Abstract
Enterovirus 71 (EV71) is the key pathogen for Hand, Foot, and Mouth Disease (HFMD) and can result in severe neurological complications and death among young children. Three inactivated-EV71 vaccines have gone through phase III clinical trials and have demonstrated good safety and efficacy. These vaccines will benefit young children under the threat of severe HFMD. However, the potential immunization-related compatibility for different enterovirus vaccines remains unclear, making it hard to include the EV71 vaccine in Expanded Program on Immunization (EPI). Here, we measured the neutralizing antibodies (NTAbs) against EV71, Coxsackievirus A16 (CA16) and Poliovirus from infants enrolled in those EV71 vaccine clinical trials. The results indicated that the levels of NTAb GMTs for EV71 increased significantly in all 3 vaccine groups (high, middle and low dosages, respectively) post-vaccination. Seroconversion ratios and Geometric mean fold increase were significantly higher in the vaccine groups (≥7/9 and 8.9~228.1) than in the placebo group (≤1/10 and 0.8~1.7, P < 0.05). But no similar NTAb response trends were found in CA16 and 3 types of Poliovirus. The decrease of 3 types of Poliovirus NTAb GMTs and an increase of CA16 GMTs post-EV71-vaccination were found in vaccine and placebo groups. Further animal study on CA16 and poliovirus vaccine co-immunization or pre-immunization with EV71 vaccine in mice indicated that there was no NTAb cross-activity between EV71 and CA16/Poliovirus. Our research showed that inactivated-EV71 vaccine has good specific-neutralizing capacity and can be included in EPI.
Introduction
Enterovirus 71 (EV71) is the main pathogen for hand, foot, and mouth disease (HFMD) and associated with severe neurological diseases in young children.Citation1-3 EV71 was responsible for the increase in the severity of HFMD onset, the incidence of severe HFMD cases, and the number of mortalities in the Asia-Pacific region.Citation4-7 Since no vaccine is available, EV71 is now considered the most dangerous neurotropic enterovirus in the post-polio era.Citation5,6 Three inactivated-EV71 vaccines had gone through Phase I-III clinical trials from December 2010 to March 2013 in China.Citation8-15 Those vaccines showed very good safety among children 6 m-5 y old. In addition, the vaccine efficacy in preventing EV71-associated HFMD was higher than 90%.Citation10,13,15 Recently, another EV71 vaccine clinical trial in Taiwan started the patient enrollment for 2-month old children (ClinicalTrials.gov No. NCT02200237). Therefore, EV71 vaccine will become another new enterovirus vaccine for infants and young children after polio vaccine.
EV71, CA16 and poliovirus all belong to the EVs genus of Picornaviridae family with similar gene and protein structures.Citation16 Exposure to and infections with multiple EVs are very common, and thus immunity should prevail in the general population.Citation17 Among those EVs, CA16 is believed to be another main pathogen of HFMD in young children. CA16 often prevails independently or co-circulates with EV71 in different regions from time to time.Citation18,19 In addition, CA16 has the highest gene sequence homology (about 70%) with EV71.Citation20,21 Poliovirus is another important virus in EVs genus. To eradicate polio globally, poliovirus vaccination has been included in routine immunization in most countries in the world. The recommended immunization schedule by WHO is 3-4 doses with 1-2 month intervals for 1.5-2 month newborns.Citation22 Some reports showed that the cross-reactive antibodies and T cellular immune responses were well conserved within each enterovirus group.Citation23,24 EV71 and CA16 did show some cross-reactions in IgG, IgM and neutralizing antibodiesCitation25,26 Cross-protection of Poliovirus vaccine on EV71 has also been reported.Citation27 Co- or pre-vaccination with CA16 or poliovirus is a challenge for inactivated-EV71 vaccine to be used in infants and young children.
Here, NTAbs against EV71, CA16 and types 1, 2, 3 Polioviruses in serum samples from 3 EV71 vaccine clinical trials were measured to investigate the impact of EV71 vaccination on NTAbs of CA16 and polioviruses. And CA16 and poliovirus vaccine pre-vaccination or co-vaccination with EV71 vaccine was carried out in mice to investigate the compatibility of inactivated-EV71 vaccine with CA16 and Poliovirus immunizations.
Results
The cross-activity of EV71 vaccination with NTAb of CA16 in infants and children
Three phase I clinical trials of EV71 inactivated vaccines were carried out in Guangxi province and Jiangsu province from December 2010 (). 101 paired sera (0d and 56d respectively) samples were collected from 3 EV71 vaccine clinical trials (Trials1-3, ). EV71 and CA16 NTAbs of every sample were measured by CPE assay ().
Table 1. Clinical trials for 3 human Enterovirus 71 (EV71) vaccines
Table 2. The change of EV71 and CA16 NTAbs in infants and children in clinical trial 1, 2 and 3
For EV71 NTAb: After 2-dose EV71 vaccinations (56d), seroconversion ratio of each vaccine group in all 3 clinical trials was higher than 7/9 and was significantly higher than that for each corresponding placebo group (all lower than 1/10, P value all <0.01). From 0d to 56d, GMTs increased from 26.9~79.7 to 1109.4~4019.4 for the high-dosage group, from 4.7~29.7 to 208.6~6762.4 for the middle-dosage group, and from 10.5~35.1 to 93.5~886.8 for the low-dosage group (P-values for all groups were <0.01), while GMTs for placebo groups were relatively flat during the same period, changing from 76.1 to 63.7, from 4 to 4, and from 27.4 to 46.3 in clinical trials 1, 2 and 3, respectively (P-values were all >0.05). Geometric mean fold increases (GMFIs) were 19.1~149.3, 41.6~228.1 and 1.7~25.3 for high-dosage, middle-dosage, and low-dosage groups respectively, which were significantly higher than those of placebo groups (0.8, 1.0 and 1.7, P value all <0.001).
For CA16 NTAb: Seroconversion ratios were 1/10~2/8, 0~2/11 and 2/9~4/10 for the vaccine groups in clinical trial 1, 2 and 3 () on 56d respectively, not significantly different from those for the corresponding placebo groups (2/11, 0/8 and 4/10, P value all >0.05). GMTs increased from 11.4~53.9 on 0d to 22.1~65.5 on 56d for high-dosage group, from 4~9.5 to 4~15.2 for the middle-dosage group, and from 6~9.3 to 11~26.3 for the low-dosage group (P values were all >0.05), while GMTs in the corresponding placebo groups increased from 39.1 to 58.3, from 4 to 4 and from 4.5 to 15.5 respectively after boosted by EV71 vaccine (P value all >0.05). GMFIs for clinical trial 1, 2 and 3 were 1.2~2.3, 1.0~1.6 and 1.9~2.8, respectively, which were not different from those in the corresponding placebo groups (1.5, 1 and 3.5, respectively; P value >0.05). CA16 GMTs increased to similar extent in both placebo group and vaccine group, while EV71 GMTs only increased in vaccine group but not in placebo group. This indicated that the increase of CA16 NTAb was not induced by EV71 vaccination but was associated with CA16 epidemic.
The cross-activity of EV71 vaccination with the NTAbs of types 1, 2 and 3 polioviruses in infants and children
One phase II clinical trials (Clinical Trial 4 of EV71 inactivated vaccines was carried out in Jiangsu Province (). 20 pairs of sera samples (0d and 56d) were collected from 6~12 month old infants in each vaccine group (dosages: 640U, 320 U, 160 U respectively) and placebo group (). EV71 NTAb and types 1, 2 and 3 Poliovirus NTAbs in all sera were measured with CPE assay ().
Table 3. The change of EV71 and Poliovirus NTAbs in infants and children from clinical trial 4
For EV71 NTAb: EV71 NTAb seroconversion ratios were 20/20, 20/20, 19/20 and 1/20 in the 640U, 320U, 160U and placebo groups respectively in clinical trial 4 on 56d (P < 0.01). GMTs increased from 8.6 on 0d to 691.7 on 56d, from 8.1 to 714.2 and from 6.1 to 689 for 640U, 320U, and 160U groups respectively (P value all <0.001), while GMTs for the placebo group increased from 11.9 on 0d to 18.2 on 56d (P = 0.285). GMFI of each vaccine group was 80.2, 88.2 and 113.8 respectively, significantly higher than that for the placebo group (1.5, P < 0.0001).
For Poliovirus NTAb: Seropositive ratios for types 1, 2 and 3 poliovirus NTAbs were all higher than 19/20 in both vaccine and placebo groups in clinical trail 4 on 0d (P > 0.05). GMTs of types 1, 2 and 3 poliovirus NTAbs were 1229-2037, 494-689.9 and 205-298.5 on 0d (P > 0.05), respectively. After the 2nd EV71 vaccination, GMTs of types 1, 2 and 3 poliovirus were 1069.9-2766.8, 380.5-761 and 128.4-282.6, respectively. The seroconversion ratios of types 1, 2 and 3 Poliovirus NTAbs were 0/20~2/20, 0/20~2/20 and 0/20~4/20 (P value all >0.05), respectively. And GMFIs of types 1, 2 and 3 Poliovirus NTAbs were 0.6~1.4, 0.7~1.1 and 0.6~0.9 (P < 0.01, >0.05 and >0.05), respectively. Poliovirus GMTs in most infants decreased after EV71 vaccination, with type 1 Poliovirus NTAb the only exception. GMT for type 1 Poliovirus increased significantly in 320U/Dose group on 56d, and the GMFI of this group was significantly higher than that in other groups (P < 0.01). This result showed that the increase of Poliovirus NTAb was associated with vaccine-derived poliovirus (VDPV), not with EV71 vaccination, because no increase of poliovirus NTAb was observed in neither 640 U/Dose group nor 160 U/Dose group.
The compatibility of CA16 and poliovirus vaccine pre-immunity with the EV71 vaccination in mice
Because no CA16 vaccine is available commercially and newborns should have the poliovirus vaccination, it is difficult to investigate the compatibility of CA16 or poliovirus pre-immunity and co-immunity with EV71 vaccination in young children. To explore whether CA16 or Poliovirus antibodies affect the inactivated-EV71 vaccine immune response, 3 groups of BALB/c mice were immunized with CA16 vaccine, Poliovirus vaccine or adjuvant without any antigen (adjuvant group) respectively (n = 10 per group) twice (day 0 and day 21). The existing antibody reached the peak one week after the vaccination before those mice were injected with inactivated-EV71 vaccine in 4 w and 7 w (200 U per mice). Sera were collected in 4 w (pre-EV71 vaccination) and 8 w (post- EV71 vaccination) from each group to measure NTAbs for EV71, CA16, and types 1, 2 and 3 Poliovirus. Control group was treated with saline. Results were listed in and . The results showed that for EV71 NTAb response, seropositive ratios were all 0/10 in CA16, poliovirus and adjuvant groups in 4 w while CA16 and poliovirus NTAbs already showed high titers. After the second EV71 vaccination, EV71 NTAb seropositive ratios were all 10/10 in CA16, Poliovirus and adjuvant groups in 8 w, and GMTs were 68.8, 84.4 and 44.8, respectively. No significant difference was observed among those groups (P > 0.05), which indicated that CA16 and poliovirus pre-immunity would not interfere with the EV71 NTAb response.
Table 4. The seropositive ratios of NTAb for inactivated-EV71 vaccination after CA16 or Poliovirus vaccination
Figure 1. The NTAb GMTs for inactivated-EV71 vaccination after CA16 or Poliovirus vaccination. BALB/c mice (n = 10 per group) were subcutaneously injected with CA16 virus (VR18, genebank no:JX481738, 4.8 × 105 PFU/mouse), inactivated-Poliovirus vaccine (Sanofi Pasteur, lot:G0510-1, Type I 20 DU/mouse, Type II 4 DU/mouse, Type III 16 DU/mouse), or aluminum adjuvant (Adjuvant). All animals were boosted in week 3 after priming. One week after the boost, all mice took the first inactivated-EV71 vaccination (SINOVAC BIOTECH CO.,LTD., 200 U/mouse), followed by the second inactivated-EV71 vaccination 3 weeks later. Negative control group was just inoculated with saline. Sera were collected on day 28 (7 days after the 2nd boost) and day 56. All the sera were stored at −20°C. Neutralization titers (NTs) of the sera for EV71 NTAb, CA16 NTAb and Polio I, II, III NTAb were determined. Data were expressed as means ± SEM. “Pre” and “Post” are equal to before (on 28 day) and after (on 56 day) EV71 vaccination. For analysis of GMTs, the data were transformed using the log 2 of the original values. Panels a-e separately show CA16, Polio I-III and EV71 neutralization titers for each group before and after EV71 vaccination and panel f shows immunization design for this experiment. Note: * means the GMTs were significantly different after vaccination (P < 0.05).*** means the GMTs were very significantly different after vaccination (P < 0.0001).
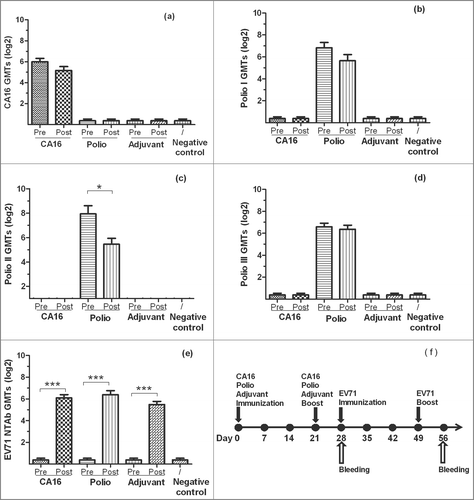
For CA16 NTAb response: Seropositive ratio of CA16 pre-immunity group was 10/10 in week 4 and 9/10 in week 8 (P > 0.05), and GMTs decreased from 64.0 in week 4 to 36.0 in week 8 (P > 0.05). These results suggested that EV71 vaccination didn't impact CA16 NTAb.
For poliovirus NTAb response: Seropositive ratios were 10/10 in week 4 vs 9/10 jn week 8, 10/10 (week 4) vs 10/10 (week 8) and 10/10 (week 4) vs 10/10 (week 8) (P > 0.05) for types 1, 2 and 3 polioviruses, respectively. And GMTs of types 1, 2 and 3 polioviruses were 113.6 vs 50.0, 244.8 vs 43.6 and 96.0 vs 82.4, during the same timeframe (week 4 vs. week 8) respectively. Except for GMT of type 2 of poliovirus which decreased significantly after EV71 vaccination (p = 0.0199), no significant difference was found for types 1 and 3 poliovirus NTAbs between pre- and post-EV71 vaccination groups (P > 0.05). Three types of poliovirus NTAbs were negative in other groups, which indicated EV71 vaccination didn't impact NTAb of poliovirus.
The compatibility of CA16 and poliovirus co-immunity with EV71 vaccination in mice
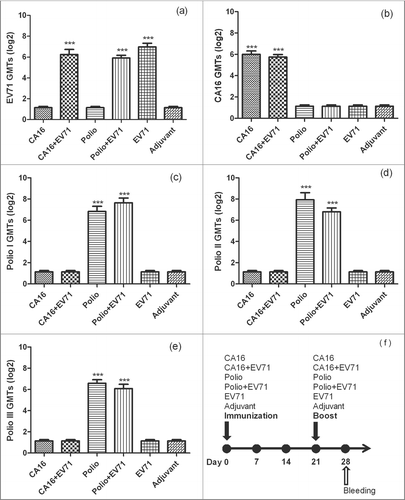
To study the compatibility of CA16 and poliovirus co-immunity with EV71 vaccination, we immunized mice with CA16 or Poliovirus vaccine alone or co-immunized mice with CA16 vaccine/EV71 vaccine or Poliovirus vaccine/EV71 vaccine. One control group was immunized with only EV71 vaccine. Sera were collected on 0d and 28d after the boost to measure the NTAbs for EV71 and CA16, types 1, 2 and 3 Polioviruses. Results were listed in and . For EV71 NTAbs, EV71 group, EV71 & CA16 group, and EV71 & Poliovirus group showed 100% seroconversion with GMTs at 126, 76, and 60.4, respectively. There was no significant difference among these groups (P > 0.05) with the single factor analysis. For CA16 NTAbs, CA16 group and CA16 & EV71 group had 100% seroconversion with GMTs at 64 and 53.6, respectively. There was no significant difference between these 2 groups (P > 0.05). For Poliovirus NTAbs, Poliovirus group and EV71 & Poliovirus group had 100% seroconversion (all 3 types of Polioviruses) in week 4 with GMTs at 200.8 and 113.6 for type 1 (P > 0.05), 112 and 244.8 for type 2 (P > 0.05), 67.6 and 96 for type 3 (P > 0.05), respectively. The above resulted showed that EV71 had no obvious impact on those neutralizing antibodies when it was co-immunized with CA16 or Poliovirus vaccine. Therefore, it is possible to have inactivated-EV71 vaccine to be co-administered with CA16 or Poliovirus vaccines.
Table 5. The Seropositive ratio of NTAb for inactivated-EV71 vaccine co-immunized with CA16 or Poliovirus
Figure 2. The NTAb GMTs for inactivated-EV71 vaccine co-immunized with CA16 or Poliovirus vaccine. 60 pathogen-free BALB/c mice (6–8 weeks,female,purchased from Vital River Lab Animal Technology Co., Ltd, Beijing, China) were used. BALB/c mice (n = 10 per group) were subcutaneously injected with inactivated-EV71 vaccine (SINOVAC BIOTECH CO.,LTD., 200 U/mouse), CA16 virus (VR18, genebank accession no:JX481738, 4.8 × 105 PFU/mouse), inactivated-Poliovirus vaccine (Sanofi Pasteur, lot:G0510-1, Type I 20 DU/mouse, Type II 4 DU/mouse, Type III 16 DU/mouse), or co-immunized with inactivated-EV71 vaccine (EV71 & CA16 group and EV71 and polio group). The control group was just inoculated with aluminum adjuvant (Adjuvant). The animals were boosted in week 3 after priming. All the sera were collected one week after the boost and stored at −20°C. Neutralization titers (NTs) of the sera were determined for EV71 NTAb, CA16 NTAb and Polio I, II, III NTAb. Data were expressed as means ± SEM. For analysis of GMTs, the data were transformed using the log 2 of the original values. Panels a-e separately show EV71, CA16 and Polio I-III neutralization titers for each group, and panel f shows immunization design for this experiment. Note: *** means this group were significantly different when compared with other groups without *** label (P < 0.0001).
Discussion
Human enteroviruses (EVs) are quite similar in compositions, structural features and gene sequences. All EVs are small non-enveloped icosahedral viruses that contain a positive-sense and single-stranded RNA genome of about 7,400 nucleotides. The conserved sequence in Poliovirus Capsid Protein VP1 is widely maintained among members of Genus Enterovirus.Citation24,28 However, Tan's study showed that the cross-reactivity with CA16 or poliovirus vaccination was limited in EV71-infected population.Citation29 Whether EV71 vaccine has a cross-neutralization or cross-immune compatibility with other EVs is important for the EV71 vaccination.
According to a study by Wu in 2007, EV71 and CA16 immune serum showed weak cross-protective phenomenon.Citation30 In 2011,Lin's study of 79 HFMD patients showed that 18.9% CA16-infected patients and 11.1% EV71-infected patients presented high cross-neutralization antibodies, which suggested that the immune reactivity to EV71 infection could be impacted by CA16, and Vice versa.Citation31 In 2013, Chou's clinical study of EV71 vaccine showed that adults with EV71 immunization had low cross antibodies against CA16.Citation26 CA16 infection, sometimes co-existing with EV71 in HFMD patients,Citation32,33 were common in Asia and other countries,Citation34-38 making it difficult to draw a conclusion for NTAb cross-activity, especially without a placebo group as the negative control. To avoid this problem, sera from children (6-month to 5-year old) in 3 inactivated-EV71 vaccine trials were collected in this research. A total of 101 paired sera from vaccine groups and the placebo group were collected and the neutralizing antibodies of EV71 and CA16 in those samples were measured. The results showed that in the 3 clinical trials, seroconversion ratios of EV71 vaccine groups (with different vaccine doses) were all significantly higher than those of the control groups after the boost. Although seroconversions of CA16 occurred in all vaccine groups too, seroconversion ratios and GMFIs showed no significant difference between vaccine groups and the placebo group (P > 0.05). The above results showed that EV71 immunization had no obvious impact on CA16 NTAb. Since those 3 clinical trials were carried out in the spring of 2011, the peak of EV71 and CA16 epidemic in China, CA16 neutralizing antibody increase in those groups might be caused by a small scale CA16 epidemic.
Further studies were carried to explore the co-immunization of EV71 and CA16 or the EV71 immunization with pre-existing antibodies of CA16, and mice were used as the research subjects. Results showed that neither pre- nor co-immunization affected EV71 or CA16 neutralizing antibody response ratios and response intensities. Therefore, there was no cross neutralization or interference between these 2 vaccines. Our research confirmed similar results from the cross-activity studies of EV71 and CA16 in rhesus monkeys.Citation39 OPV and IPV have been widely used around the world for decades. Polio cases globally dropped from 350 000 cases in 1998 to 223 cases in 2012.Citation40,41 To achieve the goal of polio eradication, since 1978 China has implemented 3-dose OPV on newborns in months 2, 3 and 4 and an extra dose at 4 year old.Citation42 And poliovirus vaccination for the infants and young children was very popular in the rest of world. If 6-month infants to 5-year old children take EV71 vaccination, high titer poliovirus NTAb should exist in these young children. In 2011, Deng's study showed that the irregularity of OPV vaccination was highly correlated to HFMD severity, especially pulmonary edema.Citation43 We measured NTAbs of EV71 and types 1, 2 and 3 polioviruses in 20 paired sera from 6-12 month old infants in each vaccine and control group. Results showed that EV71 seroconversion rate was over 95% in each vaccination group,significantly higher than control group. On the other hand, types 1,2 and 3 poliovirus NTAbs for most groups declined after the EV71-vaccination, except that type 1 Poliovirus NTAb increased significantly in 320U/Dose group. This result indicated that EV71 vaccination did not impact those 3 types poliovirus response. Because no wild poliovirus has been found in china since 2000,Citation44 poliovirus NTAb increase in several infants should be related to OPV vaccination domestically. Mouse study showed that neither pre-immunization nor co-immunization with poliovirus vaccines had any impact on NTAb response of EV71 vaccination, and confirmed that no cross-activity was found between NTAb of EV71 and 3 types of polioviruses. The studies of neutralizing linear epitopes helped us to better understand the neutralizing capability of antibodies against viruses. Most structural information about poliovirus interaction with neutralizing antibodies was revealed in the 1980s by using neutralization escape mutants. Four neutralizing antigenic sites were identified,Citation45,46 with one continuous antigenic site in BC loop of VP1 and the other 3 discontinuous sites in different capsid proteins of 3 poliovirus serotypes.Citation47,48
Six no-overlapping EV71-neutralizing linear epitopes (3 in VP1, one in VP2, 2 in VP3) and CA16-neutralizing linear epitopes within the VP1 protein were reported.Citation49-54 Among them, one EV71 epitope in VP1 (residues: 215-219) overlapped with one CA16 linear neutralizing epitope PEP71 (VP1: 211–225) and type 2 poliovirus neutralizing site 2a (VP1: 217-221), but the sequence was not conserved in CA16 or other polioviruses.Citation52 EV71 neutralizing epitope VP2-28 (VP2:136–150) showed a high degree of homology with CA16 sequence which was believed to be across-reactive epitope (). Another EV71 neutralizing epitope SP2 (VP1:145–159) also had a high degree of homology with CA16 sequence (). However, our study showed that no cross-activity of NTAb response was found between EV71 and CA16 or Polio, either pre-vaccination or co-immunity in mice or clinical trial data. Since those cross-reactive epitopes didn't have specific cross-activity with EV71 NTAb, the key neutralizing sites or conformational neutralizing epitopes might play an important role in neutralizing activities.
Table 6. Homology of 2 EV71 neutralizing epitopes with CA16 and poliovirus
In summary, although EV71, CA16 and polioviruses shared some highly conservative antigen epitopes, including cellular immune epitopes, their neutralizing antibodies demonstrated high specificity. Therefore, it is possible to include EV71 vaccination in EPI for infants and young children to prevent HFMD or other EV71-related diseases, and the combined vaccines could be developed to simplify immunization procedures in future.
Materials and Methods
The cross-activity of EV71 vaccination with NTAb of CA16 in infants and children
Paired sera from a total of 101 subjects with EV71 vaccination were collected before (0d) vaccination and after (56d) boosted and EV71 and CA16 neutralizing antibodies were measured to evaluate the impact of EV71 vaccination on CA16 neutralization antibodies. Samples were from 3 inactivated-EV71 vaccine clinical trials (Clinical Trials 1-3, ). Each trial included 3 vaccine groups based on vaccine dosage (high dose, middle dose and low dose) and the placebo group.
The cross-activity of EV71 vaccination with NTAb of types 1, 2 and 3 polioviruses in infants and children
80 paired sera were collected from 6~12-month old infants with EV71 vaccination before (0d) vaccination and after (56d) boosted. Neutralizing antibodies for EV71, Polio I, Polio II and Polio III were measured to evaluate the impact of EV71 vaccination on Polio I、Polio II and Polio III neutralizing antibody titer. Samples were from the clinical trial 4 () which includes 3 vaccine groups with high, middle and low dose and the placebo group (n = 20 for each group).
The compatibility of CA16 and poliovirus vaccine pre-immunity with EV71 vaccination in mice
All institutional (National Institutes for Food and Drug Control) guidelines for animal care and usage were strictly followed. Thirty pathogen-free BALB/c mice (6–8 week old,female,purchased from Vital River Lab Animal Technology Co., Ltd, Beijing, China) were used. BALB/c mice (n = 10 per group) were subcutaneously injected with CA16 virus (VR18, genebank no:JX481738, 4.8 × 105 PFU/mouse), inactivated-Poliovirus vaccine (Sanofi Pasteur, lot:G0510-1, Type I 20 DU/mouse, Type II 4 DU/mouse, Type III 16 DU/mouse), or aluminum adjuvant (Adjuvant). All the animals were boosted in week 3 after priming. One week after the boost, all mice were injected with the first dose of inactivated-EV71 vaccine (SINOVAC BIOTECH CO., LTD., 200 U/mouse), followed with the second dose of inactivated-EV71 vaccine 3 weeks later. The control group was just inoculated with saline. Sera were collected on day 28 (7 days after the 2nd boost) and day 56. All the sera were stored at −20°C. Neutralization titers (NT) of the sera for EV71 NTAb, CA16 NTAb and Polios I, II, III NTAb detections were determined.
The compatibility of CA16 and poliovirus vaccine co-immunity with EV71 vaccination in mice
Sixty pathogen-free BALB/c mice (6-8weeks,female,purchased from Vital River Lab Animal Technology Co., Ltd, Beijing, China) were used. BALB/c mice (n = 10 per group) were subcutaneously injected with inactivated-EV71 vaccine (SINOVAC BIOTECH CO.,LTD., 200 U/mouse), CA16 virus (VR18, genebank accession no:JX481738, 4.8 × 105 PFU/mouse), inactivated-Poliovirus vaccine (Sanofi Pasteur, lot:G0510-1, Type I 20 DU/mouse, Type II 4 DU/mouse, Type III 16 DU/mouse) respectively, or co-immunized with inactivated-EV71 vaccine (EV71 & CA16 group and EV71 and polio group). The control group was just inoculated with aluminum adjuvant (Adjuvant). The animals were boosted in week 3 after priming. All sera were collected one week after the boost and stored at −20°C. Neutralization titers (NT) of the sera were determined for EV71 NTAb, CA16 NTAb and Polio I, II, III NTAb.
EV71 or CA16 NTAb assay
The titers of NTAb against EV71 or CA16 were measured for all samples with the cytopathogenic effect (CPE) assay.Citation10,26 Blood samples were diluted 1:8, and the serum was inactivated at 56°C for 30 min. Fifty microliters of each serum dilution (ranging from 1:8 to 1:16384) were mixed with 100 TCID50 EV71 or CA16 (EV71/523-07T, C4 genotype; CA16/G10, genebank accession number: U05876, A genotype) per well in a 96-well micro-plate (Thermo Fisher Scientific, NUNC, Denmark). The resulted mixture was incubated at 37°C for 2 h, before 100 μl suspension of rhabdomyosarcoma cells (RD cells: ATCC, CCL-136, a gift from the National Vaccine & Serum Institute) (1∼2 × 105 cells/ml) was added into each well. Each assay included a cell control, a virus control (no serum) and EV71 national standard or/ a CA16 in-house reference serum.Citation55 The plate was incubated in a CO2 incubator at 35°C for 7 days before CPEs were observed with microscopy. NATb titers were defined as the highest dilution capability of 50% CPE inhibition. NTAb titers against EV71 or CA16 were defined as positive if equal to or greater than 1:8. NTAb titers equal to or greater than 1:16384 were assigned a value of 1:16384.
NTAb assay of types 1, 2, or 3 Poliovirus
To assess cross-immunity between EV71 and poliovirus, the NTAb titers of types 1, 2, or 3 Poliovirus were measured with the standard poliovirus neutralization assay recommended by WHO (an exploratory analysis based on the protocol) in all samples against Sabin strains 1, 2, and 3, respectively.Citation56 Neutralizing antibody titers of types 1, 2, or 3 Poliovirus were defined as the dilution ratios showing 50% inhibition on the cytopathogenic effect. Neutralizing antibodies equal to or greater than 1:8 were defined seropositive. NTAb titers against types 1, 2, or 3 Poliovirus were defined as positive if the neutralizing antibodies equal to or greater than 1:8. NTAb titers equal to or greater than 1:16384 were assigned a value of 1:16384.
Statistical methods
Seropositive ratios were analyzed by chi-square test. For the statistical analysis of GMTs, all data were converted from the original values using the log 2 formula form and the resulted data were analyzed with SPSS 10.0 software. This transformation was effective in stabilizing the dispersion and rendered the variances independent of the means. If the titers of neutralizing antibodies were negative, they were set as 1:4 for calculation purpose. A paired t-test with P < 0.05 was considered statistically significant. Seroconversion is defined with at least 4-fold increase on titer post-vaccination.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
supplementary_material.docx
Download MS Word (155 KB)Funding
We thank the Major Special Projects Funding Program(No. 2012ZX09304010) and National High Technology Research and Development Program (“863” program, No. 2012AA02A402) from the Ministry of Science and Technology of the People's Republic of China for their support.
References
- Abu Bakar S,Chan YF,Lam SK. Outbreaks of enterovirus 71 infection. N Engl J Med 2000; 342:355–6; PMID:10660400; http://dx.doi.org/10.1056/NEJM200002033420513
- Solomon T,Lewthwaite P,Perera D,Cardosa MJ,McMinn P,Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 2010; 10(11):778-90; PMID:20961813; http://dx.doi.org/10.1016/S1473-3099(10)70194-8
- Ooi MH,Wong SC,Lewth waite P,Cardosa MJ,Solomon T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol 2010; 9:1097-105; PMID:20965438; http://dx.doi.org/10.1016/S1474-4422(10)70209-X
- Ho M,Chen ER,Hsu KH,Twu SJ,Chen KT,Tsai SF,Wang JR,Shih SR. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med 1999; 341:929-35; PMID:10498487; http://dx.doi.org/10.1056/NEJM199909233411301
- McMinn P,Lindsay K,Perera D,Chan HM,Chan KP,Cardosa MJ. Phylogenetic analysis of enterovirus 71 strains isolated during linked epidemics in Malaysia, Singapore, and Western Australia. J Virol 2001; 75:7732-8; PMID:11462047; http://dx.doi.org/10.1128/JVI.75.16.7732-7738.2001
- Lee MS,Chang LY. Development of enterovirus 71 vaccines. Expert Rev Vaccines 2010; 9:149-56; PMID:20109026; http://dx.doi.org/10.1586/erv.09.152
- Tan X,Huang X,Zhu S,Chen H,Yu Q,Wang H,Huo X,Zhou J,Wu Y,Yan D, et al. The persistent circulation of enterovirus 71 in People's Republic of China: causing emerging nationwide epidemics since 2008. PLoS One 2011; 6(9):e25662; PMID:21980521; http://dx.doi.org/10.1371/journal.pone.0025662
- Li YP,Liang ZL,Gao Q,Huang LR,Mao QY,Wen SQ,Liu Y,Yin WD,Li RC,Wang JZ. Safety and immunogenicity of a novel human Enterovirus 71 (EV71) vaccine: a randomized, placebo-controlled, double-blind, Phase I clinical trial. Vaccine 2012; 30(22):3295-303; PMID:22426327; http://dx.doi.org/10.1016/j.vaccine.2012.03.010
- Li YP,Liang ZL,Xia JL,Wu JY,Wang L,Song LF,Mao QY,Wen SQ,Huang RG,Hu YS, et al. Immunogenicity, safety, and immune persistence of a novel inactivated human enterovirus 71 vaccine: a phase II, Randomized, double-blind, placebo-controlled Trial.J Infect Dis 2014; 209(1):46-55; http://dx.doi.org/10.1093/infdis/jit429
- Zhu F,Xu W,Xia J,Liang Z,Liu Y,Zhang X,Tan X,Wang L,Mao Q,Wu J, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med 2014; 370(9):818-28; http://dx.doi.org/10.1056/NEJMoa1304923
- Zhu FC,Wang JZ,Li XL,Liang ZL,Ge HM,Meng FY,Mao QY,Zhang YT,Zhang ZY,Ji H, et al. Reactogenicity and immunogenicity of an enterovirus 71 vaccine in chinese healthy children and infants. Pediatr Infect Dis J 2012; 31(11):1158-65; PMID:22926209; http://dx.doi.org/10.1097/INF.0b013e31826eba74
- Zhu FC,Liang ZL,Li XL,Ge HM,Meng FY,Mao QY,Zhang YT,Hu YM,Zhang ZY,Li JX, et al. Immunogenicity and safety of an enterovirus 71 vaccine in healthy Chinese children and infants: a randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet 2013; 381(9871):1037-45.
- Zhu FC,Meng FY,Li JX,Li XL,Mao QY,Tao H,Zhang YT,Yao X,Chu K,Chen QH, et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2013; 381(9882):2024-32; http://dx.doi.org/10.1016/S0140-6736(13)61049-1
- Liu L,Zhang Y,Wang J,Zhao H,Jiang L,Che Y,Shi H,Li R,Mo Z,Huang T, et al. Study of the integrated immune response induced by an inactivated EV71 vaccine. PLoS One 2013; 8(1): e54451
- Li R,Liu L,Mo Z,Wang X,Xia J,Liang Z,Zhang Y,Li Y,Mao Q,Wang J, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med 2014; 370(9):829-37; http://dx.doi.org/10.1056/NEJMoa1303224
- Van Regenmortel MHV, Fauquet CM, Bishop DHL, Carsten EB, Estes MK, Lemon SM, Maniloff J, Mayo MA, McGeoch DJ, Pringle CR, et al. (Eds.), Virus Taxonomy. Classification and Nomenclature of Viruses. Seventh Report of the ICTV. New York: Academic Press, 2000:657-73.
- Juhela S,Hyoty H,Lonnrot M,Roivainen M,Simell O,Ilonen J.1998. Enterovirus infections and enterovirus specific T-cell responses ininfancy. J Med Virol 54:226-32; PMID:9515773; http://dx.doi.org/10.1002/(SICI)1096-9071(199803)54:3%3c226::AID-JMV14%3e3.0.CO;2-F
- Liu W,Wu S,Xiong Y,Li T,Wen Z,Yan M,Qin K,Liu Y,Wu J. Co-circulation and genomic recombination of coxsackievirus A16 and enterovirus 71 during a large outbreak of hand, foot, and mouth disease in Central China. PLoS One 2014; 9(4):e96051; PMID:24776922; http://dx.doi.org/10.1371/journal.pone.0096051
- Li L,He Y,Yang H,Zhu J,Xu X,Dong J,Zhu Y,Jin Q. Genetic characteristics of human enterovirus 71 and coxsackievirus A16 circulating from 1999 to 2004 in Shenzhen, People's Republic of China. J Clin Microbiol 2005; 43:3835-9; PMID:16081920; PMID:16081920; http://dx.doi.org/10.1128/JCM.43.8.3835-3839.2005
- Oberste MS,Maher K,Kilpatrick DR,Pallansch MA. Molecular evolution 432 of the human enteroviruses: correlation of serotype with VP1 sequence and 433 application to picornavirus classification. J Virol 1999; 73:1941-8; PMID:9971773
- Yao X, Mao QY, He P, Zhou C, Zhu XF, Zhang W, Lu FM, Liang ZL, Li FX, Wang JZ. Genetic characteristics of coxsakievirus A16 complete genome isolated in Beijing, 2008. 2010, 31(12):1437-38.
- World heaith organization. WHO recommendations for routine immunization–summary tables. http://www.who.int/immunization/policy/immunization_schedules/en/
- Cello J,Strannegård O,Svennerholm B. A study of the cellular immune response to enteroviruses in humans: identification of cross-reactive T cell epitopes on the structural proteins of enteroviruses. J Gen Virol 1996; 77:2097-108; PMID:8811009; http://dx.doi.org/10.1099/0022-1317-77-9-2097
- Samuelson A,Forsgren M,Johansson B,Wahren B,Sa¨llberg M. Molecular basis for serological cross-reactivity between enteroviruses. Clin Diagn Lab Immunol 1994; 1:336-41; PMID:7496972
- Lin Y,Wen K,Pan Y,Wang Y,Che X,Wang B. Cross-reactivity of anti-EV71 IgM and neutralizing antibody in series sera of patients infected with Enterovirus 71 and Coxsackievirus A 16. J Immunoassay Immunochem 2011; 32(3):233-43; PMID:21574094; http://dx.doi.org/10.1080/15321819.2011.559297
- Chou AH,Liu CC,Chang JY,Jiang R,Hsieh YC,Tsao A,Wu CL,Huang JL,Fung CP,Hsieh SM, et al. Formalin-inactivated EV71 vaccine candidate induced cross-neutralizing antibody against subgenotypes B1, B4, B5 and C4A in adult volunteers. PLoS One 2013; 8(11):e79783. e Collection 2013; http://dx.doi.org/10.1371/journal.pone.0079783
- Leng Q,Yang C,Zhu K,Deng C,Zhu L,Wan J. Pre-existing heterologous immunity to poliovirus vaccination may mitigate severity of hand, food and mouth disease caused by EV71. BMC Proceedings 2011; 5(Suppl 1):P27; http://dx.doi.org/10.1186/1753-6561-5-s1-p27
- Hovi T,Roivainen M. Peptide antisera targeted to a conserved sequence in poliovirus capsid VP1 cross-react widely with members of the genus Enterovirus.JClinMicrobiol 1993; 31(5):1083-7.
- Tan S,Tan X,Sun X,Lu G,Chen CC,Yan J,Liu J,Xu W,Gao GF. VP2 dominated CD4+ T cell responses against enterovirus 71 and cross-reactivity against coxsackievirus A16 and polioviruses in a healthy population. J Immunol 2013; 191(4):1637-47; http://dx.doi.org/10.4049/jimmunol.1301439
- Wu TC,Wang YF,Lee YP,Wang JR,Liu CC,Wang SM,Lei HY,Su IJ,Yu CK. Immunity to avirulent enterovirus 71 and coxsackie A16 virus protects against enterovirus 71 infection in mice. J Virol 2007; 81(19):10310-5. Epub 2007 Jul 11; http://dx.doi.org/10.1128/JVI.00372-07
- Lin Y,Wen K,Pan Y,Wang Y,Che X,Wang B. Cross-reactivity of anti-EV71 IgM and neutralizing antibody in series sera of patients infected with Enterovirus 71 and Coxsackievirus A 16.J Immunoassay Immunochem 2011; 32(3):233-43; PMID:21574094; http://dx.doi.org/10.1080/15321819.2011.559297
- Zhang HM,Li CR,Liu YJ,Liu WL,Fu D,Xu LM,Xie JJ,Tan Y,Wang H,Chen XC, et al. To investigate pathogen of hand, foot and mouth disease in Shenzhen in 2008. Chin J Exp Clin Virol 2009; 23:334-6;PMID:20387478
- Pan H,Zhu YF,Qi X,Zhang YJ,Li L,Deng F,Wu B,Wang SJ,Zhu FC,Wang H. Analysis on the epidemiological and genetic characteristics of enterovirustype 71 and Coxsackie A16 virus infection in Jiangsu,China. Zhonghua Liu Xing Bing XueZaZhi 2009; 30:339-43; PMID:19731523
- Ferson MJ,Bell SM. Outbreak of CoxsackievirusA16 hand, foot, and mouth disease in a child day care center. Am J Public Health 1991; 81:1675-6; PMID:1746672; http://dx.doi.org/10.2105/AJPH.81.12.1675
- Bendig JW,Fleming DM. Epidemiological, virological, and clinical features of an epidemic of hand, foot, and mouth disease in England and Wales. Commun Dis Rep CDR Rev 1996; 6:R81-6; PMID:8664928
- Chang LY. Enterovirus 71 in Taiwan. Pediatr Neonatol 2008; 49:103-12; PMID:19054914; http://dx.doi.org/10.1016/S1875-9572(08)60023-6
- Ang LW,Koh BK,Chan KP,Chua LT,James L,Goh KT. Epidemiology and control of hand, foot and mouth disease in Singapore, 2001-2007. Ann Acad Med Singapore 2009; 38:106-12; PMID:19271036
- Zhu J,Luo Z,Wang J,Xu Z,Chen H,Fan D,Gao N,Ping G,Zhou Z,Zhang Y, et al. Phylogenetic analysis of Enterovirus 71 circulating in Beijing, China from 2007 to 2009. PLoS One 2013; 8:e56318; PMID:23418551; http://dx.doi.org/10.1371/journal.pone.0056318
- Wang J, Qi S, Zhang X, Zhang Y, Liu L, Che Y, He Z, Zhao Y, Lu S, Yu W, Li Q. Coxsackievirus A 16 infection does not interfere with the specific immune response induced by an enterovirus 71 inactivated vaccine in rhesus monkeys. Vaccine 2014; 32(35):4436-42. doi: 10.1016/j.vaccine.2014.06.062.
- Arita I,Nakane M,Fenner F. Public health. Is polio eradication realistic? Science 2006; 312(5775):852-4; http://dx.doi.org/10.1126/science.1124959
- World Health Organization. 2012, Posting date. Polio case count. http://apps.who.int/immunization_monitoring/en/diseases/poliomyelitis/afpextract.cfm
- Luo HM,Zhang Y,Wang XQ,Yu WZ,Wen N,Yan DM,Wang HQ,Wushouer F,Wang HB,Xu AQ, et al. Identification and control of a poliomyelitis outbreak in Xinjiang, China. N Engl J Med 2013; 369(21):1981-90; http://dx.doi.org/10.1056/NEJMoa1303368
- Deng C,Yang C,Wan J,Zhu L,Leng Q. Irregular poliovirus vaccination correlates to pulmonary edema of hand, foot, and mouth disease. Clin Vaccine Immunol 2011; 18(9): 1589- 90. Epub 2011 Jul 13; http://dx.doi.org/10.1128/CVI.05132-11
- Aylward B,Tangermann R. The global polio eradication initiative: lessons learned and prospects for success. Vaccine 2011; 29: Suppl 4:D80-D85; http://dx.doi.org/10.1016/j.vaccine.2011.10.005
- Minor PD,Ferguson M,Evans DM,Almond JW,Icenogle JP. Antigenic structure of polioviruses of serotypes 1, 2 and 3. J Gen Virol 1986; 67(Pt 7):1283-91; PMID:2425046; http://dx.doi.org/10.1099/0022-1317-67-7-1283
- Emini EA, Jameson BA,Lewis AJ,Larsen GR,Wimmer E. Poliovirusneutralizationepitopes: analysis and localization with neutralizing monoclonal antibodies. J Virol 1982; 43(3):997-1005.
- Lentz KN,Smith AD,Geisler SC,Cox S,Buontempo P,Skelton A,DeMartino J,Rozhon E,Schwartz J,Girijavallabhan V, et al. Structure of poliovirus type 2 Lansing complexed with antiviral agent SCH48973: comparison of the structural and biological properties of three poliovirus serotypes. Structure 1997; 5(7):961-78; PMID:9261087; http://dx.doi.org/10.1016/S0969-2126(97)00249-9
- Page GS,Mosser AG,Hogle JM,Filman DJ,Rueckert RR,Chow M. Three-dimensional structure of poliovirus serotype 1 neutralizing determinants. J Virol 1988; 62(5):1781-94; PMID:2451757
- Foo DG,Alonso S,Phoon MC,Ramachandran NP,Chow VT,Poh CL. Identification of neutralizing linear epitopes from the VP1 capsid protein of Enterovirus 71 using synthetic peptides. Virus Res 2007; 125(1):61-8; PMID:17222936; http://dx.doi.org/10.1016/j.virusres.2006.12.005
- Lee H,Cifuente JO,Ashley RE,Conway JF,Makhov AM,Tano Y,Shimizu H,Nishimura Y,Hafenstein S. A strain-specific epitope of Enterovirus 71 identified by cryoEM of the complex with Fab from neutralizing antibody. J Virol 2013; 87(21):11363-70; PMID:23946455; http://dx.doi.org/10.1128/JVI.01926-13
- Liu CC,Chou AH,Lien SP,Lin HY,Liu SJ,Chang JY,Guo MS,Chow YH,Yang WS,Chang KH, et al. Identification and characterization of a cross-neutralization epitope of Enterovirus71.Vaccine 2011; 29(26):4362-72; http://dx.doi.org/10.1016/j.vaccine.2011.04.010
- Lim XF,Jia Q,Khong WX,Yan B,Premanand B,Alonso S,Chow VT,Kwang J. Characterization of an isotype-dependent monoclonal antibody against linear neutralizing epitope effective for prophylaxis of enterovirus 71 infection. PLoS One 2012; 7(1):e29751. Epub 2012 Jan 18; PMID:22279543; http://dx.doi.org/10.1371/journal.pone.0029751
- Kirk K,Poh CL,Fecondo J,Pourianfar H,Shaw J,Grollo L. Cross-reactive neutralizing antibody epitopes against Enterovirus 71 identified by an in silicoapproach. Vaccine 2012; 30(Nov (49)):7105-10; PMID:23022400; http://dx.doi.org/10.1016/j.vaccine.2012.09.030
- Shi J,Huang X,Liu Q,Huang Z. Identification of conserved neutralizing linear epitopes within the VP1 protein of coxsackievirus A16. Vaccine 2013; 31(17):2130-6; http://dx.doi.org/10.1016/j.vaccine.2013.02.051
- Liang Z,Mao Q,Gao Q,Li X,Dong C,Yu X,Yao X,Li F,Yin W,Li Q, et al. Establishing China's national standards of antigen content and neutralizing antibody responses for evaluation of enterovirus 71 (EV71) vaccines. Vaccine 2011; 29: 9668-74; PMID:22015395; http://dx.doi.org/10.1016/j.vaccine.2011.10.018
- WHO Department of Immunization, Vaccines and Biologicals. Polio laboratory manual. 4th ed. Geneva: World Health Organization, 2004. (WHO/IVB/04.10.).
