Abstract
Because of the age-related immune system decline, 2 potentiated influenza vaccines were specifically licensed for the elderly: Fluad®, an MF59-adjuvanted vaccine administered intramuscularly (IM-MF59), and Intanza 15mcg®, a non adjuvanted vaccine administered intradermally (ID). The objective of this paper was to conduct a systematic review of studies that evaluated antibody responses in the elderly following immunization with IM-MF59 or ID vaccines. The two potentiated vaccines induced immune responses satisfying, in most instances, the European Medicine Agency immunogenicity criteria, both against vaccine antigens and heterovariant drifted strains. Considering pooled data reported in the articles analyzed and papers directly comparing the 2 vaccines, the antibody responses elicited by IM-MF59 and ID were found to be generally comparable. The use of IM-MF59 and ID vaccines can be proposed as an appropriate strategy for elderly seasonal influenza vaccination although further studies are required for a more complete characterization of the 2 vaccines.
Introduction
Annual influenza infection is a major cause of substantial morbidity and mortality, particularly in the elderly and in the other risk groups. According to previous reports in the USA, the number of annual influenza-associated hospitalizations in each influenza season between 1979 and 2001 ranged from 55,000 to 431,000,Citation1 and 90% of the 36,000 annual influenza-associated respiratory and circulatory-related deaths occurred in the 1990s among persons older than 65.Citation2 Influenza vaccines represent the major tool to reduce the burden of the disease. However, despite a high vaccination rate, vaccine efficacy among elderly is lower than among healthy adults.Citation3-5 The weakened immune response observed in elderly people, defined as immunosenescence,Citation6 is considered to be one of the main reason of the higher risk for influenza-related complications that could lead to hospitalization and death and of the reduced efficacy of influenza vaccination. The reduced immunogenicity and effectiveness of influenza vaccines in subjects with higher risk of influenza-related complications, hospitalizations and deaths, led the innovative drive to search for new strategies to implement the immune response elicited by plain influenza vaccines. Several strategies have been proposed to address the need for vaccines offering enhanced protection against homologous and drifted strains, including addition of adjuvants, use of increased dosages, multiple dose vaccination, and use of alternative and more efficient routes of antigen delivery.Citation7-9 Although most of these preparations did not meet the approval criteria or did not reach the market, 2 of these that showed higher immunogenicity and an acceptable safety profile, MF59-adjuvanted vaccine Fluad®, Novartis Vaccines and the ID vaccine Intanza 15mcg®, Sanofi Pasteur MSD SNC, were approved for the elderly population.
In this study, we analyzed data available in literature on the ability of the 2 potentiated vaccines to elicit an effective antibody response in volunteers aged over 60 y, satisfying the European Medicine Agency (EMA) immunogenicity criteria,Citation10 against vaccine antigens (homologous responses) and, when the data were available, against drifted viruses (heterologous responses).
Characteristics of the Two Enhanced Vaccines: Fluad® and Intanza 15mcg®
Fluad® (Novartis Vaccines, Italy) is an MF59-adjuvanted trivalent influenza vaccine, licensed in Italy in 1997, and administered intramuscularly into the deltoid muscle (IM-MF59). It is an inactivated subunit vaccine containing the standard dosage of 15 μg in each 0.5 ml dose of highly-purified influenza surface haemagglutinin (HA) antigen per strain and an oil-in-water emulsion of squalene, a naturally occurring biodegradable and biocompatible substance, found in the liver in a wide range of species, including humans.Citation11 The aim of the MF59 adjuvant is to increase the vaccine immunogenicity.Citation12,13 The mechanism of action of MF59 is not fully understood. Initially it was thought that the enhance of protection might be due to a depot effect: delivering the antigen in an oil-in-water emulsion would result into a slow release of the antigen and a more effective priming of the immune system. Indeed MF59 emulsion was found to induce a local immune-stimulatory environment, which is able to optimize the activation of the innate immune response at the injection site, by recruiting and activating antigen-presenting cells (APCs). APCs acquire an increased ability to capture, transport and process co-administered antigens from the peripheral tissues to local lymph nodes and consequently, stimulate an effective adaptive memory immune response specific to the vaccine.Citation13-15
Intanza 15mcg® (Sanofi Pasteur MSD SNC, France) is a split trivalent non-adjuvanted influenza vaccine administered intradermally (ID) which uses an innovative microinjection system (Soluvia, Becton Dickinson, USA) and has been available in Italy since the Winter season 2010–2011. The manufacturing processing is based on that used for the intramuscular seasonal vaccine although a lower volume is used for intradermal vaccine (15 μg HA/strain per 0.1 ml instead of 0.5 ml). Intradermal vaccination is a more efficient route for influenza vaccinesCitation16-20 and is supposed to increase vaccine immunogenicity by reliably delivering the vaccine into the immune-rich environment of the dermis where the presence of a high density of lymphatic and capillary vessels facilitates a direct contact between administered antigens and immune system.Citation9,21 The exact mechanisms involved in intradermal immunization are not fully understood, however, it is known that skin generates both innate and adaptive immune system responses. Two types of professional APCs (Langherans cells in the epidermis and dermal dendritic cells (DCs) in the dermis) play a pivotal role in skin's innate immune response and induction of adaptive immune response against pathogens.Citation21,22 These DCs favor rapid capture and movement of antigen via lymphatic vessels to lymph nodes, thus facilitating lymph node T and B cells activation/expansion and induction of antigen-specific humoral and cellular immunity.Citation21,23,24 Antigens can also drain into the lymph nodes with no involvement of peripheral tissue DCs and be captured by lymph node resident DCs from skin migratory DCs, with subsequent priming of naïve T cells.Citation25
Clearly, the immune response induced by intradermal antigen delivery is generated by a mechanism which markedly differs from that of the intramuscular route.Citation7,9
The deep muscle is relatively inefficient in capturing antigens by immune cells, and only circulating DCs are able to capture antigens and migrate to regional lymph nodes via the lymphatic conduits or bloodstream. Vaccine antigens delivered to the skin are more likely to be captured by resident APCs, such as Langherans cells and dermal DCs, and transported to the draining lymph nodes for B cells to respond.
IM-MF59 and ID influenza vaccines were repetitively examined for safety and tolerability in clinical trials. Although a number of results were available, 2 meta-analysis on IM-MF59Citation26,27 and 2 randomized controlled trials on IDCitation28,29 including safety data in adults >60 y of age with and without underlying diseases were identified. These studies included trials comparing IM-MF59 or ID with non-adjuvanted influenza vaccines; however we considered results concerning the 2 potentiated vaccines only. The analysis of solicited local and systemic reactions that occurred 0/3–7 d after vaccination revealed a constant rate in subjects receiving IM-MF59 or ID, generally higher as compared with volunteers receiving a conventional influenza vaccine. However, the majority of solicited reactions were local, and mild or moderate in intensity both after IM-MF59Citation26,27 and ID.Citation28,29 Recently, 2 different studies carried out by Van Damme et al.Citation30 and by Scheifele et al.Citation31 directly compared, in addition to immunogenicity, safety and tolerability, of IM-MF59 and ID in the elderly. Adverse events in the first 3 d post-vaccination were similar for the 2 vaccines and symptoms were mild to moderate. Injection–site reactions assessed in the first 6–7 d post-vaccination confirmed previous data and appeared to be more common in the ID group as compared with IM-MF59 group. Erythema or local inflammation were the most frequent symptoms among ID recipients and were reported in 63.1% by Van Damme et al.Citation30 and in 76.2% by Scheifele et al.Citation31 of ID volunteers, as compared with 13.4% and 13.0% of the IM-MF59 recipients, respectively. The percentages of people showing swelling and induration were lower but again more frequent following injection of ID than IM-MF59. Comparing ID with IM-MF59, swelling was found in 34.2% vs. 8.6%Citation30 and 49.2% vs. 12.0%Citation31 and induration in 32.9% vs. 10.6%Citation30 and 46.5% vs. 8.0%,Citation31 respectively. Injection site pain seemed to be similar between IM-MF59 and ID groups in the data reported by Van Damme et al.Citation30 (20.9% vs. 19.8%) and slightly higher in the IM-MF59 group as reported by Scheifele et al. (37.9% vs. 29.7%).Citation31 Systemic adverse events were generally mild, transient and similar between the two groups.
Serological Criteria for Influenza Vaccine Evaluation
For influenza vaccines there are protection correlates that although being imperfect are based on immunological criteria shared by the international scientific community and regulatory agencies that recognize that serological immune criteria may support the approval of influenza vaccines.Citation10,32
The ability to elicit HA strain-specific antibodies in the serum of immunized persons is the major indicator of influenza vaccine immunogenicity used for vaccine studies and licensure and a marker of protective efficacy. To date 2 different assays qualified for detection of anti-HA influenza strain-specific antibodies are used for vaccine licensure: the haemagglutination inhibiting assay (HI) and the single radial hemolysis test (SRH).Citation33 The HI assay is the most widely used because of its relatively simplicity. The HI test takes advantage of the influenza virus ability to agglutinate red blood cells via HA binding to sialic residues on red blood cells. Antibodies directed to HA antigen can inhibit this haemagglutination, and HI titers are expressed as the reciprocal of the highest dilution of sera that inhibits haemagglutination. Different studies suggest that HI titers ≥40 can be considered as a 50% protective titer.Citation32 However there is the need to better define correlates of protection and there are some perplexities on the identification of a single threshold HI titer for defining protection.Citation34 The SRH is a different test that can be used to detect antibodies to HA, but is less frequently reported.Citation33 The test is based on immunodiffusion of antibodies against HA in agarose gel containing influenza virus bound to red blood cells and complement. The presence of anti-HA antibodies is detected by the lysis of red blood cells and antibody titers are measured in square millimeters (mm2) of the zone of lysis.
Parameters including seroprotection rate (proportion of individuals achieving an HI titer of ≥40 or an SRH lysis area ≥25 mmCitation2), ratio of post-vaccination to pre-vaccination geometric mean titer values (GMTR) and seroconversion rate (proportion of individuals that have a fourfold or grater rise in HI titer or an SRH area increase >50% from pre- to post-vaccination titer, and seronegative individuals that achieve a titer of ≥40 or an SRH area ≥25 mm2) are conventionally used to estimate the advantages provided by vaccines and to support licensure of inactivated vaccines. Up to the present time, in Europe, for annual licensure to be granted for a specific influenza vaccine in the pre-defined age groups, EMA requires that post-vaccination titers against influenza HA antigens meet specific criteria.Citation10 For individuals ≥60 y at least one of the following values must be met for each vaccine strain: seroprotection rate ≥60%, GMTR ≥2 and seroconversion rate ≥30%.Citation10
Finally, it is important to underline that a third test, the neutralization (NT) assay, would represent a useful adjunct for detection and evaluation of antibody responses induced by influenza vaccine administration.Citation32 NT assay detects antibodies that inhibit virus replication and is considered to provide a more functional assessment of vaccine-induced immunity. Moreover, since in many instances it has been found to be more sensitive than HI test in detecting higher antibody titers, it might be preferable to confirm the immunogenicity data by using NT assay,Citation35 although to the present time NT test is not included in the assays used for vaccine licensure, since there is no known protection titer for NT antibodies.
Literature Data Search Strategy
This review includes data from male and female subjects, aged 60 or more, immunized with influenza seasonal trivalent IM-MF59 or ID vaccine who participated in well-designed controlled clinical studies and were examined for vaccine induced antibody responses.
A systematic search for studies published from 1999 to 2014 was conducted in Medline, EMBASE, Cochrane Library, BioMED, SIGN, GIMBE, and NICE. The keywords used for the search were “Fluad Influenza Vaccination Elderly,” “MF59 Influenza Vaccination Elderly,” “Intanza Influenza Vaccination Elderly,” “Intradermal Influenza Vaccination Elderly,” “Influenza Vaccines,” “Influenza vaccine immunogenicity.” To identify the studies to include in our systematic review, we followed the Meta-analysis Of Observational Studies in Epidemiology guidelines.Citation36 The relevance of the references identified was evaluated independently by the authors by reading the titles and the abstracts. The full papers of the articles considered relevant were then obtained.
Twenty five studies were included in this review.Citation4,30,31,35,Citation37-57 These studies recruited subjects who would normally be candidates for routine seasonal influenza vaccination and the strain composition of the influenza vaccines used conformed to the yearly requirements of the World Health Organization. The population consisted of elderly subjects with and without underlying diseases,Citation30,35,Citation37-42,Citation56,57 with unknown health statusCitation43-48 or healthy.Citation4,31,Citation49-55 No sub-analyses were conducted to evaluate potential differences in the outcome between these groups.
The studies were prevalently conducted with the aim of comparing the 2 enhanced vaccines with conventional vaccines. However, in this review we considered only immunogenicity data of the 2 potentiated vaccines. Although in the absence of a standardization of the existing assays, the homologous and heterologous HI antibody responses induced by IM-MF59 and ID in people aged ≥60 y were explored as reported in the studies reviewed, considering if EMA immunogenicity criteria were satisfied.Citation10 Since results from some of the studies were reported as figures instead of numerical data, only the fulfillment, if any, of EMA requirements was considered. In the description of the results obtained, when we reported the range of the values observed we considered only studies showing numerical data.
Results
Immunogenicity of FLUAD and INTANZA 15mcg® in elderly people: homologous responses
The HI antibody responses induced by vaccine administration against the 3 vaccine strains of the seasonal influenza vaccine examined are presented in for IM-MF59 vaccine and in for ID vaccine.
Table 1. Immunogenicity of IM-MF59 influenza vaccine against homologous vaccine strains in volunteers 60 y of age and older
Table 2. Immunogenicity of ID influenza vaccine against homologous vaccine strains in volunteers 60 y of age and older
As reported in , 25 studies on immune response of people aged ≥60 y after immunization with seasonal IM-MF59 were included in our systematic review for a total of 3492 subjects for A/H3N2 antigen, 3396 for A/H1N1 and 3545 for B antigen. One of the studies evaluated the results over a period of 3 winter seasons.Citation51 Three trials examined responses against A/H3N2 strain only Citation35,37,46 and one against B strain only.Citation42 The results of 13 clinical trials, representing an integrated development program of the manufacture, for a total of 2012 subjects vaccinated with IM-MF59 and 1498 with a comparator non-adjuvanted vaccine, reported in 2 meta-analysesCitation26,27 were not considered since results were reported as pooled analyses and expressed as IM-MF59 to comparator ratio. However, data from some of the 25 trials herein consideredCitation4,38,45,51 are included in the 2 meta-analyses.
For all the 25 trials, with a few exceptions, it was possible to examine numerical data or only fulfillment or not of EMA requirements for elderly people for post-vaccination seroprotection rate, GMTR (except for Baldo 2006Citation39), and seroconversion rate (except for Del Giudice et al. 2006Citation46 and Camilloni et al. 2009Citation42). Protective HI titers higher than the generally accepted value of 40 were considered by Minutello et al.Citation51 and De Donato et al.Citation45 (HI ≥128) and Gasparini et al. (HI ≥160).Citation4
With regard to responses against A/H3N2 antigen, the requested post-vaccination value of at least 60% of people with HI protective titers was always reached (range 70.8–100%) with 2 exceptions, both considering as protective titers higher than 40, Minutello et al.Citation51 (third year of observation, 51%) and Gasparini et al.Citation4 (51%). The requested values of GMTR (≥2) and of seroconversion (≥30%) were always satisfied and ranged from 2.4 to 17.6 and from 30.0 to 92.9%, respectively.
The examination of HI titers against A/H1N1 antigen showed that the values of seroprotection were >60% (range 71.6–100%) with the exclusion of the first year of observation for Minutello et al.Citation51 (22.0%, protective titers ≥128) and for Basileo et al.Citation41 (40.1%), the values of GMTR >2 (range 2.1–13.7) except for Minutello et al.Citation51 (1.8, third year of observation) and Gasparini et al.Citation4 (1.9). The requested value of at least 30% of seroconversion was not reached in 6 instancesCitation4,41,48,49,51 with percentages ranging from 20.0 to 28.3%, whereas when the percentages were satisfied, they ranged from 32.0 to 94.4%.
The responses against B antigen were somewhat lower since the 60% of seroprotected individuals in 7 of the studies (range 35.7–58.2%)Citation4,30,43,50,51,55,56 and the 30% of seroconversions in 6 of the trials (range 10.0–26.9%)Citation30,31,43,51,56,57 were not reached. When the requested criteria were satisfied the percentages of seroprotected individuals ranged from 63.0 to 100% and seroconversion percentages ranged from 32.8 to 89.0%. The GMTR was always >2 (range 2.1–16.2) with 3 exceptions, Camilloni et al. 2009Citation42 (1.8), Scheifele et al.Citation31 (1.6) and Camilloni et al. 2014Citation57 (1.6).
Considering all the studies examined, the results obtained showed that IM-MF59 vaccine met, as requested, in most instances 3 or at least 2 EMA immunogenicity criteria. The worst, but still acceptable results, were obtained by Basileo et al.Citation41 against A/H1N1 antigen and by Minutello et al.Citation51 the third year of observation and by Scheifele et al.Citation31 and Camilloni et al. 2014Citation57 against B antigen, when only one of the EMA criteria was satisfied.
reports HI antibody responses induced following administration of ID vaccine using BD's Soluvia to volunteers aged ≥60 y in the 9 different clinical studies considered in the present review.Citation28-31,Citation41,57-60 In the study by Arnou et al.Citation28 the results over 3 consecutive years were reported and in one study (Ansaldi et al. 2012Citation58) only the results for A/H3N2 strain were included. The total number of subjects examined was 5035 for A/H3N2 and 5100 for A/H1N1 and B antigens. Some of the clinical trials evaluated the immunogenicity of different doses of Intanza® and compared the results with other vaccines; however, in our review only the results obtained with Intanza 15mcg® were examined.
All the studies satisfied the 3 EMA criteria for the A/H3N2 and A/H1N1 strains, with the exception of Basileo et al.Citation41 against A/H1N1 antigen when only the GMTR value was met. For results satisfying EMA requirements, the seroprotection rate ranged from 71.0 to 98.1% and from 63.0 to 93.1%; the GMTR from 2.6 and 8.2 and from 2.0 to 8.1 and, finally, seroconversion from 33.7 to 71.8% and from 35.2 to 76.3%, respectively for A/H3N2 and A/H1N1 antigens. As found for IM-MF59, the HI antibody response induced by B strain was lower. The required 60% of seroprotected volunteers was reached in only some of the studies reporting the results for B strain with values ranging from 66.1 to 100%. Values below 60% were found in the first and second year of Arnou et al.Citation28 (55.7 and 59.9%, respectively) and in Van Damme et al.Citation30 The GMTR was in most instances >2 (range 2.5 and 3.7) except in the second year of Arnou et al.,Citation28 Scheifele et al.Citation31 (1.6) and Tsang et al.Citation60 (1.7) studies. Seroconversion rates >30% were found in 6 trials (range 36.4–46.0%). The values of the studies that did not satisfy EMA criteriaCitation28,30,31,60 ranged from 15.8 to 17.2%. In all the years studied, 3 or at least 2 of the EMA immunogenicity criteria were satisfied, except the second year of the study by Arnou et al.Citation28 when none of the immunogenicity criteria was satisfied and the studies conducted by Van Damme et al.,Citation30 Scheifele et al.Citation31 and Tsang et al.Citation60 when only one value was reached. However, Van Damme et al.,Citation30 and Scheifele et al.,Citation31 measured antibody titers using both HI and SRH methods. The study by Van Damme et al.,Citation30 showed that the results obtained by using SRH satisfied all 3 EMA immunogenicity criteria not only against A/H3N2 and A/H1N1 strains, confirming data observed using HI method, but also against B antigen, against which only one of the 3 criteria was satisfied using HI test. On the other hand, the study of Scheifele et al.,Citation31 showed that the results obtained by using HI method satisfied all 3 EMA criteria against A/H3N2 and A/H1N1 strains and only one against B strain. Conversely, by using SRH method the fulfilled criteria were 2 against A strains and the same one against B strain.
A direct comparison of immunogenicity of IM-MF59 and ID vaccine, measuring antibody titers both using HI and SRH methods, was recently reported by Van Damme et al.Citation30 and by Scheifele et al.Citation31 who examined elderly people immunized for 2007/2008 and for 2011/2012 Winter seasons, respectively.
Van Damme et al.Citation30 found that there were no differences between the 2 vaccine groups in GMTR, seroprotection and seroconversion rates for the 3 strains by either HI or SRH method with the exception of seroprotection rate for the A/H1N1 strain. Seroprotection rates were high in both groups, but significantly higher in the IM-MF59 group (differences of 5.8% (0.7–10.9) and 5.8% (1.1–10.5) by HI and SRH method respectively). Moreover, considering an arbitrary definition of non-inferiority (upper limit of the 95% confidence intervals around the post-vaccination ratios of GMT IM-MF59/ID being <1.5), Van Damme et al.Citation30 demonstrated GMT non inferiority of ID vs. IM-MF59 vaccine for A/H1N1 and B strains with HI method and for all 3 strains with SRH method. In addition, post-hoc analysis to adjust for baseline antibody titers demonstrated ID vaccine non-inferiority using both HI and SRH methods for all 3 strains.
Scheifele et al.Citation31 found that 21 d after vaccination seroprotection rates and GMT values were significantly higher after IM-MF59 vaccine vs. ID vaccine both against A/H1N1 and A/H3N2 strains when measured by HI and SRH methods, with the exception of seroprotection rates against A/H1N1 measured by SRH method that were similar in the 2 vaccine groups. However, the differences although being statistically significant, were modest. Meaningful response assessment against B vaccine strain was not possible due to the presence of very high baseline titers. Using the same arbitrary definition of non-inferiority used by Van Damme et al,Citation30 Scheifele et al.Citation31 found that HI responses against A/H3N2 and A/H1N1 antigens did not meet the non-inferiority criteria for ID relative to IM-MF59 vaccine, whereas the results were similar to all 3 vaccine antigens when assessed by SRH.
Results obtained by our research group comparing the immunogenicity evaluated as HI antibody induction of IM-MF59 and ID in elderly institutionalized volunteers during the 2011–2012Citation57 and the 2012–2013Citation41 Winter seasons were recently published. The results observed in the 2011–2012 Winter season, the same examined by Scheifele et al.,Citation31 showed that against A/H3N2 and A/H1N1 antigens both vaccines induced significant comparable increases in HI titers and the responses satisfied all 3 EMA requirements. Against B strain both vaccines stimulated responses lower as compared with the A antigens, moreover the responses were generally higher after ID than IM-MF59 and all 3 EMA criteria were met after ID and only one (seroprotection rate) after IM-MF59. Considering the results of the 2012–2013 winter seasonCitation41 both vaccines induced comparable significant HI antibody responses against the 3 vaccine strains. All three EMA criteria were satisfied against A/H3N2 and B antigens, whereas responses against A/H1N1 antigen were somewhat lower since only one criteria was met with both vaccines. Responses against B antigen tended to be higher in subjects vaccinated with ID than those immunized with IM-MF59 vaccine again, as observed in the Winter 2011–2012.Citation57
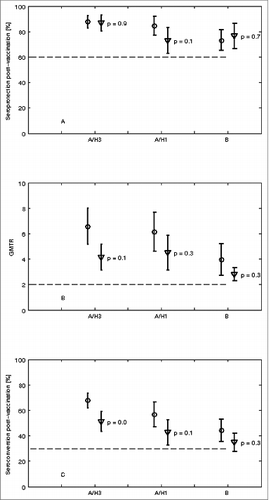
Finally, in order to try to have more information about possible differences between IM-MF59 and ID vaccine, immunogenicity data reported for the 2 vaccines in the different studies analyzed in this review were compared. Because of the different years in which IM-MF59 and ID vaccines were commercialized, 1997 and 2010 respectively, the years examined, the vaccine composition and the number of people studied, differ between the 2 vaccines. In order to analyze if differences between the pooled data from the studies analyzed for the 2 vaccines were significant statistically, an hypothesis test was applied. In particular, because of the differences in the data available and because the standard deviation values of the immunogenicity values were prevalently missing, a 2-samples pooled t-test at the α = 5% significance level was selected as the most appropriate hypothesis test. The mean and confidence interval values estimated for the 3 EMA immunogenicity criteria (seroprotection, GMTR and seroconversion) are reported in . Comparing the mean percentages of people who reached the state of seroprotection () or the mean values of GMTR () after IM-MF59 and ID vaccination, the data show that, although the mean estimates of the 2 vaccines against the 3 vaccine antigens were different, the confidence intervals were overlapping and all p values were greater than 0.05. As a consequence, the hypothesis that the corresponding true mean values are not significantly different cannot be accepted. Similar results were observed considering the mean seroconversions rate estimates against A/H1N1 and B antigens after the 2 vaccines (). Conversely, confidence intervals of the seroconversions mean values against A/H3N2 were not overlapping and the p value was smaller than 0.05, suggesting that the hypothesis that the seroconversions mean values against A/H3N2 antigens after IM-MF59 and ID vaccine are not equal, with higher values after IM-MF59 administration with respect to ID.
Figure 1. (A-C). Comparison of pooled literature data of EMA immunogenicity criteria (seroprotection, GMTR and seroconversion) against the 3 influenza antigens after immunization of people aged ≥60 y with IM-MF59 or ID influenza vaccines. The abscissa in each figure reports the antigen A/H3, A/H1 and B and each symbol represents the estimated mean value with its 95% confidence interval of the parameter indicated on the ordinate axis for the 2 vaccines (circled markers (•) represent IM-MF59; triangular markers (▾) represent ID). The horizontal dashed line indicates the EMA threshold level for each parameter.
Immunogenicity of FLUAD and INTANZA 15mcg® in elderly people: heterologous responses
In addition to the suboptimal immunogenicity of influenza vaccines in elderly people, another important limitation of influenza vaccination, during the inter-pandemic periods, is a direct consequence of the antigenic drift due to the accumulation of point mutations on genes encoding the 2 surface proteins, HA and neuraminidase, resulting in changes in the antigenic characteristics of the circulating strains, possibly determining significant mismatches between the latter and the viral variants included in the vaccine, with consequent inadequate protection of vaccines.Citation61 The availability of influenza vaccines offering a higher and broader immune response than that conferred by conventional formulations could represent a fundamental challenge in the prevention of influenza.
reports data demonstrating that IM-MF59-adjuvanted and ID vaccines have been found reproducibly to confer cross-reactivity against drifted virus strains in the elderly patients aged ≥60.Citation35,37,40,42,43,46,49,55,Citation57-59 HI antibody responses against A/H3N2 co-circulating during the winter period studiedCitation37,57,58 and A/H3N2 strains recommended as vaccine component for previous or forthcoming winter seasonsCitation35,40,43,46,49,55 were found to fulfill in most instances all the 3 or at least 2 EMA immunogenicity criteria both after IM-MF59Citation35,37,40,43,46,49,55 or IDCitation57,58 vaccine administration. Similar results were obtained studying antibody responses induced by IM-MF59Citation40,43,55 or IDCitation59 vaccines against A/H1N1 chosen as vaccine strain for forthcoming winter seasonCitation40,43,55 or A/H1N1 circulating among the population.Citation59 Induction of cross-reactive HI antibodies against influenza B virus strain was examined only after IM-MF59 vaccination in 3 different studies.Citation40,42,43 Influenza B viruses fall in 2 major antigenically and genetically distinct lineages, B/Victoria and B/Yamagata, co-circulating in some years among the population and currently in many countries only one type B strain, representing one of the 2 lineages, can be incorporated into the vaccine.Citation62,63 Increases in HI antibody titers were found against drifted B strains, both belonging to the same or different lineage as compared to vaccine strain. However, the responses prevalently satisfy, both against the vaccine and the drifted strains, only one of the 3 EMA criteria.
Table 3. Immunogenicity of IM-MF59 and ID influenza vaccines against homologous and heterologous drifted strains in volunteers 60 y of age and older
Conclusions
Previous published studies have established that the immune response induced by IM-MF59 and ID potentiated vaccines, specially licensed for elderly people and marketed in Italy in 1997 and 2010 respectively, can be considered higher in elderly people as compared to conventional vaccinesCitation4,28,29,35,Citation37-40,Citation45,46,50,51,54,56,58,59; however, it is unclear if one of the 2 potentiated vaccines offers any advantages.
In order to have more information, in this review, we examined data previously obtained and published in terms of immunogenicity, evaluated in most instances as induction of HI titers according to the 3 EMA immunogenicity requirements (i.e., seroprotection, GMTR and seroconversion), studying people aged ≥60 y immunized with the 2 potentiated vaccines.
We found evidence of a good and prevalently comparable immunogenicity profile of IM-MF59 and ID potentiated vaccines in elderly subjects both against homologous and heterologous influenza virus strains.
The homologous responses against the 3 vaccine antigens were firstly examined considering the responses reported in the different studies examined only after IM-MF59 () or ID () vaccine administration, although other vaccine preparations were studied. We found that the EMA requirements in most instances were satisfied for A/H3N2 and A/H1N1 vaccine antigens with both vaccines. The responses against the B antigen were somewhat lower, since frequently only one or 2 of the 3 EMA requirements were satisfied. The reduced responses against B influenza virus strains are in accordance with previous reports suggesting that the antibody response to inactivated conventional or potentiated vaccines is substantially lower against type B antigen than against type A antigensCitation28,42,64,65 and seem to suggest that possible strategies to control influenza B viruses should be considered separately from those of influenza A viruses. However, the interpretation of these results is complicated by the observation that the HI test is considered not to be well adapted to measure the response against B influenza viruses. Ether treatment of the viral antigen used for HI testing was found to enhance sensitivity for influenza B responses Citation66,67 but the higher HI titers observed with B strain virus treated with ether seem to be less specific and not comparable with titers obtained against A antigens not treated with ether. In the absence of international protocols or validated correlation with clinical protection, the use of ether is not considered a valid solution to the problem.
Secondly, since the results examined were obtained in different studies, in order to compare this data, we examined the pooled results by applying the 2-sample t-test that takes into account both the different sample size of the 2 vaccines and the a-priori non-knowledge of their confidence intervals. The values of HI antibody titers against the 3 homologous vaccine antigens did not significantly differ across the 2 potentiated vaccines () for the 3 EMA requirements, except for the seroconversion values against A/H3N2 antigen showing a higher value after IM-MF59 administration as compared to ID vaccine.
Thirdly, the analysis of the results of the few comparative head-to-head immunogenicity studies published in the literature to date showed that the results reported were slightly varying. An higher induction of HI antibody titers by IM-MF59 compared to ID was found by Van Damme et al.Citation30 against the A/H3N2 strain, but no differences were found after adjusting titers for baseline antibody, and by Scheifele et al.Citation31 against the 2 A strains. However, responses in the 2 studies were similar when assessed by SRH method.Citation30,31 The results of our research groupCitation41,57 showed a similar immunogenicity against A/H3N2 and A/H1N1 strains and a slightly higher immunogenicity against B strain following ID administration as compared to IM-MF59 vaccine.
Finally, some of the trials examinedCitation35,37,40,42,43,46,49,55,57-59 studied the induction of heterologous HI antibody responses, i.e., responses against influenza strains not included in the vaccine used for immunization, following immunization with IM-MF59 and ID vaccines and found results generally satisfying all or at least some of the EMA requirements ().
In conclusion, data reported in this review suggest that IM-MF59 and ID vaccines might be appropriate strategies to address the challenge of declining immune responses in older people, even during seasons when antigenic drifts occur. Because of previous good safety results, despite the higher incidence of some injection-site reactions in ID vaccinated compared with IM-MF59 vaccine,Citation26-31 data reported in this review suggest that IM-MF59 and ID vaccines can provide clinicians with an opportunity to better control influenza in aged people, although, further studies and trials are desirable because of different problems.
A significant heterogeneity was found across the studies examined for the immunogenicity outcomes. Differences between studies in terms of age, sex, health conditions and previous influenza vaccinations of the elderly volunteers immunized or the antigen vaccine composition of the vaccines used in the studies considered might have influenced the results.
Inter-laboratory variability in serological techniques and determination was previously shown suggesting the need for improved standardization of assays and study design.Citation32 Immunogenicity was evaluated as the ability of vaccines to induce HI antibody responses and inter-laboratory variability for the HI assay has been previously found to be higher as compared with SRH and NT test.Citation32 HI titers were considered according to EMA immunogenicity criteriaCitation10 although there are some disagreements on the identification of a single threshold (HI titer ≥40) for defining protection.Citation34 The HI assay has some limitations in terms of sensitivity and specificityCitation32 and recent studies show that serum HI antibody titers may not be associated with the development of influenza. Moreover, because of the importance and difficulties of evaluating efficacy and effectiveness of vaccine administration, there is the need for studies to provide estimates of vaccine effectiveness during each season and to collect protection data from laboratory-confirmed cases (PCR or virus isolation). Additionally, the antibody response is not necessarily the best predictor of clinical efficacy in older adultsCitation68 and because of this possible lack of correlation, further studies are necessary to evaluate cell-mediated immunity and the association of antibody and cellular responses with clinical outcomes, including the occurrence of influenza illness, hospitalizations, and mortality.
Disclosure of Potential Conflicts of Interest
BM has received honoraria by Sanofi Pasteur MSD Italy for scientific support, writing and critical review of the manuscript; CB, NE and IAM have declared no competing interests; VS is employed by Sanofi Pasteur MSD Italy.
References
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333-40; PMID:15367555; http://dx.doi.org/10.1001/jama.292.11.1333
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179-86; PMID:12517228; http://dx.doi.org/10.1001/jama.289.2.179
- Gaillat J, Dennetiere G, Raffin-Bru E, Valette M, Blanc MC. Summer influenza outbreak in a home for the elderly: application of preventive measures. J Hosp Infect 2008; 70:272-7; PMID:18799243; http://dx.doi.org/10.1016/j.jhin.2008.07.009
- Gasparini R, Pozzi T, Montomoli E, Fragapane E, Senatore F, Minutello M, Podda A. Increased immunogenicity of the MF59-adjuvanted influenza vaccine compared to a conventional subunit vaccine in elderly subjects. Eur J Epidemiol 2001; 17:135-40; PMID:11599686; http://dx.doi.org/10.1023/A:1017919305501
- McElhaney JE, Hooton JW, Hooton N, Bleackley RC. Comparison of single versus booster dose of influenza vaccination on humoral and cellular immune responses in older adults. Vaccine 2005; 23:3294-300; PMID:15837235; http://dx.doi.org/10.1016/j.vaccine.2005.01.080
- Grubeck-Loebenstein B, Della Bella S, Iorio AM, Michel JP, Pawelec G, Solana R. Immunosenescence and vaccine failure in the elderly. Aging Clin Exp Res 2009; 21:201-9; PMID:19571643; http://dx.doi.org/10.1007/BF03324904
- Durando P, Iudici R, Alicino C, Alberti M, de FD, Ansaldi F, Icardi G. Adjuvants and alternative routes of administration towards the development of the ideal influenza vaccine. Hum Vaccin 2011; 7 Suppl:29-40; PMID:21245655; http://dx.doi.org/10.4161/hv.7.0.14560
- Atmar RL, Patel SM, Keitel WA. Intanza(®): a new intradermal vaccine for seasonal influenza. Expert Rev Vaccines 2010; 9:1399-409; PMID:21105776; http://dx.doi.org/10.1586/erv.10.134
- Icardi G, Orsi A, Ceravolo A, Ansaldi F. Current evidence on intradermal influenza vaccines administered by Soluvia licensed micro injection system. Hum Vaccin Immunother 2012; 8:67-75; PMID:22293531; http://dx.doi.org/10.4161/hv.8.1.18419
- Committee for Medicinal Products for Human Use (CHMP). Guideline on clinical evaluation of new vaccines 2005. DHHS/FDA/CBER. Guidance for industry, clinical data needed to support the licensure of pandemic influenza vaccines, draft guidance 2006. 2013.
- Podda A, Del Giudice G. MF59-adjuvanted vaccines: increased immunogenicity with an optimal safety profile. Expert Rev Vaccines 2003; 2:197-203; PMID:12899571; http://dx.doi.org/10.1586/14760584.2.2.197.
- Durando P, Icardi G, Ansaldi F. MF59-adjuvanted vaccine: a safe and useful tool to enhance and broaden protection against seasonal influenza viruses in subjects at risk. Expert Opin Biol Ther 2010; 10:639-51; PMID:20218923; http://dx.doi.org/10.1517/14712591003724662
- O'Hagan DT. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev Vaccines 2007; 6:699-710; PMID:17931151; http://dx.doi.org/10.1586/14760584.6.5.699
- Banzhoff A, Pellegrini M, Del Giudice G, Fragapane E, Groth N, Podda A. MF59-adjuvanted vaccines for seasonal and pandemic influenza prophylaxis. Influenza Other Respi Viruses 2008; 2:243-9; PMID:19453401; http://dx.doi.org/10.1111/j.1750-2659.2008.00059.x
- El Sahly H. MF59TM as a vaccine adjuvant: a review of safety and immunogenicity. Expert Rev Vaccines 2010; 9:1135-41; PMID:20923265; http://dx.doi.org/10.1586/erv.10.111
- Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today 1993; 14:75-8; PMID:8447935; http://dx.doi.org/10.1016/0167-5699(93)90062-P
- Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med 2004; 351:2295-301; PMID:15525714; http://dx.doi.org/10.1056/NEJMoa043540
- Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity 2010; 33:464-78; PMID:21029958; http://dx.doi.org/10.1016/j.immuni.2010.10.007
- Reber AJ, Chirkova T, Kim JH, Cao W, Biber R, Shay DK, Sambhara S. Immunosenescence and challenges of vaccination against influenza in the aging population. Aging Dis 2012; 3:68-90; PMID:22500272
- Ueno H, Klechevsky E, Schmitt N, Ni L, Flamar AL, Zurawski S, Zurawski G, Palucka K, Banchereau J, Oh S. Targeting human dendritic cell subsets for improved vaccines. Semin Immunol 2011; 23:21-7; PMID:21277223; http://dx.doi.org/10.1016/j.smim.2011.01.004
- Nicolas JF, Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines 2008; 7:1201-14; PMID:18844594; http://dx.doi.org/10.1586/14760584.7.8.1201
- Lambert PH, Laurent PE. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine 2008; 26:3197-208; PMID:18486285; http://dx.doi.org/10.1016/j.vaccine.2008.03.095
- Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol 2004; 4:211-22; PMID:15039758; http://dx.doi.org/10.1038/nri1310
- Pearton M, Kang SM, Song JM, Anstey AV, Ivory M, Compans RW, Birchall JC. Changes in human Langerhans cells following intradermal injection of influenza virus-like particle vaccines. PLoS One 2010; 5: e12410; PMID:20811642; http://dx.doi.org/10.1371/journal.pone.0012410
- Allenspach EJ, Lemos MP, Porrett PM, Turka LA, Laufer TM. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity 2008; 29:795-806; PMID:18951047; http://dx.doi.org/10.1016/j.immuni.2008.08.013
- Banzhoff A, Nacci P, Podda A. A new MF59-adjuvanted influenza vaccine enhances the immune response in the elderly with chronic diseases: results from an immunogenicity meta-analysis. Gerontology 2003; 49:177-84; PMID:12679609; http://dx.doi.org/10.1159/000069172
- Podda A. The adjuvanted influenza vaccines with novel adjuvants: experience with the MF59-adjuvanted vaccine. Vaccine 2001; 19:2673-80; PMID:11257408; http://dx.doi.org/10.1016/S0264-410X(00)00499-0
- Arnou R, Icardi G, De Decker M, Ambrozaitis A, Kazek MP, Weber F, Van Damme P. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine 2009; 27:7304-12; PMID:19849996; http://dx.doi.org/10.1016/j.vaccine.2009.10.033
- Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, McKeirnan M, Salem H, Mills G, Reid J, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis 2008; 198:650-8; PMID:18652550; http://dx.doi.org/10.1086/590434
- Van Damme P, Arnou R, Kafeja F, Fiquet A, Richard P, Thomas S, Meghlaoui G, Samson SI, Ledesma E. Evaluation of non-inferiority of intradermal versus adjuvanted seasonal influenza vaccine using two serological techniques: a randomised comparative study. BMC Infect Dis 2010; 10:134-45; PMID:20504306; http://dx.doi.org/10.1186/1471-2334-10-134
- Scheifele DW, McNeil SA, Ward BJ, Dionne M, Cooper C, Coleman B, Loeb M, Rubinstein E, McElhaney J, Hatchette T, et al. Safety, immunogenicity, and tolerability of three influenza vaccines in older adults: Results of a randomized, controlled comparison. Hum Vaccin Immunother 2013; 9:2460-73; PMID:23839537; http://dx.doi.org/10.4161/hv.25580
- Katz JM, Hancock K, Xu X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert Rev Anti Infect Ther 2011; 9:669-83; PMID:21692672; http://dx.doi.org/10.1586/eri.11.51
- Schild GC, Pereira MS, Chakraverty P. Single-radial-hemolysis: a new method for the assay of antibody to influenza haemagglutinin. Applications for diagnosis and seroepidemiologic surveillance of influenza. Bull World Health Organ 1975; 52:43-50; PMID:1082381
- Coudeville L, Bailleux F, Riche B, Megas F, Andre P, Ecochard R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med Res Methodol 2010; 10:18-29; PMID:20210985; http://dx.doi.org/10.1186/1471-2288-10-18
- Ansaldi F, Bacilieri S, Durando P, Sticchi L, Valle L, Montomoli E, Icardi G, Gasparini R, Crovari P. Cross-protection by MF59-adjuvanted influenza vaccine: neutralizing and haemagglutination-inhibiting antibody activity against A(H3N2) drifted influenza viruses. Vaccine 2008; 26:1525-9; PMID:18294741; http://dx.doi.org/10.1016/j.vaccine.2008.01.019
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008-12; PMID:10789670; http://dx.doi.org/10.1001/jama.283.15.2008
- Ansaldi F, Zancolli M, Durando P, Montomoli E, Sticchi L, Del Giudice G, Icardi G. Antibody response against heterogeneous circulating influenza virus strains elicited by MF59- and non-adjuvanted vaccines during seasons with good or partial matching between vaccine strain and clinical isolates. Vaccine 2010; 28:4123-9; PMID:20433807; http://dx.doi.org/10.1016/j.vaccine.2010.04.030
- Baldo V, Menegon T, Bonello C, Floreani A, Trivello R. Comparison of three different influenza vaccines in institutionalised elderly. Vaccine 2001; 19:3472-5; PMID:11348713; http://dx.doi.org/10.1016/S0264-410X(01)00060-3
- Baldo V, Baldovin T, Floreani A, Minuzzo M, Trivello R. Response to influenza vaccine in people with non-protective HI antibody titers. Eur J Epidemiol 2006; 21:843-5; PMID:17082899; http://dx.doi.org/10.1007/s10654-006-9071-4
- Baldo V, Baldovin T, Pellegrini M, Angiolelli G, Majori S, Floreani A, Busana MC, Bertoncello C, Trivello R. Immunogenicity of three different influenza vaccines against homologous and heterologous strains in nursing home elderly residents. Clin Dev Immunol 2010; 2010:517198; PMID:20369059; http://dx.doi.org/10.1155/2010/517198
- Basileo M, Iorio A, Bartolini G, Bianchini C, Menculini G, Tozzi P, Camilloni B. Comparative study of immunogenicity of split, intradermal and MF59-adjuvanted influenza vaccines in elderly institutionalized subjects. Procedia Vaccinol 2014; 18-23; http://dx.doi.org/10.1016/j.provac.2014.07.004
- Camilloni B, Neri M, Lepri E, Iorio AM. Cross-reactive antibodies in middle-aged and elderly volunteers after MF59-adjuvanted subunit trivalent influenza vaccine against B viruses of the B/Victoria or B/Yamagata lineages. Vaccine 2009; 27:4099-103; PMID:19410623; http://dx.doi.org/10.1016/j.vaccine.2009.04.078
- Camilloni B, Neri M, Lepri E, Basileo M, Sigismondi N, Puzelli S, Donatelli I, Iorio AM. An influenza B outbreak during the 2007/2008 winter among appropriately immunized elderly people living in a nursing home. Vaccine 2010; 28:7536-41; PMID:20846530; http://dx.doi.org/10.1016/j.vaccine.2010.08.064
- de Bruijn I, Meyer I, Gerez L, Nauta J, Giezeman K, Palache B. Antibody induction by virosomal, MF59-adjuvanted, or conventional influenza vaccines in the elderly. Vaccine 2007; 26:119-27; PMID:18063446; http://dx.doi.org/10.1016/j.vaccine.2007.10.051
- De Donato S, Granoff D, Minutello M, Lecchi G, Faccini M, Agnello M, Senatore F, Verweij P, Fritzell B, Podda A. Safety and immunogenicity of MF59-adjuvanted influenza vaccine in the elderly. Vaccine 1999; 17:3094-101; PMID:10462245; http://dx.doi.org/10.1016/S0264-410X(99)00138-3
- Del Giudice G, Hilbert AK, Bugarini R, Minutello A, Popova O, Toneatto D, Schoendorf I, Borkowski A, Rappuoli R, Podda A. An MF59-adjuvanted inactivated influenza vaccine containing A/Panama/1999 (H3N2) induced broader serological protection against heterovariant influenza virus strain A/Fujian/2002 than a subunit and a split influenza vaccine. Vaccine 2006; 24:3063-5; PMID:16464520; http://dx.doi.org/10.1016/j.vaccine.2006.01.015
- Della Cioppa G, Nicolay U, Lindert K, Leroux-Roels G, Clement F, Castellino F, Galli G, Groth N, Del Giudice G. Superior immunogenicity of seasonal influenza vaccines containing full dose of MF59 (®) adjuvant: results from a dose-finding clinical trial in older adults. Hum Vaccin Immunother 2012; 8:216-27; PMID:22426371; http://dx.doi.org/10.4161/hv.18445
- Iorio AM, Camilloni B, Basileo M, Guercini F, Conti S, Ferrante F, Paccamiccio E, Iaboni S, Vecchioli M, Iorio A. Influenza vaccination in patients on long-term anticoagulant therapy. Vaccine 2006; 24:6624-8; PMID:16828939; http://dx.doi.org/10.1016/j.vaccine.2006.05.036
- Iorio AM, Neri M, Lepri E, Camilloni B, Basileo M, Sigismondi N, Fabiani C, Calzoletti L, Puzelli S, Donatelli I. An influenza A/H3 outbreak during the 2004/2005 winter in elderly vaccinated people living in a nursing home. Vaccine 2006; 24:6615-9; PMID:16828941; http://dx.doi.org/10.1016/j.vaccine.2006.05.037
- Li R, Fang H, Li Y, Liu Y, Pellegrini M, Podda A. Safety and immunogenicity of an MF59-adjuvanted subunit influenza vaccine in elderly Chinese subjects. Immun Ageing 2008; 5:2-10; PMID:18289372; http://dx.doi.org/10.1186/1742-4933-5-2
- Minutello M, Senatore F, Cecchinelli G, Bianchi M, Andreani T, Podda A, Crovari P. Safety and immunogenicity of an inactivated subunit influenza virus vaccine combined with MF59 adjuvant emulsion in elderly subjects, immunized for three consecutive influenza seasons. Vaccine 1999; 17:99-104; PMID:9987141; http://dx.doi.org/10.1016/S0264-410X(98)00185-6
- Pregliasco F, Mensi C, Serpilli W, Speccher L, Masella P, Belloni A. Immunogenicity and safety of three commercial influenza vaccines in institutionalized elderly. Aging (Milano) 2001; 13:38-43; PMID:11292151
- Ruf BR, Colberg K, Frick M, Preusche A. Open, randomized study to compare the immunogenicity and reactogenicity of an influenza split vaccine with an MF59-adjuvanted subunit vaccine and a virosome-based subunit vaccine in elderly. Infection 2004; 32:191-8; PMID:15293073; http://dx.doi.org/10.1007/s15010-004-3204-z
- Sindoni D, La Fauci V, Squeri R, Cannavo G, Bacilieri S, Panatto D, Gasparini R, Amicizia D. Comparison between a conventional subunit vaccine and the MF59-adjuvanted subunit influenza vaccine in the elderly: an evaluation of the safety, tolerability and immunogenicity. J Prev Med Hyg 2009; 50:121-6; PMID:20099444
- Song JY, Cheong HJ, Noh JY, Seo YB, Choi WS, Cho GJ, Hwang TG, Kim WJ. Long-term and cross-reactive immunogenicity of inactivated trivalent influenza vaccine in the elderly: MF59-adjuvanted vaccine versus unadjuvanted vaccine. J Med Virol 2013; 85:1591-7; PMID:23852684; http://dx.doi.org/10.1002/jmv.23630
- Squarcione S, Sgricia S, Biasio LR, Perinetti E. Comparison of the reactogenicity and immunogenicity of a split and a subunit-adjuvanted influenza vaccine in elderly subjects. Vaccine 2003; 21:1268-74; PMID:12559808; http://dx.doi.org/10.1016/S0264-410X(02)00401-2
- Camilloni B, Basileo M, Di Martino A, Donatelli I, Iorio AM. Antibody responses to intradermal or intramuscular MF59-adjuvanted influenza vaccines as evaluated in elderly institutionalized volunteers during a season of partial mismatching between vaccine and circulating A(H3N2) strains. Immun Ageing 2014; 11:10; PMID:24860610; http://dx.doi.org/10.1186/1742-4933-11-10
- Ansaldi F, Canepa P, Ceravolo A, Valle L, de Florentiis D, Oomen R, Vogel FR, Denis M, Samson SI, Icardi G. Intanza (®) 15 mcg intradermal influenza vaccine elicits cross-reactive antibody responses against heterologous A(H3N2) influenza viruses. Vaccine 2012; 30:2908-13; PMID:22342501; http://dx.doi.org/10.1016/j.vaccine.2012.02.003
- Ansaldi F, Orsi A, de Florentiis D, Parodi V, Rappazzo E, Coppelli M, Durando P, Icardi G. Head-to-head comparison of an intradermal and a virosome influenza vaccine in patients over the age of 60: Evaluation of immunogenicity, cross-protection, safety and tolerability. Hum Vaccin Immunother 2013; 9:591-8; PMID:23295262; http://dx.doi.org/10.4161/hv.23240
- Tsang P, Gorse GJ, Strout CB, Sperling M, Greenberg DP, Ozol-Godfrey A, Diazgranados C, Landolfi V. Immunogenicity and safety of Fluzone intradermal and high-dose influenza vaccines in older adults >/=65 years of age: A randomized, controlled, phase II trial. Vaccine 2013; 32:2507-17; PMID:24120672; http://dx.doi.org/10.1016/j.vaccine.2013.09.074
- Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine 2007; 25:6852-62; PMID:17719149; http://dx.doi.org/10.1016/j.vaccine.2007.07.027
- McCullers JA, Saito T, Iverson AR. Multiple genotypes of influenza B virus circulated between 1979 and 2003. J Virol 2004; 78:12817-28; PMID:15542634; http://dx.doi.org/10.1128/JVI.78.23.12817-12828.2004
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993-8; PMID:22226861; http://dx.doi.org/10.1016/j.vaccine.2011.12.098
- Iorio AM, Camilloni B, Basileo M, Neri M, Lepri E, Spighi M. Effects of repeated annual influenza vaccination on antibody responses against unchanged vaccine antigens in elderly frail institutionalized volunteers. Gerontology 2007; 53:411-8; PMID:17975317; http://dx.doi.org/10.1159/000110579
- Keitel WA, Cate TR, Couch RB. Efficacy of sequential annual vaccination with inactivated influenza virus vaccine. Am J Epidemiol 1988; 127:353-64; PMID:3337087
- Kendal AP, Cate TR. Increased sensitivity and reduced specificity of hemagglutination inhibition tests with ether-treated influenza B/Singapore/222/79. J Clin Microbiol 1983; 18:930-4; PMID:6630472
- Monto AS, Maassab HF. Ether treatment of type B influenza virus antigen for the hemagglutination inhibition test. J Clin Microbiol 1981; 13:54-7; PMID:7462419
- McElhaney JE. Influenza vaccine responses in older adults. Ageing Res Rev 2011; 10:379-88; PMID:21055484; http://dx.doi.org/10.1016/j.arr.2010.10.008
- Public Health Agency of Canada. Statement on seasonal influenza vaccine for 2012–2013. Canada Communicable Disease Report 38 ACS-2. 2012.
