Abstract
In 2002, a pneumococcal conjugate vaccine (PCV) was introduced to French infants and toddlers. A change has been witnessed in the incidence of pneumococcal diseases in adults: the incidence of invasive pneumococcal disease (IPD) of serotypes covered by PCV decreased, and serotypes not covered by PCV increased. This study aimed to quantify the public health and budget impact of pneumococcal vaccination strategies in at-risk adults in France over 5 years. A previously published population-based Markov model was adapted to the French situation. At-risk adults received either PPV23 (pneumococcal polysaccharide vaccine; for the immunocompetent) or PCV13 (for the immunosuppressed). The strategy was compared to PCV13 alone. Uncertainty was addressed using extreme scenario analyses. Between 2014 and 2018, vaccination with PPV23/PCV13 led to a higher reduction in terms of IPD and non-bacteremic pneumococcal pneumonia cases avoided in most scenarios analyzed when compared to PCV13 alone. For budget impact, none of the scenarios was in favor of PCV13. Under conservative coverage assumptions, the total incremental budget impact ranged from € 39.8 million to € 69.3 million if PCV13 were to replace PPV23 in the immunocompetent. With the epidemiological changes of pneumococcal diseases and the broader serotype coverage of PPV23, the current program remains an optimal strategy from public health perspective. Given the additional budget required for the use of PCV13 alone and its uncertain public health benefits, vaccination with PPV23 remains the preferred strategy.
Abbreviations
| ATIH | = | Agence Technique de l'Information sur l'Hospitalisation |
| BdM IT | = | Base des Médicaments et Informations Tarifaires |
| CNRP | = | Center National de Référence des Pneumocoques |
| COPD | = | chronic obstructive pulmonary disease |
| GHM | = | groupes homogènes de malades |
| GP | = | general practitioner |
| HCSP | = | Haut Conseil de la Santé Publique |
| HIV | = | human immunodeficiency virus |
| ICD | = | International Classification of Diseases |
| INED | = | Institut National d'Etudes Démographiques |
| INSEE | = | Institut National de la Statistique et des Etudes Economiques |
| IPD | = | invasive pneumococcal pneumonia |
| NBPP | = | non-bacteremic pneumococcal pneumonia |
| PCV7/13 | = | 7-/13-valent pneumococcal conjugate vaccine |
| PMS | = | post-meningitis sequelae |
| PMSI | = | Program de médicalisation des systèmes d'information |
| PPV23 | = | 23-valent pneumococcal polysaccharide vaccine |
| WHO | = | World Health Organization |
Introduction
Streptococcus pneumoniae
Streptococcus pneumoniae is a gram-positive bacterium, of which more than 90 distinct serotypes have been identified.Citation1 Infection with the bacterium can lead to life-threating diseases, such as pneumonia, meningitis and bacteremia. S. pneumoniae is also associated with non-invasive form of diseases, including non-bacteremic pneumococcal pneumonia (NBPP), which accounted for approximately 80% of the burden of pneumococcal pneumonia.Citation2-5
Pneumococcal diseases lead to approximately 1.6 million deaths annually in the world, most in infants, children and the elderly.Citation6 In France, the annual number of pneumococcal infection cases was estimated at 455,000, and deaths from these diseases were between 3,500 and 11,000. In terms of invasive pneumococcal diseases (IPD), the number of cases was reported to range between 6,000 and 7,000.Citation7
Individuals with predisposing factors are more likely to contracting pneumococcal infection. Chronic pulmonary, cardiac, renal and hepatic diseases and immunosuppression are considered as risk factors.Citation2-4,6,8
Vaccination against pneumococcal diseases
Vaccination is the only public health measure that is likely to reduce the burden of pneumococcal diseases.Citation4 Currently, 2 types of vaccines are licensed, pneumococcal polysaccharide vaccines (PPV) and pneumococcal conjugate vaccines (PCV).
In France, the 23-valent PPV (PPV23; Pneumo 23®, Sanofi Pasteur MSD) is indicated in the prevention of pneumococcal infections caused by serotypes included in the vaccines in individuals aged 2 years and older who are at risk.Citation9 The 13-valent PCV (PCV13; Prevenar 13®, Pfizer), which replaced the 7-valent vaccine (PCV7; Prevenar®, Pfizer) in 2010, is indicated in the prevention of IPD, pneumonia and acute otitis media in those aged between 6 weeks and 17 years (in those between 6 weeks and 5 years from 2010 and expanded to between 6 weeks and 17 years from 2013), as well as in the prevention of IPD in those aged 18 years old or older (in those aged 50 years and above from 2011 and expanded to 18 years and above from 2013).Citation10-12
In Europe, recommendation on the use of PPV in at-risk adults and/or the elderly dates back to the 1980s.Citation13 For infants and toddlers, many European countries introduced routine childhood vaccination programs using PCVs.Citation13 In 2013, HCSP (Haut Conseil de la Santé Publique; High Council of Public Health) updated its recommendation to introduce the sequential use of PCV and PPV in certain at-risk populations aged 5 years and older: the immunosuppressed, those with nephrotic syndrome, those with a cerebrospinal fluid leak, those with a cochlear implant and those who are candidates for such implant. The recommendation for the immunosuppressed and the asplenic was made based on immunogenicity data of PCVs and hyporesponsiveness risk assumed for PPV23.Citation14 Nevertheless, the recommendation has not considered the epidemiological change induced by childhood vaccination with PCVs (see the following section). PPV23 is recommended in other high-risk populations, including those with hearth, pulmonary and renal failure, those with chronic hepatic diseases, those with severe asthma under continuous treatment, and those with diabetes not sufficiently by diet.Citation15 Revaccination with the same vaccine is not recommended, regardless of risk group.
The impact of vaccinating infants and toddlers on the epidemiology in adults
In France, infants and toddlers are vaccinated with 3 or 4 doses of PCV13. It is estimated that, in those born in 2011, 96.1% received the first vaccine dose at 6 months of age and 88.8% received at least 3 doses at 2 years of age (end of 2013 data).Citation16
The routine vaccination of infants and toddlers with PCV not only affects the epidemiology in children, changes have also been observed in adults in the UK,Citation17 the US,Citation18 GermanyCitation19 and Spain.Citation20
As a herd immunity effect, the incidence of IPD associated with the serotypes covered in PCV decreased in adults. However such reduction has been offset by serotype replacement: an increase in the incidence of IPD associated with the serotypes not covered in PCV.Citation17-20 In France, the data from CNRP (Center National de Référence des Pneumocoques; National Reference Center for Pneumococcus) showed a marked increase in the proportion of IPD associated with non-PCV serotypes overtime (Emmanuelle Varon, personal communication; the data have been reported graphically in the CNRP Report 2011Citation21).
Objectives
With the move from PCV7 to PCV13 in France in 2010, it is expected that the epidemiology of pneumococcal diseases may be further affected. In light of the expansion of the indication of PCV13, the current study aimed to assess the comparative public health and budget impact of vaccinating at-risk adults in France with different strategies (i.e. PPV23 for the immunocompetent, PCV13 for the immunosuppressed; PCV13 alone), in the context of epidemiological changes. Several scenario analyses were carried out in order to address the uncertainties around vaccine effectiveness, costs and epidemiology.
Results
At-risk population and vaccination coverage
French at-risk population eligible for vaccination against pneumococcal diseases (9.5 million) was tracked over time. Approximately 80% of these individuals were aged 50 years and older. Between 2014 and 2018, a total of 1.95 million people received the vaccination.
Public health impact
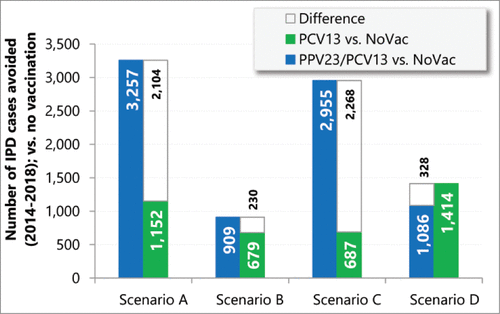
Vaccinating the immunocompetent individuals with PPV23 and the immunosuppressed individuals with PCV13 (referred to as PPV23/PCV13 hereafter) was associated with the greatest decrease in the total number of IPD cases over the period between 2014 and 2018, in all 4 scenarios except scenario D (; also Table S6). When compared to vaccination with PCV13 alone, the strategy led to favorable outcomes in 3 of 4 scenarios, ranging from 230 (−1.1%; calculated as the difference divided by the total number of IPD cases under PCV13 only strategy, the same below) additional cases avoided in scenario B to 2,268 (−8.4%) additional IPD cases avoided in scenario C. In scenario D, where higher efficacy was assumed for PCV13, the additional IPD cases avoided was 328 (1.6%) under the PCV13 only strategy.
Table 1. Definition of at-risk adults.15
In terms of the total number of NBPP cases, PPV23/PCV13 led to a decreased or unchanged number when compared to PCV13 alone in scenarios A, B and C. In scenario D, where higher efficacy was assumed for PCV13, the number of cases avoided increased by 10,145 (+9.7%) when comparing PCV13 to PPV23/PCV13. The cumulative number of PMS (post-meningitis sequelae) cases followed the same pattern as that of IPD.
Budget impact analysis
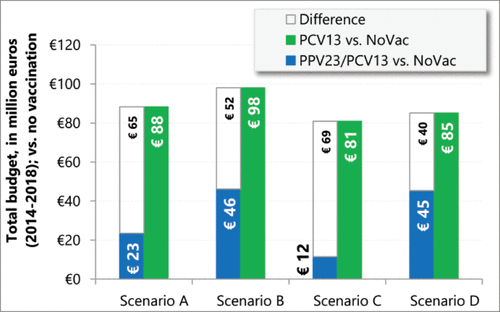
From 2014 to 2018, vaccination with PCV13 alone was associated with the highest costs across 4 scenarios. Even in scenario D, the most favorable scenario for PCV13, the incremental cost was estimated at € 39.8 million (+15.2%; calculated as the difference divided by the total budget under PPV23/PCV13 strategy). In other scenarios, such increment ranged between € 51.9 million (+19.7%; scenario B) and € 69.3 (+19.0%; scenario C), when compared to the use of PPV23/PCV13 (; Table S7).
Table 2. Clinical and demographical parameters.
Sensitivity analyses
Sensitivity analyses have been carried out for costs except for the price of vaccines (doubling or halved), vaccination coverage (1.25 times or halved) and the prevalence of risk factors (using the UK sourceCitation22). The results did not change qualitatively but only quantitatively (Table S8).
Table 3. Scenario analysis.
Discussion
Summary of findings
In the current analysis, the public health and budget impact of using different vaccination strategies were assessed in the French context. Epidemiological changes of IPD induced by routine infant and toddler vaccination were taken into account in the analysis. Due to uncertainties around such changes, as well as around efficacy due to a lack of head-to-head studies, 4 scenario analyses were devised, with one extreme scenario (D) that favors PCV13.
The strategy with PCV13 alone was only found to render more public health benefits in scenario D, where higher efficacy was assumed. It was associated with the highest cost across scenarios. The additional cost (when compared to PPV23/PCV13) amounted to between € 39.8 million and € 69.3 million.
Strengths
As our previous studies,Citation23,24 the current analysis considered the epidemiological changes of IPD stemmed from routine infant and toddler vaccination. Indeed, a clear trend has been witnessed across countries that the use of PCVs in infants and toddlers would provide an indirect herd effect on adults, leading to a reduced value of using such vaccines in the adult population.
Secondly, the study employed recently published data from CAPiTA study of PCV13,Citation25 which improved the scientific evidence. However, there is still a lack of head-to-head studies comparing the 2 vaccines. In order to address uncertainties, 4 scenarios were considered, with some of them favoring PCV13.
Lastly, French data were used whenever possible, especially the data on the prevalence of risk factors. Although English and Welsh national dataCitation22 were also used to fill the data gaps, the comparable estimates from the 2 populations followed the same trend.
Limitations
Firstly, the current analysis did not fully reflect the French recommendation since it was not possible to model the sequential use of PPV and PCV in the immunosuppressed due to a lack of data and the limitation of the model structure. Nevertheless the size of the immunosuppressed population was small and the conclusion will not be changed qualitatively. Furthermore, the target population was included only adults (vs. those aged 5 years or older in the recommendation) and proxies were used to calculate the size of the target population.
As an infectious disease, the current analysis only adopted a static approach and it did not capture the dynamics of transmission of pneumococcal infections therefore the benefits of vaccination may not be reflected wholly.
Thirdly, the model has not distinguished case-fatality rates across serotypes. A Danish study has reported that serotypes 10A, 11A, 15B and 17F are associated with an elevated case-fatality rate, to which PPV23 was shown to induce a robust immune response.Citation26,27
Fourthly, although best efforts were made to identify French data, there was still missing information that could not be found. This related to the incidence of NBPP, which contributes to a large proportion of the burden of pneumococcal diseases. Similarly, the prevalence of risk factors was retrieved from various sources, which might lack consistencies. More research is required to update current study.
Fifthly, the current analysis adopted a time horizon of 5 years, which is arguably not long enough to forecast the future public health benefits from vaccination. However the epidemiology will further change due to continued serotype replacement and herd effect, which may make the forecast inaccurate. Therefore it might be prudent to have a limited time horizon, and update the analysis when future information is made available.
Additionally, the estimation of the absolute number of IPD cases did not involve any back calculation, and the actual burden was -therefore conservatively underestimated.
Concluding remarks
This study has demonstrated that a combination of PPV23 for the at-risk immunocompetent adults and PCV13 for the at-risk immunosuppressed adults is likely to afford better protection to these populations compared to the use of PCV13 alone. With the switch from PCV7 to PCV13 in the routine vaccination of infants and toddlers, further changes in the epidemiology of IPD are expected. The extended use of PCV13 in children has substantially restricted its public health impact in adults because of the change in the serotype distribution of pneumococcal diseases.Citation28 Therefore the value of broader serotype coverage by PPV23 is of the most importance. Considering the additional budget required for the use of PCV13 alone, vaccination of immunocompetent individuals with PPV23 remains the preferred strategy.
Close and continued monitoring of the disease is required to update the current analysis. Other epidemiological parameters are also needed to refine the results.
Materials and Methods
Model structure
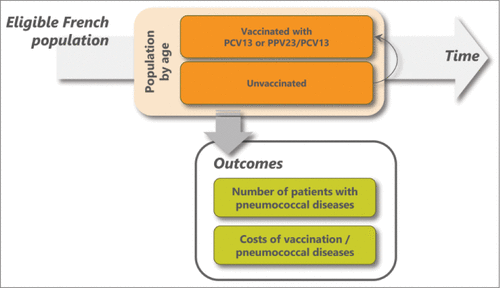
The current model was adapted from the previous analysis developed for Germany.Citation23 It was a population-based, multiple cohort Markov model which tracked at-risk French adults. They could receive a vaccine or remain unvaccinated.
Incident cohorts of the adults who are at-risk in the first calendar year were tracked until they reached 100 years old, die, or until 2018. The model recorded the number of disease cases and costs. The model schematics are presented in .
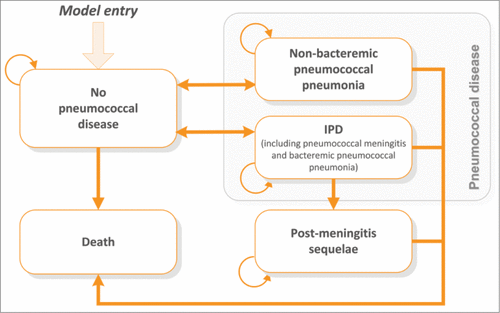
describes the model structure. For all cohorts, with or without vaccination, adults with no pneumococcal disease could develop IPD (pneumonia or meningitis) or NBPP, or stay without pneumococcal disease. Those with meningitis might develop PMS if they survived meningitis. Those who recovered from IPD or NBPP may contract pneumococcal diseases for a second time. Due to the rarity, those with PMS were assumed not to develop another episode of IPD or NBPP. Excess mortality (expressed as case-fatality rate) associated with IPD and NBPP was considered in the model. The mortality rate was assumed to be the same for all other health states, as for the general population.
The model had a cycle length of one year and the results were presented for the period between 2014 and 2018. In order to account for those vaccinated before 2014, the model was initiated in 2005.
Target population and assessed strategies
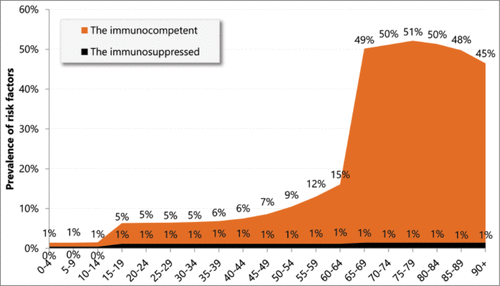
The analyses considered at-risk adults (immunocompetent or immunosuppressed) and did not take into account those aged below 18 years. The risk factors included followed the HCSP recommendation,Citation15 and are presented in .
Table 4. Waning function.
Three strategies were considered in the analysis, vaccination with PPV23/PCV13, vaccination with PCV13 alone and no vaccination. Sequential vaccination (with 2 different vaccines over a short period of time) was not considered due to the lack of data on efficacy and the limitation of the model structure. Following the study question, the incremental results are presented for PPV23/PCV13 vs. PCV13 alone. The results of the no vaccination strategy are presented in the Online Appendix.
Revaccination (with the same vaccine over a long period of time) was not considered since not recommended by HCSP.Citation15
Invasive pneumococcal diseases
As noted earlier, the analysis considered changes in the epidemiology of IPD. Since the introduction of PCV vaccination in infants and toddlers, a herd immunity effect has been witnessed in adults, as well as a serotype replacement effect.Citation21,29
To model such changes, the incidence of IPD between 2005 and 2011 was retrieved from the EPIBAC (Agnès Lepoutre, personal communication) and the serotype split in 2005, 2007, 2009, 2010 and 2011 from CNRP (Emmanuelle Varon, personal communication; the CNRP Report 2012Citation29).
In France, PCV13 replaced PCV7 in 2010 in the French vaccination program of infants and toddlers.Citation30 Since the last dose of PCV was given at the 24 months old, the analysis assumed that the effect of PCV7 will last until 2011. Therefore the effect of PCV7 on the epidemiology of IPD was assumed to be captured by the CNRP data. From 2012, it was assumed that the incidence of IPD associated with 6 serotypes covered by PCV13 but not PCV7 would decrease, of IPD associated with other serotypes would increase and of IPD associated with 7 serotypes covered by PCV7 would remain stable. Using the same approach as in previous studies,Citation23,24 it was assumed that the proportion change in the incidence of IPD was a function of the cumulative vaccination rate in children,Citation16 following the same pattern as observed for PCV7. A cumulative gamma distribution was selected because of the goodness of fit. The aforementioned changes were applied for a period of 6 years, as per the observed data of PCV7. The analysis applied constant incidence beyond this period. The incidence between 2005 and 2020 is presented in and the parameters in (Emmanuelle Varon, personal communication; the CNRP Report 2012Citation29).
Table 5. Costs.
Figure 5. Observed and projected epidemiological changes among adults and elderly in serotype-specific incidence of IPD induced by vaccination of infants and toddlers.
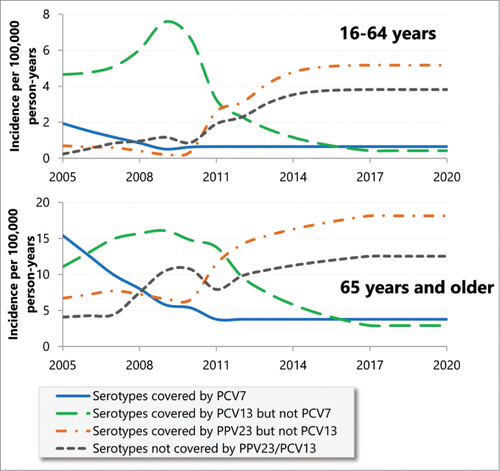
It should be noted that no back calculation has been performed to account for existing PPV23 vaccination. Nevertheless the approach was conservative because there was an underestimation of the number of IPD cases in the no vaccination scenario, leading to a downwards bias in the effectiveness of vaccination.
The incidence of IPD was adjusted for the at-risk population, since the aforementioned incidence was for the overall population. Due to a lack of information, a national study conducted in England and Wales was utilized.Citation22 The case-fatality rate from IPD was also based on this study due to a lack of French data. In the model, 7.6% of IPD cases were meningitis, based on average incidence from the French EPIBAC dataset (Agnès Lepoutre, personal communication). The rate of post-meningitis sequelae came from an international meta-analysis.Citation31
Natural course of other pneumococcal diseases
No publication was found reporting the incidence of NBPP in France. The estimation was done based on the incidence of hospitalized pneumonia and the hospitalization rate (32%) from the UK.Citation32 The incidence of hospitalized pneumonia was calculated using the French hospital database ATIH (Agence Technique de l'Information sur l'Hospitalisation; Technical Information Agency on Hospitalization).Citation33 The data consisted of all pneumonia due to S. pneumoniae (ICD-10 J13) and pneumonia, organism unspecified (ICD-10 J18), of which 37.2% were attributable to S. pneumoniae.Citation34 The case-fatality rate was also based on the UK data, which reported that 9.6% for hospitalized cases and 0% for outpatient cases.Citation32
Demography
Population size and life tables (by age and over time) were obtained from the INSEE (Institut National de la Statistique et des Etudes Economiques; National Institute for Statistics and Economic Studies) and INED (Institut National d'Etudes Démographiques; National Institute for Demographic Studies).Citation35-38
The proportion of the at-risk population for vaccination by age group was estimated from French sources.Citation33,39-52 When French data were not available, the data from England and Wales were used.Citation22 The prevalence of risk factors is presented in .
Vaccine coverage was derived using the actual and projected sales data of PPV23,Citation53 and the size of the target population. Between 2014 and 2020, approximately 4.0% of the at-risk adults would receive the vaccination annually.
Vaccine efficacy and effectiveness
Four scenarios have been considered for vaccine efficacy and effectiveness due to a lack of head-to-head comparison between PPV23 and PCV13 (). In scenario A (point estimates derived from published literature), absolute vaccine efficacy of PCV13 against IPD vaccine type-related in the immunocompetent population was based on recently published results from the CAPiTA study.Citation25 For PPV23, its comparative efficacy versus PCV13 was derived using an indirect comparison (placebo as the common comparator), utilizing the data synthesized in a Cochrane review.Citation54 For efficacy against IPD in the immunosuppressed, the estimate came from a clinical trial of patients infected with HIV.Citation55 The effectiveness against NBPP in the immunocompetent was retrieved from the Spanish EVAN-65 study.Citation56 No effectiveness was assumed against NBPP in the immunosuppressed. In scenario B, favorable efficacy estimates were assumed for PCV13 while in scenario C, for PPV23 (assuming vaccination provides limited protection in both scenarios). In scenario D, the highest estimates from the previous 3 scenarios were used for PCV13 while lowest for PPV23.
The aforementioned vaccine efficacy waned over time for both vaccines since there is no long-term follow-up date for PCV13. It was assumed that there was no more protection 8 years after the initial vaccination ().Citation57,58
Costs
Costs were estimated from the perspective of the French health insurance, Assurance Maladie. All costs were estimated in 2013/4 and expressed in euros (). The costs were not discounted due to the nature of the study.
The unit price of PPV23 and PCV13 were retrieved from BdM IT (Base des Médicaments et Informations Tarifaires; Basic Drugs and Tariff Information), € 13.56 per one dose of PPV23 and € 56.72 per one dose of PCV13 (retail price, VAT included).Citation59 Both vaccines were reimbursed at 65%. One visit to a general practitioner (GP) was assumed for the administration, based on a cost-effectiveness analysis of meningococcal B vaccine published by HCSP.Citation60 One GP visit cost € 23, of which 70% was reimbursed and the patients were required to pay € 1 flat fee (participation forfairaire) in addition.Citation61
All IPD cases were hospitalized and the cost was retrieved from the PMSI (Program de médicalisation des systèmes d'information; Program of Medicalization of Information Systems) database,Citation62,63 using the weight average cost of pneumonia and common pleuritis in adults (GHM 04M05) for pneumonia, € 3,600.85 per case, and central nervous system infection except viral meningitis (GHM 01M05) for meningitis, € 5,636.37 per case.
For hospitalized NBPP, the above cost of invasive pneumonia was used. For outpatient cases, 2 GP visits and 10 day of co-amoxiclav (1g t.i.d.; € 7.28 for 8 sachets of 1g co-amoxiclav, reimbursement rate 65%) were assumed.Citation64 The cost was therefore calculated at €32.85 from the perspective of health insurance.Citation59,61
Lastly, a case of PMS would incur an annual cost of € 8,000, based on the aforementioned HCSP study on the cost-effectiveness of a meningococcal vaccine.Citation60
Public health and budget impact
In the analysis, the number of IPD and NBPP cases over time, the cumulative number of PMS cases, total costs and costs by category over time were reported. The public health and budget impact were estimated as the difference between the assessed strategies, i.e., between PPV23/PCV13 and PCV13 only. The public health and budget impact was estimated for 4 scenarios with different assumptions on efficacy, costs and epidemiological changes (). Additional deterministic sensitivity analyses were also conducted for costs (doubling or halved; except for the price of vaccines), vaccination coverage (1.25 times or halved) and the prevalence of risk factors (UK sourceCitation22).
Disclosure of Potential Conflicts of Interest
This study was conducted by Amaris and funded by Sanofi Pasteur MSD. Yiling Jiang, Frédéric Gervais and Aline Gauthier are employees of Amaris, which received consulting fees from Sanofi Pasteur MSD. Charles Baptiste, Prescilla Martinon and Xavier Bresse are employees of Sanofi Pasteur MSD. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Supplemental_Tables_1011957.zip
Download Zip (129.6 KB)Acknowledgments
The authors would like to thank Murielle Cornier, Mathieu Uhart and Karine Bloch (employees of Sanofi Pasteur MSD) for their constructive inputs to the analyses. The authors would also like to thank Emmanuelle Varon (Center National de Référence des Pneumocoques) and Agnès Lepoutre (Institut de Veille Sanitaire) for providing data that are crucial for the current study.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Jackson LA. Pneumococcal polysaccharide vaccines. Vaccines In: Plotkin S, Orenstein W, Offit P, eds. Philadelphia, PA: Saunders Elsevier; 2013:542-72.
- Fedson DS. Pneumococcal vaccination for older adults: the first 20 years. Drugs Aging 1999; 15(Suppl 1):21-30; PMID:10690792; http://dx.doi.org/10.2165/00002512-199915001-00003
- Lynch JP, III, Zhanel GG. Streptococcus pneumoniae: epidemiology, risk factors, and strategies for prevention. Semin Respir Crit Care Med 2009; 30:189-209; PMID:19296419; http://dx.doi.org/10.1055/s-0029-1202938
- Prato R, Tafuri S, Fortunato F, Martinelli D. Why it is still important that countries know the burden of pneumococcal disease. Hum Vaccin 2010; 6:918-21; PMID:21045538; http://dx.doi.org/10.4161/hv.6.11.13352
- Said MA, Johnson HL, Nonyane BA, Deloria-Knoll M, O'Brien KL, Andreo F, Beovic B, Blanco S, Boersma WG, Boulware DR, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One 2013; 8:e60273; PMID:23565216; http://dx.doi.org/10.1371/journal.pone.0060273
- 23-valent pneumococcal polysaccharide vaccine. WHO position paper. Wkly Epidemiol Rec 2008; 83:373-84; PMID:18927997
- Institut National de Prévention et d'Education pour la Santé. Vaccination contre les infections invasives à pneumocoque. Guide des vaccinations. Édition 2012. 2012. Available at http://www.inpes.sante.fr/10000/themes/vaccination/guide-vaccination-2012/pdf/GuideVaccinations2012_Vaccination_contre_les_infections_invasives_pneumocoque.pdf, accessed 7 April 2014
- Cartwright K. Pneumococcal disease in western Europe: burden of disease, antibiotic resistance and management. Eur J Pediatr 2002; 161:188-95; PMID:12014384; http://dx.doi.org/10.1007/s00431-001-0907-3
- Agence Nationale de Sécurité du Médicament. Résumé des caractéristiques du produit. Pneumo 23. 17 October 2011. Available at http://agence-prd.ansm.sante.fr/php/ecodex/frames.php?specid=65861879&typedoc=R&ref=R0199189.htm, accessed 7 April 2014
- European Medicines Agency. Summary of product characteristics: Prevenar 13. 19 February 2014. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001104/WC500057247.pdf, accessed 7 April 2014.
- Haut Conseil de la Santé Publique. Avis 10 mars 2010. 10 March 2010. Available at http://www.has-sante.fr/portail/upload/docs/application/pdf/2010-04/prevenar13_-_ct-7346.pdf, accessed 7 April 2014.
- European Medicines Agency. Prevenar 13: Changes since initial authorisation of medicine. 29 October 2014. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001104/human_med_001220.jsp&mid=WC0b01ac058001d124, accessed 29 October 2014.
- Pebody RG, Leino T, Nohynek H, Hellenbrand W, Salmaso S, Ruutu P. Pneumococcal vaccination policy in Europe. Euro Surveill 2005; 10:174-8; PMID:16280609
- Haut Conseil de la Santé Publique. Vaccinations des personnes immunodéprimées ou aspléniques. Recommandations. 12 July 2012. Available at http://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=322, accessed 7 April 2014.
- Haut Conseil de la Santé Publique. Infections invasives à pneumocoque : recommandations vaccinales pour les personnes à risque. 25 April 2013. Available at http://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=355, accessed 7 April 2014.
- Institut de veille sanitaire. Pneumocoque. Données par groupe d'âge. 2013. Available at http://www.invs.sante.fr/Dossiers-thematiques/Maladies-infectieuses/Maladies-a-prevention-vaccinale/Couverture-vaccinale/Donnees/Pneumocoque, accessed 7 April 2014.
- Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 2011; 11:760-8; PMID:21621466; http://dx.doi.org/10.1016/S1473-3099(11)70090-1
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010; 201:32-41; PMID:19947881; http://dx.doi.org/10.1086/648593
- van der Linden M, Imohl M. R2762 Serotype distribution among bacteraemic pneumococcal pneumonia in adults in Germany. Clin Microbiol Infect 2011; 17:S832.
- Ardanuy C, Domenech A, Rolo D, Calatayud L, Tubau F, Ayats J, Martín R, Liñares J. Molecular characterization of macrolide- and multidrug-resistant Streptococcus pyogenes isolated from adult patients in Barcelona, Spain (1993-2008). J Antimicrob Chemother 2010; 65:634-43; PMID:20118164; http://dx.doi.org/10.1093/jac/dkq006
- Varon E, Janoir C, Gutmann L. Rapport d'activité 2011. Epidémiologie 2010. Centre National de Référence. 2011. Available at http://www.invs.sante.fr/content/download/48913/210452/version/1/file/Rapport_activites_CNRP2011.pdf, accessed 7 April 2014.
- van Hoek AJ, Andrews N, Waight PA, Stowe J, Gates P, George R, Miller E. The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J Infect 2012; 65:17-24; PMID:22394683; http://dx.doi.org/10.1016/j.jinf.2012.02.017
- Jiang Y, Gauthier A, Annemans L, van der Linden M, Nicolas-Spony L, Bresse X. A public health and budget impact analysis of vaccinating at-risk adults and the elderly against pneumococcal diseases in Germany. Expert Rev Pharmacoecon Outcomes Res 2012; 12:631-43; PMID:23025421; http://dx.doi.org/10.1586/erp.12.55
- Jiang Y, Gauthier A, Keeping S, Carroll S. A public health and budget impact analysis of vaccinating the elderly and at-risk adults with the 23-valent pneumococcal polysaccharide vaccine or 13-valent pneumococcal conjugate vaccine in the UK. Expert Rev Pharmacoecon Outcomes Res 2014; 14(6):901-11; [Epub ahead of print]; PMID:25186657; http://dx.doi.org/10.1586/14737167.2014.953932
- Bonten M, Bolkenbaas M, Huijts S, Webber C, Gault S, Grubber W, et al. Community acquired pneumonia immunisation trial in adults (CAPiTA). Presented at: 9th International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD). Hyderabad, India, 9-13 March. 2014. Available at http://isppd.meetingxpert.net/ISPPD_433/poster_90453/program.aspx/anchor90453, accessed 7 April 2014.
- Harboe ZB, Thomsen RW, Riis A, Valentiner-Branth P, Christensen JJ, Lambertsen L, Krogfelt KA, Konradsen HB, Benfield TL. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLoS Med 2009; 6:e1000081; PMID:19468297; http://dx.doi.org/10.1371/journal.pmed.1000081
- Ciprero KL, Marchese RD, Richard P, Baudin M, Musey LK, Manoff SB, et al. Immune responses of Pneumovax™ II to pneumococcal serotypes associated with increased mortality and meningitis in adults 50 years of age and older: an extension of Study U05-PnPS-403. Proceedings of the 9th International Symposium on Pneumococci and Pneumococcal Diseases 2014 March 9-13; Hyderabad, India.
- Fedson DS. Pneumococcal conjugate vaccination for older adults: Reply letter to Hollingsworth. Hum Vaccin Immunother 2014; 10:47-51; PMID:24030320; http://dx.doi.org/10.4161/hv.26422
- Varon E, Janoir C, Gutmann L. Rapport d'activité 2012. Epidémiologie 2011. Centre National de Référence 2012. Available at http://cnr-pneumo.com/docs/rapports/CNRP2012.pdf, accessed 7 April 2014
- Institut de veille sanitaire. Impact de la vaccination par le vaccine pneumococcique conjugué sur l'épidémiologie des infections invasives à pneumocoques en France – 1998-2010. 2010. Available at http://www.invs.sante.fr/surveillance/epibac/donnees_2010/Pneumocoque_impact_2010.pdf, accessed 7 April 2014.
- Jit M. The risk of sequelae due to pneumococcal meningitis in high-income countries: a systematic review and meta-analysis. J Infect 2010; 61:114-24; PMID:20433866; http://dx.doi.org/10.1016/j.jinf.2010.04.008
- Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, Macfarlane JT, Read RC, Roberts HJ, Levy ML, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009; 64(Suppl 3):iii1-55; PMID:19783532; http://dx.doi.org/10.1136/thx.2009.121434
- Agence Technique de l'Information sur l'Hospitalisation. Statistiques - utilisation des codes diagnostics principaux ou actes classants dans les bases. 2014. Available at http://www.atih.sante.fr/statistiques-utilisation-des-codes-diagnostics-principaux-ou-actes-classants-dans-les-bases, accessed 7 April 2014.
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012; 67:71-9; PMID:20729232; http://dx.doi.org/10.1136/thx.2009.129502
- Institut National d'Etudes Démographiques. Tables de mortalité depuis 1977. July 2012. Available at http://www.ined.fr/fichier/t_telechargement/65115/telechargement_fichier_fr_sd2011_t68_fm.xls, accessed 7 April 2014.
- Institut National de la Statistique et des Etudes Economiques. Population totale par sexe, âge et état matrimonial au 1er janvier. 2011. Available at http://www.insee.fr/fr/themes/detail.asp?ref_id=ir-sd2011&page=irweb/sd2011/dd/sd2011_population.htm, accessed 7 April 2014.
- Institut National de la Statistique et des Etudes Economiques. Bilan démographique 2013. 14 January 2014. Available at http://www.insee.fr/fr/themes/detail.asp?reg_id=0&ref_id=bilan-demo, accessed 7 April 2014.
- Institut National de la Statistique et des Etudes Economiques. Évolution et structure de la population. Données détaillées. December 2010. Available at http://www.insee.fr/fr/themes/detail.asp?ref_id=ir-projpop0760, accessed 7 April 2014.
- Pauline Ricci P, Blotière P-O, Weill A, Simon D, Tuppin P, Ricordeau P, et al. Diabète traité: quelles évolutions entre 2000 et 2009 en France ? Bull Epidemiol Hebd (Paris) 2011; 42-43:425-31
- Jezewski-Serra D, Korribi-Meribai W, Ganne N, Gelsi E, Michel L, Moirand R, et al. Morbidité et létalité hospitalières liées aux maladies alcooliques du foie en 2008 en France. Bull Epidemiol Hebd (Paris) 2013; 16-17-18:191-4
- Institut de veille sanitaire. Prévalence des hépatites B et C en France en 2004. March 2007. Available at http://www.invs.sante.fr/publications/2006/prevalence_b_c/vhb_france_2004.pdf, accessed 7 April 2014.
- Haute Autorité de Santé. Maladie rénale chronique de l'adulte. February 2012. Available at http://www.soc-nephrologie.org/PDF/enephro/recommandations/HAS/2012/MRC-2012.pdf, accessed 7 April 2014.
- Institut de Recherche et de Documentation en Economie de la Santé. L'asthme en France en 2006 : prévalence, contrôle et déterminants. January 2011. Available at http://www.irdes.fr/Publications/Rapports2011/rap1820.pdf, accessed 7 April 2014.
- Ministère des Affaires sociales et de la Santé. Qu'est-ce que la BPCO ? 13 August 2009. Available at http://www.sante.gouv.fr/qu-est-ce-que-la-bpco.html, accessed 7 April 2014.
- Institut de veille sanitaire. L'insuffisance cardiaque. 27 May 2013. Available at http://www.invs.sante.fr/Dossiers-thematiques/Maladies-chroniques-et-traumatismes/Maladies-cardio-vasculaires/L-insuffisance-cardiaque, accessed 7 April 2014.
- Agence de la biomédecine. Activité de greffe. 2012. Available at http://www.agence-biomedecine.fr/annexes/bilan2011/donnees/cellules/03-greffe/pdf/greffe.pdf, accessed 7 April 2014.
- Fédération des Associations pour le Don d'Organes et de Tissus Humains. Greffés. 2014. Available at http://www.france-adot.org/images/pj/Chiffres-Greffes-prelevements.pdf, accessed 7 April 2014.
- Institut National du Cancer. Situation de la chimiothérapie des cancers en 2011. June 2012. Available at http://www.e-cancer.fr/component/docman/doc_download/9420-situation-de-la-chimiotherapie-des-cancers-en-2011, accessed 7 April 2014.
- UNAIDS. France. HIV and AIDS estimates. 2012. Available at http://www.unaids.org/en/regionscountries/countries/france/, accessed 7 April 2014.
- Le Centre de Référence Déficits Immunitaires Héréditaires. Estimation de la prévalence des patients avec DIH en France. 13 January 2014. Available at http://www.ceredih.fr/documents/PrevRegionale.pdf, accessed 7 April 2014.
- Bardakdjian J, Wajcman H. [Epidemiology of sickle cell anemia]. Rev Prat 2004; 54:1531-3; PMID:15558961
- Legrand A, Bignon A, Borel M, Zerbib P, Langlois J, Chambon JP, Lebuffe G, Vallet B. [Perioperative management of asplenic patients]. Ann Fr Anesth Reanim 2005; 24:807-13; PMID:15967628; http://dx.doi.org/10.1016/j.annfar.2005.05.002
- Sanofi Pasteur MSD data on file.
- Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2013; 1:CD000422; PMID:23440780
- Rodriguez-Barradas MC, Goulet J, Brown S, Goetz MB, Rimland D, Simberkoff MS, Crothers K, Justice AC. Impact of pneumococcal vaccination on the incidence of pneumonia by HIV infection status among patients enrolled in the Veterans Aging Cohort 5-Site Study. Clin Infect Dis 2008; 46:1093-100; PMID:18444830; http://dx.doi.org/10.1086/529201
- Vila-Corcoles A, Ochoa-Gondar O, Hospital I, Ansa X, Vilanova A, Rodriguez T, Llor C; EVAN Study Group. Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin Infect Dis 2006; 43:860-8; PMID:16941367; http://dx.doi.org/10.1086/507340
- Middleton DB, Lin CJ, Smith KJ, Zimmerman RK, Nowalk MP, Roberts MS, Fox DE. Economic evaluation of standing order programs for pneumococcal vaccination of hospitalized elderly patients. Infect Control Hosp Epidemiol 2008; 29:385-94; PMID:18521990; http://dx.doi.org/10.1086/587155
- Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, Adair RK, Clemens JD. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med 1991; 325:1453-60; PMID:1944423; http://dx.doi.org/10.1056/NEJM199111213252101
- Assurance Maladie. Base des Médicaments et Informations Tarifaires. 2014. Available at http://www.codage.ext.cnamts.fr/codif/bdm_it/index_presentation.php?p_site=AMELI, accessed 7 April 2014.
- Haut Conseil de la Santé Publique. Vaccination contre les infections invasives à méningocoque B. Place du vaccin Bexsero®. 25 October 2013. Available at http://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=386, accessed 7 April 2014.
- Assurance Maladie. Les consultations en métropole. 25 February 2014. Available at http://www.ameli.fr/assures/soins-et-remboursements/combien-serez-vous-rembourse/consultations/les-consultations-en-metropole/dans-le-cadre-du-parcours-de-soins-coordonnes.php, accessed 7 April 2014.
- T2A Conseil. Les tarifs GHS 2013 publics et privés au format Excel avec bornes hautes et basses. 5 March 2013. Available at http://www.t2a-conseil.com/blog/les-tarifs-ghs-2013-publics-et-prives-au-format-excel-avec-dms-bornes-hautes-et-bornes-basses/, accessed 7 April 2014.
- Agence Technique de l'Information sur l'Hospitalisation. Référentiel de coûts MCO 2011. 24 May 2013. Available at http://www.atih.sante.fr/referentiel-de-couts-mco-2011, accessed 7 April 2014.
- Martin M, Quilici S, File T, Garau J, Kureishi A, Kubin M. Cost-effectiveness of empirical prescribing of antimicrobials in community-acquired pneumonia in three countries in the presence of resistance. J Antimicrob Chemother 2007; 59:977-89; PMID:17395688; http://dx.doi.org/10.1093/jac/dkm033
- Maruyama T, Taguchi O, Niederman MS, Morser J, Kobayashi H, Kobayashi T, D'Alessandro-Gabazza C, Nakayama S, Nishikubo K, Noguchi T, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ 2010; 340:c1004; PMID:20211953; http://dx.doi.org/10.1136/bmj.c1004