Abstract
The threat posed by the 2009 pandemic H1N1 virus emphasized the need for new influenza A virus vaccines inducing a broad cross-protective immune response for use in both humans and pigs. An effective and broad influenza vaccine for pigs would greatly benefit the pork industry and contribute to public health by diminishing the risk of emerging highly pathogenic reassortants. Current inactivated protein vaccines against swine influenza produce only short-lived immunity and have no efficacy against heterologous strains. DNA vaccines are a potential alternative with advantages such as the induction of cellular and humoral immunity, inherent safety and rapid production time. We have previously developed a DNA vaccine encoding selected influenza proteins of pandemic origin and demonstrated broad protective immune responses in ferrets and pigs. In this study, we evaluated our DNA vaccine expressed by next-generation vectors. These new vectors can improve gene expression, but they are also efficiently produced on large scales and comply with regulatory guidelines by avoiding antibiotic resistance genes. In addition, a new needle-free delivery of the vaccine, convenient for mass vaccinations, was compared with intradermal needle injection followed by electroporation. We report that when our DNA vaccine is expressed by the new vectors and delivered to the skin with the needle-free device in the rabbit model, it can elicit an antibody response with the same titers as a conventional vector with intradermal electroporation. The needle-free delivery is already in use for traditional protein vaccines in pigs but should be considered as a practical alternative for the mass administration of broadly protective influenza DNA vaccines.
Abbreviations
| DNA | = | DeoxyriboNucleic Acid |
| IDAL® | = | IntraDermal Application of Liquids® |
| i.d. | = | intra-dermal |
| tPA | = | tissue plasminogen activator |
| HA | = | hemagglutinin |
| NA | = | neuraminidase |
| NP | = | nucleoprotein |
| M | = | matrix protein |
| BSA | = | bovine serum albumin |
| FCS | = | fetal calf serum |
| IgG | = | immunoglobulin G |
| HAI | = | hemagglutination inhibition assay |
| HI | = | hemagglutination inhibition |
| HAU | = | hemagglutination units |
| RDE | = | receptor destroying enzyme |
| WHO | = | world health organization |
| NZW | = | New Zealand White |
| ELISA | = | Enzyme Linked Immunosorbent Assay |
| bp | = | base pair |
| MDCK cells | = | Madin-Darby Canine Kidney epithelial cells |
| PBS | = | phosphate buffered saline |
| TMB | = | tetramethylbenzidine |
| DK | = | Denmark |
| US | = | the United States |
| EP | = | electroporation |
| SEM | = | standard error mean |
Introduction
The use of nucleic acid-based vaccines is appealing for the field of influenza vaccination.Citation1,2 The current seasonal inactivated influenza vaccine for humans has many drawbacks: no long-lasting antibody titers, no T-cell immunity, time-consuming preparation from fertilized egg-propagated virus and a narrow target spectrum that has to be predicted yearly.Citation3,4 DNA vaccines have benefits such as rapid production and ease of immunogen exchange, which make the technology a potential candidate for an influenza vaccine. The theory of the DNA vaccine was proven in the early 1990s,Citation5,6 and the influenza virus was an early target for vaccination with gene immunization.Citation7,8 Many influenza plasmid DNA vaccine candidates have demonstrated promising results in animal models, and improved DNA vaccines have reached sufficient potency in clinical trials.Citation9-13
One improvement to increase both the antigenicity and the immune response of a DNA vaccine is codon optimization, where the antigen coding sequence is optimized for efficient expression in the mammalian host cellsCitation14-16; this has demonstrated beneficial effects in several influenza DNA vaccine studies.Citation9,11,17-21 A complementary method to increase the immunogenicity is to optimize the DNA vaccine vector backbone. So-called ‘next generation’ vectors under development will increase antigen expression, manufacturing yield and quality and follow regulatory compliance standards.Citation16 In addition, the delivery mode of a DNA vaccine is a relevant factor in eliciting the proper immune response. A needle and syringe is the conventional method of vaccination, including for DNA-based vaccines. The administration of a brief electrical pulse, electroporation, after the injection of a DNA vaccine has proven effective in enhancing uptake and immunogenicity of DNA vaccines.Citation22–25 However, the fine balance between gaining high efficacy of DNA uptake and minimizing tissue damageCitation26 is a weak point of this method. Perhaps the major obstacle to in vivo electroporation is the practical difficulty of the method with regard to its tolerability in humans, animal welfare and inconvenience in mass vaccinations. One additional mode of delivery demonstrating the effective generation of an immune response is particle-mediated delivery to the skin by a gene gun.Citation25,27-29 Needle-free jet injection is an alternative approach with proven effectiveness and benefits such as ease of use during mass vaccinations and excellent safety and tolerability profiles.Citation30,31 The needle-free jet uses a high velocity stream of liquid containing the vaccine. The stream enables the fluid to penetrate across the skin's physical barriers, resulting in vaccine delivery into the different layers of the skin. Different needle-free injection systems have been tested successfully with influenza DNA plasmids in animalsCitation21,32 and humans.Citation11 The needle-less IDAL® vaccinator (MSD Animal Health, Summit, NJ, US) is already used routinely for vaccination in swine herds for attenuated and inactivated protein vaccines against the Porcine Reproductive and Respiratory Syndrome virus,Citation33 Aujeszky's disease virusCitation34 and Mycoplasma hyopneumoniaeCitation35,36 with promising results.
Previously, we have demonstrated cross-protective immunity in ferrets and pigs from DNA vaccines with pandemic genes of the 1918 H1N1-, 1968 H3N2- and pdm09 H1N1-influenza virus.Citation25,37 In the present study, we examined immunogenicity when the previously used genes were mixed and administered to rabbits. We also investigated the potential of this polyvalent influenza DNA in an optimized setting with codon-optimized influenza genes inserted into next generation vectors and delivered with the needle-free jet-injector IDAL® in the rabbit model. Vaccination was standardized to equimolar amounts of conventional and next generation vectors; this reduced the mass of the DNA dosage from 200 μg to 125 μg. Despite the reduced mass dosage, the next generation vectors, with a smaller plasmid backbone and antibiotic-free production, induced an immune response equivalent to that of the conventional vector. The needle-free delivery demonstrated that the IDAL® device can be used in rabbits, even though it was developed for use in pig skin, and the delivery method preforms equally well as the previous intradermal (i.d.) injection with electroporation with regard to induced immune response. These optimizations, next generation vectors and convenient needle-free delivery, would greatly enhance a realistic mass-vaccination of pigs with an efficient broad influenza DNA vaccine.
Materials and Methods
Construction of the DNA vaccines
Influenza DNA vaccine genes were designed from nucleotide sequences published in GenBank (1918 NP: A/Brevig Mission/1/18(H1N1) AY744935, 1918 M: A/Brevig Mission/1/18(H1N1) AY130766, 2009 HA: A/California/04/2009(H1N1)pdm09 ACP41105, 2009 NA: A/California/04/2009(H1N1)pdm09 ACP41107, 1968 HA: A/Aichi/2/1968(H3N2) AB295605, 1968 NA: A/Aichi/2/1968(H3N2) AB295606). The genes were made synthetically and designed to include the appropriate restriction enzymes and Kozak sequence (GCCACC), -1 base upstream from the start codon, for efficient cloning and transcription into the expression vectors pSSI (Statens Serum Institut, DK), NTC8385-VA1Citation16,38 (Nature Technologies Corporation, Lincoln, NE, US) and NTC9385RCitation16 (Nature Technologies Corporation, Lincoln, NE, US). The genes were synthesized using only codons from highly expressed human or mammalian genes (codon optimized). The pSSI expression vector backbone (3665 bp) contains a kanamycin resistance gene, cytomegalovirus immediate-early promoter, intron A and polyadenylation signal. Endogenous signal sequences from the influenza HA and NA genes were used for secretory expression. The pSSI vaccine construct was produced in the E. coli strain DH5α, using kanamycin as the selection antibiotic. Endotoxin-free DNA purification of the vaccine clones was prepared by the EndoFree Plasmid Giga Kit (QIAGEN, cat. no. 12391). The NTC8385-VA1 (3025 bp) and the NTC9385R (1700 bp) vectors are minimalized antibiotic-free plasmid vectors with the expression enhancers human T-lymphotropic virus type I (HTLV-I) R region and adenovirus serotype-5 virus-associated (VA) RNA interference (only in NTC8385-VA1).Citation38 Both the NTC8385-VA1 and the NTC9385R vaccine constructs have been produced in the HyperGROTM fermentation process.Citation39
Vaccine delivery mode
The vaccine constructs were delivered in 2 different modes to the animals (). One) An i.d. needle injection of naked DNA in PBS in a volume of 0.1 ml was divided into 2 sites on shaved abdominal skin. This was followed by electroporation using 2 rows of 6 skin electrodes in the OncoVet™ system (CytoPulse Sciences/Cellectis, Romainville, France) over each injected area. The electroporation condition was 10 pulses of 2 × 450 V and 8 × 110 V of 2 × 0.05 ms and 8 × 10 ms pulse length, with a 0.2, 50 and 7 × 20 ms interval between each pulse. The use of electroporation in rabbit skin has been evaluated for efficacyCitation40 and safetyCitation41 and the method has been used successfully in previous studies with DNA vaccines in rabbits.Citation42,43 For the vaccination procedure, the rabbits were anesthetized using intramuscular-administered Hypnorm® (fentanyl citrate:fluanisone mix) (Skanderborg Pharmacy, Denmark) 0.3 ml/kg. 2). A needle-free i.d. injection using the IntraDermal Application of Liquids (IDAL®) immunization technique (MSD Animal Health, Summit, NJ, US) was distributed at 2 injection sites on the back. With this immunization technique there is no need for anesthesia. For the practical use of the IDAL® device, the vaccine constructs were premixed at a 1:1 volume ratio with a α-tocopherol-based aqueous solution (Diluvac Forte®, MSD Animal Health), and the total injection volume was 0.2 ml at each site. The IDAL® device is successfully used for the vaccination of swine.Citation33,34,36
Table 1. Overview of rabbit vaccinations
DNA immunization of New Zealand White (NZW) rabbits
Ten-week-old female nulliparous NZW rabbits were housed at Statens Serum Institute Animal Facility (Copenhagen, Denmark). Animal experiments were performed by certified animal handlers and according to the Animal Experimentation Act of Denmark and European Convention ETS 123. Animal acclimatization was at least 10 d prior to any experimental procedures. The rabbits were divided into 4 groups with 4 or 5 rabbits in each group, and they were all vaccinated at weeks 0 and 3 of the experiment. Both vaccinations contained an identical mix formulation of 10 pmol of each of the 6 influenza gene plasmids (). Blood serum was collected before the first vaccination (week 0), at week 2, before second vaccination (week 3) and at week 5.
Serum antibody responses by ELISA
ELISA plates (96 wells) were coated with 100 μl of recombinant influenza hemagglutinin protein antigen from A/California/04/09(H1N1)pdm09, A/Aichi/2/1968(H3N2) or A/swine/Guangxi/13/2006(H1N2) (Sino Biological, cat. no. 11055-V08B, 11707-V08H and 11703-V08H, respectively), 2 μg/ml in 50 mM carbonate buffer, overnight at 4°C. Wells were blocked with 2% skim milk powder in PBS (1% BSA, 10% FCS and 1% Triton X-100) for one hour at room temperature. Plates were washed with 1% Triton X-100/PBS. Rabbit sera, diluted in 2% skim milk powder blocking buffer, were added and incubated for one hour at room temperature. The plates were again washed and incubated with horseradish peroxidase-conjugated anti-rabbit-IgG antibody (Sigma, cat. no. A1949) for one hour. Following washing, the color was developed with TMB (Kem-En-Tec, cat. no. 4380) for 30 minutes, and the reaction was stopped by adding 0.2 M H2SO4. The absorbance was read at OD450 nm.
Hemagglutination inhibition assay (HAI)
The HAI assay measures how well sera from vaccinated rabbits inactivate influenza virus binding to red blood cells. Rabbit sera were treated with a receptor-destroying enzyme (RDE(II), Seiken, cat. no. 370013) as described by the manufacturer. Virus was titrated using the hemagglutination assay according to the protocols of the WHOCitation44 with 0.75% guinea pig red blood cells in U-bottom plates (U96 MicroWell Plates, Nunc) and incubated for one hour. The virus isolates tested were 2 homologs to the vaccine, A/California/07/09(H1N1v) and A/Aichi/02/68(H3N2, and 4 heterologs to the vaccine, A/New Caledonia/20/99(H1N1), A/swine/DK/102586/2010(H1N1), A/swine/DK/16525/2008(H1N2) and A/swine/DK/101501/2010(H3N2). The isolates were produced in MDCK cells and standardized to a 100% hemagglutination endpoint titer of 8 hemagglutination units (HAU). The HAI assay was performed according to the protocols of the WHOCitation44 with 0.75% guinea pig red blood cells in U-bottom plates (U96 MicroWell Plates, Nunc) and the HI-titers were read as the reciprocal of the last dilution of sera that completely inhibited hemagglutination.
Statistical analysis
Figures and statistical analysis were prepared using GraphPad Prism statistical software version 5 (GraphPad Software) according to the statistical tests described in the figure legends.
Results
Immunogenicity of a hexavalent DNA vaccine
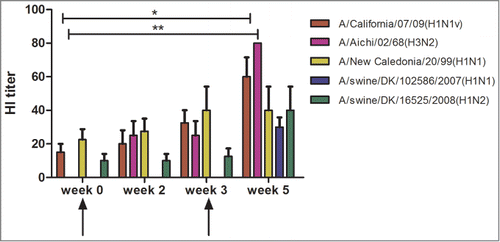
In our previous challenge studies of ferretsCitation37 and pigs,Citation25 the 6 influenza genes (pandemic 1918 nucleoprotein (NP) and matrix protein (M), pandemic H1N1pdm09 and 1968 H3N2 surface antigens hemagglutinin (HA) and neuraminidase (NA)) were not given as a single mixed dose. In the current study, 4 rabbits (Group I, ) were immunized with the same genes as used previously but in a hexavalent vaccine mix, given at 2 immunization time points. The vaccine was delivered with i.d. needle injection followed by electroporation. This mix of plasmids induced HI antibody responses against both human and swine influenza isolates, homologous and heterologous to the vaccine genes, which is especially observed after the 2nd vaccination ().
Figure 1. Hemagglutination inhibition (HI) antibody responses in rabbit sera (n = 4, Group I) immunized with a hexavalent DNA vaccine mix (1918-NP-M, 1968-HA3-NA2, pdm09-HA1-NA1). HI antibody responses were measured against the human isolates H1N1v A/California/07/09 and H3N2 A/Aichi/02/68, homologous to the vaccine, and H1N1 A/New Caledonia/20/99, heterologous to the vaccine. HI antibody responses were also measured against 2 isolates from swine, H1N1 A/swine/DK/102586/2007 and H1N2 A/swine/DK/16525/2008. Arrows indicate vaccination time points. Titers are provided as geometric mean titers. Significant values are indicated by *P < 0.05, **P < 0.01 using one-way ANOVA, Freidman's test with Dunn's multiple-comparison test.
Needle-free delivery of DNA vaccine elicits antibody responses equal to injection with electroporation
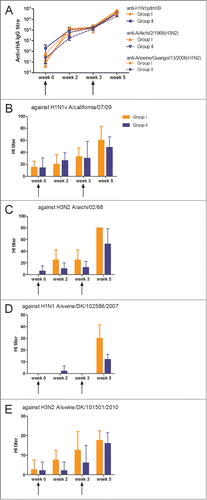
To compare the previously used delivery method, i.d. needle injection plus electroporation, with the needle-free IDAL® device, sera from 2 groups of rabbits were evaluated; Groups I and II were immunized according to . The IDAL® device is developed for use in pigs and has previously only been used in pig studies.Citation33-36 The immunization of rabbits with the IDAL® device was successful in the current study and the desired deposition of the vaccine into the dermis layers was evaluated immediately after injections with colored liquid in a single rabbit (). The induced immune response by needle-free delivery and electroporation was evaluated after 2 DNA immunizations at weeks 0 and 3, respectively. HA-specific antibody responses against the vaccine components from A/California/04/09(H1N1) and A/Aichi/2/1968(H3N2) were measured by ELISA (). In addition, the antibody response was also detected against the heterologous HA of A/swine/Guangxi/13/2006(H1N2) (). Positive HA-specific antibodies were already detected after the 1st immunization for both vaccinated rabbit groups and the response levels were further boosted following the 2nd immunization. Levels of serum HI antibodies were tested against homologous vaccine viruses A/California/04/09(H1N1) and A/Aichi/02/68(H3N2), and this increased for both groups after 2nd vaccination, independent of the delivery mode of the vaccine (). To determine the cross-reactivity, we also measured HI titers against 2 heterologous swine virus strains, one H1N1 and one H3N2 (). Both vaccinated rabbit groups developed cross-reactive IgG against the antigenically different swine strains after vaccination, but only to low HI titers. To summarize, the needle-free delivery of the DNA vaccine was equally effective as the i.d. injection followed by electroporation with regard to the induction of an antibody response. The use of pandemic genes as DNA vaccines, via both delivery systems, could induce a cross-reactive response detected both by HA-specific antibodies and the inhibition of hemagglutination.
Figure 2. Needle-free delivery of influenza DNA vaccine into New Zealand White rabbits. One rabbit was sacrificed immediately after injection in order to evaluate deposition of delivered liquid from IDAL® into the rabbit skin. (A) Colored liquid was injected with IDAL® onto shaved skin. (B) The red arrow indicates the injection site on intact skin. (C) Incision of the skin minutes after injection at the site of delivery. The colored liquid is observed deposited though all skin layers until the muscle fascia.
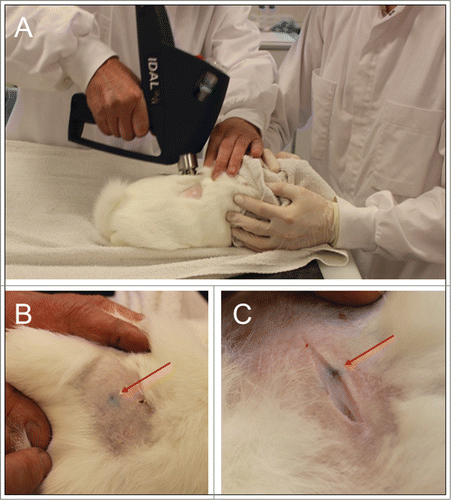
Figure 3. Antibody response induced by 2 different delivery mode of vaccination. (A) Serum anti-HA IgG responses measured by ELISA against HA as the coating antigen from H1N1pdm09, 1968 H3N2 and a swine H1N2 isolate, heterologous to the DNA vaccine. Group I was immunized by i.d. and electroporation with influenza genes inserted into the pSSI vector. Group II was immunized by needle-free IDAL® with influenza genes inserted into the pSSI vector. (B-E) Vaccine induced hemagglutination inhibition antibody responses in rabbit sera against (B) H1N1pdm09, (C) 1968 H2N3, (D) swine H1N1 and (E) swine H3N2 isolates were measured. The HI titers are given as the geometric mean titer. The arrows indicate vaccination time points and each curve or bar represents the mean with SEM of each group of 4–5 rabbits.
Antibody responses induced by different DNA vaccine vectors
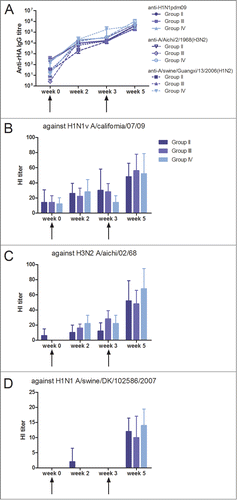
Next generation vectors with small backbones and no antibiotic resistance genes, vector NTC8385-VA1 and NTC9385R, were inserted with our influenza vaccine genes. Two groups of rabbits were immunized with the next generation vectors, Group III and IV (), and the induced immune response was compared to the conventional pSSI DNA vector used in Group II. All three groups of animals were vaccinated with needle-free delivery at equimolar doses. The HA-specific antibody response was measured by ELISA () and the serum levels of the HI antibodies were detected against the homologous virus strains A/California/04/09(H1N1) and A/Aichi/02/68(H3N2) () and a heterologous swine H1N1 virus strain (). All antibody responses measured demonstrated indistinguishable titers for the 3 different DNA vaccine vectors. All groups of rabbits developed HA-specific antibodies already after the first vaccination against both homologous and heterologous HA protein. HI antibodies were observed after the second vaccination with the highest HI titers against the homologous vaccine virus and lower but detectable titers against the heterologous swine H1N1 virus strain. Thus, the new generation of vectors perform as well as a conventional DNA vaccine vector at a lower mass dosage (125 μg versus 200 μg), which is promising for future DNA vaccine development.
Figure 4. Induced antibody response by 3 different DNA vaccine vectors. (A) Serum anti-HA IgG responses measured by ELISA against HA as the coating antigen from H1N1pdm09, 1968 H3N2 and a swine H1N2 isolate, heterologous to the DNA vaccine. Group II was immunized with influenza genes inserted into the conventional pSSI vector. Group III and IV were immunized with influenza genes inserted into the new vectors NTC8385-VA1 and NTC9385R, respectively. (B-D) Vaccine-induced hemagglutination inhibition antibody responses in rabbit sera against (B) H1N1pdm09, (C) 1968 H2N3 and (D) swine H1N1 isolates were measured. The HI titers are provided as the geometric mean titer. The arrows indicate vaccination time points and each curve or bar represents the mean with SEM of each group of 4–5 rabbits.
Discussion
In the present study, we have mixed 6 different influenza genes of pandemic origin into a single mixed-dose DNA vaccine. The six influenza genes have in our previous DNA vaccine studies induced cross-protective immunity in ferrets and pigs.Citation25,37 We demonstrate herein the possibility of inducing a humoral immune response when using this hexavalent DNA vaccine mix. We could detect high HA-specific IgG titers against both homologous and heterologous viral proteins. High HI titers against homologous viruses were observed after vaccination and detectable but lower titers against heterologous viruses. Indeed, even though HI antibody titers higher than 40 correlates with the seroprotection rate after vaccination,Citation45 it is not the only factor determining vaccine efficacy.Citation46,47 Although not assessed in this study, potent influenza DNA vaccines have been demonstrated to induce a cellular immune response in parallel to the antibody response.Citation48,49 A cellular immune response to the conserved NP and M internal influenza components of the mixed DNA vaccine may contribute to cross-protective immunity.Citation37
Influenza is a major infectious disease affecting both humans and animals globally. Swine influenza is endemic in pigs and affects the majority of herds in modern swine production.Citation50,51 The recent 2009 emergence of the pandemic influenza virus (pdm09H1N1v) highlighted the potential human health threat posed by infection in pigs.Citation52 Genes from human, classic swine and North American avian influenza viruses in the pdm09H1N1v strainCitation53 reinforced the role of pigs as a “mixing vessel”Citation54 with the potential for the development of novel virus strains to which humans have no immunity. The risk of a sudden outbreak of pandemic influenza in humans highlights the importance of developing efficient vaccines that can be produced in sufficient doses. The DNA vaccine approach offers many advantages for the strategy of controlling a pandemic influenza and a DNA vaccine is a valid candidate for protecting both humans and pigs from influenza. Vaccination of swineherds will not only minimize the risk of reassortants with zoonotic potential but also save costs in swine productionCitation55 and decrease the use of antibiotics against diseases associated with swine influenza infection. We have previously demonstrated the cross-protective effects of our polyvalent DNA vaccine in pigs delivered with i.d. electroporation.Citation25 However, large-scale vaccination of pigs with our DNA vaccine and electroporation would be impractical because the electroporation technique requires the sedation of the animals. In a mass vaccination situation, the convenient delivery with the IDAL® device, without the requirements for the chemical or physical restraint of the pigs, provides an easy and safe alternative for vaccine administration. In the current study, we have evaluated the needle-free IDAL® device for easy delivery of vaccine to rabbits. We have used the rabbit model as an initial examination of the vaccine immunogenicity. The benefits of using rabbits are the production of high antibody titers, large volume of sera and the zero-negative background, which can be difficult to find in pigs. To our knowledge, this is the first time the IDAL® device has been used in a rabbit study or with a DNA vaccine, and our results demonstrate the possibility of using this animal model and the IDAL® device with DNA vaccines even though the IDAL® device was developed for use with protein vaccines in pigs. Because the needle-free delivery could induce same levels of immune response in our study as the electroporation method, we speculate that vaccinating pigs with our influenza DNA genes with the same needle-free delivery will induce the potent response in a more user-friendly, safe and convenient manner than needle injection with electroporation.
The use of antibiotic selection during DNA plasmid production is problematic and raises concerns regarding environmental contamination and the transfer of antibiotic resistance.Citation16 There are regulatory guidelines recommending the elimination of antibiotic-resistance genes,Citation56,57 and in addition, these selection markers can have a negative impact on gene expression.Citation15 Inserting our DNA vaccine genes into 2 next generation vectors, the antibiotic-free vectors NTC8385-VA1 and NTC9385R, resulted in a similar antibody response compared to vaccine genes in conventional pSSI expression vector with antibiotic-resistance gene using equimolar dosages. These new vectors have in previous studies demonstrated increased in vivo gene expression compared to conventional vectors.Citation16 In this study, we have not assessed the expression level from the vectors, but we could not see a clear increase in immunogenicity of these new vectors with equimolar dosage. This may in part be due to the reduced DNA amount with new vectors (e.g., 125 μg vs. 200 μg for NTC9385R versus pSSI, respectively) because the adjuvant activity of the plasmid backbone (through activation of cytoplasmic DNA sensing innate immune receptors) may be dependent on the total amount of DNA. There are other superior features of these new generation vectors. The smaller plasmid backbone of these vectors, in particular NTC9385R, is trimmed to remove unnecessary gene material,Citation16 according to regulatory guidelines. The smaller size also lowers the necessary amount of DNA plasmids in the vaccine. Conventional DNA vectors are not optimized for large-scale manufacturing, whereas the new vectors used in this study are developed for high-quality, high-yield production.Citation16 Thus, the use of next generation plasmids in DNA vaccines, such as vector NTC8385-VA1 and NTC9385R, seem to generate equal immune response as conventional plasmids at lower mass dosage and they offer several advantages for large-scale production and compliance to regulatory guidelines.
In conclusion, we believe that our optimized influenza DNA vaccine mix has potential in both humans and pigs, based on current findings and previous challenge studies.Citation25 The data reported herein demonstrate that a hexavalent influenza DNA vaccine can be efficiently mass-delivered with the needle-free IDAL® device. The optimized DNA vaccine and delivery will greatly aid in the large-scale production of safe DNA mass vaccinations with broader protection against influenza virus.
Disclosure of Potential Conflicts of Interest
James Williams has an equity interest in Nature Technology Corporation. The other authors declare that there are no other conflicts of interest.
Acknowledgments
We thank the skilled technical assistance of Randi Thøgersen. We also gratefully acknowledge the staff at the animal facility at Statens Serum Institut for their excellent care of the animals.
Funding
This study was supported in part with research grants from UNISEC, which has been made possible by contributions from the European Commission DG-research and the European member states, and NIH grant R43 GM102972–01 given to J.A.W.
References
- Liu MA. DNA vaccines: An historical perspective and view to the future. Immunol Rev 2011; 239:62-84; PMID:21198665; http://dx.doi.org/10.1111/j.1600-065X.2010.00980.x
- Kutzler MA, Weiner DB. DNA vaccines: Ready for prime time? Nat Rev Genet 2008; 9:776-88; PMID:18781156; http://dx.doi.org/10.1038/nrg2432
- Wong SS, Webby RJ. Traditional and new influenza vaccines. Clin Microbiol Rev 2013; 26:476-92; PMID:23824369; http://dx.doi.org/10.1128/CMR.00097-12
- Lambert LC, Fauci AS. Influenza vaccines for the future. N Engl J Med 2010; 363:2036-44; PMID:21083388; http://dx.doi.org/10.1056/NEJMra1002842
- Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature 1992; 356:152-4; PMID:1545867; http://dx.doi.org/10.1038/356152a0
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science 1990; 247:1465-8; PMID:1690918; http://dx.doi.org/10.1126/science.1690918
- Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, DeWitt CM, Friedman A, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 1993; 259:1745-9; PMID:8456302; http://dx.doi.org/10.1126/science.8456302
- Robinson HL, Hunt LA, Webster RG. Protection against a lethal influenza virus challenge by immunization with a haemagglutinin-expressing plasmid DNA. Vaccine 1993; 11:957-60; PMID:8212843; http://dx.doi.org/10.1016/0264-410X(93)90385-B
- Jones S, Evans K, McElwaine-Johnn H, Sharpe M, Oxford J, Lambkin-Williams R, Mant T, Nolan A, Zambon M, Ellis J, et al. DNA vaccination protects against an influenza challenge in a double-blind randomised placebo-controlled phase 1b clinical trial. Vaccine 2009; 27:2506-12; PMID:19368793; http://dx.doi.org/10.1016/j.vaccine.2009.02.061
- Girard MP, Tam JS, Pervikov Y, Katz JM. Report on the first WHO integrated meeting on development and clinical trials of influenza vaccines that induce broadly protective and long-lasting immune responses: Hong Kong SAR, China, 24-26 January 2013. Vaccine 2013; 31:3766-71; PMID:23810374; http://dx.doi.org/10.1016/j.vaccine.2013.06.047
- Smith LR, Wloch MK, Ye M, Reyes LR, Boutsaboualoy S, Dunne CE, Chaplin JA, Rusalov D, Rolland AP, Fisher CL, et al. Phase 1 clinical trials of the safety and immunogenicity of adjuvanted plasmid DNA vaccines encoding influenza A virus H5 hemagglutinin. Vaccine 2010; 28:2565-72; PMID:20117262; http://dx.doi.org/10.1016/j.vaccine.2010.01.029
- Ledgerwood JE, Wei CJ, Hu Z, Gordon IJ, Enama ME, Hendel CS, McTamney PM, Pearce MB, Yassine HM, Boyington JC, et al. DNA priming and influenza vaccine immunogenicity: two phase 1 open label randomised clinical trials. Lancet Infect Dis 2011; 11:916-24; PMID:21975270; http://dx.doi.org/10.1016/S1473-3099(11)70240-7
- Ledgerwood JE, Hu Z, Gordon IJ, Yamshchikov G, Enama ME, Plummer S, Bailer R, Pearce MB, Tumpey TM, Koup RA, et al. Influenza virus h5 DNA vaccination is immunogenic by intramuscular and intradermal routes in humans. Clin Vaccine Immunol 2012; 19:1792-7; PMID:22956656; http://dx.doi.org/10.1128/CVI.05663-11
- Corbet S, Vinner L, Hougaard DM, Bryder K, Nielsen HV, Nielsen C, Fomsgaard A. Construction, biological activity, and immunogenicity of synthetic envelope DNA vaccines based on a primary, CCR5-tropic, early HIV type 1 isolate (BX08) with human codons. AIDS Res Hum Retroviruses 2000; 16:1997-2008; PMID:11153083; http://dx.doi.org/10.1089/088922200750054738
- Li L, Saade F, Petrovsky N. The future of human DNA vaccines. J Biotechnol 2012; 162:171-82; PMID:22981627; http://dx.doi.org/10.1016/j.jbiotec.2012.08.012
- Williams JA. Vector design for improved DNA vaccine efficacy, safety and production. Vaccines 2013; 1:225-49; http://dx.doi.org/10.3390/vaccines1030225
- Shan S, Jiang Y, Bu Z, Ellis T, Zeng X, Edwards J, Tian G, Li Y, Ge J, Chen H, et al. Strategies for improving the efficacy of a H6 subtype avian influenza DNA vaccine in chickens. J Virol Methods 2011; 173:220-6; PMID:21333689; http://dx.doi.org/10.1016/j.jviromet.2011.02.008
- Li J, Jiang Y, Zhao S, Chang X, Liu J, Zeng X, Li Y, Chen H. Protective efficacy of an H5N1 DNA vaccine against challenge with a lethal H5N1 virus in quail. Avian Dis 2012; 56:937-9; PMID:23402115; http://dx.doi.org/10.1637/10150-040812-ResNote.1
- Patel A, Dong JC, Trost B, Richardson JS, Tohme S, Babiuk S, Kusalik A, Kung SK, Kobinger GP. Pentamers not found in the universal proteome can enhance antigen specific immune responses and adjuvant vaccines. PLoS One 2012; 7:e43802; PMID:22937099; http://dx.doi.org/10.1371/journal.pone.0043802
- Wang S, Hackett A, Jia N, Zhang C, Zhang L, Parker C, Zhou A, Li J, Cao WC, Huang Z, et al. Polyvalent DNA vaccines expressing HA antigens of H5N1 influenza viruses with an optimized leader sequence elicit cross-protective antibody responses. PLoS One 2011; 6:e28757; PMID:22205966; http://dx.doi.org/10.1371/journal.pone.0028757
- Ault A, Zajac AM, Kong WP, Gorres JP, Royals M, Wei CJ, Bao S, Yang ZY, Reedy SE, Sturgill TL, et al. Immunogenicity and clinical protection against equine influenza by DNA vaccination of ponies. Vaccine 2012; 30:3965-74; PMID:22449425; http://dx.doi.org/10.1016/j.vaccine.2012.03.026
- Otten G, Schaefer M, Doe B, Liu H, Srivastava I, zur Megede J, O'Hagan D, Donnelly J, Widera G, Rabussay D, et al. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine 2004; 22:2489-93; PMID:15193413; http://dx.doi.org/10.1016/j.vaccine.2003.11.073
- Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M, Leung L, Otten GR, Thudium K, Selby MJ, et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol 2000; 164:4635-40; PMID:10779767; http://dx.doi.org/10.4049/jimmunol.164.9.4635
- Hirao LA, Wu L, Khan AS, Satishchandran A, Draghia-Akli R, Weiner DB. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine 2008; 26:440-8; PMID:18082294; http://dx.doi.org/10.1016/j.vaccine.2007.10.041
- Bragstad K, Vinner L, Hansen MS, Nielsen J, Fomsgaard A. A polyvalent influenza A DNA vaccine induces heterologous immunity and protects pigs against pandemic A(H1N1)pdm09 virus infection. Vaccine 2013; 31:2281-8; PMID:23499598; http://dx.doi.org/10.1016/j.vaccine.2013.02.061
- van Drunen Littel-van den Hurk S, Babiuk SL, Babiuk LA. Strategies for improved formulation and delivery of DNA vaccines to veterinary target species. Immunol Rev 2004; 199:113-25; PMID:15233730; http://dx.doi.org/10.1111/j.0105-2896.2004.00140.x
- Wang S, Zhang C, Zhang L, Li J, Huang Z, Lu S. The relative immunogenicity of DNA vaccines delivered by the intramuscular needle injection, electroporation and gene gun methods. Vaccine 2008; 26:2100-10; PMID:18378365; http://dx.doi.org/10.1016/j.vaccine.2008.02.033
- Yager EJ, Dean HJ, Fuller DH. Prospects for developing an effective particle-mediated DNA vaccine against influenza. Expert Rev Vaccines 2009; 8:1205-20; PMID:19722894; http://dx.doi.org/10.1586/erv.09.82
- Drape RJ, Macklin MD, Barr LJ, Jones S, Haynes JR, Dean HJ. Epidermal DNA vaccine for influenza is immunogenic in humans. Vaccine 2006; 24:4475-81; PMID:16150518; http://dx.doi.org/10.1016/j.vaccine.2005.08.012
- McAllister L, Anderson J, Werth K, Cho I, Copeland K, Le Cam Bouveret N, Plant D, Mendelman PM, Cobb DK. Needle-free jet injection for administration of influenza vaccine: A randomised non-inferiority trial. Lancet 2014; 384:674-81; PMID:24881803; http://dx.doi.org/10.1016/S0140-6736(14)60524-9
- Mitragotri S. Current status and future prospects of needle-free liquid jet injectors. Nat Rev Drug Discov 2006; 5:543-8; PMID:16816837
- Gorres JP, Lager KM, Kong WP, Royals M, Todd JP, Vincent AL, Wei CJ, Loving CL, Zanella EL, Janke B, et al. DNA vaccination elicits protective immune responses against pandemic and classic swine influenza viruses in pigs. Clin Vaccine Immunol 2011; 18:1987-95; PMID:21918118; http://dx.doi.org/10.1128/CVI.05171-11
- Martelli P, Cordioli P, Alborali LG, Gozio S, De Angelis E, Ferrari L, Lombardi G, Borghetti P. Protection and immune response in pigs intradermally vaccinated against porcine reproductive and respiratory syndrome (PRRS) and subsequently exposed to a heterologous European (Italian cluster) field strain. Vaccine 2007; 25:3400-8; PMID:17276558; http://dx.doi.org/10.1016/j.vaccine.2006.12.050
- Ferrari L, Borghetti P, Gozio S, De Angelis E, Ballotta L, Smeets J, Blanchaert A, Martelli P. Evaluation of the immune response induced by intradermal vaccination by using a needle-less system in comparison with the intramuscular route in conventional pigs. Res Vet Sci 2011; 90:64-71; PMID:20546827; http://dx.doi.org/10.1016/j.rvsc.2010.04.026
- Martelli P, Saleri R, Cavalli V, De Angelis E, Ferrari L, Benetti M, Ferrarini G, Merialdi G, Borghetti P. Systemic and local immune response in pigs intradermally and intramuscularly injected with inactivated Mycoplasma hyopneumoniae vaccines. Vet Microbiol 2014; 168:357-64; PMID:24332702; http://dx.doi.org/10.1016/j.vetmic.2013.11.025
- Tassis PD, Papatsiros VG, Nell T, Maes D, Alexopoulos C, Kyriakis SC, Tzika ED. Clinical evaluation of intradermal vaccination against porcine enzootic pneumonia (Mycoplasma hyopneumoniae). Vet Record 2012; 170:261; PMID:22262700; http://dx.doi.org/10.1136/vr.100239
- Bragstad K, Martel CJ, Thomsen JS, Jensen KL, Nielsen LP, Aasted B, Fomsgaard A. Pandemic influenza 1918 H1N1 and 1968 H3N2 DNA vaccines induce cross-reactive immunity in ferrets against infection with viruses drifted for decades. Influenza Other Respir Viruses 2011; 5:13-23; PMID:21138536; http://dx.doi.org/10.1111/j.1750-2659.2010.00177.x
- Luke JM, Vincent JM, Du SX, Gerdemann U, Leen AM, Whalen RG, Hodgson CP, Williams JA. Improved antibiotic-free plasmid vector design by incorporation of transient expression enhancers. Gene Ther 2011; 18:334-43; PMID:21107439; http://dx.doi.org/10.1038/gt.2010.149
- Carnes AEW, J. A. Process for plasmid DNA fermentation. US Patent 7943377 2011
- Medi BM, Hoselton S, Marepalli RB, Singh J. Skin targeted DNA vaccine delivery using electroporation in rabbits. I: efficacy. Int J Pharm 2005; 294:53-63; PMID:15814230; http://dx.doi.org/10.1016/j.ijpharm.2004.12.014
- Medi BM, Singh J. Skin targeted DNA vaccine delivery using electroporation in rabbits II. Safety. Int J Pharm 2006; 308:61-8; http://dx.doi.org/10.1016/j.ijpharm.2005.10.035
- Borggren M, Vinner L, Andresen BS, Grevstad B, Repits J, Melchers M, Elvang T, Sanders RW, Martinon F, Dereuddre-Bosquet N, et al. Optimization of HIV-1 envelope DNA vaccine candidates within three different animal models, guinea pigs, rabbits and cynomolgus macaques. Vaccines 2013; 1:305-27; http://dx.doi.org/10.3390/vaccines1030305
- Heyndrickx L, Stewart-Jones G, Jansson M, Schuitemaker H, Bowles E, Buonaguro L, Grevstad B, Vinner L, Vereecken K, Parker J, et al. Selected HIV-1 Env trimeric formulations act as potent immunogens in a rabbit vaccination model. PLoS One 2013; 8:e74552
- WHO Recommendations for Influenza Vaccine Composition [http://www.who.int/influenza/vaccines/virus/en/
- Coudeville L, Bailleux F, Riche B, Megas F, Andre P, Ecochard R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med Res Methodol 2010; 10:18; PMID:20210985; http://dx.doi.org/10.1186/1471-2288-10-18
- Ohmit SE, Petrie JG, Cross RT, Johnson E, Monto AS. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J Infect Dis 2011; 204:1879-85; PMID:21998477; http://dx.doi.org/10.1093/infdis/jir661
- Tamura S, Tanimoto T, Kurata T. Mechanisms of broad cross-protection provided by influenza virus infection and their application to vaccines. Japan J Infect Dis 2005; 58:195-207; PMID:16116250
- Pillet S, Kobasa D, Meunier I, Gray M, Laddy D, Weiner DB, von Messling V, Kobinger GP. Cellular immune response in the presence of protective antibody levels correlates with protection against 1918 influenza in ferrets. Vaccine 2011; 29:6793-801; PMID:21211587; http://dx.doi.org/10.1016/j.vaccine.2010.12.059
- Xu K, Ling ZY, Sun L, Xu Y, Bian C, He Y, Lu W, Chen Z, Sun B. Broad humoral and cellular immunity elicited by a bivalent DNA vaccine encoding HA and NP genes from an H5N1 virus. Viral Immunol 2011; 24:45-56; PMID:21319978; http://dx.doi.org/10.1089/vim.2010.0056
- Choi YK, Goyal SM, Joo HS. Retrospective analysis of etiologic agents associated with respiratory diseases in pigs. Can Vet J 2003; 44:735-7; PMID:14524628
- Loeffen WL, Kamp EM, Stockhofe-Zurwieden N, van Nieuwstadt AP, Bongers JH, Hunneman WA, Elbers AR, Baars J, Nell T, van Zijderveld FG. Survey of infectious agents involved in acute respiratory disease in finishing pigs. Vet Record 1999; 145:123-9; PMID:10466829; http://dx.doi.org/10.1136/vr.145.5.123
- Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 2009; 459:1122-5; PMID:19516283; http://dx.doi.org/10.1038/nature08182
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009; 325:197-201; PMID:19465683; http://dx.doi.org/10.1126/science.1176225
- Crisci E, Mussa T, Fraile L, Montoya M. Review: influenza virus in pigs. Mol Immunol 2013; 55:200-11; PMID:23523121; http://dx.doi.org/10.1016/j.molimm.2013.02.008
- Holtkamp DJ, Kliebenstein JB, Neumann EJ, et al. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J Swine Health Prod. 2013; 21(2):72-84
- (EDQM) EDftQoM. Gene Transfer Medical Products for Human Use. In European Pharmacopoeia, 7.0 ed. Council of Europe: Strasbourg, France 2011:648
- Agency EM. Presence of the Antibiotic Resistance Marker Gene nptII in GM Plants and Food and Feed Uses. London, UK 2007; EMEA/CVMP/56937/2007
