Abstract
Pertussis remains a challenging public health problem with many aspects of infection, disease and immunity poorly understood. Initially controlled by mass vaccination, pertussis resurgence has occurred in some countries with well-established vaccination programs, particularly among adolescents and young adults. Several studies have used mathematical models to investigate drivers of pertussis epidemiology and predict the likely impact of different vaccination strategies. We reviewed a number of these models to evaluate their suitability to answer questions of public health importance regarding optimal vaccine scheduling. We critically discuss the approaches adopted and the impact of chosen model structures and assumptions on study conclusions. Common limitations were a lack of contemporary, population relevant data for parameterization and a limited understanding of the relationship between infection and disease. We make recommendations for future model development and suggest epidemiologic data collections that would facilitate efforts to reduce uncertainty and improve the robustness of model-derived conclusions.
Abbreviations:
- S, susceptible compartment
- I, infectious compartment
- R, removed/immune compartment
- E, infected but not yet infectious compartment
- W, waned immunity compartment
- λ or FOI, force of infection
- WAIFW, who acquires infection from whom
- US, United States
- AIC, Akaike information criterion
- POLYMOD, European Union funded project
- UK, United Kingdom
- WHO, World Health Organization
Introduction
Pertussis, also known as whooping cough, is a highly contagious respiratory disease caused by infection with the bacterium Bordetella pertussis. Although well researched, pertussis presents a challenging public health problem as many aspects of infection, disease and immunity remain uncertain. While the reduction in case notifications following the introduction of mass vaccination from the 1940s was dramatic, pertussis has not been eliminated. In the 1980s and early 1990s, pertussis notifications began increasing in many, but not all, countries with high-coverage vaccination programs, particularly among adolescents and young adults. There has been much debate over whether the increase in cases represents a true increase in incidence or whether it is due to increased awareness by medical practitioners,Citation1 improved diagnostics,Citation2 or greater reporting fidelity.Citation3 Potential explanations for a genuine resurgence of pertussis include waning immunity,Citation4 reduction in opportunities for immunity boosting,Citation5 legacy of historically lower vaccination rates,Citation6 and evolution of the B. pertussis bacterium.Citation7 The timing and magnitude of resurgence, or lack thereof, in different settings may contain clues as to which of these explanations, if any, is responsible for increasing incidence.
Mathematical models of infection and transmission describe a population and the spread of a pathogen (here, pertussis) within that population by a set of equations. These equations “model” the biological and epidemiological mechanisms hypothesized to be responsible for observations (in this case, of pertussis incidence over time). A model breaks the population into a number of sub-populations (known as “compartments” or “states”), each defined by an infection status such as “susceptible,” “infectious” or “recovered.” The equations capture the processes by which individuals transition from one state to another and these transitions are governed by the model's parameters. Parameters with biological, demographic and epidemiologic interpretations, in combination with the chosen compartmental break-down, define the model structure and the model as a whole reflects the assumptions made about the population under study and the infection and transmission processes occurring within it.
A number of mathematical models of pertussis transmission, tracking sub-populations with different infection and immunity characteristics over time, have been published since the 1980s. Authors have used these models to provide insights into drivers of pertussis epidemiology, explore alternative explanations of the observed resurgence and predict potential effects of different vaccination strategies. We reviewed the assumptions, reflected in alternative model structures and parameterizations, used in a selection of these models to evaluate their suitability to answer current questions regarding optimal vaccine formulation and scheduling to mitigate the burden of pertussis disease. Models were selected for review where they characterized an aspect of pertussis infection or immunity differently to other models; although not an exhaustive list, we consider these models to be a good representation of published pertussis models. We critically discuss the approaches adopted in the reviewed models and the impact of chosen structures and assumptions on model conclusions. Additionally, we make recommendations for future model development and suggest epidemiologic data collections that would facilitate efforts to reduce uncertainty and improve the robustness of model-derived conclusions.
Basics of Infectious Disease Modeling
Compartmental infectious disease models divide the population between infection states: susceptible to infection (S), infectious (I) or immune (R); and establish a set of rules governing transfer between these compartments. These are commonly referred to as SIR models (), and are suitable for diseases with permanent immunity. Models for diseases with temporary protection often return individuals to the susceptible state when immunity wanes, an SIRS model, also represented in , by the addition of a dashed line indicating the return to susceptibility following a period of immunity. Extensions to this basic model structure are sometimes necessary, with the inclusion of compartments to capture additional or more nuanced classifications of the status of sub-populations, for example, to account for a latent period (adding an exposed (E) compartment) or varying levels of immunity (). Extra compartments may also be included to represent different infection processes, such as boosting of immunity upon re-exposure, without transmissible infection ().
Figure 1. The classic SIR epidemiologic model for infections inducing permanent immunity (solid lines). Susceptibles acquire infection and move to the I state at a rate λ, with the population in I losing infectiousness at a rate γ. The dashed line represents the SIRS variant, for infections inducing only temporary immunity, with immunity lost at a rate ω.
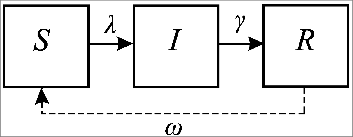
Figure 2. An SIRS model variant for infections with different characteristics for naïve and repeat infections. Naïve susceptibles (S1) acquire infection and move to the I1 state at a rate λ, with the population in I1 losing infectiousness at a rate γ and moving to the R compartment. Immunity is lost at a rate ω, after which individuals become susceptible to repeat infections (S2). This model structure assumes no return to the naïve state.
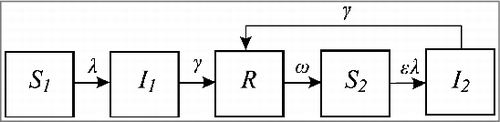
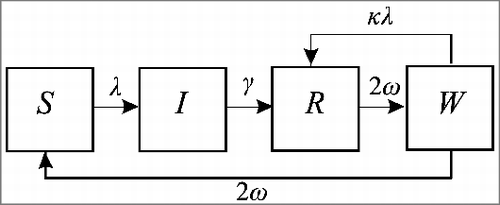
Figure 3. The boosting SIRWS model. Naïve susceptibles (S) acquire infection and move to the I state at a rate λ, with the population in I losing infectiousness at a rate γ and moving to the R compartment. Immunity is lost at a rate 2 ω, after which individuals can have their immunity boosted at a rate, κλ, proportional to the force of infection, without experiencing a transmissible infection (W). If not exposed, individuals in W return to the naïve susceptible state at a rate 2 ω.
It is common in the research field to further subdivide each of the compartments into discrete age groups, where necessary, to model vaccination schedules or differences in behavior and infection characteristics by age.
Models may be either deterministic or stochastic. Deterministic models, usually suitable for studying endemic infections in large populations, describe the average behavior of the population and for a fixed set of parameter values will always produce the same model results. Stochastic models describe transition between compartments (e.g. susceptible and infectious) as a random process and so model results vary with each simulation.
Considerations for Construction of a Pertussis Transmission Model
Variable severity (by age) and ascertainment (by age and over time) as well as the lack of a consistently applied, sensitive diagnostic gold standard mean the true infection and disease burden of pertussis is not well known.Citation8 Data from the pre-vaccine era are scarce and include few cases in adults, who rarely presented with typical symptoms.Citation4,9 Even contemporary data generally reflect disease burden, whereas transmission models capture infectious processes, of which observed disease comprises only a proportion. Infants and unvaccinated individuals usually experience the most severe pertussis symptoms and teasing out the relationship between infections and surveillance data is not straightforward.Citation10 Observed disease is a function of severity, treatment seeking behavior, diagnostic testing and reporting infrastructure.Citation3 These complexities in available data have led to uncertainty in the characteristics of infection and immunity, reflected in the chosen structure of models and by a range of assumptions made about key infection and immunity processes.
Transmission rates
The per capita rate at which susceptibles acquire infection, the force of infection (λ or FOI), depends on how frequently individuals make contact with other individuals sufficient to transmit infection (the transmission rate) and how many of these contacts occur with infectious individuals. In turn, the transmission rate is a composite measure accounting for contact rates between individuals and transmission likelihood given contact, which may further be influenced by host dependent characteristics such as prior immunity and infectiousness. While early models tended to parameterize the transmission rate as a single value, more recent models break transmission down into its constituent parts. Here we consider how these various options may be modeled.
Models of direct transmission require either explicit or implicit parameterization of transmission between sub-groups of the population, known as the ‘Who-Acquires-Infection-From-Whom’ (WAIFW) matrix. The simplest option for characterizing transmission is to assume susceptible individuals acquire infection from all infected individuals in the population at the same rate. This assumption is known as homogeneous mixing and is implicit in non-age-structured models, where the WAIFW matrix is replaced with a single numerical value. Age-structured models, in which compartments (e.g., S, I or R) are further subdivided into age specific compartments (e.g. S(0–1 y olds), S(1–5 y olds) etc.), are not necessarily constrained by the assumption of homogeneous mixing and can account for different transmission rates within and between age-groups.
Methods for estimating the transmission parameters of the WAIFW matrix using seroprevalence or case data are well described in several excellent resources, for example Vynnycky and WhiteCitation11 or Anderson and May,Citation12 and are thus not fully detailed here. Of note, however, is that these methods require contact within and between subgroups to be heuristically configured a priori to match expected mixing patterns of the population. Configurations that have been used include mixing proportional to the size and activity levels of the age groups involved (‘proportionate mixing’), mixing only within age groups (‘assortative mixing’) and ‘selective mixing’ to capture known features of mixing patterns, such as higher rates of contact between primary school children. Assumed configurations must be constrained to ensure parameter identifiability,Citation11,12 limiting the ability for the matrix to capture all mixing patterns in a population.
With the increasing availability of social contact data collected in different populations,Citation13,14 published models recently have begun to incorporate empirical contact data instead of heuristically configured mixing structures.Citation15-17 Published contact data are available in 5 y age bandsCitation14 and their use within models implies homogeneity of contact patterns within the band. While this is likely to be a good approximation for many age bands, it is highly unlikely to be true for under 5 y olds. The missing piece of the contact puzzle, arguably the most important for pertussis, is the contact patterns of infants too young to be protected by vaccination.
Seasonality and periodicity of pertussis
In addition to seasonal variation in case numbers,Citation18 a common feature of pertussis incidence is the occurrence of persistent 2–5 yearly epidemics.Citation19 While the underlying mechanisms for these cyclic patterns have not been determined, it has been shown that seasonal changes in transmission rates for infectious diseases may result not only in annual peaks but also in multiennial oscillations in incidence.Citation12 One option for producing ongoing pertussis cycles in a model is therefore the inclusion of seasonal variation in transmissibility, known as ‘seasonal forcing’, to mimic environmental or sociological changes.Citation20 A further mechanism for generating ongoing cyclic behavior in models is the incorporation of stochasticity that captures the probabilistic nature of transmission and demographics.Citation11 Finally, it has also been shown that under some circumstances, ongoing cycles may be a natural consequence of transmission dynamics without inclusion of either seasonal forcing or stochasticity.Citation21
Modification of infection and immunity characteristics by prior infection or vaccination
A key question for pertussis transmission yet to be completely resolved is how previous infection and vaccination modify infection and immunity characteristics and thus the outcome of exposure to pertussis. Major uncertainties exist around the importance of repeat infections in the transmission process and how response to pertussis exposure depends on, for example age, time since last exposure and vaccine composition.
The most straightforward assumption for susceptibility is that individuals are either fully protected from, or fully susceptible to, infection (). Other options include partial protection, whereby susceptibility to infection is reduced for individuals with prior experience of pertussis (), or enhanced boosting, whereby partially waned individuals are more likely to mount an immune response to exposure than naïve individuals are to become infected and infectious ().
The simplest assumption for transmissibility is that all infections contribute equally to the force of infection (). At the other extreme is the assumption that some infections are not infectious at all (). Alternatively, infectiousness may be linked to an individual's immune state prior to infection, although there are very limited data to support such assumptions ().Citation22,23 Infections with more severe symptoms, including cough, may well be more infectious, but importantly cases with less severe symptoms may be responsible for greater transmission as activities such as attendance at work and school are not curtailed to the same extent and so the total amount of contact may be higher.Citation24
Parameterization of models
Our limited understanding about how well observed pertussis incidence reflects the level of infection in a population makes direct comparison between model results and data problematic for pertussis, leading to a number of different methods being used for parameterization.
Parameter values may be adopted from previous epidemiologic or modeling studies, possibly a valid approach for parameters describing pathogen biology that we expect to be consistent across populations. An alternative is to choose parameters to qualitatively match the general features of observed data, such as the inter-epidemic period or the average age of infection. A third option is to formally fit the model by statistical comparison of model generated and observed epidemiology. The most credible models are those which are biologically plausible and are able to make successful predictions of data not used for parameterization.
Published Models of Pertussis Transmission
Since the 1980s, compartmental transmission models have been widely used to study pertussis dynamics and predict the impact of vaccination programs. We reviewed a selection of these models, broadly grouping them by their primary aim.
Exploring the impact of childhood vaccination
Pertussis vaccines are typically administered over a 3–6 dose schedule, with a multi-dose infant series and booster doses provided in early childhood and adolescence. Not all of the reviewed models had a structure appropriate to study the impact of multiple vaccinations, with model configuration limiting how vaccination could be applied. For models without age structure,Citation19,25,26 vaccination could only be applied at birth, meaning that indirect protection of infants too young to be vaccinated could not be studied. The additional complexity of the 3 dose primary course for pertussis forces assumptions about the immunological response to each dose. In some models, the effect of vaccination is applied to a proportion of the age group when each dose is due,Citation24,27-32 while in others it is applied to all members of the age group after a specified number of doses.Citation5,15,22,33-35 Given evidence of reduced hospitalisations following one dose,Citation36 a further option would be to allow each vaccine dose to have different effects on infection and disease. To adequately assess the impact of vaccination on vulnerable infants and be consistent with epidemiologic evidence of the effect of vaccination, vaccination in pertussis models should not be applied at birth, nor left until all doses of the primary course have been received.
A major obstacle to understanding the impact of vaccination is that infections in a previously vaccinated individual may arise through primary vaccine failure, low vaccine efficacy, waned immunity or some combination of all 3.Citation37 These different mechanisms may be included in transmission models and comparisons between modeled incidence and observed data used to infer the importance of each to infection dynamics.
Grenfell and Anderson's classic model of pertussis transmission, published in 1989, was used to explore the impact of different vaccine failure mechanisms (incomplete protection against infection and/or disease; and, waning immunity) on summary measures of disease incidence aggregated across all age groups.Citation29 The authors qualitatively compared the inter-epidemic period of pertussis between the pre-vaccine and vaccine eras and estimated the reduction in cases following vaccination under different scenarios. It is worth noting that deterministic, unforced models such as Grenfell and Anderson's normally settle to a point equilibrium in which the proportion of the population present in each model state (e.g., S, I or R) is constant over time, even though individuals change status. In this model, oscillatory behavior, in the form of temporal fluctuation in the prevalence of infection, was created by perturbing the model away from the equilibrium such that the timing and magnitude of epidemics matched observations. Comparisons between this model and those which explicitly include mechanisms for periodicity, such as seasonal forcing,Citation6,15,19,38 are thus not straightforward.
Estimation of transmission parameters between age groups in the Grenfell and Anderson model was performed with an FOI derived, under the assumption of permanent immunity, from pre-vaccine era case notifications in England and Wales.Citation29 The assumed mixing configuration had very high levels of contact within the 5–9 y age group and high levels between all children under 15, but did not capture mixing between parents and children, which may have underestimated the role of adults in the spread of pertussis. Teasing apart the impact of the different assumptions used in this model is difficult, as most of the results presented in the paper are not stratified by age.
All infected individuals in Grenfell and Anderson's model were equally infectious.Citation29 More recently, varying levels of infectiousness have been applied to infected individuals depending on their immune state at infection (similar to ),Citation19,30,31,33-35 with a number of models incorporating a non-infectious cycle which boosts immunity without creating a transmissible infection (similar to ).Citation5,24,26-28 Milder symptoms may be associated with reduced infectiousness, even though there is little available evidence for estimating this effect. The role of asymptomatic infections in pertussis transmission is yet to be convincingly resolved, although it is worth noting that minimal transmission from repeat infections may produce equivalent effects in case data as do long durations of immunity.Citation19
Hethcote's model published in 1997 was used to investigate the impact of the historical vaccination schedule on pertussis incidence in the US and predict likely incidence into the future.Citation27 This model has become the basis of many subsequent models exploring the impact of vaccination.Citation27 Major differences between the Hethcote,Citation27 and Grenfell and Anderson,Citation29 models are the inclusion in Hethcote's model of varying levels of immunity and infectiousness, incremental increases to immunity with each vaccine dose, and distinction of naïve and experienced individuals. With multiple levels of susceptibility, this model strongly linked both disease severity and infectiousness to an individual's immune state at the point of infection. While logical, compelling data to support such an assumption remains lacking.
Model parameters were chosen to be broadly consistent with available epidemiologic studies, albeit conducted in different settings, with many such as FOI and infectiousness chosen under advice from experts. Model-generated incidence reacted quickly to changes in vaccination coverage, with the timing of peak incidence not matching that of the observed incidence data. Reported vaccination coverage was thus modified for inclusion in the model under the assumption that coverage data contained measurement errors.Citation27 Importantly, the reduction in coverage due to the vaccine scare of the mid 1970s-early 1980s was not included. For this reason, Hethcote did not attempt to validate the model through comparison to data.Citation27
With computer simulations not predicting the observed rise in incidence in the US in the 1990s, Hethcote concluded that the observed increase may have been due to a higher reported percentage of cases.Citation27 While increased reporting may have had an impact, an alternative explanation is that historical changes in vaccine coverage, excluded from the model, may have altered the population immunity profile. With the recent modeling study of Riolo and colleagues finding reduced coverage and imperfect vaccines may explain pertussis resurgence,Citation6 the assumption of monotonically increasing vaccine coverage may have driven some of Hethcote's conclusions.
Luz and colleagues used a simplified form of Hethcote's 1997 model with fewer compartments to explore the transmission dynamics of pertussis in a model of a large city, using demographic and vaccination coverage data from Rio de Janeiro.Citation31 Only individuals in the lowest immunity state could be infected, with no allowance for immune boosting from higher immunity states. Unlike Hethcote's early models,Citation24,27,28 experienced susceptibles had a reduced risk of infection. Parameters were selected from previous epidemiologic and modeling studies, and modeled incidence was qualitatively compared to surveillance data under a number of assumptions about reporting likelihood.Citation31 Although the introduction of vaccination in the model had a major impact on primary infections, the reduction in infections of 0–1 month infants was modest, reflecting a consistent limitation of models to capture observed herd effects in this age group.Citation27,28 Among other possible causes, this limitation could be due to inadequately capturing the mixing patterns of young infants, or not including the effect of maternal immunity, albeit that this is known to be of limited duration.Citation8,39
Interventions aimed at older age groups
Increasing notifications in adolescents and adults in some countries with long-standing vaccination programs has focused attention on estimating the impact of interventions targeted beyond the first decade of life. These strategies include adolescent vaccination, one-off or regular adult vaccination and the “cocoon” strategy, whereby regular contacts of infants (e.g. parents, grandparents) are vaccinated. Many of the reviewed models which considered vaccination outside the usual childhood vaccination schedule,Citation24,28,30,32 were based on Hethcote's 1997 age-structured model,Citation27 with Rohani and colleagues’ model published in 2010 a notable exception.Citation15
In 1999, Hethcote considered 2 variants of his 1997 model to study the effect of adult boosters in the United States.Citation28 In the first variant, individuals primed by vaccination or infection waned back to the naïve susceptible state, from which they would, if infected, experience typical disease. As this structure generated unrealistically high numbers of typical cases, the second variant removed waning back to the naïve susceptible state, as well as enhancing the response to vaccine boosters.Citation28 As for the 1997 model, features of simulated incidence were broadly compared to surveillance data rather than the model being validated through formal statistical methods. In the second model variant, which provides temporary full protection following vaccine boosters, decennial adult boosters were found to be effective at reducing modeled infections in adults, but had little impact on infant incidence.
With slight changes to waning of vaccinated infants, the second model structure used in Hethcote's 1999 paper was applied to the Australian population to estimate the impact of moving an 18 month dose to the adolescent years.Citation24 It was predicted that overall incidence would reduce and although cases were predicted to increase in the 2–4 y age group, the authors found this was more than offset by large reductions in cases in under 2 y olds and adolescents.Citation24 These results hinged on adolescents having relatively low pre-vaccination immunity due to waning, while toddlers were assumed still well protected from the primary course, based on vaccine effectiveness from an Italian study.Citation24 As for the previous Hethcote models of pertussis in the US, this model was not calibrated to Australian incidence data, which are scarce for the period 1950–1990.Citation40 Again, only increasing vaccine coverage was applied to the model, despite falls in coverage through the late 1970s and early 1980s.Citation3 Many parameters were used from other studies and locations, although it is interesting to note that the infectiousness of different classes of infective were different in this iteration of the model compared to the US version.Citation28 There may be insights to be gained by examining why the predicted reduction in incidence following the switch from a toddler to an adolescent booster has not been reflected in Australian data, with a large epidemic occurring from 2008–2012.Citation41
In a further modification of the Hethcote model, van Rie and Hethcote removed the rigid binding of infection outcomes to prior immune states, and instead distributed infection severity across several levels based on a range of different data sources.Citation30 This model was the first to estimate the likely impact of a cocoon strategy, an intervention focused on the family unit. This was achieved by subdividing the infant population into those protected and not protected by a household cocoon. A strong impact of cocooning on infants was predicted, with results unsurprisingly sensitive to the assumed proportion of infant infections arising from adults in the household.Citation30 Coudeville and colleagues updated this model with higher values of vaccine efficacy and a much lower proportion of infant cases attributable to households.Citation32 Notably, these changes led to a more conservative impact of the cocoon strategy and a more favorable impact of regular adult vaccination. Such variation in conclusions, directly reflecting the alternative assumptions, highlights the importance of developing well designed field studies to measure the effects of cocooning. Models will then provide the means through which to assess the broader implications of such a strategy.
These studies show the estimated impact of vaccine interventions targeted at older age groups is particularly sensitive to assumed mixing rates between adults and children. Comparison of heuristically configured WAIFW matrices such as those used by Hethcote,Citation42 with empirical contact matrices from large populations reveals that the former generally have insufficient flexibility to adequately capture the mixing between adults and children found in social contact studies.Citation13,14
Interventions aimed at specific age groups are readily modeled using age-structured, compartmental models. Although this type of model has been used to assess the effectiveness of the cocoon strategy in a rudimentary way,Citation30,32 compartmental models are not well suited to the study of fertility-targeted immunisation approaches, as the dependence of infant protection on their mother's or family's immune status cannot easily be captured. Individual based models, which explicitly characterize immunity and exposure of individuals rather than subgroups of the population, and allow allocation of individuals to social structures such as households, may be better able to capture the likely impact of such closely targeted strategies.Citation43 Advances in computing capability make these models, if accurately parameterized, a practical tool for assessing the impact of household-based interventions,Citation43 including schedules currently under evaluation such as maternal antenatal immunisation.
Investigation of pertussis resurgence
One of the earliest models investigating higher than expected pertussis incidence was van Boven and colleagues’ modeling of the 1996/7 epidemic in the Netherlands.Citation33 Both waning immunity and subclinical infections were included, to explore the contribution of subclinical infections to the epidemic, as the authors noted evidence suggesting that circulation of pertussis remains high even in well-vaccinated populations.Citation33 Vaccinated and naturally immune populations, initially kept distinct in the model, were indistinguishable once waned, a feature shared by many models.Citation6,19,22,26,27,29 Further, individuals were fully protected from infection or fully susceptible, similar to with ϵ = 1.
A novel feature was the use of current incidence data to estimate the FOI, rather than using historical data from England and Wales, as was the practice in earlier models.Citation27,29
This estimation method, which took the model structure into account, found the FOI to be highly sensitive to waning immunity and any bias in case reporting. Although requiring infection rates to be stable over time and so not necessarily suitable for all populations, estimating the FOI in this way meant that the model was parameterized using contemporary, population-specific data which is likely to be an advantage over the use of pre-vaccine era data from populations that differed in terms of their demographic characteristics and social structure.
Van Boven and colleagues determined the 1996/7 epidemic was due to a sudden decline in the duration of vaccine protection,Citation33 and further used the model to compare incidence under scenarios that might have led to this outcome.Citation22 The authors concluded that a change in the circulating strain had a greater capacity to spark a large epidemic than a decrease in vaccine potency, as it would render all with vaccine immunity susceptible rather than just the newly vaccinated.
Using a similar model structure to van Boven and colleagues,Citation22 Wearing and Rohani investigated the duration of natural immunity consistent with historical incidence data from England and Wales.Citation26 Unlike van Boven,Citation22 upon exposure immunity could be boosted in a non-infectious boosting cycle, rather than all infections being infectious to some degree. Major features were the division of the infectious compartment, I, into multiple stages to provide less variance in the duration of infectiousness, and the use of robust epidemiologic signatures, such as the average age of infection in the pre-vaccine era, to constrain parameters.
Wearing and Rohani's model could only produce a significant change in the inter-epidemic period following vaccination, as observed in data from England and Wales,Citation44 if natural immunity was long lived and vaccine immunity generally shorter. With most repeat infections in the model boosting immunity rather than contributing to transmission, the authors concluded that waning natural immunity is not a primary driver of pertussis resurgence and that further research should focus on other likely dynamic drivers such as changes in vaccine formulation and uptake.
In the mid-2000s, reduced circulation of pertussis, with consequences for natural boosting, was proposed as an alternative explanation for the observed resurgence. Using a simple non age-structured model, Gomes and colleagues identified a ‘re-infection threshold’, below which transmission is dominated by primary infections and above which repeat infections prevail.Citation25 Aguas and colleagues developed this model further, hypothesizing that immunity has a greater impact on disease than on transmission.Citation34 Both waning immunity and partial protection were included, with the authors concluding that above the re-infection threshold reported disease and age of infection both increase as transmission falls.Citation34 Reduced transmission is associated with more severe disease due to the greater proportion of infections acquired from the low immune state. As the authors sought to characterize transmission rather than realistically model a specific population, parameters were selected from previous studies and modeled incidence was not compared to any data. Whether these assumptions are able to capture observed infection patterns is yet to be seen.
Most of the models reviewed assumed exposed individuals with waned immunity had either the same,Citation15,19,22,24,26-29 or a reduced,Citation30-32,34,35 risk of infection compared to naïve individuals. Lavine and co-authors incorporated the unusual, but biologically plausible, assumption that a primed immune system responds more readily to subsequent exposure than a naïve one, the SIRWS structure of .Citation5 To account for the patterns of age-specific pre-vaccine and vaccine era incidence data in Massachusetts, primed individuals had to respond at least 10 times more readily than naïve individuals (the ‘boosting coefficient’), otherwise there was a poor qualitative match between model generated incidence and reported adult infections in the pre-vaccine era.
Other than the value of the boosting coefficient, parameters used for the base case of Lavine's model were selected from previous studies, although the average lifespan of 50 y was short (and arguably unrealistically so) compared to other models. In an analysis of the model structure used by Lavine,Citation5 Dafilis and colleagues showed that lifespan and the boosting coefficient were critical determinants of pertussis dynamics and that oscillatory incidence could be explained equally well by increasing lifespan as it could by vaccination.Citation21 As with a number of other models,Citation22,26,33 the placement of newly vaccinated and recovered individuals in the same compartment meant vaccine and naturally acquired immunity were not distinguished, requiring selection of a duration of protection compatible with data for both natural and vaccine immunity, chosen as 10 y in this model.Citation5 This choice may have inflated the fitted value of the boosting coefficient (equal to 10 in this model). With a longer duration of immunity, fewer boosts are required to prevent waning to the naïve state, and this would have been reflected in a lower value for the boosting coefficient.
Lavine and colleagues later fit their SIRWS model () to pre-vaccine era incidence data from Copenhagen, comparing the fit to that of SIRS and SIR models () using the Akaike information criterion (AIC).Citation45 Of the 3 model structures compared, the SIRWS provided the best explanation of the observed dynamics. Rather than using parameters derived from literature as in the earlier model, parameters were fit to the Copenhagen data using maximum likelihood, yielding estimates consistent with those in other models, although somewhat different to those used in the earlier iteration of the model.Citation45 Notably, the boosting coefficient was lower at 6.6, with a confidence interval showing that enhanced boosting (greater than the force of infection) was not necessarily required to explain dynamics. With a 95% confidence interval of 17 to 66 years, the estimated immune period was substantially longer than the 10 y used to model pertussis in Massachusetts.
As incidence patterns generated by most pertussis models were sensitive to the choice of mixing pattern, Rohani and colleagues explored the impact of contact patterns on age-stratified incidence.Citation15 Parameterization of the WAIFW matrix was undertaken by first estimating the age–specific number of contacts occurring with infective individuals (‘risky contacts’) using POLYMOD contact data,Citation14 and Swedish incidence data prior to the reintroduction of vaccination. These ‘risky contacts’ were then compared to the FOI, calculated using the age distribution of cases. From this analysis, the authors concluded that the probability of infection given contact was reasonably independent of age, although assumptions of lifelong immunity and a constant reporting probability across all ages likely influenced this result, as shown in the supplementary material.Citation15 The authors then constructed a stochastic, seasonally forced SEIR model under the assumption of an age independent probability of infection.Citation15 Parameters were estimated by fitting the model to Swedish incidence data during the period 10 y prior to the reintroduction of vaccination, and the model was run forward in time to predict the incidence for the following 10 y. Quantitative comparison between simulated and observed incidence was undertaken for both the pre-vaccine and vaccine eras, highlighting that the order of magnitude difference between modeled and observed incidence varied by age.Citation15 It is worth noting that the 10 y period over which predictions were made may not have been sufficiently long for the impact of waning vaccine derived immunity and reduced circulation following reintroduction to have had a major impact on incidence and the situation in Sweden should be monitored for signs of resurgence.
To investigate whether pertussis resurgence in the UK could be due to historical fluctuations in coverage and the use of imperfect vaccines, Riolo and colleagues developed a stochastic, seasonally forced variant of an SIRS model.Citation6 While vaccinated and naturally immune were initially kept distinct in this model, following waning, individuals reverted to naïve susceptibility. The model used many parameter values from the study by Rohani,Citation15 and qualitatively compared incidence with observed patterns. Various durations of natural and vaccine immunity were simulated, with all predicting the gradual increase in adult cases except those in which vaccine induced and natural immunity were of equal duration.Citation6
The impact of ongoing circulation and repeat infections on population dynamics
Concerns regarding whole cell pertussis vaccine reactogenicity through the late 1970s and 1980s led to a number of countries experiencing considerable dips in coverage, or complete cessation of pertussis vaccination. Models developed by Hethcote and colleagues only ever include increasing vaccination coverage, even though available vaccination records show downward trends through the late 1970s and 1980s.Citation24,27,28,30 As described above, decreases in coverage were not included in the models as they resulted in immediate increases in modeled incidence not observed in data, however, this strong assumption may have discarded important trends.Citation27 Given highly variable and time-dependent reporting practices we cannot be certain whether resurgence following periods of low incidence may have been missed.
Recent studies have suggested reduced circulation may impact on disease,Citation5,34 and with some modeling studies finding natural immunity lasts decades,Citation15,19,26,45 increased transmission associated with decreased coverage may have implications for population immunity many years into the future. Models that fail to include fluctuations in vaccine coverage may not capture cohort effects, which could be important under some parameterizations, such as a long duration of natural immunity.
Undertaking an epidemiologic study of World Health Organization (WHO) data for 64 countries, Broutin and co-authors showed that pertussis periodicity was affected by the birth rate and vaccination coverage.Citation19 A non-age-structured model was then used to examine the transmission characteristics of repeat infections.Citation19 The authors found that when experienced infectives were assigned low infectiousness in the model, the duration of immunity had little influence on the inter-epidemic period, but the reverse was true when repeat infections were large contributors to the FOI.Citation19 This result highlights that parameters that act together on transmission may not be individually identifiable, yet change our predictions for future epidemiologic behavior.
Lavine and colleagues’ non-infectious boosting cycle,Citation5 whereby partially waned individuals have their immunity boosted without contributing to the force of infection, would make the average duration of immunity appear longer during periods of high circulation. In a setting of reduced boosting opportunities, the observed duration of protection following exposure would be expected to fall concordant with the current perception of waning immunity as a key driver of resurgence.
To investigate the impact of vaccination on transmission and immunity, Blackwood and colleagues used statistical inference to compare incidence generated by competing stochastic models with surveillance data from Thailand.Citation38 Although models including waning immunity were simulated, the authors found the SIR model (with lifelong naturally acquired and vaccine induced immunity) outperformed all other models using AIC, as well as providing a good qualitative fit to surveillance data.Citation38 Sensitivity analyses for this model showed incidence patterns strongly dependent on vaccine coverage, a similar finding to that of Hethcote with his original model.Citation27
Whether pertussis dynamics are more SIR- or SIRS-like is a perplexing question. Evidence for an SIRS-like structure includes the large number of cases reported in vaccinated individuals in some populations,Citation46-48 and the high prevalence of adults with serological evidence of recent infection in pertussis serosurveys.Citation41 On the other hand, recent models in which parameters have been estimated from incidence data using statistical inference point to a limited role for repeat infections in transmission and/or a long duration of protection, a more SIR-like structure.Citation15,38 Although we may expect subtle differences in models in different settings, we might expect that underlying characteristics, such as whether or not immunity is permanent, would be consistent across different settings. Notably, the Blackwood model,Citation38 and the Rohani model,Citation15 were fit to incidence data from countries with shorter vaccination histories than most other models (Thailand) or a break in coverage (Sweden), respectively. Reduced boosting opportunities may gradually erode population immunity under high coverage vaccination programs and cause a shift from SIR- to SIRS-like dynamics.Citation5 If natural immunity is long-lived, as found in a number of models, it may be that this effect is yet to be observed in Thailand and Sweden. As longer time series of data become available, it will be important that models include provision for waning immunity, to examine its influence. With the aforementioned difficulties in interpreting available data, we also caution against pure reliance on statistical measures of fit such as the AIC when evaluating which of simple or more complex models are most well supported. Such rigorous statistical assessments cannot reflect the (largely unquantifiable) uncertainty associated with the available epidemiologic data.
Conclusion
While simple models may provide insights into the underlying mechanisms driving pertussis transmission, additional realism is required when the aim is to match observed dynamics and predict the outcome of control measures. Our review clearly shows it is important that models include age structure, without which it is impossible to realistically model pertussis vaccination. Further, although the duration of vaccine and natural immunity is yet to be convincingly determined, it is apparent that neither is permanent, and thus models must allow for reinfection to occur. We recommend contemporary data, relevant to the population being modeled, are used for parameterization wherever possible and caution against the use of pre-vaccine era forces of infection, especially in countries with long vaccination histories. As incidence patterns generated using most models were sensitive to mixing patterns, we prefer the use of appropriate empirical contact data over heuristically configured contact matrices, as they are more likely to reflect true population mixing.
It is a common problem that there are insufficient data to parameterize pertussis transmission models, particularly those that adequately capture the generally understood complexities of available epidemiologic data sources. Although some authors state that parameters such as the average duration of infectiousness are well known, there is great variation in the values used in the reviewed models. While one might expect that biological parameters remain relatively constant across populations, there is inherent difficulty separating environmental and biological aspects of transmission. Estimating appropriate transmission parameters is particularly difficult, with traditional methods relying on ad hoc assumptions about mixing structures and the use of pre-vaccine era force of infection data, which most likely do not capture a significant majority of infection events. These traditional methods are likely to underestimate the contribution by adults to the spread of pertussis.
While the way forward with pertussis modeling may well be to fit models to incidence data using statistical inference, a major obstacle for this approach is the poorly quantified relationship between infection and disease. There are many layers involved in an infection becoming a notified case: development of clinical symptoms; seeking treatment; physician awareness; testing likelihood and accuracy; and notification infrastructure. Formal fitting processes should be capturing these time-varying aspects in addition to transmission characteristics, rather than assuming simple relationships between infection and disease.
Estimates of under-reporting vary widely between studies and are necessarily population specific due to differences in diagnostic capability and the reporting infrastructure. The age distribution of prior vaccination schedules and its effect on the pertussis immunity profile also should be considered. Under-reporting values from a study in one population would therefore not generally be applicable to another and every effort should be made to use local data, or undertake a sensitivity analysis on this aspect of a model.
Estimation of herd protection of infants too young to be vaccinated is currently limited by the lack of suitable data on their contact patterns. It is often the case that mixing for this group is extrapolated from data for pre-school children, who may in reality have substantially different patterns due to differences in childcare usage and social interaction. Robustness of predictions for the vulnerable infant age group would be enhanced through incorporation of detailed contact data and household structure into models.
Decreases in vaccination coverage provide an opportunity for the susceptible pool to increase more quickly, while increases may reduce transmission and natural boosting. Models can be very sensitive to the assumed level of vaccination coverage, with changes to coverage causing oscillations in the system that may take years to subside and possibly creating cohort effects with varying impact on epidemiology. Many countries appear to have periods of unreliable pertussis vaccination coverage records and since assumptions made about coverage during these periods may impact the immune profile of the population, they should be discussed alongside results.
Sensitivity analyses in published models of pertussis often vary only one or a few parameters at a time, which may miss critical dependencies between parameters. Finding a set of parameters which fits the data does not preclude the existence of other, equally plausible, parameter combinations. Simultaneous variation of parameter values over plausible ranges may overcome this limitation and provide a deeper understanding of relationships between parameters.
This review of existing models has revealed the many uncertainties and evidence gaps in our understanding of pertussis drivers and trends, leading to parallel development of plausible alternative model structures based on diverse assumptions. Structures of future models to address pressing policy questions must necessarily be guided by the key issues to be explored, within real world information constraints. Given the sensitivity of model conclusions to long term immunisation and disease trends, relevant local data should be used, wherever available, to inform model parameters for evaluation of setting-specific interventions. Such detailed evaluation of pertussis behavior in multiple contexts affords opportunity to identify unifying drivers of disease, reduce uncertainty regarding model interpretation and extrapolation and provide support for local and global decision-making regarding improved vaccine approaches.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Patricia Campbell was the recipient of an Australian Postgraduate Award during this study. James McCaw is supported by an Australian Research Council Future Fellowship (FT110100250). Jodie McVernon is supported by a National Health and Medical Research Council Career Development Fellowship.
References
- Cherry JD. Pertussis: challenges today and for the future. PLoS Pathog 2013; 9:e1003418; PMID:23935481; http://dx.doi.org/10.1371/journal.ppat.1003418
- Jackson DW, Rohani P. Perplexities of pertussis: recent global epidemiological trends and their potential causes. Epidemiol Infect 2014; 142:672-84; PMID:23324361; http://dx.doi.org/10.1017/S0950268812003093
- McIntyre P. Lessons from surveillance: solving the pertussis puzzle. N S W Public Health Bull 2003; 14:69-71; PMID:12806403; http://dx.doi.org/10.1071/NB03022
- Wendelboe AM, Van Rie A, Salmaso S, Englund JA. Duration of immunity against pertussis after natural infection or vaccination. Pediatr Infect Dis J 2005; 24(5 Suppl):S58-61; PMID:15876927; http://dx.doi.org/10.1097/01.inf.0000160914.59160.41
- Lavine JS, King AA, Bjørnstad ON. Natural immune boosting in pertussis dynamics and the potential for long-term vaccine failure. Proc Natl Acad Sci U S A 2011; 108:7259-64; PMID:21422281; http://dx.doi.org/10.1073/pnas.1014394108
- Riolo MA, King AA, Rohani P. Can vaccine legacy explain the British pertussis resurgence? Vaccine 2013; 31:5903-8; PMID:24139837; http://dx.doi.org/10.1016/j.vaccine.2013.09.020
- Rohani P, Drake JM. The decline and resurgence of pertussis in the US. Epidemics 2011; 3:183-8; PMID:22094341; http://dx.doi.org/10.1016/j.epidem.2011.10.001
- Crowcroft NS, Pebody RG. Recent developments in pertussis. Lancet 2006; 367:1926-36; PMID:16765762; http://dx.doi.org/10.1016/S0140-6736(06)68848-X
- Cherry JD. Pertussis in the preantibiotic and prevaccine era, with emphasis on adult pertussis. Clin Infect Dis 1999; 28 Suppl 2:S107-11; PMID:10447027; http://dx.doi.org/10.1086/515057
- Teunis PFM, Van Der Heijden OG, De Melker HE, Schellekens JFP, Versteegh FGA, Kretzschmar MEE. Kinetics of the IgG antibody response to pertussis toxin after infection with B. pertussis. Epidemiol Infect 2002; 129:479-89; PMID:12558330; http://dx.doi.org/10.1017/S0950268802007896
- Vynnycky E, White RG. An Introduction to Infectious Disease Modelling. Oxford: Oxford University Press, 2010.
- Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. New York: Oxford University Press, 1991.
- Wallinga J, Teunis P, Kretzschmar M. Using data on social contacts to estimate age-specific transmission parameters for respiratory-spread infectious agents. Am J Epidemiol 2006; 164:936-44; PMID:16968863; http://dx.doi.org/10.1093/aje/kwj317
- Mossong J, Hens N, Jit M, Beutels P, Auranen K, Mikolajczyk R, Massari M, Salmaso S, Tomba GS, Wallinga J, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med 2008; 5:e74; PMID:18366252; http://dx.doi.org/10.1371/journal.pmed.0050074
- Rohani P, Zhong X, King AA. Contact network structure explains the changing epidemiology of pertussis. Science 2010; 330:982-5; PMID:21071671; http://dx.doi.org/10.1126/science.1194134
- Ogunjimi B, Hens N, Goeyvaerts N, Aerts M, Van Damme P, Beutels P. Using empirical social contact data to model person to person infectious disease transmission: an illustration for varicella. Math Biosci 2009; 218:80-7; PMID:19174173; http://dx.doi.org/10.1016/j.mbs.2008.12.009
- Kretzschmar M, Teunis PFM, Pebody RG. Incidence and reproduction numbers of pertussis: estimates from serological and social contact data in five European countries. PLoS Med 2010; 7:e1000291; PMID:20585374; http://dx.doi.org/10.1371/journal.pmed.1000291
- de Greeff SC, Dekkers ALM, Teunis P, Rahamat-Langendoen JC, Mooi FR, de Melker HE. Seasonal patterns in time series of pertussis. Epidemiol Infect 2009; 137:1388-95; PMID:19327200; http://dx.doi.org/10.1017/S0950268809002489
- Broutin H, Viboud C, Grenfell BT, Miller MA, Rohani P. Impact of vaccination and birth rate on the epidemiology of pertussis: a comparative study in 64 countries. Proc R Soc Lond B Biol Sci 2010; 277:3239-45.
- Dafilis MP, Frascoli F, McVernon J, Heffernan JM, McCaw JM. The dynamical consequences of seasonal forcing, immune boosting and demographic change in a model of disease transmission. J Theor Biol 2014; 361:124-32; PMID:25106793; http://dx.doi.org/10.1016/j.jtbi.2014.07.028
- Dafilis MP, Frascoli F, Wood JG, Mccaw JM. The influence of increasing life expectancy on the dynamics of SIRS systems with immune boosting. ANZIAM J 2012; 54:50-63; http://dx.doi.org/10.1017/S1446181113000023
- van Boven M, de Melker HE, Schellekens JFP, Kretzschmar M. A model based evaluation of the 1996-7 pertussis epidemic in The Netherlands. Epidemiol Infect 2001; 127:73-85.
- Préziosi M-P, Halloran ME. Effects of pertussis vaccination on transmission: vaccine efficacy for infectiousness. Vaccine 2003; 21:1853-61; http://dx.doi.org/10.1016/S0264-410X(03)00007-0
- Hethcote HW, Horby P, McIntyre P. Using computer simulations to compare pertussis vaccination strategies in Australia. Vaccine 2004; 22:2181-91; PMID:15149775; http://dx.doi.org/10.1016/j.vaccine.2003.11.053
- Gomes MGM, White LJ, Medley GF. Infection, reinfection, and vaccination under suboptimal immune protection: epidemiological perspectives. J Theor Biol 2004; 228:539-49; PMID:15178201; http://dx.doi.org/10.1016/j.jtbi.2004.02.015
- Wearing HJ, Rohani P. Estimating the duration of pertussis immunity using epidemiological signatures. PLoS Pathog 2009; 5:e1000647; PMID:19876392; http://dx.doi.org/10.1371/journal.ppat.1000647
- Hethcote HW. An age-structured model for pertussis transmission. Math Biosci 1997; 145:89-136; PMID:9309930; http://dx.doi.org/10.1016/S0025-5564(97)00014-X
- Hethcote HW. Simulations of pertussis epidemiology in the United States: effects of adult booster vaccinations. Math Biosci 1999; 158:47-73.
- Grenfell BT, Anderson RM. Pertussis in England and Wales: an investigation of transmission dynamics and control by mass vaccination. Proc R Soc Lond B Biol Sci 1989; 236:213-52; PMID:2567004; http://dx.doi.org/10.1098/rspb.1989.0022
- Van Rie A, Hethcote HW. Adolescent and adult pertussis vaccination: computer simulations of five new strategies. Vaccine 2004; 22:3154-65; PMID:15297068; http://dx.doi.org/10.1016/j.vaccine.2004.01.067
- Luz PM, Codeço CT, Werneck GL, Struchiner CJ. A modelling analysis of pertussis transmission and vaccination in Rio de Janeiro, Brazil. Epidemiol Infect 2006; 134:850-62; PMID:16316489; http://dx.doi.org/10.1017/S095026880500539X
- Coudeville L, Van Rie A, Andre P. Adult pertussis vaccination strategies and their impact on pertussis in the United States: evaluation of routine and targeted (cocoon) strategies. Epidemiol Infect 2008; 136:604-20; PMID:17612417; http://dx.doi.org/10.1017/S0950268807009041
- van Boven M, de Melker HE, Schellekens JFP, Kretzschmar M. Waning immunity and sub-clinical infection in an epidemic model: implications for pertussis in The Netherlands. Math Biosci 2000; 164:161-82; PMID:10748285; http://dx.doi.org/10.1016/S0025-5564(00)00009-2
- Águas R, Gonçalves G, Gomes MGM. Pertussis: increasing disease as a consequence of reducing transmission. Lancet Infect Dis 2006; 6:112-7; PMID:16439331; http://dx.doi.org/10.1016/S1473-3099(06)70384-X
- Roberts MG. Can we prevent the next epidemic? The elimination of childhood diseases by mass vaccination. J App Math Decision Sci 2000; 4:175-82; http://dx.doi.org/10.1155/S1173912600000134
- Quinn HE, Snelling TL, Macartney KK, McIntyre PB. Duration of protection after first dose of acellular pertussis vaccine in infants. Pediatrics 2014; 133:e513-9; PMID:24515514; http://dx.doi.org/10.1542/peds.2013-3181
- McLean AR. Vaccines and their impact on the control of disease. Br Med Bull 1998; 54:545-56; PMID:10326283; http://dx.doi.org/10.1093/oxfordjournals.bmb.a011709
- Blackwood JC, Cummings DAT, Broutin H, Iamsirithaworn S, Rohani P. Deciphering the impacts of vaccination and immunity on pertussis epidemiology in Thailand. Proc Natl Acad Sci U S A 2013; 110:9595-600.
- Mooi FR, de Greeff SC. The case for maternal vaccination against pertussis. Lancet Infect Dis 2007; 7:614-24; PMID:17537674; http://dx.doi.org/10.1016/S1473-3099(07)70113-5
- Hall R. Notifiable diseases surveillance, 1917 to 1991. Communicable Dis Intell 1993; 17:226-36.
- Campbell P, McIntyre P, Quinn H, Hueston L, Gilbert GL, McVernon J. Increased population prevalence of low pertussis toxin antibody levels in young children preceding a record pertussis epidemic in Australia. PLoS One 2012; 7:e35874; PMID:22558249; http://dx.doi.org/10.1371/journal.pone.0035874
- Hethcote HW. Modeling heterogeneous mixing in infectious disease dynamics. In: Isham V, Medley G, eds. Models for Infectious Human Diseases. Press Syndicate of the University of Cambridge, Cambridge, 1996:215-38.
- Geard N, McCaw JM, Dorin A, Korb KB, McVernon J. Synthetic population dynamics: a model of household demography. J Artif Soc Social Simul 2013; 16:8.
- Rohani P, Earn DJD, Grenfell BT. Impact of immunisation on pertussis transmission in England and Wales. Lancet 2000; 355:285-6; PMID:10675078; http://dx.doi.org/10.1016/S0140-6736(99)04482-7
- Lavine JS, King AA, Andreasen V, Bjørnstad ON. Immune boosting explains regime-shifts in prevaccine-era pertussis dynamics. PLoS One 2013; 8:e72086
- Van Buynder PG, Owen D, Vurdien JE, Andrews NJ, Matthews RC, Miller E. Bordetella pertussis surveillance in England and Wales: 1995-7. Epidemiol Infect 1999; 123:403-11; PMID:10694150; http://dx.doi.org/10.1017/S0950268899003052
- Senzilet LD, Halperin SA, Spika JS, Alagaratnam M, Morris A, Smith B, Sentinel Health Unit Surveillance System Pertussis Working Group. Pertussis is a frequent cause of prolonged cough illness in adults and adolescents. Clin Infect Dis 2001; 32:1691-7; PMID:11360208; http://dx.doi.org/10.1086/320754
- Tartof SY, Lewis M, Kenyon C, White K, Osborn A, Liko J, Zell E, Martin S, Messonnier NE, Clark TA, et al. Waning immunity to pertussis following 5 doses of DTaP. Pediatrics 2013; 131:e1047-e52; PMID:23478868; http://dx.doi.org/10.1542/peds.2012-1928
