with isopropanol, following the guidelines indicated by Kees et al.Citation68 Next, 2 ml fractions were collected and analyzed by SDS-PAGE and stained with Coomassie blue, and rPorA was identified by Western blotting, using custom-made rabbit polyclonal or anti-His6 monoclonal antibodies against PorA. To remove urea, the PorA-containing fractions were pooled and dialyzed overnight at 4°C with 10 mM Tris HCl, pH 8.0 containing 0.1% Triton X-100. Finally, a Detoxi-GelTM Endotoxin Removing Gel column (Thermo Scientific, Waltham, MA 02451 USA) was used for pyrogen removal. The protein concentration in the fractions was determined using the Bradford method.Citation69
For protease degradation studies, PorA was obtained from 2 liters of PorA-expressing cultures (split in 4 one-liter flasks), using the procedure described previously, under denaturing conditions (8 M urea) during Ni-NTA column elution.
PorB purification for protease degradation studies
PorB was purified under denaturing conditions, using an Ni-NTA column (Invitrogen). Elution was performed with 250 mM imidazole, according to the instructions from the vendor with some modifications. Briefly, 2 liters of culture split in 4 flasks (LB medium, 100 μg/ml ampicillin) were inoculated (1:100), cultured overnight, grown until the OD600 reached 1.1-1.4 and induced with 0.5 mM IPTG for 4 hours. The cells were washed (0.16 M NaCl) and separated by centrifugation (Beckman J21, rotor J10, 4000 rpm, 4°C) for 30 min. The pellet was suspended in 8 ml of 0.15 M NaCl and washed again. The pellet was re-suspended in 20 ml of buffer 1 (8 M urea, 0.01 M TrisHCl, 0.1 M NaH2PO4, 1% Triton X-100, pH 8.0), split into 3 15-ml Falcon tubes and sonicated for 22 min at 40% power and 70% pulse frequency on ice (small probe, Omni sonicator, model Omni-Ruptor 250, Kennesaw, GA 30144, USA), until a clear lysate was observed. This lysate was filtered through a 0.2 μm GyroDisc CA-PC filter and kept frozen at −20°C, if necessary. Twenty milliliters of lysate were mixed with 2 ml of Ni-NTA-agarose and incubated at room temperature for 10 min. Next, a 5-ml syringe with glass fiber at the bottom was loaded with 2 ml of resin. The column was washed with 10 ml buffer 1, 10 ml buffer 2 (buffer 1 containing 20 mM imidazole) and 10 ml of buffer 3 (buffer 2 adjusted at pH 6.3), and the bound protein was eluted with 10 ml of buffer 4 (buffer 3 containing 250 mM imidazole). The elute was collected in 0.5 ml fractions, and 20 μl aliquots of each were analyzed using SDS-PAGE, as described for PorA. The fractions with higher amount of PorB were pooled and kept frozen at −20°C, until use.
Immunization of animals
BALB/c (HdCitation2) female mice (6–8 weeks old) used for the immunization studies with the purified PorA were provided by the Instituto de Salud Pública de Chile (ISP). The animals were housed under controlled conditions (12/12 h photoperiod) at 22°C and received water and food ad libitum. The BALB/c mice used for the oral immunization with PorA-HpaA hybrids were provided by the animal facilities of the Faculty of Biological Sciences (Pontificia Universidad Católica de Chile). In both cases, the animals were treated and sacrificed following guidelines of the Guide for the Care and Use of Laboratory Animals, National Research Council, Canada, as recommended by the institutional Bioethics and Biosafety Committees of the sponsor institutions. The immunization protocols were previously approved by these committees.
Immunizations with purified rPorA and with rPorA mixed with AbISCO TM-100® were performed. Groups of 8 mice of 6–8 weeks of age were orally immunized by gavages (0.5 × 19 mm in diameter) with 5 and 10 μg of rPorA mixed with 24 μg of AbISCO TM-100® adjuvant in a final volume of 200 μl per dose. The AbISCO TM-100® adjuvant was obtained from Isconova AB, Uppsala, Sweden. The AbISCO TM-100® adjuvants were saponin-containing ISCOM-Matrix preparations.Citation70
Group 1 was immunized with a mixture of 5 μg of the rPorA protein combined with 50 μL of AbISCO TM-100® (containing 0.48 mg/ml of Quillaja saponins), group 2 with 5 μg of rPorA alone, group 3 with a mixture of 10 μg of rPorA and 50 μl of AbISCO TM-100® and group 4 with 10 μg of the rPorA protein alone. Group 5, the control group, was immunized with preparations containing AbISCO TM-100® alone, suspended in PBS (136 mM NaCl, 1.5 mM KH2PO4, 10 mM Na2HPO4, 2.7 mM KCl, pH 6.8). All immunizations were performed on days 1, 3, 5 and 20. Blood samples were taken before the first immunization and on day 28. Simultaneously, splenocytes were obtained, and IL-4 and IFN-γ secretion were determined. All sera were stored at −20°C. This study complied with the animal care and animal experimental guidelines of the ISP Ethics and Biosafety Committee.
Oral immunizations using Lactococcus lactis expressing PorA fused to the N-terminus of HpaA, a Helicobacter pylori antigen
The protocol was adapted from Gu et al.Citation71 and Scavone et al.Citation72 Five groups of 7 female BALB/c mice each, aged 7–9 weeks, were separated as follows: Control group 1, PBS group orally immunized with 6 doses of 200 μl of buffer (PBS:7.5% NaHCO3 in 4:1 ratio); Control group 2, pSEC group immunized with L. lactis carrying only the pSEC cloning vector lacking the nisin promoter, with 3 primary daily doses (approximately 1- 5 × 10Citation9 CFU/mice) given in the first week and 3 similar booster doses one days 15, 16 and 17; HpaA control group 3 immunized with L. lactis expressing the H. pylori HpaA antigen cloned in pSEC and induced with 10 ng/ml nisin; PorA351-HpaA hybrid group 4 immunized with L. lactis expressing the larger hybrid cloned in pSEC; and PorA222-Hpa hybrid group 5 expressing the smaller hybrid. All oral immunizations, except group 1, were performed using the L. lactis grown under the same conditions, induced with nisin, suspended in a solution of PBS:7.5% NaHCO3 (4:1), administered in a volume of 200 μl using an orogastric Tygon tubing. At week 6, after the primary immunization, mice were challenged with H.pylori SS-1 (a 200μl single 1 × 10Citation8 CFU/mice dose), and at week 9, the mice were sacrificed by cervical dislocation. Blood samples were taken at days 1, 14, 41 and 62.
Evaluation of serum IgG immune response against rPorA by ELISA
Pools of murine antisera from each experimental group were analyzed using ELISA to test anti-PorA antibodies. The ELISA plates were coated by incubating with 1 μg of rPorA for 1 h at 37ºC or overnight at 4°C. The plates were blocked with 1% BSA-PBS for 1 h at 37°C. The plates were washed with PBS-0.02% Tween 20 solution, and the mice sera were added to the wells in triplicates at 2-fold consecutive dilutions in 1% BSA-PBS solution. The plates were washed after incubating for 1 h at 37°C. Next, for detecting bound IgG, alkaline phosphatase-conjugated goat polyclonal antibody against mouse IgG (SIGMA, St. Louis, MO 63103) (diluted 1:1000 in 1% BSA-PBS) was added and incubated at 37°C for 1 h. The alkaline phosphatase activity was detected by incubating with 100 μl of 1 mg/ml of p-nitrophenyl phosphate in carbonate buffer (pH 9.8). The reaction was stopped by adding 50 μl of 3 M NaOH, and the absorbance was read at 405 nm in an ELISA plate reader. The ELISA data for sera IgG were expressed relative to the absorbance obtained for the control mice group.
Evaluation of serum IgG immune response against H. pylori HpaA by ELISA
The IgG anti-HpaA antibodies induced by the oral administration of L. lactis expressing the HpaA antigen were determined in 96-well ELISA plates. Each well was activated by coating with 100 μl solution containing 500 ng of purified His-tagged HpaA antigen by a 17-hour incubation at 4°C in rotary shaker. Next, after 3 washes with PBS, the plate was blocked with 200 μl PBS-1% BSA solution per well for 1 hr at room temperature with shaking. The plates were washed 3 times as described previously. A 100 μl volume of each mouse serum was added at 1:64 dilution (in PBS-1% BSA solution) and the plates were incubated at 37°C for 2 hours and washed 3 times, as described previously. Next, 50 μl of the secondary anti-mouse IgG antibody conjugated to HRP (1:3000 dilution in PBS-1% BSA) was added and incubated for 1 h at 37°C. The plates were washed as described previously. The reaction was detected using a mixture of 1 mg/ml TMB (in DMSO) diluted 1:10 in 35 mM citrate/67 mM phosphate buffer, pH 5.0 and a 1:1000 dilution of 30% v/v H2O2. Fifty microliters of this mix were added per well, and the plate was incubated for 10 min at 25°C. The reaction was stopped with 100 μl 4N H2SO4 per well. The plate was read at 450 nm. To obtain a standard curve, an anti-HpaA polyclonal antibody (GrupoBios-Chile) was used in a serial dilution starting from 1:1000 to 1:28000. For simplicity, the first point in the curve was given the value of 1000 arbitrary units. The standard curve was adjusted to a log equation, which was then applied to analyze the samples. To facilitate comparison, the pre-immune values were subtracted from all sample absorbance values.
ELISA analysis of IgG1 and IgG2a from mice sera antibodies against rPorA
The analysis of specific IgG1 and IgG2a directed against rPorA were performed using a modification of Mouse Immunoglobulin Isotyping ELISA Kit (#550487 BD PharMingen, San Diego, CA, USA). Briefly, the ELISA plates were coated overnight with 1 μg of rPorA and blocked for 30 minutes with 1% BSA at room temperature. The experimental animal sera (pool of serum from each experimental group) were diluted 1:50 in 1% of BSA, and 100 μl were added to the wells in triplicates. The plates were washed with the PBS-0.05% Tween 20 solution, and 50 μl of either the mouse monoclonal antibody against IgG1 or that against IgG2a were added (#550487 BD PharMingen, San Diego, CA, USA) and incubated for 1 h at room temperature. The wells were washed and incubated with the HRP-conjugated goat polyclonal antibody against mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA 95060, USA) diluted 1:200 in 1% BSA at 37°C for 1 h. HRP was detected by incubation at room temperature with the reagents available in the BD kit. The absorbance was read at 450 nm. The ELISA data of both IgG1 and IgG2a sera were analyzed in triplicates and expressed relative to the absorbance values obtained in the mice experimental control group.
Measurement of IL-4 and IFN-γ secretion in splenocytes
Intact splenocytes were obtained from immunized and sacrificed mice. After erythrocyte lysis with 0.15 M NH4Cl pH 7.4 buffer, the splenocytes were seeded in 1 ml RPMI 1640 medium (Thermo Fisher Scientific, Waltham, MA 02451 USA) in 24-well flat-bottom plates in triplicates, at a concentration of 5 × 10Citation6 cells/ml. The cells were pulsed with 5 μg of rPorA and incubated for 72 h (37°C and 5% C02). IL-4 and IFN-γ were evaluated in the cell culture supernatants using ELISA, with Quantikine kit (R&D System, Minneapolis, MN 55413, USA), specific for each cytokine, according to the manufacturer's protocol.
Serum bactericidal assays
Serum bactericidal activity assays were performed by a standard method using human serum as complement source, at biosafety level 2 facilities in the meningococcal reference laboratory of the ISP. The serum bactericidal activity (SBA) titers obtained at the lowest dilution to kill ≥ 50% of the target strain were recorded as described.Citation73
Statistical analysis
All PorA data were analyzed using Prism software (GraphPad Software Inc., San Diego, CA). The anti-HpaA IgG mice response was statistically analyzed by ANOVA followed by the multiple comparison Bonferroni test. The statistical significance was defined as a P value of less than 0.05.
Conclusion
In conclusion, this is the first report to describe an oral immunization with rPorA that induced protective bactericidal antibodies with or without an adjuvant. In addition, when PorA is used as a fused protein bound to a non-related antigen and delivered by L. lactis probiotic, it induced an enhanced IgG response against the H. pylori antigen after oral immunization. Finally, our results with rPorA as a potential adjuvant are similar to those reported for Neisseria PorB porin;Citation74 however, regarding this last report, the PorB administration routes are different, because this protein has been administered by subcutaneous (s.c.)Citation74 or intra-peritoneal (i.p.)Citation75 routes. Nevertheless, our data support PorA behavior as a potential oral adjuvant. PorB has been extensively studied at the structural and functional levels,Citation76 and its mechanism as an adjuvant involves TLR2/TLR1 hybrid recognitionCitation77 and enhancing cell survival and continued activation through mitochondrial interactions, protecting these cells from apoptosis.Citation78 We compared the resistances of PorA and PorB against known gastrointestinal proteases and observed that PorA performed better than PorB when both porins were submitted to proteolytic degradation. Although differences in their mechanism as adjuvant molecules have been established, a TLR molecule involved in PorA binding has not been described yet. These PorA features strengthen the idea that this protein may be a new and interesting adjuvant molecule, useful to improve orally administered vaccines. Currently, our group is actively working to define the putative TLR involved in the recognition of this antigen by the innate immune system.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors’ Contributions
Conception and design of the experiments: AEV, AV; performance of PorA and PorB cloning and expression experiments: AEV, AA, EB, MM and RM; purification of proteins and protease degradation assays: AA, MJB, and JHT; PorA hybrid constructions and transfer to pSEC vector: MM; expression of PorA hybrid in L. lactis: MM and EB; mice immunization assays: RM, EB, DS, MM and JHT; bactericidal assays: AEM; statistical analysis and manuscript writing: AEV, PRH and AV.
Acknowledgments
We acknowledge Claudio Figueroa, Bárbara Riveros and América Abarca from the ISP laboratory for helpful discussions and technical assistance and Shelton Wright and Hans Jensen for critical review of the manuscript.
Funding
This research was funded by FONDEF DO2I-1067 (CONICYT-CHILE), FONDECYT 1085232 (CONICYT-CHILE) and BMRC CTU-06 Area 5 (Pontificia Universidad Católica, Laboratorio Recalcine, CONICYT-CHILE), granted to A. Venegas and an institutional grant to A. E. Vásquez from the Instituto de Salud Pública de Chile.
References
- Rosenstein NE, Bradleya MD, Perkins MD, Stephens DS, Popovic T, Hughes MD. Meningococcal Disease. N Engl J Med 2001; 344:1378-88; PMID:11333996; http://dx.doi.org/10.1056/NEJM200105033441807
- Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009; 27 (Suppl. 2):B51-63; PMID:19477562
- Swartley JS, Marfin AA, Edupuganti S, Liu LJ, Cieslak P, Perkins B, Wenger JD, Stephens DS. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci USA 1997; 94:271-6.; PMID: 8990198
- Halperin SA, Bettinger JA, Greenwood B, Harrison LH, Jelfs J, Ladhani SN, McIntyre P, Ramsay ME, Sáfadi MA. The changing and dynamic epidemiology of meningococcal disease. Vaccine 2012; 30 Suppl 2:B26-36; PMID:22178525; http://dx.doi.org/10.1016/j.vaccine.2011.12.032
- Snape MD, Pollard AJ. Meningococcal polysaccharide-protein conjugate vaccines. Lancet Infect Dis. 2005; 5:21-30; PMID:15620558; http://dx.doi.org/10.1016/S1473-3099(04)01251-4
- Girard MP, Preziosi M-P, Aguado M-T, Kieny MP. A review of vaccine research and development: Meningococcal disease. Vaccine 2006; 24:4692-700; PMID:16621189; http://dx.doi.org/10.1016/j.vaccine.2006.03.034
- Richmond P, Borrow R, Goldblatt D, Findlow J, Martin S, Morris R, Cartwright K, Miller E. Ability of 3 different meningococcal C conjugate vaccines to induce immunologic memory after a single dose in UK toddlers. J Infect Dis 2001; 183:160-3; PMID:11078484; http://dx.doi.org/10.1086/317646
- Pichichero M, Casey J, Blatter M, Rothstein E, Ryall R, Bybel M, Gilmet G, Papa T. Comparative trial of the safety and immunogenicity of quadrivalent (A, C, Y,W-135) meningococcal polysaccharide-diphtheria conjugate vaccine versus quadrivalent polysaccharide vaccine in two- to ten-year-old children. Pediatr Infect Dis J 2005; 24:57-62; PMID:15665711
- Pace D, Pollard AJ, Messonier NE. Quadrivalent meningococcal conjugate vaccines. Vaccine. 2009; 27 Suppl 2:B30-41; PMID:19477560; http://dx.doi.org/10.1016/j.vaccine.2009.05.003
- Griffiss JM, Yamasaki R, Estabrook M, Kim JJ. Meningococcal molecular mimicry and the search for an ideal vaccine. Trans R Soc Trop Med Hyg 1991; 85(Suppl 1):32-6; PMID:1725072
- Zimmer SM, Stephens DS. Serogroup B meningococcal vaccines. Curr Opin Investig Drugs 2006; 7:733-9; PMID:16955685
- Panatto D, Amicizia D, Lai PL, Gasparini R. Neisseria meningitidis B vaccines. Expert Rev Vaccines 2011; 10:1337-51
- Santolaya ME, O'Ryan ML, Valenzuela MT, Prado V, Vergara R, Muñoz A, Toneatto D, Graña G, Wang H, Clemens R., et al., Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled study. Lancet 2012; 379: 617-24; PMID:22260988; http://dx.doi.org/10.1016/S0140-6736(11)61713-3
- Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, Evans A, Telford KL, Ypma E, Toneatto D, et al., Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant Meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis 2010; 51:1127-37; PMID:20954968
- Pizza M, Scarlato V, Masignani V, Giuliani MM, Aricò B, Comanducci M, Jennings GT, Baldi L, Bartolini E, Capecchi B, et al. Identification of vaccine candidates against serogroup B by whole-genome sequencing. Science 2000; 287:1816-20; PMID:10710308; http://dx.doi.org/10.1126/science.287.5459.1816
- Moe GR, Dave A, Granoff DM. Molecular analysis of anti-N-propionyl Neisseria meningitidis group B polysaccharide monoclonal antibodies. Mol Immunol 2005; 43:1424-31; PMID:16140379
- Giuliani M, Adu-Bobie J, Comanducci M, Aricò B, Savino S, Santini L, Brunelli B, Bambini S, Biolchi A, Capecchi B, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci USA 2006; 103:10834-9.
- Jodar L, Feavers MI, Saliisbury D, Granoff DM. Development of vaccines against meningococcal disease. Lancet 2002; 359:1499-508; PMID:11988262; http://dx.doi.org/10.1016/S0140-6736(02)08416-7
- Boslego J, Garcia J, Cruz C, Zollinger W, Brandt B, Ruiz S, Martinez M, Arthur J, Underwood P, Silva W, et al. Efficacy, safety, and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Vaccine 1995; 13:821-9; PMID:7483804; http://dx.doi.org/10.1016/0264-410X(94)00037-N
- De Moraes JC, Perkins BA, Camargo MC, Hidalgo NT, Barbosa HA, Sacchi CT, Gral IL, Gattas VL, Vasconcelos HG, Plikaytis BD, et al. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet 1992; 340:1074-8; PMID:1357461; http://dx.doi.org/10.1016/0140-6736(92)93086-3
- O’Hallahan J, Lennon D, Oster P. The strategy to control New Zealand's epidemic of group B meningococcal disease. Pediatr Infect Dis J 2004; 23:S293-8; PMID:15597072
- Oster P, Lennon D, O’Hallahan J, Mulholland K, Reid S, Martin N. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine 2005; 23:2191-6.
- Holst J, Feiring B, Næss LM, Norheim G, Kristiansen P, Høiby EA, Bryn K, Oster P, Costantino P, Taha M-K, e al. The concept of “tailor-made”, protein-based, outer membrane vesicle vaccines against meningococcal disease. Vaccine 2005; 23:2191-6
- Derrick JP, Urwin R, Suker J, Feavers IM, Maiden IM. MCJ. Structural and evolutionary inference from molecular variation in Neisseria porins. Infect Immun 1999; 67:2406-13; PMID:10225902
- Idanpaan-Heikkila I, Wahlstrom E; Nurminen M, Makela HP, Sarvas M. The Neisseria meningitidis outer membrane protein P1 produced in Bacillus subtilis and reconstituted into phospholipid vesicles elicits antibodies to native P1 epitopes. Microb Pathog 1995; 18:423-36
- Van der Voort ER, Van der Ley P, Van der Biezen J, Steohen G, Tunnela O, Van der Diejken H, Kuipers B, Poolman J. Specificity of human bactericidal antibodies against PorA P1.7,16 Induced with a hexavalent meningococcal outer membrane vesicle vaccine. Infect Immun 1996; 64:2745-51; PMID:8698504
- Neisseria PorA typing [Internet]. UK: Keith Jolley and sited at the University of Oxford. 2010 – [cited 2014 september 12]. Available from: http://pubmlst.org/neisseria/PorA/
- Luijkx TA, van Dijken H, Hamstra H-J, Kuipers B, van der Ley P, van Alphen L, van den Dobbelsteen G. Relative immunogenicity of PorA subtypes in a multivalent Neisseria meningitidis vaccine is not dependent on presentation form. Infect Immun 2003; 71: 6367-71; PMID:14573657; http://dx.doi.org/10.1128/IAI.71.11.6367-6371.2003
- Holst J. Strategies for development of universal vaccines against meningococcal serogroup B disease. Hum Vaccines 2007; 3: 290-4; PMID:17712231
- van den Dobbelsteen GP, van Dijken HH, Pillai S, van Alphen L. Immunogenicity of a combination vaccine containing pneumococcal conjugates and meningococcal PorA OMVs. Vaccine 2007; 25: 2491-6; PMID:17023098; http://dx.doi.org/10.1016/j.vaccine.2006.09.025
- Kaaijk P, van Straaten I, van de Waterbeemd B, Boot EPJ, Levels LMAR, van Dijken HH, van den Dobbelsteen GPJM. Preclinical safety and immunogenicity evaluation of a nonavalent PorA native outer membrane vesicle vaccine against serogroup B meningococcal disease. Vaccine 2013; 31:1065-71; PMID:23273968; http://dx.doi.org/10.1016/j.vaccine.2012.12.031
- Cartwright K, Morris R, Rümke H, Fox A, Borrow R, Begg N, Richmond P, Poolman J. Immunogenicity and reactogenicity in UK infants of a novel meningococcal vesicle vaccine containing multiple class 1 (PorA) outer membrane proteins. Vaccine 1999; 17:2612-9; PMID:10418910; http://dx.doi.org/10.1016/S0264-410X(99)00044-4
- Longworth E, Borrow R, Goldblatt D, Balmer P, Dawson M, Andrews N, Miller E, Cartwright K. Avidity maturation following vaccination with a meningococcal recombinant hexavalent PorA OMV vaccine in UK infants. Vaccine 2002; 20:2592-6; PMID:12057617; http://dx.doi.org/10.1016/S0264-410X(02)00151-2
- Arigita C, Kersten GFA, Hazendonk T, Hennink WE, Crommelin DJA, Jiskoot W. Restored functional immunogenicity of purified meningococcal PorA by incorporation into liposomes. Vaccine 2003; 21: 950-60; PMID:12547608; http://dx.doi.org/10.1016/S0264-410X(02)00546-7
- Pinto VB, Moran EE, Cruz F, Wang X-M, Fridman A, Zollinger WD, Przysiecki CT, Burden R. An experimental outer membrane vesicle vaccine from N. meningitidis serogroup B strains that induces serum bactericidal activity to multiple serogroups. Vaccine 2011; 29:7752-8
- Morein B, Sundquist B, Höglund S, Dalsgaard K, Ostehaus A. Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. Nature 1984; 308:457-60; PMID:6709052; http://dx.doi.org/10.1038/308457a0
- Sjölander A, Cox JC. Uptake and adjuvant activity of orally delivered saponin and ISCOM vaccines. Adv Drug Del Rev 1998; 34:321-38; PMID:10837684ND; http://dx.doi.org/10.1016/S0169-409X(98)00046-5
- Peeters CC, Claassen IJ, Schuller M, Kersten GF, Van der Voort EM, Poolman JT. Immunogenicity of various presentation forms of PorA outer membrane protein of Neisseria meningitidis in mice. Vaccine 1999; 17:2702-12; PMID:10418921
- Humphries HE, Williams JN, Christodoulides M, Heckels J. Recombinant meningococcal PorA protein, expressed using a vector system with potential for human vaccination, induces a bactericidal immune response. Vaccine 2004; 22:5164-9; PMID:15063582; http://dx.doi.org/10.1016/j.vaccine.2003.09.042
- Humphries HE, Williams JN, Blackstone R, Jolley KA, Yuen HM, Christodoulides M, Heckels JE. Multivalent liposome-based vaccines containing different serosubtypes of PorA protein induce cross-protective bactericidal immune responses against Neisseria meningitidis. Vaccine 2006; 24:36-44; PMID:16105711
- Lövgren K, Morein B. The requirement of lipids for the formation of immunostimulating complexes (iscoms). Biotechnol Appl Biochem 1988; 10:161-72; PMID:2838046
- Morein B, Bengtsson LK. Immunomodulation by Iscoms, immunostimulating complexes. Methods (San Diego, Calif.) 1999; 19:94-102; PMID:10525444
- Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol 2008; 30:23-32; PMID:19059004
- Organización Panamericana de la Salud. Informe regional de SIREVA II 2000-2005. 2005: 108-14.
- Saunders NB, Zollinger WD, Rao VB. A rapid and sensitive PCR strategy employed for amplification and sequencing of porA from a single colony-forming unit of Neisseria meningitidis. Gene 1993; 137:153-62; PMID:8299943
- Sacchi CT, Lemos AP, Brandt ME, Whitney AM, Melles CE, Solari CA, Frasch CE, Mayer LW. Proposed standardization of Neisseria meningitidis PorA variable-region typing nomenclature. Clin Diagn Lab Immunol. 1998; 5(6):845-55; PMID:9801347
- Rokbi B, Renauld-Mongenie G, Mignon M, Danve B, Poncet D, Chabanel C, Caugant DA, Quentin-Millet MJ. Allelic diversity of the two transferrin binding protein B gene isotypes among a collection of Neisseria meningitidis strains representative of serogroup B disease: implication for the composition of a recombinant TbpB-based vaccine. Infect Immun. 2000; 68(9):4938-47; PMID:10948108; http://dx.doi.org/10.1128/IAI.68.9.4938-4947.2000
- Kashiwagi Y, Maeda M, Kawashima H, Nakayama T. Inflammatory responses following intramuscular and subcutaneous immunization with aluminum-adjuvanted or non-adjuvanted vaccines. Vaccine. 2014 5; 32(27):3393-401; PMID:24768634; http://dx.doi.org/10.1016/j.vaccine.2014.04.018
- Liu X, Wetzler LM, Massari, P. The PorB porin from commensal Neisseria lactamica induces Th1 and Th2 immune responses to ovalbumin in mice and is a potential immune adjuvant. Vaccine 2008; 26:786-96; PMID:18191311; http://dx.doi.org/10.1016/j.vaccine.2007.11.080
- Perrett KP, Pollard AJ. Towards an improved serogroup B Neisseria meningitidis vaccine. Expert Opin Biol Ther 2005; 5:1611-25; PMID:16318425
- Sorhouet PC, Regueira M, Mollerach M. PorA types in Neisseria meningitidis serogroup B isolated in Argentina from 2001 to 2003: implications for the design of an outer membrane protein-based vaccine. J Med Microbiol 2008; 57:338-42PMID:18287297; http://dx.doi.org/10.1099/jmm.0.47631-0
- Zhu D, Williams JN, Rice J, Stevenson FK, Heckels JE, Christodoulides M. A DNA fusion vaccine induces bactericidal antibodies to a peptide epitope from the PorA porin of Neisseria meningitidis. Infect Immun 2008; 76:334-8; PMID:17967859
- O’Dwyer CA, Reddin K, Martin D, Taylor SC, Gorringe AR, Hudson MJ, Brodeur BR, Langford PR, Kroll JS. Expression of heterologous antigens in commensal Neisseria spp: preservation of conformational epitopes with vaccine potential. Infect Immun 2004; 72:6511-8; PMID:15501782
- Danve B, Lissolo L, Mignon M, Dumas P, Colombani S, Schryvers AB, Quentin-Millet MJ. Transferrin-binding proteins isolated from Neisseria meningitidis elicit protective and bactericidal antibodies in laboratory animals. Vaccine 1993; 11:1214-20; PMID:8256502
- West D, Reddin K, Matheson M, Heath R, Funnell S, Hudson M, Robinson A, Gorringe A. Recombinant Neisseria meningitidis transferrin binding protein A protects against experimental meningococcal infection. Infect Immun 2001; 69:1561-7; PMID:11179327
- Pillai S, Howell A, Alexander K, Bentley BE, Jiang HQ, Ambrose K, Zhu D, Zlotnick G. Outer membrane protein (OMP) based vaccine for Neisseria meningitidis serogroup B. Vaccine 2005; 23:2206-9; PMID:15755596
- Welsch JA, Moe GR, Rossi R, Adu-Bobie J, Rappuoli R, Granoff DM. Antibody to genome-derived neisserial antigen 2132, a Neisseria meningitidis candidate vaccine, confers protection against bacteremia in the absence of complement-mediated bactericidal activity. J Infect Dis 2003; 188:1730-40; PMID:14639545
- Comanducci M, Bambini S, Caugant DA, Mora M, Brunelli B, Capecchi B, Ciucchi L, Rappuoli R, Pizza M. NadA diversity and carriage in Neisseria meningitidis. Infect Immun 2004; 72: 4217-23; PMID:15213166
- Holst J, Martin D, Arnold R, Huergo CC, Oster P, O’Hallahan J, Rosenqvist E. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 2009; 27(Supp 2):B3-B12; PMID:19481313; http://dx.doi.org/10.1016/j.vaccine.2009.04.071
- de Kleijn E, van Eijndhoven L, Vermont C, Kuipers B, van Dijken H, Rümke H, de Groot R, van Alphen L, van den Dobbelsteen G. Serum bactericidal activity and isotype distribution of antibodies in toddlers and schoolchildren after vaccination with RIVM hexavalent PorA vesicle vaccine. Vaccine 2001; 20:352-8; PMID:11672897; http://dx.doi.org/10.1016/S0264-410X(01)00371-1
- Gentle IE, Burri L, Lithgow T. Molecular architecture and function of the Omp85 family of proteins. Mol Microbiol 2005; 58:1216-25; PMID:16313611; http://dx.doi.org/10.1111/j.1365-2958.2005.04906.x
- Werner J, Misra R. Yae T (Omp85) affects the assembly of lipid-dependent and lipid-independent outer membrane proteins of Escherichia coli. Mol Microbiol 2005; 57:1450-9; PMID:NOT_FOUND
- Robert V, Volokhina EB, Senf F, Bos MP, Van Gelder P, Tommassen J. Assembly factor Omp85 recognizes its outer membrane protein substrates by a species-specific C-terminal motif. PloS Biol 2006; 4:e377; PMID:17090219
- Jansen C, Wiese A, Reuvsaet L, Dekker N, de Cook H. Seydel U, Tommassen J. Biochemical and biophysical characterization of in vitro folded outer membrane porin PorA of Neisseria meningitidis. Biochim Biophys Acta 2000; 1464:284-98; PMID:10727615
- Næss L, Tanja N, Aarvaka A, Oftung F, Hoiby E, Sandin R, Michaelsen TE. Human IgG subclass responses in relation to serum bactericidal and opsonic activities after immunization with three doses of the Norwegian serogroup B meningococcal outer membrane vesicle vaccine. Vaccine 1999; 17:754-64; http://dx.doi.org/10.1016/S0264-410X(98)00259-X
- Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection serum bactericidal antibody activity. Vaccine 2005; 23:2222-7; PMID:15755600; http://dx.doi.org/10.1016/j.vaccine.2005.01.051
- Santos GF, Deck R, Donnelly J, Blackwelder W, Granoff DM. Importance of complement source in measuring meningococcal bactericidal titers. Clin Diag Lab Immunol 2001; 8:616-23; PMID:11329468
- Kees LM, Franken KL, Hiemstra HS, van Meijgaarden KE, Subronto Y, Den Hartigh J, Ottenhoff TH, Drijfhout JW. Purification of His-tagged proteins by immobilized chelate affinity chromatography: The benefits from the use of organic solvent. Proc Natl Acad Sci USA 2000; 97:2029-34.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248-54; PMID:942051
- Bengtsson LK, Sjölander A. Adjuvant activity of iscoms; effect of ratio and co-incorporation of antigen and adjuvant. Vaccine 1996; 14:753-60; PMID:8817821
- Gu Q, Song D, Zhu M. Oral vaccination of mice against Helicobacter pylori with recombinant Lactococcus lactis expressing urease subunit B. FEMS. Immunol Med Microbiol 2009; 56:197-203; PMID:19453750
- Scavone P, Miyoshi A, Rial A, Chabalgoity A, Langella P, Azevedo V, Zunino P. Intranasal immunisation with recombinant Lactococcus lactis displaying either anchored or secreted forms of Proteus mirabilis MrpA fimbrial protein confers specific immune response and induces a significant reduction of kidney bacterial colonisation in mice. Microbes Infect 2007; 9:821-8; PMID:17540603
- Hoiby E, Rosenqvist E, Froholm L, Bjune G, Fiering B, Nokleby H, Ronnild E. Bactericidal antibodies after vaccination with the Norwegian meningococcal serogoup B outer membrane vesicle vaccine: a brief survey. NIPH Ann 1991; 14:147-56; PMID:1812429
- Wetzler LM. Innate immune function of the neisserial porins and the relationship to vaccine adjuvant activity. Future Microbiol 2010; 5:749-58; PMID:20441547; http://dx.doi.org/10.2217/fmb.10.41
- Burke JM, Gangley-Leal LM, Khatri A, Wetzler LM. Neisseria meningitidis PorB, a TLR2 ligand, induces an antigen-specific eosinophil recall response: potential adjuvant for helminth vaccines? J Immunol 2007; 179:3222-30; PMID:17709538
- Tanabe M, Nimigean CM, Iverson TM. Structural basis for solute transport, nucleotide regulation, and immunological recognition of Neisseria meningitidis PorB. Proc Natl Acad Sci USA 2010; 107:6811-6; PMID:20351243; http://dx.doi.org/10.1073/pnas.0912115107
- Massari P, Visintin A, Gunawardana J, Halmen KA, King CA, Golenbock DT, Wetzler LM. Meningococcal porin PorB binds to TLR2 and requires TLR1 for signaling. J Immunol. 2006; 176:2373-80; PMID:16455995; http://dx.doi.org/10.4049/jimmunol.176.4.2373
- Massari P, Ho Y, Wetzler LM. Neisseria meningitidis porin PorB interacts with mitochondria and protects cells from apoptosis. Proc Natl Acad Sci USA 2000; 97:9070-5; PMID:10922061
