Abstract
BACKGROUND: Influenza vaccine production capacity is still insufficient to meet global demand in case of a pandemic. To expand worldwide influenza vaccine production capacity, a solid and transferable egg-based influenza vaccine production process was established that is suitable for upscaling and technology transfer to vaccine manufacturers in low- and middle-income countries. As a proof-of-concept, the safety and immunogenicity of a pandemic whole inactivated virus (WIV) vaccine (H5N1) and a monovalent seasonal WIV vaccine (H3N2) were evaluated in a phase I clinical trial in adults. METHODS: Subjects were vaccinated with 2 doses of pandemic WIV vaccine (pWIV), or one dose of either seasonal WIV vaccine (sWIV) or a commercially available trivalent comparator vaccine followed by a placebo dose. Haemagglutination inhibiting antibody titres against the influenza strains were determined before and 21 d after each vaccination. RESULTS: The frequency and severity of adverse reactions were comparable between groups. No serious adverse events were reported. After a single dose of sWIV the seroconversion rate was 91% (Committee for Proprietary Medicinal Products (CPMP) criterion >40%), the seroprotection rate was 100% (CPMP criterion >70%), and the mean geometric mean titre (GMT) increase was 24.9 (CPMP criterion >2.5). After two doses of pWIV, seroconversion rate and seroprotection rate were both 71%, and the mean GMT increase was 7.8. CONCLUSIONS: Both pWIV and sWIV were equally well-tolerated as the comparator vaccine, and both vaccines complied with all 3 CPMP criteria. EudraCT 2011-000159-17. Netherlands National Trial Register 2695.
Introduction
Influenza seasonal epidemics continue to be a major cause of high morbidity and mortality worldwide.Citation1-3 Currently, vaccination against influenza remains the most effective measure for reducing the impact of influenza virus infection.Citation2-5 In addition, the 2009 influenza H1N1 pandemic has shown that with modern levels of global travel as well as urbanization, and overcrowded conditions, a new influenza virus is able to quickly take hold around the world.Citation6
The threat of an imminent influenza pandemic has been felt closely, since in 1997 a highly pathogenic avian influenza A (H5N1) virus became infectious in humans. It triggered World Health Organization (WHO) to evaluate pandemic preparedness, eventually resulting in the Global Pandemic Influenza Action Plan (GAP) initiated by WHO in 2006.Citation7 At that time, estimated worldwide vaccine production capacity for influenza vaccines was 350 million doses per year.Citation8 Currently, influenza vaccine production capacity is still insufficient to meet global demand in case of a pandemic. Major influenza vaccine producers operate and supply largely in Europe and North America. During a pandemic, unavailability of a vaccine would put the majority of the population in low- and middle-income countries, mostly in Africa, Middle-East and Asia, seriously at risk of a high death toll and morbidity burden. WHO currently estimates worldwide production capacity for pandemic vaccines at 3 billion doses per year,Citation9 which will be quite inadequate to cover a world population of 6.8 billion people in which virtually everyone is susceptible to infection by a new and readily contagious virus. The main objective of GAP is to increase the supply of pandemic influenza vaccine, and thereby reduce the current gap between demand and supply that is anticipated during an influenza pandemic.Citation5,10 One of the approaches is to expand worldwide influenza vaccine production capacity by transfer of influenza vaccine production technology to low- and middle-income countries. Netherlands Vaccine Institute (NVI), now Intravacc, has been selected by WHO in 2007 to establish an expertise center (“hub”) for technology transfer of all aspects of production and quality control of influenza vaccines within an International Technology Platform for Influenza Vaccines (ITPIV).Citation11
Primary objective of the ITPIV project is to increase availability and affordability of (pandemic) influenza vaccines for low- and middle-income countries by transferring technology that is needed to establish their own production facilities. As it is not economically feasible for manufacturers to focus only on production of vaccines that are needed in the occasional event of an influenza pandemic, the technology to be transferred should also be applicable for production of seasonal influenza vaccines. A solid and transferable egg-based influenza vaccine production process was established, that is suitable for upscaling, and technology transfer to vaccine manufacturers in low- and middle-income countries meeting WHO defined quality/viability criteria, and in compliance with WHO guidelines, as outlined in the recommendations for the production and control of influenza vaccine,Citation12 and the standards published in the European Pharmacopoeia (5th Edition, 01/2005). The use of the egg-based production method was chosen at the request of WHO, for which the reasons as well as the possible limitations and logistic aspects have been described and discussed elsewhere.Citation11
Although most vaccine manufacturers nowadays produce split virus or subunit influenza vaccines, in this study a whole inactivated virus (WIV) vaccine was developed instead. This decision was guided by the main purpose of the ITPIV project to set up an affordable and robust production process for a potent influenza vaccine, being less complicated to produce and without the need for addition of adjuvants. A production process without splitting, further purification and adjuvation procedures has a number of beneficial consequences including a decrease in number of production steps and corresponding analytical tests required and thus a shorter production time and lower production costs. In our laboratory, product consistency did not differ between split and WIV vaccines when produced at pilot scale. Besides the benefits of a simpler and shorter production process, evidence exists that immunogenicity induced by WIV vaccines is superior to split virus or subunit vaccines, especially in the naive human population,Citation13-15 likely due to the adjuvating nature of whole virus particlesCitation16,17 and viral RNA.Citation16,Citation18-20 In addition, virus inactivation was performed with β-propiolactone (BPL) instead of formalin to preserve the fusion activity of the virus which is associated with immunogenicity in the mouse model.Citation21 Using the production process developed in the technology transfer project, 2 WIV vaccines were produced, one with a prototype pandemic influenza strain and one with a seasonal influenza strain. In the phase I study presented here the safety and immunogenicity of these WIV vaccines were investigated to establish proof-of-concept and to explore whether the criteria for acceptance of influenza vaccines according to the European Agency for the Evaluation of Medicinal Products were met.
Results
Subject disposition
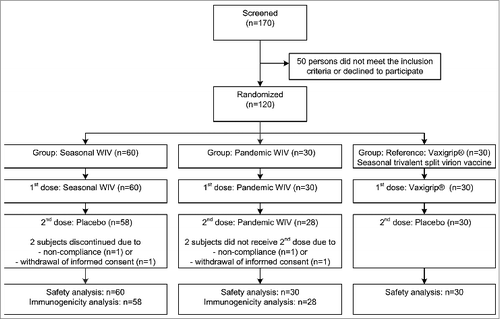
In total, 170 subjects were screened, of which 120 subjects fulfilled all inclusion criteria and were randomized either to pWIV (30 subjects), sWIV (60 subjects) or seasonal split comparator vaccine (30 subjects) (). All eligible subjects received the first vaccination and were included in the safety evaluation. Because of 2 drop-outs in each WIV group, immunogenicity evaluation was performed with data from 58 subjects in the sWIV group, and 28 subjects in the pWIV group (). No subjects discontinued the study due to an adverse event. No major differences in age, gender or Body Mass Index (BMI) were observed between the different treatment groups ().
Table 1. Summary of demographic data
Safety
Solicited local and systemic adverse events during the first 5 d following each vaccination are shown in . In the pWIV group, occurrence of at least one solicited local reaction was reported by 24 subjects (80%) after the first, and by 13 subjects (46%) after the second vaccination. In the sWIV group and the comparator vaccine group, at least one local reaction occurred in 47 subjects (78%) and 21 subjects (70%), respectively. After placebo injection the occurrence of at least one local reaction was significantly lower: 4 subjects (13%) in the comparator group and 10 subjects (17%) in the sWIV group respectively (). In all groups the most frequently reported local reaction was pain at injection site, followed by reduced movement of the injected arm. Almost all local adverse events were of mild or moderate intensity. Of five subjects reporting induration, one also reported swelling of severe intensity (≥5 cm) after sWIV vaccine administration, which had completely resolved on day 2. The majority of local reactions in all groups resolved within 3 d. Injection site swelling and erythema were less often reported in sWIV and pWIV groups than in the comparator vaccine group (p-values <0.05).
Table 2. Solicited and non-solicited adverse events up to 5 d after vaccination
At least one solicited systemic reaction after the first vaccination occurred in 19 subjects (63%) in the pWIV group, in 48 subjects (80%) in the sWIV group, and in 20 subjects (67%) in the comparator group. After the second injection, 9 subjects (32%) who received pWIV reported at least one solicited systemic reaction, compared with 6 subjects (20%) in the comparator group and 23 subjects (40%) in the sWIV group who both received placebo at day 21 (). Myalgia was the most frequently reported systemic adverse event after pWIV and sWIV administration. Headache, fatigue and malaise were also frequently reported in most groups. Of 11 subjects reporting headache in the sWIV group, there was one severe case 2 d after the first dose (vaccine), and one severe case one day after the second dose (placebo). Both cases had resolved within one day. All other reported systemic adverse events were of mild, or moderate intensity. Axillary temperature was measured 4–6 h after vaccination, and thenceforth daily until day 5. No cases of fever (temperature of ≥37 .5°C) were reported in any of the groups. Fatigue and myalgia were less often reported after the second pWIV dose compared to the comparator vaccine (p-values <0.05).
In both WIV vaccine groups as well as the comparator vaccine group, the numbers of subjects with any solicited local or systemic event were significantly higher than after a placebo dose. The largest differences were observed in the incidence of injection site pain, decreased mobility of the injected limb and myalgia as shown in . The reported differences in incidence fall within the expected variability for this type of vaccine and the sample size used in this study. No differences between groups were observed for unsolicited adverse events. No serious adverse events were reported during the study period.
Immunogenicity
As reported in , haemagglutination inhibition (HI) antibodies to the H5N1 strain were undetectable before vaccination in all subjects except one, confirming that the study population was immunologically naive for the H5N1 virus. One subject had an HI titre against H5N1 of 30, which is below the cut-off indicative for protection (≥40). In contrast, 65% of subjects had detectable HI antibody titres against the H3N2 strain, and 52% had levels of HI titres ≥40, indicating previous exposure to H3N2 influenza viruses.
Table 3. Immunogenicity of sWIV and pWIV
In the sWIV (H3N2) group (n=60), 65% of subjects had detectable HI titres (≥10) before vaccination, and 57% had protective HI titres (≥40) for H3N2 (). Administration of the seasonal vaccine effectively boosted the immune response against the H3N2 strain: the seroconversion rate was 91%, and the seroprotection rate after the first dose increased to 100%. The GMT for H3N2 before vaccination was 32.2, and increased to 788.8 after 21 d. The GMT increase was 24.9 at 21 d after vaccination ().
In the pWIV (H5N1) group, the proportion of subjects with a measurable antibody titre (≥10) increased to 86% (n=24) after the first dose, and to 100% after the second dose. The seroconversion rate was equal to the seroprotection rate and was 50% (n=14) after one dose, and 71% (n=20) after 2 doses. The GMT increased from 5.3 at pre-vaccination to 33.7 at 21 d after the first dose, and further increased to 48.5 at 21 d after the second dose, with a mean GMT increase of the pre-vaccination HI titre of 6.3 and 9.1 respectively ().
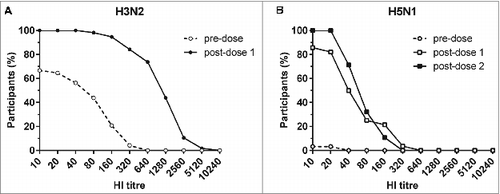
shows the reverse cumulative distribution (RCD) curves of HI titres before and after vaccination for the sWIV group (one dose, ) and for the pWIV group (2 doses, ). The RCD curves clearly illustrate that all HI titres were higher after one dose in both WIV vaccine groups. It also shows that the second dose of pWIV elicited a further increase of HI titres and notably enhanced the proportion of subjects with an HI titre ≥40.
Figure 2. Reverse cumulative distribution curves of HI titres against H3N2 before and after one dose of sWIV (A) and against H5N1 before and after one or 2 doses of pWIV (B). The curves were obtained by calculating the percentage of subjects (Y-axis) with an HI titre equal to or greater than the HI titre shown in a logarithmic scale along the X-axis.
Both the pWIV (2 doses) and the sWIV (one dose) met all criteria on seroprotection, seroconversion and GMT increase as defined for licensing of influenza vaccines in European Guideline CPMP/BWP/214/96.
Discussion
We showed that influenza WIV vaccines in this study were safe in adults. The observed frequencies of adverse events in the WIV vaccine groups were similar to or lower than those of the comparator vaccine group. It should be noted that sample sizes for phase I studies are not suitable to reliably determine these frequencies. Reported local reactions were mostly mild and of short duration, as normally seen after influenza vaccination, and were mainly caused by reactogenicity of the vaccine and partly by the injection procedure. Systemic adverse events were frequently reported but also of short duration and in majority mild or moderate. Two cases of severe headache, defined as preventing daily activity, were observed, one after sWIV and one after a placebo injection. Headache is a commonly reported adverse event after influenza vaccination in general (1–10%). A causal relation to the vaccination is possible, but may also be coincidental as is demonstrated by the case in the placebo group.
A limitation of this study is that the total dose of HA per vaccine in the monovalent WIV vaccines was lower than that in the comparator trivalent split vaccine: 15 vs 45 μg HA. However, in previous studies with monovalent pandemic influenza vaccines (either WIV, split or subunit vaccine) the total HA dose appeared to have no effect on tolerability in most studiesCitation13,Citation22-25 and in 2 studies was correlated only with the incidence of local pain.Citation26,27 Another limitation was that H3N2 strain A/Uruguay/716/2007 had been replaced by H3N2 A/Victoria/210/2009 in the licensed vaccine for 2010–2011 that was used as comparator vaccine. Immunogenicity is known to be influenza sub-type specific whereas differences in virus strain-specific antigens included in the vaccine is believed not to be of influence on reactogenicity, which is caused by vaccine type and formulation. Therefore immunogenicity of the sWIV could not be compared with that of the licensed vaccine. No H5N1 pandemic vaccine was available in the European Union (EU).
Nevertheless, in this study, the proof-of-principle of the investigated influenza WIV vaccines was established. The results showed that both the monovalent seasonal (H3N2) and the pandemic (H5N1) WIV vaccine were immunogenic in adults. The sWIV vaccine induced sufficient antibodies to meet all 3 (only one required) European immunogenicity criteria (). The pWIV vaccine met all 3 criteria after 2 vaccinations as is required for pandemic influenza vaccines, although the sample size of the pWIV group was not intended for formal assessment of the immunogenicity criteria. Since formalin-treatment of influenza virus was shown to severely reduce fusion activity of the virus, BPL was selected instead for inactivation of the WIV vaccines.Citation21 The concept of preservation of fusion activity upon BPL inactivation was demonstrated in our laboratory using a hemolysis assay.Citation28 This analysis was performed on the influenza A/PR8/34 H1N1 strain but not repeated with the strains used for production of the WIV vaccines in this study. All the same, after one dose of the non-adjuvanted BPL-inactivated pWIV vaccine already 50% of the subjects were seroprotected, which was comparableCitation29 or betterCitation30-32 than in studies with formalin-inactivated H5N1 WIV vaccine adjuvanted with aluminum hydroxide. The immunogenicity after one and 2 doses of pWIV was also comparableCitation33 or betterCitation34 than is published for H5N1 subunit vaccines with low HA dose but adjuvanted with MF59.
Immunogenicity of the vaccines was assessed by measuring HI titres because the HI antibody titre is the only correlate of protection against influenza that is accepted worldwide for licensing of influenza vaccines. It has been recognized that this correlate is less useful for predicting protection in for example elderly and young children or upon intranasal vaccination. Therefore, during further clinical development, immunogenicity assessment of WIV vaccines should also include assays for other responses that might relate to protection, such as cell mediated immune responses.Citation35,36
Conclusion
This phase I study has shown that the production process for BPL-inactivated WIV vaccines chosen for technology transfer is suitable for production of safe and immunogenic seasonal and pandemic influenza vaccines. Without the use of an adjuvant, the immunogenicity criteria for seasonal vaccines were met convincingly. Moreover, for the pWIV vaccine all criteria were met after 2 doses and 2 out of 3 criteria were met after one dose. This is a promising result especially for the H5N1 strain and warrants further development of WIV vaccines. Importantly, the technology can easily be transferred to other parties, including those in middle- and low-income countries, to achieve increased influenza vaccine production capacity worldwide.
Materials and Methods
Study design
A phase I, double-blind, parallel, randomized, controlled trial (n=120) was conducted at the facility of Quest Pharmaceutical Services (QPS) Netherlands, Groningen, the Netherlands between June 14th and October 11th 2011 to avoid interference of influenza infection during the study period.
The safety of seasonal and pandemic monovalent influenza WIV vaccine was compared with a commercially available trivalent seasonal split influenza vaccine. For the pWIV group and the comparator group, the sample size was 30. The sWIV group consisted of 60 subjects to enable exploratory immunogenicity evaluation according to requirements for influenza vaccines (European Guideline CPMP/BWP/214/96). Group sizes were in accordance with WHO Technical Report Series No. 924, 2004: Guidelines on clinical evaluation of vaccines: regulatory expectations, annex 1Citation37.
Vaccines
Two strains were used for the formulation of influenza vaccines for the clinical trial: a seasonal strain (NYMC-X175C reassortant derived from influenza A/Uruguay/716/2007 (H3N2)) and a prototype pandemic strain (NIBRG23 reassortant derived from influenza A/turkey/Turkey/1/2005 (H5N1)). Seed viruses were obtained from the National Institute for Biological Standards and Control. The vaccines were produced as described previously.Citation11 Briefly, virus was cultured in embryonated eggs. The virus-containing fluid was harvested and clarified by a combination of centrifugation and filtration. The virus was first purified and concentrated by sucrose gradient ultracentrifugation, and then filtrated and inactivated with BPL. The product was sterilized using 0.22 μm filtration and formulated in a dosis of 15 μg HA to obtain monovalent WIV vaccine. The placebo used in the clinical trial consisted of the vaccine formulation buffer. Each vaccine dose of 0.5 ml was administered intramuscularly. The comparator vaccine was Vaxigrip® 2010/2011, (Sanofi Pasteur MSD) containing the following strains: A/California/7/2009 (H1N1) – like strain, A/Perth/16/2009 (H3N2) – like strain and B/Brisbane/60/2008.
Participants
Subjects were recruited from the QPS volunteer database and website. People were directed to the website through advertisement materials approved by the Medical Ethics Committee. Subjects were eligible if they were between 18 and 49 y of age, were in good health as determined by the outcome of medical history, physical examination screening/baseline labs and clinical judgment of the investigator. Specifically, subjects should have had no known or suspected disease that affects the immune system, use medication that may influence the immune system, have a history of any neurological disorder including epilepsy or febrile seizures or any known or suspected allergy to any of the vaccine components. Female subjects should not be pregnant and willing to use contraceptives for the duration of the study. Subjects should not have been vaccinated with an influenza vaccine in the previous 3 winter seasons (2008–2009, 2009–2010, 2010–2011).
The study was conducted in compliance with good clinical practice guidelines and the provisions of the Declaration of Helsinki. The protocol was approved by the Dutch Central Committee on Research involving Human Subjects and by the WHO Research Ethics Review Committee. Written informed consent was obtained from all participants. The trial was registered in EU Clinical Trials Register with EudraCT 2011-000159–17 and in the Netherlands National Trial Register under number 2695. The study was included in the WHO table on clinical evaluation of influenza vaccines at http://www.who.int/immunization/diseases/influenza/clinical_evaluation_tables/en/.
Randomization and blinding
Subjects were randomly assigned to one of the 3 groups taking into account the different group sizes based on a computer-generated randomization schedule prepared before the study. Vaccines and placebo were packaged in identical syringes and labeled with the randomization code by the pharmacist at the site. Subjects, investigator and sponsor were blinded to treatment allocation until database lock.
Procedures
Subjects in the pWIV group received a vaccination on day 0 and 21, whereas the sWIV and comparator vaccine groups received the vaccine only on day 0 and a placebo on day 21 in order to keep subjects as well as the investigator blinded to treatment. All subsequent procedures including blood draws were identical to maintain the blind throughout the entire study period. Subjects were vaccinated intramuscularly into the deltoid muscle of the upper arm. During screening and on day 42 blood samples were taken for biochemistry and haematology analysis. Physical exam including vital signs was performed at each visit. Blood samples were collected for immunogenicity analysis on day 0 (pre-vaccination), before the second administration (day 21) and on day 42. Subjects were kept under observation for 30 minutes after each vaccination. Adverse events following immunisation and concomitant medication, if any, were recorded in a diary that the subjects completed at home daily during the 5 d following vaccination and by the investigator at each visit. Subjects were requested to inform the investigator of any serious adverse event occurring until 6 months after vaccination.
Laboratory tests
All sera were tested by Viroclinics, Rotterdam, the Netherlands, for anti-haemagglutinin antibodies against the WIV vaccine strains using HI assays following standard procedures.Citation38 Briefly, serum samples were treated with cholera filtrate and heat-inactivated at 56°C for 1 h. Duplicate 2-fold serial dilutions of pretreated serum samples were incubated with 4 haemagglutination units of an influenza virus or phosphate-buffered saline for 30 min at 37°C, and subsequently, 1% turkey erythrocytes were added. Haemagglutination patterns were read after incubation for 1 h at 4°C. The highest dilution of serum that still gave complete inhibition of haemagglutination was recorded as the titre, and when duplicate results were different, mean titres were calculated. Pre- and post-vaccination sera were titrated simultaneously.
For the purposes of calculation, any HI titre <10(=undetectable at a serum dilution 1:10) was expressed as 5.
Statistical analysis
All data were analyzed according to a pre-established statistical analysis plan. All evaluations were explorative in nature. For evaluation of vaccine safety all subjects who received at least one vaccination were included. Safety data were reported in terms of the number and proportion of individuals who had an injection site or systemic reaction within 5 d after each vaccination in each group, and a 2-sided Fisher's exact test was used to compare groups when relevant. All reported p-values are 2-sided with an α value of 0.05 considered to indicate statistical significance. Immunogenicity analysis was performed on subjects that completed the study per protocol. For GMTs all valid HI titres per group for each time point were used. Seroconversion rates were calculated if subjects had a valid pre- and post-vaccination titre. Seroconversion was defined as a pre-vaccination HI titre <10 and post-vaccination titre ≥40 or, when subject had a pre-vaccination titre of 10 or higher, a minimum fourfold rise in HI antibody titre upon vaccination. Seroprotection was defined as an HI antibody titre above the cut-off value indicative for protection (≥40). Because the influenza strains in the licensed seasonal vaccine were different from the strains in the investigational vaccines, data from the comparator group were not included in the immunogenicity analysis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The H3N2 influenza virus strain was a kind gift from Solvay. We thank Marie-Paule Kieny, Erin Sparrow, Martin Friede of WHO and Mariska Beukers-Reuvers of QPS for their support and organization of the study. Patrick de Jong is acknowledged for excellent process development.
Funding
This work was financed by WHO (grant reference V21-TSA-008).
References
- Monto AS. Influenza: quantifying morbidity and mortality. Am J Med 1987; 82:20-5; PMID:3591814; http://dx.doi.org/10.1016/0002-9343(87)90556-0
- Palese P, Garcia-Sastre A. Influenza vaccines: present and future. J Clin Invest 2002; 110:9-13; PMID:12093881; http://dx.doi.org/10.1172/JCI0215999
- Poland GA, Ovsyannikova IG, Jacobson RM. Immunogenetics of seasonal influenza vaccine response. Vaccine 2008; 26:D35-40; PMID:19230157; http://dx.doi.org/10.1016/j.vaccine.2008.07.065
- Nichol KL. Efficacy and effectiveness of influenza vaccination. Vaccine 2008; 26:D17-22; PMID:19230153; http://dx.doi.org/10.1016/j.vaccine.2008.07.048
- Oshitani H, Kamigaki T, Suzuki A. Major issues and challenges of influenza pandemic preparedness in developing countries. Emerg Infect Dis 2008; 14:875-80; PMID:18507896; http://dx.doi.org/10.3201/eid1406.070839
- World Health Organization. Pandemic preparedness and response. WHO Guidance document 2009. [Last viewed on March 10, 2015] Available at http://www.who.int/influenza/resources/documents/pandemic_guidance_04_2009/en/
- Kieny MP, Costa A, Hombach J, Carrasco P, Pervikov Y, Salisbury D, Greco M, Gust I, LaForce M, Franco-Paredes C, et al. A global pandemic influenza vaccine action plan. Vaccine 2006; 24:6367-70; PMID:17240560; http://dx.doi.org/10.1016/j.vaccine.2006.07.021
- World Health Organization. Global pandemic influenza action plan to increase vaccine supply. 2006. [Last viewed on march 10, 2015] Available at http://www.who.int/csr/resources/publications/influenza/WHO_CDS_EPR_GIP_2006_1/en/
- World Health Organization. Pandemic influenza vaccines: current status. Pandemic (H1N1) 2009 11. 2009
- Jadhav S, Datla M, Kreeftenberg H, Hendriks J. The developing countries vaccine manufacturers' network (DCVMN) is a critical constituency to ensure access to vaccines in developing countries. Vaccine 2008; 26:1611-5; PMID:18294742; http://dx.doi.org/10.1016/j.vaccine.2008.01.034
- Hendriks J, Holleman M, de Boer O, de Jong P, Luytjes W. An international technology platform for influenza vaccines. Vaccine 2011; 29 1:A8-11; PMID:21684431; http://dx.doi.org/10.1016/j.vaccine.2011.04.124
- World Health Organization. Recommendations for the production and control of influenza vaccine (inactivated). WHO Tech Rep Series, No. 927, 2005, 3. 2005
- Stephenson I, Nicholson KG, Gluck R, Mischler R, Newman RW, Palache AM, Verlander NQ, Warburton F, Wood JM, Zambon MC. Safety and antigenicity of whole virus and subunit influenza A/Hong Kong/1073/99 (H9N2) vaccine in healthy adults: phase I randomised trial. Lancet 2003; 362:1959-66; PMID:14683655; http://dx.doi.org/10.1016/S0140-6736(03)15014-3
- Parkman PD, Hopps HE, Rastogi SC, Meyer HM, Jr. Summary of clinical trials of influenza virus vaccines in adults. J Infect Dis 1977; 136 S722-30; PMID:606797; http://dx.doi.org/10.1093/infdis/136.Supplement_3.S722
- Beyer WE, Palache AM, Osterhaus AD. Comparison of serology and reactogenicity between influenza subunit vaccines and whole virus or split vaccines: a review and meta-analysis of the literature. Clin Drug Invest 1998; 15:1-12; PMID:18370460; http://dx.doi.org/10.2165/00044011-199815010-00001
- Zabel F, Kundig TM, Bachmann MF. Virus-induced humoral immunity: on how B cell responses are initiated. Curr Opin Virol 2013; 3:357-62; PMID:23731601; http://dx.doi.org/10.1016/j.coviro.2013.05.004
- Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol 2010; 10:787-96; PMID:20948547; http://dx.doi.org/10.1038/nri2868
- Geeraedts F, Bungener L, Pool J, ter Veer W, Wilschut J, Huckriede A. Whole inactivated virus influenza vaccine is superior to subunit vaccine in inducing immune responses and secretion of proinflammatory cytokines by DCs. Influenz Other Res Virus 2008; 2:41-51; PMID:19453471; http://dx.doi.org/10.1111/j.1750-2659.2008.00038.x
- Geeraedts F, Goutagny N, Hornung V, Severa M, de Haan A, Pool J, Wilschut J, Fitzgerald KA, Huckriede A. Superior immunogenicity of inactivated whole virus H5N1 influenza vaccine is primarily controlled by Toll-like receptor signalling. PLoS Pathog 2008; 4:e1000138; PMID:18769719; http://dx.doi.org/10.1371/journal.ppat.1000138
- Hovden AO, Cox RJ, Haaheim LR. Whole influenza virus vaccine is more immunogenic than split influenza virus vaccine and induces primarily an IgG2a response in BALB/c mice. Scandinavian j Immunol 2005; 62:36-44; PMID:16092921; http://dx.doi.org/10.1111/j.1365-3083.2005.01633.x
- Budimir N, Huckriede A, Meijerhof T, Boon L, Gostick E, Price DA, Wilschut J, de Haan A. Induction of heterosubtypic cross-protection against influenza by a whole inactivated virus vaccine: the role of viral membrane fusion activity. PLoS One 2012; 7:e30898; PMID:22303469; http://dx.doi.org/10.1371/journal.pone.0030898
- Beigel JH, Voell J, Huang CY, Burbelo PD, Lane HC. Safety and immunogenicity of multiple and higher doses of an inactivated influenza A/H5N1 vaccine. J Infect Dis 2009; 200:501-9; PMID:19569973; http://dx.doi.org/10.1086/599992
- Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, Stephenson I. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med 2009; 361:2424-35; PMID:19745215; http://dx.doi.org/10.1056/NEJMoa0907650
- Nolan T, McVernon J, Skeljo M, Richmond P, Wadia U, Lambert S, Nissen M, Marshall H, Booy R, Heron L, et al. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA 2010; 303:37-46; PMID:20026597; http://dx.doi.org/10.1001/jama.2009.1911
- Plennevaux E, Sheldon E, Blatter M, Reeves-Hoche MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet 2010; 375:41-8; PMID:20018365; http://dx.doi.org/10.1016/S0140-6736(09)62026-2
- Bernstein DI, Edwards KM, Dekker CL, Belshe R, Talbot HK, Graham IL, Noah DL, He F, Hill H. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis 2008; 197:667-75; PMID:18260764; http://dx.doi.org/10.1086/527489
- Leroux-Roels I, Borkowski A, Vanwolleghem T, Drame M, Clement F, Hons E, Devaster JM, Leroux-Roels G. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 2007; 370:580-9; PMID:17707753; http://dx.doi.org/10.1016/S0140-6736(07)61297-5
- Soema PC, Rosendahl Huber SK, Willems GJ, Jiskoot W, Kersten GF, Amorij JP. Influenza T-cell Epitope-Loaded Virosomes Adjuvanted with CpG as a potential Influenza vaccine. Pharm Res 2014; PMID:25344321 Open Acess. Available at http://link.springer.com/article/10.1007/s11095-014-1556-3/fulltext.html
- Sansyzbay AR, Erofeeva MK, Khairullin BM, Sandybayev NT, Kydyrbayev ZK, Mamadaliyev SM, Kassenov MM, Sergeeva MV, Romanova JR, Krivitskaya VZ, et al. An inactivated, adjuvanted whole virion clade 2.2 H5N1 (A/chicken/astana/6/05) influenza vaccine is safe and immunogenic in a single dose in humans. Clin Vaccine Immunol 2013; 20:1314-9; PMID:23803900; http://dx.doi.org/10.1128/CVI.00096-13
- Wu J, Fang HH, Chen JT, Zhou JC, Feng ZJ, Li CG, Qiu YZ, Liu Y, Lu M, Liu LY, et al. Immunogenicity, safety, and cross-reactivity of an inactivated, adjuvanted, prototype pandemic influenza (H5N1) vaccine: a phase II, double-blind, randomized trial. Clin Infect Dis 2009; 48:1087-95; PMID:19281330; http://dx.doi.org/10.1086/597401
- Lin J, Zhang J, Dong X, Fang H, Chen J, Su N, Gao Q, Zhang Z, Liu Y, Wang Z, et al. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet 2006; 368:991-7; PMID:16980114; http://dx.doi.org/10.1016/S0140-6736(06)69294-5
- Ikeno D, Kimachi K, Kino Y, Harada S, Yoshida K, Tochihara S, Itamura S, Odagiri T, Tashiro M, Okada K, et al. Immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1, NIBRG-14) vaccine administered by intramuscular or subcutaneous injection. Microbiol Immunol 2010; 54:81-8; PMID:20377741; http://dx.doi.org/10.1111/j.1348-0421.2009.00191.x
- Beran J, Abdel-Messih IA, Raupachova J, Hobzova L, Fragapane E. A phase III, randomized, open-label study to assess the tolerability and immunogenicity of an H5N1 influenza vaccine administered to healthy adults with a 1-, 2-, 3-, or 6-week interval between first and second doses. Clin Ther 2010; 32:2186-97; PMID:21316535; http://dx.doi.org/10.1016/S0149-2918(11)00024-5
- Vesikari T, Forsten A, Herbinger KH, Cioppa GD, Beygo J, Borkowski A, Groth N, Bennati M, von Sonnenburg F. Safety and immunogenicity of an MF59((R))-adjuvanted A/H5N1 pre-pandemic influenza vaccine in adults and the elderly. Vaccine 2012; 30:1388-96; PMID:22192847; http://dx.doi.org/10.1016/j.vaccine.2011.12.009
- van Els C, Mjaaland S, Naess L, Sarkadi J, Gonczol E, Smith Korsholm K, Hansen J, de Jonge J, Kersten G, Warner J, et al. Fast vaccine design and development based on correlates of protection (COPs): Influenza as a trendsetter. Hum Vaccin Immunother 2014; 10:1935-48; PMID:25424803; http://dx.doi.org/10.4161/hv.28639
- Reber A, Katz J. Immunological assessment of influenza vaccines and immune correlates of protection. Expert Rev Vaccines 2013; 12:519-36; PMID:23659300; http://dx.doi.org/10.1586/erv.13.35
- World Health Organization. Guidelines on clinical evaluation of vaccines: regulatory expectations. WHO Tech Rep Ser No. 924, 2004, 1. 2004.
- Palmer D, Dowle, W., Coleman,M., and Schild, G. Haemagglutination inhibition test. Advanced laboratory techniques for influenza diagnosis: procedural guide. Atlanta, GA: US. Dep Health Edu Welfare 1975:25-62.