Abstract
To identify distinct antibody profiles among adults 50-to-74 years old using influenza A/H1N1 HI titers up to 75 days after vaccination. Healthy subjects 50 to 74 years old received the 2010-2011 trivalent inactivated influenza vaccine. We measured venous samples from Days 0, 28, and 75 for HI and VNA and B-cell ELISPOTs. Of 106 subjects, HI titers demonstrated a ceiling effect for 11 or 10% for those with a pre-vaccination HI titer of 1:640 where no subject post-vaccination had an increase in titer. Of the remaining 95 subjects, only 37 or 35% overall had at least a 4-fold increase by Day 28. Of these 37, 3 waned at least 4-fold, and 13 others 2-fold. Thus 15% of the subjects showed waning antibody titers by Day 75. More than half failed to respond at all. The profiles populated by these subjects as defined by HI did not vary with age or gender. The VNA results mimicked the HI profiles, but the profiles for B-cell ELISPOT did not. HI titers at Days 0, 28, and 75 populate 4 biologically plausible profiles. Limitations include lack of consensus for operationally defining waning as well as for the apparent ceiling. Furthermore, though well accepted as a marker for vaccine response, assigning thresholds with HI has limitations. However, VNA closely matches HI in populating these profiles. Thus, we hold that these profiles, having face- and content-validity, may provide a basis for understanding variation in genomic and transcriptomic response to influenza vaccination in this age group.
Abbreviations:
- ASC, Antibody-Secreting Cells
- ELISPOT, Enzyme-Linked ImmunoSpot
- Et al., Et alia (and others)
- HI, Hemagglutination-Inhibition
- IgG, Immunoglobulin G
- IQR, Interquartile Range
- MDCK, Madin-Darby Canine Kidney
- μl, Microliters
- p, p-value
- PFU, Plaque-Forming Units
- RBC, Red Blood Cells
- TCID50, Tissue Culture Infectious Dose 50
- VNA, Virus Neutralization Assay
- WHO, World Health Organization
Clinicians and public health workers struggle to reduce the impact of influenza upon the elderly with the current vaccines. In a previous study, we found only 17% of elderly subjects seroconverted to all 3 antigens, and age negatively correlated with antibody titers achieved.Citation1 Studies indicate that vaccines, while less immunogenic in older adults, convey benefits, but the responses are poorer than in younger adults and the responses vary widely among individuals.Citation1-5
As investigators seek to determine the genetic contribution to the inter-individual variability in vaccine immunogenicity, we have observed diverse approaches to generate suitable targets for categorizing the immunogenicity responses. Bucasas et al. constructed a “Titer Response Index” that utilizes prevaccination titers and integrates the responses across the 3 components of the trivalent influenza vaccine.Citation6 Henn et al. combined vaccination history, prevaccination immunity (as defined as hemagglutinin antibodies measured by hemagglutination-inhibition, HI, as ≤1:10), and protective titers of antibody obtained daily on Days 1 through 10 and Day 21 (as defined as a titer >1:40 again as measured by HI).Citation7 Furman et al. reported the number of strains for which seroconversion, defined as a Day 28 4-fold increase, occurred as measures of the breadth of seroconversion, and reported geometric mean titers obtained post-vaccination as measures of the strength of seroconversion.Citation8 These fail to address waning, however.Citation5
Using the antibody titers for hemagglutination-inhibition and examining the variation post-vaccination (Days 0, 28, and 75), we sought to determine the distribution of different hemagglutinin antibody profiles in adults 50 to 74 years old. We also examined, for each profile generated, how the antibody titers (as measured by virus neutralization assay, VNA), and how B-cell activity (as measured by influenza-specific B-cell ELISPOTs) behaved at each of the 3 time points for each profile.
Results
Of the 106 subjects, 41 were male and 65 female. Median age was 59-years, 8-months old; the IQR was 55-years, 3-months to 67-years, 7-months old. All 106 identified themselves as non-Hispanic; 104 identified themselves as white, one as Asian, and one as more than one race. displays the distribution of pre-vaccination measures for HI, VNA, and B cell ELISPOT by sex and age category.
Table 1. Baseline (Day 0) Measures of Immune Response in the 106 Subjects by Sex, Age, and Race
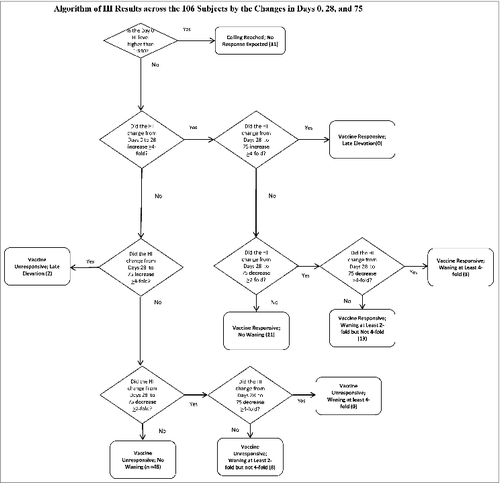
Using the currently accepted threshold of 1:40 as seropositive, at Day 0, 78 (73.6%) of our subjects were seropositive. At Day 28, 98 (92.5%) were seropositive. At Day 75, 96 (90.6%) were seropositive. We sought to determine if a ceiling titer existed for which no subject increased their titers from Day 0 to Day 28. That ceiling appeared to be 1:640. We observed this in 11 subjects. We then examined the remaining 95 for those who had an increase in HI titer from Day 0 to Day 28 of at least 4-fold. Of the 95 subjects, 37 did.
Across these 37 subjects, we examined the behavior of the Day 75 titer for changes from Day 28. None had a second 4-fold increase from Days 28 to 75. The majority—21—did not wane; 3 had at least 4-fold waning, and 13 had 2-fold waning. demonstrates how the titers of the 106 subjects populated the various, biologically plausible profiles.
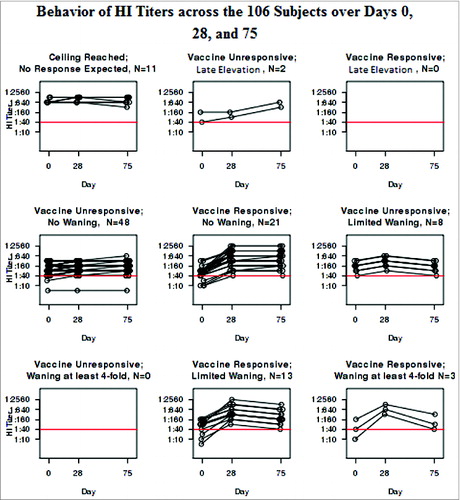
Fifty-eight subjects remained who did not respond with an increase in their HI titers from Day 0 to Day 28. Two had a 4-fold increase from Days 28 to 75, suggesting that they were inadvertently either immunized between Days 28 and 75 (despite the subjects reporting otherwise to us), or exposed to wild-type virus without recognizing the flu-like illness. Of the remaining 56, 8 had a 2-fold, but not a 4-fold, decrease. shows the distribution of the cohort with the median and IQR for age as well as the percent female. illustrates the behavior of the HI assay in each of the groups categorized in . The HI profiles vary neither with age or gender statistically (p = 0.39 and 0.62 respectively) or visually.
Table 2. Distribution of the 106 Subjects by Changes at Days 0, 28, and 75
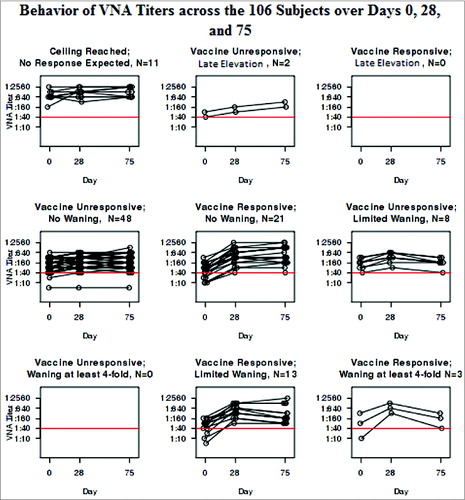
As illustrates, the VNA at Days 0, 28, and 75 mimicked the profiles of the HI results that led to the categorization. In fact, the 2 assays are highly correlated with Spearman coefficients of 0.94 each at Days 0, 28, and 75 (all P < 0.001). illustrates the behavior of the VNA within each group categorized by HI. On the other hand, the B-cell ELISPOT responses failed to correlate with the HI results at any of the time points. The Spearman coefficient between the HI and the B-cell ELISPOT was 0.08 at Day 0 (p = 0.43), 0.13 at Day 28 (p = 0.20), and 0.17 at Day 75 (p = 0.089).
Table 3. Distribution of the Hemagglutination-Inhibition Assay, Viral Neutralization Assay and B-cell ELISPOT Results
Discussion
We categorized our subjects across a number of biologically plausible profiles constructed from current understandings of seroconversion and waning. Approximately 10% had pre-vaccination titers that represent an apparent ceiling effect—titers so high that they apparently could not further respond to the vaccine. Another 35% responded to the vaccine, but approximately a third demonstrated some waning (2-fold or more) in the first 75 days following vaccination. More than half did not respond. Our review of the literature indicated no one has previously sought to create such a comprehensive classification scheme of profiles in influenza vaccine response with hemagglutination inhibition.
Labeling the profiles poses some difficulties as the mechanisms for the variation are not necessarily shared across one profile. Therefore, we refer to those subjects whose antibody titers demonstrated a 4-fold increase between Days 28 and 75 as having a “late elevation.” Investigators may wish to treat subjects in this category as “contaminated” in the sense that they may have inadvertently been exposed to either the wild influenza H1N1 virus (subway boosting) or a virus with cross-reactivity for this antibody. Perhaps some of the subjects deviated from instructions and underwent re-vaccination without reporting it. Although it is possible that some or all of them truly are only responding to the vaccination after Day 28, it is much more likely something else caused the change in antibody status.
Demonstration of HI response is requisite for licensure of influenza vaccines in the USCitation9-11 Nonetheless, assessment of neutralization of influenza infectivity through VNA and IgG secreting memory-like B-cells through B-cell ELISPOT adds to the characterization of the humoral response to influenza vaccination.Citation11-13 In our study, we compared HI to these other correlates of influenza humoral immunity and found correlation and agreement with VNA. As correlates of protection other than HI are explored for assessing immunologic response to existing and novel influenza vaccines as well as for their licensure, our results provide context of HI and VNA response in older adults receiving inactivated influenza vaccine.
Although we observed a qualitative rise in influenza-specific memory B-cell ELISPOT from baseline at Day 28 for each of the response profiles, we failed to find a correlation between HI and influenza-specific memory B-cell ELISPOT in this study. We previously reported our observed differences over time with the B-cell ELISPOT response,Citation13 but here we report these differences do not mirror those of HI. Because of our study design (Days 0, 28, and 75 blood draws), we were not able to detect significant numbers of influenza-specific antibody-secreting cells (ASC)/plasmablasts that typically peak 5–10 days following influenza vaccination. In our study we utilized an in vitro PBMC culture with polyclonal unspecific stimulation and influenza virus-specific ELISPOT assay to quantify memory-like IgG B cells that have differentiated into ASCs.Citation14 In the absence of clinical outcomes, it is not clear whether our results reflect that this assay is suboptimal for assessing protective humoral responses in adults 50 to 74 years of age or whether the lack of correlation between HI and B-cell activation measured by the assay is a potential explanation for the reduced vaccine efficacy observed in older adults despite seroprotective HI titers. The polyclonal affinity of antibodies has also been shown to play a role in age-specific antibody responses following influenza vaccination.Citation15 As more investigators include B-cell ELISPOT in their assessment of humoral response to influenza vaccine, our results can help provide context in terms of response.
Petrie et al. discusses the concept of a ceiling effect where “once antibody titers were increased in response to the vaccine, they could go no higher in response to infection,” and speculates how that might interfere with evidence of immunologic response to inactivated influenza vaccine.Citation16 Ohmit et al. reported a paradoxical finding following a placebo-controlled trial of inactivated and live attenuated vaccines that might be explained by a ceiling phenomenon.Citation17 In these subjects, 90.9% of subjects who subsequently developed H3N2 influenza disease that season actually seroconverted (4-fold increase in HI titers) following vaccination, as contrasted to only 75.1% of controls. In this study, both prevaccination and postvaccination titers of HI were protective against developing influenza.
As for limitations to our work, the literature lacks accepted operational definitions for the waning of influenza immunity, and we are the first to articulate a numerical value for a ceiling effect as evidenced by our own data for influenza vaccination. In addition, we only have the testing against influenza A/H1N1 and not A/H3N2 or B viruses. We have focused our efforts on seasonal influenza A/H1N1 vaccine for the following reasons: 1) Novel influenza A/H1N1 strains represent a potentially devastating global public health threat and are category C bioagents; 2) Influenza A/California/H1N1 virus is the H1N1 component of the 2010-2011 trivalent seasonal vaccine for the Southern and Northern Hemisphere when subjects were recruited for this study. While A/H3N2 poses more of a threat to older adults, our laboratory efforts that led to this inquiry resulted from our focus on the recent pandemic. Also, our choice of Day 75 was a compromise. While four months or the end of the season might represent better time points for waning, we wanted to avoid our research subjects’ exposure to influenza disease and have learned from previous trials that unrecognized exposure and disease could interfere with interpretation of immune measures at that later time. We also lacked the histories for the individual subjects for their previous influenza vaccinations. While we suspect all had repeatedly received influenza vaccination throughout their lives, we do not have the data to support that suspicion and such detail is not possible in adults of these ages. Furthermore, our measures of immunity used here are not based on actual immunity as demonstrated from viral challenge. HI is well accepted as a marker for influenza vaccine response and has been used to predict vaccine efficacy in healthy adults and children.Citation11,18-20 It does, however, have several limitations.Citation17 The correlation between HI titer and vaccine efficacy in adults 65 years and older is not well established, although higher titers are associated with lower risk of influenza infection from H3N2 and B strains.Citation11,21 HI is able to quantify the antibody response to the globular head of influenza hemagglutinin, but HI cannot assess the quality of the antibodies to neutralize infection. In older adults who have likely had natural exposure to numerous influenza strains of the same subtype, protective humoral responses are best directed at the initial infecting strain due to “original antigenic sin,” and would likely vary by age due to potential infection by H1N1 viruses before 1957. As such, HI may not be an ideal correlate of humoral immunity in older adults.Citation11,22 However, HI and functional VNA generally are highly correlated, even in the data presented here. Nonetheless, new data suggests that the correlation between HI thresholds and vaccine efficacy is also suboptimal in healthy adults; for example, Ohmit et al. found in a study of the 2007-2008 season that all 22 trial participants receiving inactivated vaccine (n = 259) who subsequently developed H3N2 influenza infection had HI titers above traditional thresholds for seroprotection (both ≥1:32 and ≥1:64).Citation17 Antibody titers pre- and post-vaccination of course vary as a result of a variety of factors, including previous influenza infections and vaccinations, and genetic differences, as well as past heterologous infections.Citation23-25
It is not necessarily that a persisting relationship between HI titer and vaccine efficacy fails to exist. Instead, it may be the relationship between defining a titer as seroprotective (e.g., 1:40) that is problematic. Ohmit et al.'s results demonstrate a relationship between HI titer and infection risk,Citation17 as do the classic studies of HI titer as a correlate of protection.Citation18,26 Further, in Ohmit et al., inactivated vaccine failures occurred above these thresholds because all inactivated vaccine recipients achieved titers ≥1:32 30 days post-vaccination and 97% achieved titers ≥1:64. In a study where fewer of the recipients achieved seroprotective titers, higher levels of efficacy were demonstrated for inactivated vaccine compared to placebo and live attenuated vaccine, with only 63% and 85% of respective subjects demonstrating ≥1:32 titers 30 days post-vaccination.Citation27
This illustrates a problem with using an arbitrary, absolute HI titer as an endpoint for seroconversion. While we chose to avoid using an absolute value to define seroprotectiveness or seroconversion, the ceiling effect we ascribe to titers of 1:640 may also occur at lower titers. As shown in , the majority of subjects had Day 0 HI titers ≥1:40, and only one had a titer <1:40 on Days 28 and 75. In terms of 4-fold seroconversion, which we did use, we note that those who responded to the vaccine had lower pre-vaccination titers on average than those who did not, even if one does not consider those in the ceiling group. Given this observation, and the fact that there is likely individual variation in the level of antibody ceiling, we must consider that among those who appeared not to respond by titer to the vaccine, a significant proportion may very well be immune.
We believe these profiles will have applicability as conceptual categories of outcomes of vaccine response. We would hold that these have face and content validity and that the distributions of responses advance others’ work observing responsiveness decreasing with pre-existing strain-specific immunity. Furthermore, our study describes the natural history of humoral response to influenza vaccine in adults 50 to 74 years of age through broad immunologic assessment. As the FDA requires HI for licensure of influenza vaccines, the results of this study are important considerations (especially with regard to the ceiling) for vaccine manufacturers and regulatory scientists. Also, our work provides further insight in understanding VNA and B-cell ELISPOT activity following vaccination. We intend to use these profiles in our efforts with the genomic and transcriptomic products of influenza vaccination to better understand the mechanisms underlying inter-individual variability in vaccine response.
Methods
We recruited 200 subjects in generally good health, 50-74 years of age, to participate in August and September 2010 in Rochester, MN. The Mayo Clinic Institutional Review Board approved our protocol. We excluded subjects if they already received the 2010-2011 seasonal influenza vaccine, had a severe reaction associated with past influenza vaccination, or were allergic to egg proteins or latex. We also excluded subjects with a suspected immunodeficiency, a serious chronic disorder, a bleeding diathesis that contraindicated intra-muscular injection or a history of Guillain-Barré. We also excluded those who had a receipt of any blood products within 6 months of enrollment, of systemic corticosteroids in the last 30 days, or of anticoagulant therapy. We also excluded subjects from enrollment or subsequent participation if they were diagnosed with influenza, a flu-like illness, or exhibited symptoms consistent with influenza at any time from the beginning of the influenza season in our state, as defined by the first reported cases through the length of the individual subject's participation. We have previously published details of this study's recruitment and the study participants.Citation28,29
We vaccinated subjects with an intramuscular dose of the 2010-2011 trivalent inactivated seasonal influenza vaccine Fluarix (GlaxoSmithKline), containing A/Christchurch/16/2010 NIB-74XP (H1N1) (an A/California/7/2009-like virus), A/Texas/50/2012 NYMC X-223A (H3N2) (an A/Victoria/361/2011-like virus), and B/Brisbane/60/2008 strains.Citation30 Subjects received the vaccine during their baseline visits, and underwent blood draws of 100 mL before, at 28 ± 2 days, and 75 ± 7 days after vaccination. At the conclusion of the study, 159 subjects had met all study criteria and had adequate blood draws at all 4 study visits. We set aside a third of the subjects (n = 53) for other purposes, with that third determined by the Pocock-and-Simon dynamic random allocation,Citation31 using age and gender as stratification factors, leaving 106 subjects for analysis.
Influenza Hemagglutination-Inhibition Assay
We have previously described the HI assay.Citation29 In brief, we performed the HI assays in a single run for each subject on samples from Days 0, 28, and 75 simultaneously using a standard WHO protocol. We measured HI titers against the influenza vaccine strain A/California/7/2009/H1N1 using a 0.5% solution of turkey red blood cells (RBC).Citation32-35 We propagated the virus in the allantoic cavity of embryonated chicken eggs. We pretreated subjects’ sera with Vibrio cholerae receptor-destroying enzyme (1:4 dilution; Accurate Chemical and Scientific, Westbury, NY) to inactivate non-specific inhibitors of hemagglutination.Citation32 We permitted serial dilutions of treated serum samples to react with virus at a fixed dose of 8 hemagglutinin units per 50 μl, followed by the addition of 0.5% turkey RBC (Lampire Biological Laboratories, Pipersville, PA). We tested all samples in triplicate. We read HI titers after a 45-minute incubation time. We reported the HI titer as the reciprocal of the highest dilution in which complete inhibition occurred. The average coefficient of variation (CV) for this assay was 2.9%.
Influenza Virus Neutralization Assay
We assessed virus-specific neutralizing antibody (VNA) titers using a standard protocol, as described previously in a single run for each subject on samples from Days 0, 28, and 75.Citation36 We conducted influenza VNA with the influenza A virus strain A/California/07/2009/H1N1.Citation37-39 Briefly, we mixed 2-fold dilutions of receptor-destroying enzyme-treated serum samples with 200 PFU of the A/H1N1 virus. We then incubated these samples at room temperature for 60 minutes to allow any HA-specific antibodies existing in the serum to neutralize the virus. We added serial dilutions of serum samples and virus onto MDCK cell (ATCC®) monolayers cultured in a 96-well flat-bottom microwell plate and incubated the plate to determine titers required to prevent virus growth. We defined the neutralization titer as the reciprocal of the highest dilution of serum that neutralizes 200 PFU of virus. We defined seroconversion to the virus vaccine as a 4-fold increase in antibody titers between the pre-vaccination Day 0 and the post-vaccination Day 28 serum samples.Citation10,38,40 The average CV for this assay was 4.7%.
B-cell ELISPOT Assay
We have previously published these methods.Citation13,29 In brief, we quantified influenza virus-specific IgG-secreting memory B-cells in peripheral blood mononuclear cells (PBMCs) in a single run for each subject on samples from Days 0, 28, and 75 using Human IgG ELISpotPLUS kit (Mabtech, Inc.., Mariemont, OH). We performed ELISPOT assays following the manufacturer's protocol after coating plates with influenza A/California/7/2009/H1N1 virus (50,000 TCID50 per well) and analyzed plates on an ImmunoSpot® S4 Pro Analyzer (Cellular Technology Ltd., Cleveland, OH) using ImmunoSpot® version 4.0 software (Cellular Technology Ltd.). We reported the results in spot-forming counts (SFC) per 2 × 105 PBMCs as the median of influenza-specific values from 4 replicates, minus the single unstimulated value. The 95% confidence interval for intra-class correlation coefficients for the baseline samples stimulated with influenza virus was 0.86 to 0.91.
Analytic Plan
We considered biologically plausible categories (See ) based on published definitions of vaccine response including definitions of seroconversion (4-fold increase), the accepted timing of seroconversion (Day 28), waning (2-fold and 4-fold) changes (Day 75), as well as for late (apparently non-vaccine-related) elevations (Day 75). We sought evidence for a ceiling effect by examining Day 0 titers associated with subjects’ having no increase in titers post-vaccination.
We did conduct our measures at Day 3, as well as Days 0, 28, and 75, but when we compared HI for Days 0 and 3, we found no differences (p = 0.57, Wilcoxon signed rank test). Thus, we excluded Day 3 results from our analysis, and we examined for each of the 106 subjects their trajectories by HI for Days 0, 28, and 75. We then compared distributions statistically and, acknowledging small sample sizes leading to low power for these comparisons, visually of age [median, interquartile range (IQR), Kruskal Wallis test] and gender (percent, Pearson's chi-square test) between profiles. We compared distributions of VNA and B-cell ELISPOT responses among various biologically plausible profiles, in terms of values (median and IQR) for Day 0, 28, and 75, as well as persistent membership by profile (with VNA, attempting to replicate the behavior of HI) via similar non-parametric methods.
Disclosure of Potential Conflicts of Interest
Dr. Poland is the chair of a safety evaluation committee for novel investigational vaccine trials being conducted by Merck Research Laboratories. Dr. Poland offers consultative advice on vaccine development to Merck & Co. Inc.., CSL Biotherapies, Avianax, Sanofi Pasteur, Dynavax, Novartis Vaccines and Therapeutics, PAXVAX Inc., Emergent Biosolutions, Vaxness, and Adjuvance. Dr. Jacobson serves as a member on a safety review committee and on a data monitoring committee concerning several non-influenza vaccines in studies funded by Merck Research Laboratories. These activities have been reviewed by the Mayo Clinic Conflict of Interest Review Board and are conducted in compliance with Mayo Clinic Conflict of Interest policies. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies.
Acknowledgments
We would like to acknowledge the work of Krista Goergen who served as our statistical programmer analyst in this effort. We would also like to thank our patients who volunteered to participate in our study.
Funding
This study was funded by the National Institute of Allergic and Infectious Diseases of the National Institutes of Health (Bioinformatics Approach to Influenza A/H1N1 Vaccine Immune Profiling, HIPC, U01 AI 089859). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol 2001; 75:12182-7; PMID:11711609; http://dx.doi.org/10.1128/JVI.75.24.12182-12187.2001
- Bernstein E, Kaye D, Abrutyn E, Gross P, Dorfman M, Murasko DM. Immune response to influenza vaccination in a large healthy elderly population. Vaccine 1999; 17:82-94; PMID:10078611; http://dx.doi.org/10.1016/S0264-410X(98)00117-0
- Nicholls S, Carroll K, Crofts J, Ben-Eliezer E, Paul J, Zambon M, Joseph CA, Verlander NQ, Goddard NL, Watson JM. Outbreak of influenza A (H3N2) in a highly-vaccinated religious community: a retrospective cohort study. Commun Dis Public Health 2004; 7:272-7; PMID:15779788
- Engler RJ, Nelson MR, Klote MM, VanRaden MJ, Huang CY, Cox NJ, Klimov A, Keitel WA, Nichol KL, Carr WW, et al. Half- vs full-dose trivalent inactivated influenza vaccine (2004-2005): age, dose, and sex effects on immune responses. Arch Intern Med 2008; 168:2405-14; PMID:19064822; http://dx.doi.org/10.1001/archinternmed.2008.513
- Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis 2008; 197:490-502; PMID:18275271; http://dx.doi.org/10.1086/524146
- Bucasas KL, Franco LM, Shaw CA, Bray MS, Wells JM, Nino D, Arden N, Quarles JM, Couch RB, Belmont JW. Early patterns of gene expression correlate with the humoral immune response to influenza vaccination in humans. J Infect Dis 2011; 203:921-9; PMID:21357945; http://dx.doi.org/10.1093/infdis/jiq156
- Henn AD, Wu S, Qiu X, Ruda M, Stover M, Yang H, Liu Z, Welle SL, Holden-Wiltse J, Wu H, et al. High-resolution temporal response patterns to influenza vaccine reveal a distinct human plasma cell gene signature. Sci Rep 2013; 3:2327; PMID:23900141; http://dx.doi.org/10.1038/srep02327
- Furman D, Jojic V, Kidd B, Shen-Orr S, Price J, Jarrell J, Tse T, Huang H, Lund P, Maecker HT, et al. Apoptosis and other immune biomarkers predict influenza vaccine responsiveness (vol 9, pg 659, 2013). Mol Syst Biol 2013; 9; 659; PMID:23591775; http://dx.doi.org/10.1038/msb.2013.15
- Center for Biologics Evaluation and Research, Food and Drug Administration, U.S. Department of Health and Human Services. Guidance for Industry: Clinical Data Needed to Support the Licensure of Pandemic Influenza Vaccines. In: Food and Drug Administration, ed. Guidelines for Industry. Rockville, MD: Food and Drug Administration; 2007: PDF.
- Center for Biologics Evaluation and Research, Food and Drug Administration, U.S. Department of Health and Human Services. Guidance for Industry: Clinical Data Needed to Support the Licensure of Seasonal Inactivated Influenza Vaccines. In: Food and Drug Administration, ed. Guidelines for Industry. Rockville, MD: Food and Drug Administration; 2007: PDF.
- Reber A, Katz J. Immunological assessment of influenza vaccines and immune correlates of protection. Expert Rev Vaccines 2013; 12:519-36; PMID:23659300; http://dx.doi.org/10.1586/erv.13.35
- Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu XH, Lim W, Fukuda K, Cox NJ, Katz JM. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol 1999; 37:937-43; PMID:10074505
- Painter SD, Haralambieva IH, Ovsyannikova IG, Grill DE, Poland GA. Detection of influenza A/H1N1-specific human IgG-secreting B cells in older adults by ELISPOT assay. Viral Immunol 2014; 27:32-8; PMID:24605786; http://dx.doi.org/10.1089/vim.2013.0099
- Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods 2004; 286:111-22; PMID:15087226; http://dx.doi.org/10.1016/j.jim.2003.12.015
- Khurana S, Frasca D, Blomberg B, Golding H. AID Activity in B Cells Strongly Correlates with Polyclonal Antibody Affinity Maturation in-vivo Following Pandemic 2009-H1N1 Vaccination in Humans. Plos Pathog 2012; 8; e1002920; PMID:23028320; http://dx.doi.org/10.1371/journal.ppat.1002920
- Petrie JG, Ohmit SE, Johnson E, Cross RT, Monto AS. Efficacy Studies of Influenza Vaccines: Effect of End Points Used and Characteristics of Vaccine Failures. J Infect Dis 2011; 203:1309-15; PMID:21378375; http://dx.doi.org/10.1093/infdis/jir015
- Ohmit SE, Petrie JG, Cross RT, Johnson E, Monto AS. Influenza Hemagglutination-Inhibition Antibody Titer as a Correlate of Vaccine-Induced Protection. J Infect Dis 2011; 204:1879-85; PMID:21998477; http://dx.doi.org/10.1093/infdis/jir661
- Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972; 70:767-77; PMID:4509641; http://dx.doi.org/10.1017/S0022172400022610
- Ndifon W, Dushoff J, Levin SA. On the use of hemagglutination-inhibition for influenza surveillance: Surveillance data are predictive of influenza vaccine effectiveness. Vaccine 2009; 27:2447-52; PMID:19368786; http://dx.doi.org/10.1016/j.vaccine.2009.02.047
- Ng S, Fang VJ, Ip DKM, Chan KH, Leung GM, Peiris JSM, Cowling BJ. Estimation of the association between antibody titers and protection against confirmed influenza virus infection in children. J Infect Dis 2013; 208:1320-4; PMID:23908481; http://dx.doi.org/10.1093/infdis/jit372
- Gorse GJ, O'Connor TZ, Newman FK, Mandava MD, Mendelman PM, Wittes J, Peduzzi PN. Immunity to influenza in older adults with chronic obstructive pulmonary disease. J Infect Dis 2004; 190:11-9; PMID:15195238; http://dx.doi.org/10.1086/421121
- Webster RG, Kasel JA, Couch RB, Laver WG. Influenza-Virus Subunit Vaccines .2. Immunogenicity and Original Antigenic Sin in Humans. J Infect Dis 1976; 134:48-58; PMID:59787; http://dx.doi.org/10.1093/infdis/134.1.48
- Selin LK, Lin MY, Kraemer KA, Pardoll DM, Schneck JP, Varga SM, Santolucito PA, Pinto AK, Welsh RM. Attrition of T cell memory: Selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity 1999; 11:733-42; PMID:10626895; http://dx.doi.org/10.1016/S1074-7613(00)80147-8
- Welsh RM, Selin LK. No one is naive: The significance of heterologous T-cell immunity. Nat Rev Immunol 2002; 2:417-26; PMID:12093008
- Chen HD, Fraire AE, Joris I, Welsh RM, Selin LK. Specific history of heterologous virus infections determines anti-viral immunity and immunopathology in the lung. Am J Pathol 2003; 163:1341-55; PMID:14507643; http://dx.doi.org/10.1016/S0002-9440(10)63493-1
- Salk JE, Pearson HE, et al. Immunization against influenza with observations during an epidemic of influenza A one year after vaccination. Am J Hyg 1945; 42:307-22; PMID:21005133
- Monto AS, Ohmit SE, Petrie JG, Johnson E, Truscon R, Teich E, Rotthoff J, Boulton M, Victor JC. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med 2009; 361:1260-7; PMID:19776407; http://dx.doi.org/10.1056/NEJMoa0808652
- Ovsyannikova IG, White SJ, Albrecht RA, Garcia-Sastre A, Poland GA. Turkey versus guinea pig red blood cells: hemagglutination differences alter hemagglutination inhibition responses against influenza A/H1N1. Viral Immunology 2014; 27:174-8; PMID:24787023; http://dx.doi.org/10.1089/vim.2013.0111
- Ovsyannikova IG, White SJ, Larrabee BR, Grill DE, Jacobson RM, Poland GA. Leptin and leptin-related gene polymorphisms, obesity, and influenza A/H1N1 vaccine-induced immune responses in older individuals. Vaccine 2014; 32:881-7; PMID:24360890; http://dx.doi.org/10.1016/j.vaccine.2013.12.009
- U.S. Food and Drug Administration. Influenza Virus Vaccine for the 2010-2011 Season. Silver Spring, MD 2014.
- Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975; 31:103-15; PMID:1100130; http://dx.doi.org/10.2307/2529712
- Burnet FM, Stone JD. The receptor-destroying enzyme of V. cholera. Austr J Exper Med Sci 1947; 25:227-33; PMID:20270643; http://dx.doi.org/10.1038/icb.1947.33
- Robinson RQ, Dowdle WR. Influenza viruses. In: Lennette EH, Schmidt NJ, eds. Diagnostic Procedures for Viral and Rickettsial Diseases. New York: American Public Health Association; 1969:414-33.
- Wang S, Taaffe J, Parker C, Solorzano A, Cao H, Garcia-Sastre A, Lu S. Hemagglutinin (HA) proteins from H1 and H3 serotypes of influenza A viruses require different antigen designs for the induction of optimal protective antibody responses as studied by codon-optimized HA DNA vaccines. J Virol 2006; 80:11628-37; PMID:16987975; http://dx.doi.org/10.1128/JVI.01065-06
- WHO Global Influenza Surveillance Network, World Health Organization. Manual for the laboratory diagnosis and virological surveillance of influenza. Geneva: World Health Organization; 2011.
- Cottey R, Rowe CA, Bender BS. Unit 19:11 Influenza Virus. Curr Protoc Immunol 2001; 42(19.11):19.11.1-.32. PMID:18432752
- Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flano E, Mejias A, Albrecht RA, Blankenship D, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med 2013; 5:176ra32; PMID:23486778; http://dx.doi.org/10.1126/scitranslmed.3005191
- Brady RC, Treanor JJ, Atmar RL, Keitel WA, Edelman R, Chen WH, Winokur P, Belshe R, Graham IL, Noah DL, et al. Safety and immunogenicity of a subvirion inactivated influenza A/H5N1 vaccine with or without aluminum hydroxide among healthy elderly adults. Vaccine 2009; 27:5091-5; PMID:19577636; http://dx.doi.org/10.1016/j.vaccine.2009.06.057
- Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011; 470:543-U136; PMID:21350488; http://dx.doi.org/10.1038/nature09737
- Keitel WA, Campbell JD, Treanor JJ, Walter EB, Patel SM, He F, Noah DL, Hill H. Safety and immunogenicity of an inactivated influenza A/H5N1 vaccine given with or without aluminum hydroxide to healthy adults: results of a phase I-II randomized clinical trial. J Infect Dis 2008; 198:1309-16; PMID:18808338; http://dx.doi.org/10.1086/592172