Abstract
Although pancreatic cancer is but the eleventh most prevalent cancer in the US, it is predicted that of all the patients newly diagnosed with this disease in 2014, only 27% will still be alive at the end of the first year, which is reduced to 6% after 5 years. The choice of chemotherapy in the treatment of pancreatic cancer is dependent on disease stage and patient performance status but, in general, the most widely used approved regimens include 5-fluorouracil (5-FU) combinations and gemcitabine combinations. Recent therapeutic strategies have resulted in an improvement in survival of patients with pancreatic cancer but the magnitude of change is disappointing and vast improvements are still needed. The goal of immunotherapy is to enhance and guide the body's immune system to recognize tumor-specific antigens and mount an attack against the disease. Among newer immune therapies, GI-4000 consists of 4 different targeted molecular immunogens, each containing a different Ras protein (antigen) encoded by the most commonly found mutant RAS genes in solid tumors—RAS mutations exist in over 90% of pancreatic ductal adenocarcinomas. We will review pancreatic cancer epidemiology and its current treatment options, and consider the prospects of immunotherapy, focusing on GI-4000. We discuss the potential mechanism of action of GI-4000, and the performance of this vaccination series thus far in early phase clinical trials.
Pancreatic Cancer – Surgery, Chemotherapy, and Radiotherapy
In general usage, the term “pancreatic cancer” refers to pancreatic adenocarcinoma, which arises from the exocrine portion of the pancreas. It is currently estimated to be the eleventh most common cancer diagnosed in the US but the fourth leading cause of death.Citation1 Thus, it is approximated that 46,420 patients will have been diagnosed with pancreatic cancer in the United States by the end of 2014, and that the majority of these will sooner or later die from their disease.Citation1
Traditional TNM staging is used for pancreatic cancer but, in the clinic, for practical purposes, patients are divided into 3 groups according to their tumor's operability: surgically resectable tumor, locally advanced unresectable disease, and metastatic cancer. Surgery remains the only known curative approach but unfortunately, few patients present with local disease and have a surgical option.
In studies carried out by Cameron (2006) and Geer (1993), it was observed that patients with operable pancreatic cancer but with lymph-node involvement had a 5-year survival rate of 10% following surgery, whereas those without lymph-node involvement had a 25-30% 5-year survival rate.Citation2,3 Due to an observed survival advantage, it is now an established standard to treat patients with post-operative chemotherapy including either gemcitabine or 5-FU. In a study by Lim et al., 2003, survival rates were found to be significantly higher in patients receiving adjuvant chemotherapy compared with those who had no chemotherapy after surgery; 3 year survival rates were 45% (adjuvant chemotherapy) versus 30% (no chemotherapy).Citation4 Two meta-analyses support these findings in that they show statistically significant improvement in survival benefit with adjuvant chemotherapy.Citation5,6
Locally advanced unresectable cancer is treated with either chemotherapy or chemoradiation, although the role of radiation is more controversial. Treatment choices are often dependent on a patient's performance status and symptoms at presentation.
Krzyzanowska et al. (2003) used the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database to carry out a retrospective study in 1,696 patients diagnosed with locally advanced pancreatic cancer between 1991 and 1996. The investigators calculated the median survival (adjusted for age, sex, and comorbidity) of patients who had received a range of different standard treatments. Thus, patients who received no therapy, chemotherapy alone, radiation therapy alone, or chemoradiotherapy (chemotherapy plus radiation therapy) were calculated to have median survival times of 15, 27, 29, and 47 weeks, respectively, thereby suggesting that chemoradiotherapy provides the highest survival benefit.Citation7
Metastatic disease is typically treated with chemotherapy. The evolution of systemic treatment has resulted in some survival advantage for patients. Originally, treatment was limited to single agent therapies including 5-FU and gemcitabine. For more than a decade, no real advances were made despite significant effort; only erlotinib (an EGFR-targeted agent) was shown to add a very small survival advantage when administered alongside gemcitabine.Citation8 Eventually, FOLFIRINOX (a 3 drug regimen of 5-FU, oxaliplatin and irinotecan) was tested in the ACCORD 11 trial,Citation9 and was shown to significantly increase survival compared with gemcitabine alone (11.1 months vs. 6.8 months). As might be expected, FOLFIRINOX treatment-related toxicities were markedly greater than those related to single agent gemcitabine therapy.Citation9
A recent Phase III clinical trial carried out by Von Hoff et al. (2013-2014) brought forward another gemcitabine-based treatment option to the table for patients with metastatic pancreatic cancer.Citation10,11 In this study—the MPACT study—patients with metastatic pancreatic cancer for which they had received no prior chemotherapy were randomly assigned to receive either a combination of nab-paclitaxel (paclitaxel protein-bound particles) + gemcitabine or gemcitabine treatment alone. Initially, it was hypothesized that the albumin bound formulation would increase the cancer targeting based on SPARC expression, but this theory has not held up in further studies. A total of 861 patients with pancreatic cancer were randomly assigned to nab-paclitaxel plus gemcitabine or gemcitabine alone. The median overall and progression-free survivals were 8.5 months and 5.5 months, respectively, in the nab-paclitaxel + gemcitabine arm, and 6.7 months and 3.7 months in the gemcitabine-only arm. The differences in survival measures between the 2 treatment arms were statistically significant. At one year, the overall survival rate was 35% in the nab-paclitaxel + gemcitabine arm, and 22% in the gemcitabine arm. This was reduced to 9% and 4% at 2 years. The most common adverse events in both groups of grade 3 or higher disease were neutropenia, fatigue, and neuropathy. It was concluded that in patients with metastatic pancreatic adenocarcinoma, nab-paclitaxel + gemcitabine significantly improved survival compared with gemcitabine alone. However, rates of peripheral neuropathy and myelosuppression were increased and some have countered that the increase in side-effects as well as cost of treatment negated the improvement in survival.Citation12 However, due to the lack of treatment options available to patients with metastatic pancreatic cancer and the lethality of disease, on September 6, 2013, the Food and Drug Administration (FDA) approved paclitaxel protein-bound particles (albumin-bound—Abraxane®; nab-paclitaxel) in combination with gemcitabine for the first-line treatment of patients with metastatic adenocarcinoma of the pancreas.Citation13 An updated survival analysis showed that the significant difference in overall survival of patients on the Abraxane® + gemcitabine arm of the study compared to the gemcitabine alone arm has been sustained for 3 years.Citation11 These data confirm and expand upon the initial 2013 reportCitation10 and support the greater efficacy of the combination treatment. It was concluded that these results should “encourage efforts to build upon this well tolerated backbone to further extend survival.”Citation11
Despite the gains from FOLFIRINOX and Gemcitabine/nab-paclitaxel combinations, there is a clear, urgent unmet need for additional effective treatments for pancreatic cancer.
Pancreatic Cancer – Novel Treatment Approaches
Several novel approaches to the treatment of cancer have emerged; focused on signal transduction, angiogenesis and immune therapy (immunotherapy). Immunotherapy is the least mature of the group but has enjoyed a recent explosion of success in cancers such as melanoma and kidney cancer. The basic concept is to help the body's innate immune system recognize tumor-specific antigens and mount an attack against the disease.
In the past decade, several novel immune therapy approaches have been tested in human trials. Agents such as IL-2 and interferon-α were tested as non-specific enhancers of the immune response but these were effective only in renal cancers and melanomas.Citation14-16 A second approach involves direction of the immune system to recognize a specific antigen that is particular to the cancer of interest, and by “immunizing” the patient, antigen specific responses may be generated, resulting in a cytotoxic T cell response.Citation17 As an example of this, we and others performed a series of clinical trials testing CEA targeted vaccines, which demonstrated significant CEA-specific T cell responses in many patients, clinical benefit in some patients, and a very good safety profile.Citation18,19 Unfortunately, a phase III randomized trial of a CEA-targeted vaccine in second line pancreatic cancer failed to show any benefit.Citation20,21
More recently, checkpoint inhibitors have emerged as therapies with increased impact in certain cancers and are being rapidly developed and approved for use in melanomaCitation22 and renal cancer,Citation23-25 as well as being tested in other cancers, including those of the GI tract.
In this review, we are focused on GI-4000, a vaccine consisting of targeted molecular immunogens that contain a modified human Ras protein. GI-4000 is designed to target specific RAS mutations commonly found in pancreatic cancer.
KRAS Oncogene and Pancreatic Cancer Development
Oncogenes are modified, or mutated, genes that have the potential to cause cancer by coding for aberrant proteins. Humans have 3 different RAS genes: HRAS, KRAS and NRAS, and mutations in these genes are commonly found in many different cancers. The KRAS gene encodes the human K-ras protein. Of all cancer-related mutations in the RAS gene family, 85% are the result of KRAS gene mutations, 12% are the result of mutations in the NRAS gene, and only 3% are the result of mutations in HRAS.Citation26
In pancreatic cancers, KRAS mutations account for over 90% of RAS gene mutationsCitation26-28 and this gene is thus the focus of a number of possible pancreatic cancer treatments.
The protein product of the normal KRAS gene is K-ras, a small membrane-bound GTPase. Circulating growth factors activate extracellular EGFR, which then acts down-stream to activate K-ras’ GTPase activity.Citation29 K-ras behaves as a “molecular on/off switch.”Citation30 In its active state, it binds to GTP and catalyzes the cleavage of the terminal phosphate from the GTP molecule, converting it to GDP, at which point K-ras is switched off. Guanine nucleotide exchange factors (RasGEFs) and GTPase activating proteins (RasGAPs) facilitate the conversion from GTP to GDP and back again.Citation26,31
Abnormal K-ras (the protein product of a mutated KRAS gene) becomes locked into the GTP bound “on” position and is thus persistently active; in this state, cell proliferation and survival become independent of EGFR signaling.Citation32
The Ras protein binds to the surface receptors of cells, leading to protein phosphorylation and activation of signal transduction pathways that are linked to growth and development. Ras-signaling occurs through both the mitogen activated protein kinase cascade pathway (MAP) and the PI3K/AKT (AKT) pathway.Citation28,33 Activation of the MAP pathway promotes transcription of genes involved in cellular growth and determination of a cell's inheritable functionality and fate. Activation of the AKT pathway inhibits apoptosis.
The majority of KRAS mutations in pancreas tumor cells are located in codon 12,Citation26,34 and although mutations in codon 13 and 61 can also exist, they are much less common. Four different mutations are observed within the K-ras protein at codon 12, which result from the following amino acid substitutions: glycine to valine (G12V), glycine to cysteine (G12C), glycine to aspartate (G12D), and glycine to arginine (G12R).Citation35 These mutations result in conformational changes that can render the interaction of K-ras with RasGAPs impossible, thus preventing the return of K-ras to its inactive state.Citation27 Conformational changes of K-ras also occur due to codon 13 mutations and the result is the same—persistent K-ras activation. Codon 61 mutations (of which 3 are known) lead to an interference of GTP conversion back to GDP, again resulting in continuous activation of K-ras. All codon 12 and codon 61 mutations studied thus far are seen as “non-self.” This makes them ideal targets for immune-directed therapy.
While likely playing a central role in carcinogenesis, it is unlikely that a single KRAS mutation alone can completely transform normal cells to cancer cells. Thus additional genetic and/or environmental factors are needed. Molecular profiling has shown that KRAS mutations are frequently followed by the mutation and inactivation of tumor suppressor genes such as p53, p16, and SMAD4. Continuous activation of the K-ras pathway and inactivation of suppressor genes freely promote cancer cell development by encouraging tumor maintenance.Citation26,35-39
Hence, while ras mutations and their corresponding proteins are known to be causative in a number of human cancers, making them an obvious target for drug development, targeting RAS directly using traditional pharmaceutical approaches has been difficult; efforts to block the mutated protein have been mostly unsuccessful leading to its characterization as “undruggable.”Citation40 Thus, an immune approach is a highly attractive alternative therapeutic angle to test.
Vaccine Therapy
In general, tumor-vaccine research has to focus on raising a sufficiently specific host immune response to tumors. This is not an easy task, partly due to the fact that cancer is predisposed to protecting itself by immunoevasion, where a dampening of both T and natural killer (NK) cell responses provide the tumor with a route of escape from the host's immune system,Citation42-45 and immunoediting, where changes in tumor immunogenicity occur as a response to a host immune system attack, leading to the emergence of immune-resistant variants [www.springerreference.com/docs/html/chapterdbid/174644.html].
The immune system is equipped with 2 fundamental modes of attack: cellular and humoral immunity. While the 2 are complementary, most believe that anti-tumor immunity is predominantly mediated through the cellular arm. Tumors express novel proteins and peptide fragments that are “processed” and contextualized in such a way as to either stimulate an immune response or, in fact, stimulate tolerance. One of the goals of vaccine-based therapies is to shift this stimulation more toward an immune response.
In more detail, the recognition of tumor cells by the body's immune system begins with the secretion of an antigenic substance by the tumor cells—termed a tumor antigen— and ideally this substance is recognized by the body's immune system. This antigen is then “consumed” by an antigen-presenting cell (APC), such as a dendritic cell, which processes the antigen via membrane-bound vesicles, called endosomes.Citation46 These endosomes contain proteolytic enzymes that break down the protein antigen into individual peptides. These peptides are subsequently associated with “major histocompatibility complex II proteins” (MHC-II) within the endosomes, which are then transported to the cell surface. The dendritic APCs subsequently take the processed antigens to a draining lymph node and present them to the lymphocytes therein.Citation47 The MHC-peptide complex can now be recognized by CD4+ helper T-cells. This process activates the humoral arm of the immune system (an immune response mediated by B-cells), which leads to the formation of antibodies to the “foreign” antigen. Another major histocompatibility complex, MHC-I, a cell-surface complex, mediates elimination of malignant cells by interacting with CD8+ cytotoxic T-cell surfaces. This arm of the immune system is termed cellular immunity.Citation48 Depending on the presence of other co-stimulatory molecules, the peptide/MHC presentation between the APC and the T-cell can generate either a cytotoxic T-cell response or tolerance. Vaccines such as GI-4000 are engineered to actuate the desired cytotoxic response through stimulation of co-stimulatory molecules and “danger” signals.
Vaccine Therapy in Pancreatic Cancer
Mutated KRAS appears to play a role in the ability of tumors to evade the body's immune response. Mice endogenously expressing a single mutated KRAS allele in progenitor cells of the pancreas were studied and the presence of mutated KRAS was shown to create an immunosuppressive environment comprising B-cell entities—macrophages, myeloid-derived suppressor cells and T-regulator cells—while effector T-cells (CD8 and others) seemed to be practically nonexistent.Citation49-52 It turns out that this immunosuppressive environment is uniquely useful for tumor angiogenesis, growth and invasion but the absence of prior T-cell selection is useful regarding possible anti-tumor immunotherapeutic strategies in the form of vaccinations.Citation50,51
Pancreatic cancer seems to be a good candidate for immune therapy,Citation53 especially as the majority (88%) of patient pancreas cancers express mutated KRAS—53 out of 63 (84%),Citation54 28 out of 30 (93%),Citation55 and 286 of 325 (88%) [unpublished data, 2014]—as well as other potentially useful immune therapy targets such as mucin1 (MUC1; over 85%), survivin (approx. 77%), CEA (over 90%), and others such as p53 and human telomerase reverse transcriptase (hTRT).Citation56 Immune suppressive molecules such as VEGF, PD-L1 and a range of cytokines (e.g., TGF-β, IL-10, and IL-6) also play a role in pancreatic cancer growth and survival.Citation57
Although the factors listed above are, and have been, very worthy of consideration, they also present several challenges, as they are non-specific to pancreatic cancer. When it comes to vaccine development, tumor cell specificity of antigenic markers is of high importance if one is to avoid the attack of normal cells and increase the risk of autoimmunity or other side effects.
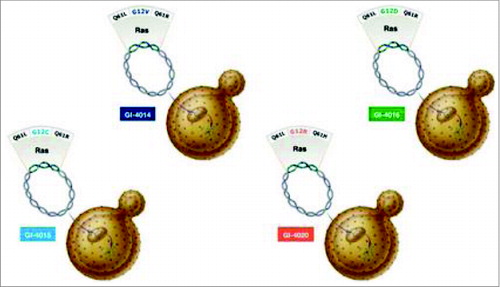
Vaccines such as GI-4000 are engineered to stimulate the cytotoxic response through stimulation of co-stimulatory molecules and “danger” signals. GI-4000 consists of tarmogens (targeted molecular immunogens), which are intact, heat-inactivated yeast (Saccharomyces cerevisiae) containing a modified human Ras protein (a target protein).Citation58 These Ras proteins are modified according to the mutated RAS gene encoding them and are representative of known sequenced tumor mutations (it should be remembered that KRAS mutations account for the vast majority of RAS mutations in pancreatic cancer). GI-4000 is made up of 4 different tarmogens: GI-4014, GI-4015, GI-4016, and GI-4020 and each tarmogen expresses a different combination of 3 of the 7 most common RAS mutations found in tumors ().Citation59 A patient's RAS-mutated tumor is sequenced to identify its specific RAS mutation and then a tarmogen with the same RAS mutation is selected for treatment of that patient. Upon systemic introduction of the tarmogen, it is taken up by an APC, processed, and then presented by both MHC-I and MHC-II. Both CD4+ helper T-cells and CD8+ killer T-cells are activated upon successful presentation of the modified Ras protein. This stimulates the immune system to target the modified Ras protein, which is considered alien to the human body. It is then intended that a specific immune response be raised against cells containing this abnormal protein without leading to the death of healthy cells; this reduces the possibility of any treatment-related side effects.Citation48 Indeed, GI-4000 has been well tolerated without any significant adverse findings.Citation60
Figure 1. GI-4000 Series: Four different strains: GI-4014, -4015, -4016, -4020 of heat-inactivated S. Cerevisiae; each express a mutated Ras fusion protein. The mutations of RAS represent the most common mutations found in solid tumors via genotype analysis.Each strain bares a fusion protein that is inclusive of 3 different RAS mutation combinations; 2 of the mutations are found on codon 61 and the other on codon 12. (http://meetinglibrary.asco.org/content/40075?format=posterImg) – Permission required.
A phase 1 clinical trial assessed the effect of GI-4000 treatment in patients with advanced pancreatic and colorectal cancer. All patients had previously failed first-line chemotherapy. The tumors were first sampled and sequenced for mutations in KRAS, HRAS, and NRAS genes. If a target mutation was detected, the patient received 5 subcutaneous weekly doses of the tarmogen that contained the same mutation. Out of 32 patients, only 9 had tumors that harbored RAS mutations and 7 of these mutations were contained in the 3 available tarmogens. Six patients were treated—3 with a low dose—and cellular assay data were available for these low-dose patients: Of these 3 patients, 2 showed mutation-specific T-cell responses (analyzed using proliferation and cytokine secretion assays).Citation61 Results have not yet been published but it was observed that GI-4000 was well tolerated by all patients treated (mild fever and malaise was observed in one patient) and 72% of subjects had antigen specific cellular responses. One pancreatic cancer subject with stage 4 disease survived longer than 2 years and 4 CRC subjects, also with stage 4 disease, survived 1–3 years. A GI-4000 tarmogen is thus capable of inducing a specific T-cell response to a mutant-gene encoded Ras protein and results thus far are promising.
In a Phase II study recently presented at the 2014 AACR-RAS Oncogene conference,Citation34 176 subjects with Ras mutant + pancreatic adenocarcinoma, post-tumor resection, were randomized 1:1 to receive either a combination of GI- 4000 and gemcitabine or gemcitabine with placebo; patients were stratified by resection status (R0 [negative resection margins] vs. R1 [microscopic tumor infiltration at margins—post-operative residual disease]). They first received 3 weekly priming injections of GI-4000 or placebo, which were followed by 6 cycles of adjuvant gemcitabine 1000 mg/m2 IV. Monthly GI-4000 or placebo injections were given to patients during the 2 week holiday between cycles of gemcitabine, and continued monthly until disease recurrence, intolerable toxicity or death. Blood taken from patients at sufficient time-points throughout the study was assayed for patient immune response by interferon-γ (IFNγ) ELISpot assay. Frequencies of regulatory T-cells (Tregs)—measured at baseline (before priming injections) and then at time-points after GI-4000/placebo treatment began but before gemcitabine was administered—were measured by flow cytometry (n = 76).Citation34
Study result analysis showed that in R1 subjects, GI-4000 treatment led to a significantly greater rate of immune response to mutated K-Ras than placebo: 7/15 (46.7%) vs. 1/12 (8.3%) had an IFNγ response to their K-Ras codon 12 (G12) mutation (p = 0.043). However, in contrast, GI-4000 treatment of R0 subjects showed no increase in Ras mutation-specific IFNγ responses compared to placebo: 16/52 (30.8%) vs. 22/50 (44.0%) respectively. However, R0 patients treated with GI-4000 had naive Tregs (CD4+/CD45RA+/Foxp3low) that were significantly decreased compared with placebo: 11/42 (26.2%) vs. 3/34 (8.8%) subjects had a >2-fold decrease in this fraction (p = 0.048 Fisher's exact test), suggesting an immunotherapeutic effect in R0 as well as R1 patients.Citation34
An unexpected finding was that R0 patients with a glycine to arginine (G12R) K-ras mutation appeared to have a better prognosis overall—with and without GI-4000 treatment—when compared with all other mutations (median OS = 335 days longer; ).
Figure 2. Overall Survival (OS) of patients with a G12R mutation compared with all other Ras mutations, regardless of treatment.Citation34
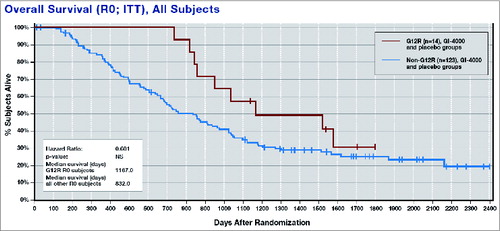
Median OS was also greatly improved in GI-4000-treated G12R R0 subjects compared with placebo-treated G12R R0 subjects (median OS = 568 days longer), as was the case for G12R subjects vs. non-G12R subjects receiving GI-4000 (median OS was 773 days longer in G12R subjects). When placebo subjects were taken alone, there was only a 94-day improvement in median OS for G12R vs. non-G12R subjects.Citation34
These observations suggest that the presence of the Ras G12R mutation results in a survival advantage for pancreatic cancer patients, and that treatment with the GI-4000 can further improve immune response and survival for this subset of patients. Thus, immune targeting of the activating RAS mutation certainly seems to be a promising approach to treating RAS mutated cancers such as pancreatic cancer.
Taking things a step further, data were recently presented regarding a proteomic marker that appears to predict response to GI-4000.Citation41 A novel technique has been developed that allows for deeper exploration into the proteome. The employment of half a million laser shot Deep MALDI spectra led to the identification of more than 700 mass spectral features within the proteome. Multivariate classifiers were created using a subset of these 700 and were filtered for performance and combined using dropout regularization. A test set was thus developed that could be used to independently assess individual proteome signatures. The resulting test (BDX-001) can identify, based on the proteomic signature, which patients are likely to benefit from the addition of GI-4000 to gemcitabine (BDX-001(+)) and which are not likely to benefit (BDX-001(-)).
Taking pretreatment plasma samples from 90 of the 176 patients in the Coeshott study (2014),Citation34 and using BDX-001 for stratification, subjects who were BDX-001(+) demonstrated a 499 day advantage in median OS, and a 351 day improvement in regression-free survival (RFS) when treated with GI-4000/gemcitabine vs. placebo/gemcitabine. Although sample size was too small to proclaim statistical significance between test groups, the test is worthy of further validation in a larger population. Additionally, it is possible that the test should be performed in combination with checkpoint inhibitors, for example.Citation41
Over the past 5 years, focus has been on the discovery and development of product candidates for the treatment of a range of cancers. GI-4000 itself has been studied in non-small cell lung cancer and colorectal cancer, as well as pancreatic cancer.Citation62 In late 2011, the investigation of tarmogens for the treatment of chronic hepatitis B infection began.Citation63
In overall conclusion, immunotherapy has great promise in the treatment of pancreatic cancer. GI-4000 for RAS-mutated pancreatic cancer is proof-of-principle of an approach investigating immune response to a specific tumor mutation. If this approach is successful in pancreatic cancer then it should be successful in other Ras-mutated tumors. It will be interesting to see whether future development of GI-4000 and related agents will result in targeted therapy that can be successfully used in our smarter war against this lethal cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Timothy Rodell, President and CEO of GlobeImmune, Inc. for his expert knowledge and insight while composing this review.
References
- American Cancer Society. American Cancer Society. Cancer Facts & Figures 2014. Atlanta; 2014.
- Cameron JL, Riall TS, Coleman J, Belcher K A. One thousand consecutive pancreaticoduodenectomies. Ann Surg 2006; 244(1):10-5; PMID:16794383; http://dx.doi.org/10.1097/01.sla.0000217673.04165.ea
- Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg 1993; 165:68-72; discussion 72-73; PMID:8380315; http://dx.doi.org/10.1016/S0002-9610(05)80406-4
- Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg 2003; 237(1):74-85; PMID:12496533; http://dx.doi.org/10.1097/01.SLA.0000041266.10047.38
- Liao WC, Chien KL, Lin YL, Lin JT, Wang HP, Tu YK. Adjuvant treatments for resected pancreatic adenocarcinoma: a systematic review and network meta-analysis. Lancet Oncol 2013; 14:1095-103; PMID:24035532; http://dx.doi.org/10.1016/S1470-2045(13)70388-7
- Yu Z, Zhong W, Tan ZM, Wang LY, Yuan YH. Gemcitabine adjuvant therapy for resected pancreatic cancer: a meta-analysis. Am J Clin Oncol 2013; Aug 7 (Epub ahead of print); http://dx.doi.org/10.1097/COC.0b013e3182a46782
- Krzyzanowska MK, Weeks JC, Earle CC. Treatment of locally advanced pancreatic cancer in the real world: population-based practices and effectiveness. J Clin Oncol 2003; 21(18):3409-14; PMID:12972517; http://dx.doi.org/10.1200/JCO.2003.03.007
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the national cancer institute of Canada clinical trials group. J Clin Oncol 2007; 25:1960-66; PMID:17452677; http://dx.doi.org/10.1200/JCO.2006.07.9525
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364(19):1817-25; http://dx.doi.org/10.1056/NEJMoa1011923
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369(18):1691-703; PMID:24131140; http://dx.doi.org/10.1056/NEJMoa1304369
- Goldstein D, Hassan R, Maraghi El, RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, Scheithauer W, Siena S, Tabernero J et al. Updated survival from a randomized phase III trial ( MPACT ) of nab -paclitaxel plus gemcitabine versus gemcitabine alone for patients ( pts ) with metastatic adenocarcinoma of the pancreas. J Clin Oncol. 2014; 32(3): Abstract 178.
- Von Hoff DD, Goldstein D, Renschler MF. Albumin-bound paclitaxel plus gemcitabine in pancreatic cancer. N Engl J Med 2014; 370(5):479-80; PMID:24476438; http://dx.doi.org/10.1056/NEJMc1314761
- American Society of Clinical Oncology (ASCO). FDA approves Abraxane plus gemcitabine for metastatic pancreatic cancer. Available at: http://www.asco.org/advocacy/fda-approves-abraxane-plus-gemcitabine-metastatic-pancreatic-cancer
- Hauschild A, Volkenandt M. [Adjuvant therapy of malignant melanoma]. Ther Umsch 1999; 56(6):324-9. Available at: http://www.mrw.interscience.wiley.com/cochrane/clcentral/articles/636/CN-00165636/frame.html; http://dx.doi.org/10.1024/0040-5930.56.6.324
- Hauschild A. Adjuvant interferon alfa for melanoma: new evidence-based treatment recommendations? Curr Oncol 2009; 16(3):3-6. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2695706&tool=pmcentrez&rendertype=abstract; PMID:19526078; http://dx.doi.org/10.3747/co.v16i3.447
- Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, Seipp CA, Rogers-Freezer L, Morton KE, White DE, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal Cancer. J Clin Oncol 2003; 21(16):3127-32; http://dx.doi.org/10.1056/NEJMc1314761; 10.1200/JCO.2003.02.122
- Tsang KY, Zaremba S, Nieroda CA, Zhu MZ, Hamilton JM, Schlom J. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst 1995; 87:982-90; PMID:7629885; http://dx.doi.org/10.1093/jnci/87.13.982
- Marshall JL, Hawkins MJ, Tsang KY, Richmond E, Pedicano JE, Zhu MZ, Schlom J. Phase I study in cancer patients of a replication-defective avipox recombinant vaccine that expresses human carcinoembryonic antigen. J Clin Oncol 1999; 17(1):332-7.
- Bilusic M, Heery CR, Arlen PM, Rauckhorst M, Apelian D, Tsang KY, Tucker JA, Jochems C, Schlom J, Gulley JL MR. Phase I trial of a recombinant yeast-CEA vaccine (GI-6207) in adults with metastatic CEA-expressing carcinoma. Cancer Immunol Immunother. 2014; 63(3):225-34; PMID:24327292; http://dx.doi.org/10.1007/s00262-013-1505-8
- Nordqvist C. Therion reports results of phase 3 PANVAC-VF trial and announces plans for company sale [press release]. PR Newswire. http://www.lifesciencesworld.com/news/view/7586. Published 2006
- Rahma OE, Khleif SN. Therapeutic vaccines for gastrointestinal cancers. Gastroenterol Hepatol 2011; 7(8):517-64.
- Azijli K, Stelloo E, Peters GJ, VAN DEN Eertwegh AJM. New developments in the treatment of metastatic melanoma: immune checkpoint inhibitors and targeted therapies. Anticancer Res 2014; 34(4):1493-505. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24692676; PMID:24692676
- Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel T, Harrison MR, Vaishampayan UN, Drabkin HA, George S, Logan TF, et al. Nivolumab for metastatic renal cell carcinoma (mRCC): results of a randomized, dose-ranging phase II trial. ASCO Meet Abstr Jun 11, 2014:5009 Available at: http://meeting.ascopubs.org/cgi/content/abstract/32/15_suppl/5009
- Amin A, Plimack ER, Infante JR, Ernstoff MS, Rini BI, McDermott DF, Knox JJ, Pal SK, Voss MH, Sharma P et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC). ASCO Meet Abstr 2014; 32 (15 suppl):5010. Available at: http://meeting.ascopubs.org/cgi/content/abstract/32/15_suppl/5010
- Hammers HJ, Plimack ER, Infante JR, Ernstoff MS, Rini BI, McDermott DF, Razak ARA, Pal SK, Voss MH, Sharma P et al. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC). ASCO Meet Abstr 2014; 32 (15 suppl):4504. Available at: http://meeting.ascopubs.org/cgi/content/abstract/32/15_suppl/4504
- Bryant KL, Mancias JD, Kimmelman AC, Der CJ. KRAS: feeding pancreatic cancer proliferation. Trends Biochem Sci 2014; 39(2):91-100; PMID:24388967; http://dx.doi.org/10.1016/j.tibs.2013.12.004
- Zorde Khvalevsky E, Gabai R, Rachmut IH, Horwitz E, Brunschwig Z, Orbach A, Shemi A, Golan T, Domb AJ, Yavin E, et al. Mutant KRAS is a druggable target for pancreatic cancer. Proc Natl Acad Sci U S A 2013; 110(51):20723-8; PMID:24297898; http://dx.doi.org/10.1073/pnas.1314307110
- Eser S, Schnieke A, Schneider G, Saur D. Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer 2014; 111:817-22; http://dx.doi.org/10.1038/bjc.2014.215
- Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res 2012; 5(1):19-27. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24603996; PMID:22574233
- Goodsell DS. The molecular perspective: the ras oncogene. Oncologist 1999; 4(3):263-4; PMID:10394594
- Alberts SR. K-ras inhibitors and pancreatic cancer. M. D. Anderson Solid Tumor Oncol Ser 2008:601-7 http://link.springer.com/chapter/10.1007%2F978-0-387-69252-4_35
- Wang JY, Wang YH, Jao SW, Lu CY, Kuo CH, Hu HM, Hsieh JS, Chong IW, Cheng TL, Lin SR. Molecular mechanisms underlying the tumorigenesis of colorectal adenomas: correlation to activated K-ras oncogene. Oncol Rep 2006; 16:1245-52; PMID:17089045
- Slebos RJC, Hoppin JA, Tolbert PE, Holly EA, Brock JW, Zhang RH, Bracci PM, Foley J, Stockton P, McGregor LM, et al. K-ras and p53 in Pancreatic Cancer : Association with Medical History, Histopathology, and Environmental Exposures in a Population-based Study. 2000; 9 11:1223-32
- Coeshott C, Holmes T, Mattson A, Bellgrau D, King T, Guo Z, Roder H, Roder J, Cohn A, Rodell TC. Immune Responses To Mutated Ras - Development Of A Yeast Based Immunotherapeutic. In: AACR-RAS Oncogene Conference 2014.
- Rozenblum E, Schutte M, Goggins M, Hahn SA, Panzer S, Zahurak M, Goodman SN, Sohn TA, Hruban RH, Yeo CJ, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res 1997; 57:1731-4; PMID:9135016
- Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer 2002; 2(12):897-909; PMID:12459728; http://dx.doi.org/10.1038/nrc949
- Collins MA, Bednar F, Zhang Y, Brisset JC, Galbán S, Galbán CJ, Rakshit S, Flannagan KS, Adsay NV, Pasca di Magliano M. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. 2012; 122(2):639-53; PMID:22232209; http://dx.doi.org/10.1172/JCI59227DS1
- Di Magliano MP, Logsdon CD. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology 2013; 144(6):1220-9; PMID:23622131; http://dx.doi.org/10.1053/j.gastro.2013.01.071
- Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, et al. Oncogenic kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012; 149(3):656-70; PMID:22541435; http://dx.doi.org/10.1016/j.cell.2012.01.058
- Berndt N, Hamilton AD, Sebti SM, Targeting protein prenylation for cancer therapy. Nat Rev Cancer 2011; 11:775-91; PMID:22020205.
- Richards DA, Muscarella P, Bekaii-Saab T, Wilfong LS, Velanovich V, Raynov J, Flynn PJ, Fisher WE, Whiting SH, Timcheva C, et al. A proteomic signature predicts response to a therapeutic vaccine in pancreas cancer; analysis from the GI-4000-02 trial. In: AACR Annual Meeting 2014, Abstract # 5314.
- Fruci D, Lo Monaco E, Cifaldi L, Locatelli F, Tremante E, Benevolo M, Giacomini P. T and NK cells: two sides of tumor immunoevasion. J Transl Med 2013; 11:30. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3621684&tool=pmcentrez&rendertype=abstract; PMID:23379575; http://dx.doi.org/10.1186/1479-5876-11-30
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144(5):646-74; PMID:21376230; http://dx.doi.org/10.1016/j.cell.2011.02.013
- Yang L, Pang Y, Moses HL. TGF-Beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol 2010; 31(6):220-7; PMID:20538542; http://dx.doi.org/10.1016/j.it.2010.04.002
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 2009; 182(8):4499-506; PMID:19342621; http://dx.doi.org/10.4049/jimmunol.0802740
- Chatterjee B, Smed-Sörensen A, Cohn L, Chalouni C, Vandlen R, Lee BC, Widger J, Keler T, Delamarre L, Mellman I. Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells. Blood 2012; 120(10):2011-20; PMID:22791285; http://dx.doi.org/10.1182/blood-2012-01-402370
- Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol 2009; 10(12):1237-44. Available at: http://www.nature.com.proxy.library.georgetown.edu/ni/journal/v10/n12/full/ni.1822.html. Accessed May 2, 2014; PMID:19915624; http://dx.doi.org/10.1038/ni.1822
- Fehres CM, Unger WWJ, Garcia-Vallejo JJ, van Kooyk Y. Understanding the biology of antigen cross-presentation for the design of vaccines against cancer. Front Immunol 2014; 5:149. Available at: http://www.frontiersin.org/Journal/10.3389/fimmu.2014.00149/abstract. Accessed May 1, 2014; PMID:24782858; http://dx.doi.org/10.3389/fimmu.2014.00149
- Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res 2007; 67(19):9518-27; PMID:17909062; http://dx.doi.org/10.1158/0008-5472.CAN-07-0175
- Collins MA, Pasca di Magliano M. Kras as a key oncogene and therapeutic target in pancreatic cancer. Front Physiol 2013; 4(January):407; PMID:24478710; http://dx.doi.org/10.3389/fphys.2013.00407
- Vonderheide RH, Bayne LJ. Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr Opin Immunol 2013; 25(2):200-5; PMID:23422836; http://dx.doi.org/10.1016/j.coi.2013.01.006
- Steele CW, Jamieson NB, Evans TR, McKay CJ, Sansom OJ, Morton JP, Carter CR. Exploiting inflammation for therapeutic gain in pancreatic cancer. Br J Cancer 2013; 108(5):997-1003; PMID:23385734; http://dx.doi.org/10.1038/bjc.2013.24
- Soares KC, Zheng L, Edil B, Jaffee EM. Vaccines for pancreatic cancer. Cancer J 2012; 18(6):642-52. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3539747&tool=pmcentrez&rendertype=abstract; PMID:23187853; http://dx.doi.org/10.1097/PPO.0b013e3182756903
- Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell 1988; 53(4):549-54; PMID:2453289; http://dx.doi.org/10.1016/0092-8674(88)90571-5
- Smit VT, Boot AJ, Smits AM, Fleuren GJ, Cornelisse CJ, Bos JL. KRAS codon 12 mutations occur very frequently in pancreatic adenocarcinomas. Nucleic Acids Res 1988; 16(16):7773–82; http://dx.doi.org/10.1093/nar/16.22.10952
- Niccolai E, Prisco D, D’Elios MM, Amedei A. What is recent in pancreatic cancer immunotherapy? Biomed Res Int 2013; 2013:492372; PMID:23509731
- Koido S, Homma S, Takahara A, Namiki Y, Tsukinaga S, Mitobe J, Odahara S, Yukawa T, Matsudaira H, Nagatsuma K, et al. Current immunotherapeutic approaches in pancreatic cancer. Clin Dev Immunol 2011; 2011:267539; PMID:21922022
- Shahda S, O’Neil B. GI-4000 in KRAS mutant cancers. Expert Opin Investig Drugs 2014; 23(2):273-8. Available at: http://informahealthcare.com/doi/abs/10.1517/13543784.2014.876408. Accessed May 5, 2014
- Sam HW, Muscarella P, Rosenmurgy E, Fisher WE, Richards DA, Harrell F Jr., Ferraro J, Speyer S, Cohn A. A randomized, placebo-controlled, multicenter phase II adjuvant trial of the efficacy, immunogenicity, and safety of GI-4000 plus gemcitabine versus gemcitabine alone in patients with resected pancreatic cancer with activating ras mutations. J Clin Oncol 2010; 28 15s (suppl; abstr TPS226). Available at: http://meetinglibrary.asco.org/content/42584-74 (accompanying poster).
- Globoimmune. GlobeImmune power point presentation: recombinant DNA advisory committee – GI-6301. Available at: http://osp.od.nih.gov/sites/default/files/1_1107_1119_Gulley.pdf
- Cohn A, Morse MA, O’Neil B, Bellgrau D, Duke RC, Franzusoff AJ, Munson S, Ferraro J, Rodell TC. Treatment of Ras mutation-bearing solid tumors using whole recombinant S. cerevisiae yeast expressing mutated Ras: preliminary safety and immunogenicity results from a phase 1 trial. J Clin Oncol 2005; 23(16 Suppl):2571
- Chaft JE, Litvak A, Arcila ME, Patel P, D’Angelo SP, Krug LM, Rusch V, Mattson A, Coeshott C, Park B, et al. Phase II study of the GI-4000 KRAS vaccine after curative therapy in patients with stage I-III lung adenocarcinoma harboring a KRAS G12C, G12D, or G12V mutation. Clin Lung Cancer 2014; 15 6:405-10. PMID:25044103; http://dx.doi.org/10.1016/j.cllc.2014.06.002
- GlobeImmune, Inc. GlobeImmune Announces Presentation of GI-4000 Phase 2 Data at IASLC World Conference on Lung Cancer, GlobeNewswire Europe, October 27, 2013 4:02 PM (Sydney, Australia, October 28, 2013).
