Abstract
Our objective was to develop a multivalent prophylactic HPV vaccine that protects against infection and disease caused by HPV16/18 (oncogenic types in existing prophylactic vaccines) plus additional oncogenic types by conducting 3 Phase II studies comparing the immunogenicity (i.e., anti-HPV6/11/16/18 geometric mean titers [GMT]) and safety of 7 vaccine candidates with the licensed quadrivalent HPV6/11/16/18 vaccine (qHPV vaccine) in young women ages 16–26. In the first study (Study 1), subjects received one of 3 dose formulations of an 8-valent HPV6/11/16/18/31/45/52/58 vaccine or qHPV vaccine (control). In Study 2, subjects received one of 3 dose formulations (termed low-, mid-, and high-dose formulations, respectively) of a 9-valent HPV6/11/16/18/31/33/45/52/58 vaccine (9vHPV vaccine) or qHPV vaccine (control). In Study 3, subjects concomitantly received qHPV vaccine plus 5-valent HPV31/33/45/52/58 or qHPV vaccine plus placebo (control). All vaccines were administered at day 1/month 2/month 6. In studies 1 and 3, anti-HPV6/11/16/18 GMTs at month 7 were non-inferior in the experimental arms compared with the control arm; however, there was a trend for lower antibody responses for all 4 HPV types. In Study 2, this immune interference was overcome with the mid- and high-dose formulations of the 9vHPV vaccine by increasing antigen and adjuvant doses. In all 3 studies, all vaccine candidates were strongly immunogenic with respect to HPV31/33/45/52/58 and were well tolerated. Based on the totality of the results, the middle dose formulation of the 9vHPV vaccine was selected for Phase III evaluation. Each 0.5mL dose contains 30μg/40μg/60μg/40μg/20μg/20μg/20μg/20μg/20μg of HPV6/11/16/18/31/33/45/52/58 virus-like particles, and 500μg of amorphous aluminum hydroxyphosphate sulfate adjuvant.ClinicalTrials.gov numbers NCT00260039, NCT00543543, and NCT00551187.
Introduction
HPV infection causes benign and malignant dysplastic disease localized primarily in the anogenital area and aerodigestive tract.Citation1-3 Persistent HPV infection significantly increases the risk of cervical cancer, other anogenital cancers, and oropharyngeal cancer.Citation4 Nearly 100% of cervical cancer cases are caused by HPV infection. Cervical cancer is the second most common cancer in women worldwide with approximately 530,000 new cases diagnosed annually.Citation4 Licensed prophylactic HPV vaccines, including the quadrivalent HPV type 6/11/16/18 virus-like particle (VLP) vaccine (qHPV vaccine) and the bivalent HPV16/18 VLP vaccine, are based on VLPs made of L1 major capsid proteins.Citation5 These 2 vaccines include 2 oncogenic HPV types (16 and 18) which are responsible for approximately 70% of cervical cancers worldwide.Citation6,7 Partial cross-protection against non-vaccine HPV types has been reported for both licensed vaccines although its clinical significance remains uncertain.Citation5 Oncogenic HPV types 31/33/45/52/58 cause approximately an additional 20% of cervical cancers worldwide. Thus, a multivalent HPV vaccine including HPV16/18 plus these 5 additional HPV types has the potential to prevent approximately 90% of cervical cancers worldwide.Citation6,7
Adding additional antigen types to an existing vaccine can potentially impact the vaccine's immunogenicity and safety. Therefore, Phase II evaluation needs to be designed to select a vaccine dose formulation that has an acceptable immunogenicity and safety profile. The aim of this Phase II clinical program was to identify a vaccine candidate that would provide greater cervical cancer coverage. The key objective was to identify a vaccine candidate that: (1) generates anti-HPV6, anti-HPV11, anti-HPV16, and anti-HPV18 responses that are non-inferior to those induced by the licensed qHPV vaccine with no evidence of overall negative trend in immunogenicity, especially for the oncogenic HPV types 16/18; (2) elicits robust antibody responses to additional HPV types not included in the currently licensed HPV vaccines; and (3) is generally well tolerated.
Three Phase II studies, evaluating 7 multivalent HPV vaccine formulations, were conducted to facilitate the selection of the optimal formulation for Phase III evaluation. The initial study (Study 1) evaluated 3 dose formulations of an 8-valent HPV type 6/11/16/18/31/45/52/58 vaccine (8vHPV vaccine); however, a lead vaccine candidate was not selected. Two additional studies were subsequently conducted. For these 2 studies (Studies 2 and 3), changes were made in vaccine formulation; also, L1 VLPs for another oncogenic HPV type (HPV33) became available and was included in the candidate vaccines. Study 2 evaluated 3 dose formulations of a 9-valent HPV type 6/11/16/18/31/33/45/52/58 vaccine (9vHPV vaccine) (termed low-, mid-, and high-dose formulations) with increased antigen and adjuvant doses. Study 3 evaluated concomitant administration of qHPV vaccine and a 5-valent HPV type 31/33/45/52/58 (5vHPV vaccine) in separate limbs. The results of the 3 studies are described in this report. Based on the totality of the results, the middle dose formulation of the 9vHPV vaccine was selected for Phase III evaluation.
Results
Study population
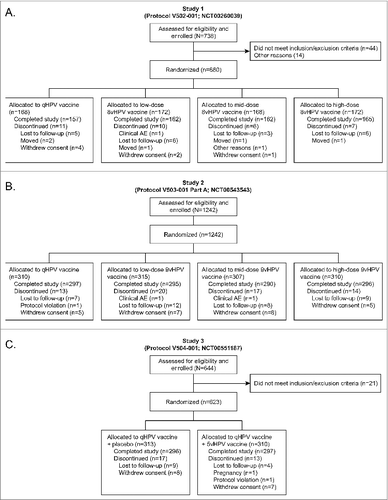
A total of 680 subjects were randomized in Study 1 from 9 sites in Latin America (Colombia, Peru) and North America (United States). A total of 1242 subjects were randomized in Study 2 from 33 sites in Asia (Taiwan), Europe (Denmark, Norway), Latin America (Colombia, Mexico, Peru) and North America (United States). A total of 623 subjects were randomized in Study 3 from 20 sites in Europe (Austria, Denmark, Sweden) and North America (Canada, United States [including Puerto Rico]). A summary of the number of subjects who were randomized, vaccinated, and who completed or discontinued during the study is shown in . A summary of baseline subject characteristics is provided in . Within each study the vaccination groups were equally distributed across geographic region.
Table 1. Characteristics of randomized study participants
Vaccine formulations
shows the formulations of the 7 vaccine candidates. As shown in , the 8vHPV vaccine dose formulations evaluated in Study 1 contained the same amounts of HPV6/11/16/18 VLPs as the qHPV vaccine; however, due to the additional antigen, the adjuvant to total antigen ratios in all the 8vHPV vaccine dose formulation were lower than in the qHPV vaccine. In Study 2, both the amounts of antigen in the 9vHPV vaccine and the amount of adjuvant were changed to give variable absolute concentrations of the different antigens and varying antigen to adjuvant ratios. The mid- and high-dose formulations of the 9vHPV vaccine contained higher amounts of VLPs 6, 16, and 18 than the qHPV vaccine, and all dose formulations of the 9vHPV vaccine contained the same higher amount of adjuvant. In Study 3, the 5vHPV vaccine had the same amount of antigen as the high-dose formulation of the 9vHPV vaccine for HPV31/33/45/52/58.
Table 2. Antigen and adjuvant composition of vaccines administered
Immunogenicity analyses: HPV6/11/16/18
displays the month 7 HPV competitive Luminex Immunoassay (cLIA) geometric mean titers (GMTs) in the Study 1 cohorts for HPV6/11/16/18 in the per-protocol immunogenicity (PPI) population. The lower bound of the 95% confidence interval (CI) of the GMT ratio exceeded 0.5 for all 4 HPV types, with p-values <0.001, indicating cLIA GMT responses in subjects administered any of the 3 dose formulations of the 8vHPV vaccine were non-inferior to those in subjects administered the qHPV vaccine alone. Seroconversion rates at month 7 were 100% for each of these 4 HPV types in all vaccination groups (data not shown). However, the GMTs were numerically lower for HPV types 6/11/16/18 in the 8-valent HPV (8vHPV) vaccine cohorts compared with the qHPV vaccine cohort. Therefore, additional Phase II studies were conducted to identify a vaccine candidate that did not reduce immunogenicity of the original HPV types. The vaccine candidates evaluated in Studies 2 and 3 addressed the same HPV types as the 8vHPV vaccine plus an additional oncogenic HPV type, HPV33.
Table 3. Immunogenicity analyses: HPV 6, 11, 16, and 18
In Study 2, a post-dose 2 (month 3) interim immunogenicity analysis was conducted to identify the most promising vaccine candidate for use in the Phase III program. As shown in , the GMTs in the low-dose 9vHPV vaccine group were numerically lower than those in the qHPV vaccine group. Compared with the qHPV vaccine control, the GMTs in the mid-dose and high-dose 9vHPV vaccine groups were numerically similar for HPV6 and HPV16, higher for HPV18, and lower for HPV11. Seroconversion rates at month 3 were greater than 98% for each of the original 4 HPV types in all vaccination groups (data not shown). A non-inferiority analysis was conducted at the end of the Phase II/III study with respect to anti-HPV6/11/16/18 responses in the PPI population for the mid-dose which had been selected for Phase III testing. As shown in , the lower bound 95% CI GMT ratio exceeded 0.5 for all 4 HPV types, with p-values <0.001, indicating cLIA GMT responses in subjects administered mid-dose 9vHPV vaccine were non-inferior to those in subjects administered qHPV vaccine.
Study 3 tested whether the immune interference noted in Study 1 could be overcome by administering the qHPV vaccine and the 5vHPV vaccine concomitantly in different limbs. As shown in , anti-HPV6/11/16/18 GMTs were non-inferior in subjects administered qHPV vaccine concomitantly with 5vHPV vaccine compared with subjects administered qHPV vaccine concomitantly with placebo. However, GMTs were numerically lower for all 4 HPV types in the qHPV vaccine + 5vHPV vaccine group vs. the qHPV vaccine + placebo group. Therefore, this approach was not pursued further.
Immunogenicity analyses: HPV31/33/45/52/58
In Study 1, all 3 8vHPV vaccine dose formulations induced robust anti-HPV31/45/52/58 responses at Month 7 (). The GMT response was dose dependent within the dose range tested (5 μg to 40 μg). For each HPV type, ≥97.7% subjects seroconverted at month 7 (data not shown).
Figure 2. Anti-HPV31, 45, 52, and 58 GMTs in Study 1 at one month post-dose 3; B) Interim analysis of anti-HPV31, 33, 45, 52, and 58 GMTs in Study 2 at one month post-dose 2.
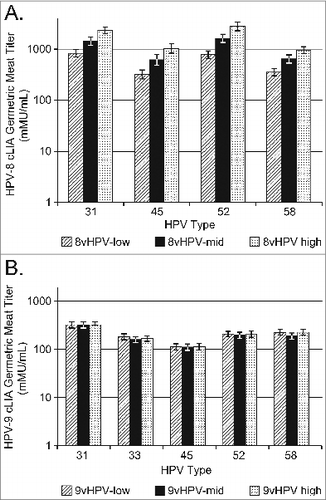
In Study 2, the post-dose 2 interim immunogenicity analysis showed that all 3 9vHPV vaccine dose formulations induced robust anti-HPV31/33/45/52/58 responses at month 3 (). For each HPV type, over 95% subjects seroconverted at month 3 (data not shown).
In Study 3, administration of qHPV vaccine + 5vHPV vaccine induced robust anti-HPV31/33/45/52/58 responses at month 7 (data not shown). For each HPV type, ≥99.6% subjects seroconverted at month 7 (data not shown).
Safety
The vaccines administered as a 3-dose regimen in Studies 1, 2, and 3 were generally well tolerated in each participant group (). Serious adverse events (AEs) and discontinuations as a result of an AE were rare (0–1.2% and 0-0.6%, respectively). No deaths or serious vaccine-related AEs were reported. The proportions of participants who reported at least one injection-site AEs were numerically higher among subjects who received 8vHPV or 9vHPV vaccines than among subjects who received qHPV vaccine. The proportions of subjects who reported systemic AEs were generally similar between vaccination groups for all 3 studies.
Table 4. Adverse event (AE) summary
Discussion
It is generally accepted that prophylactic HPV vaccination prevents HPV infection and disease by generating type specific neutralizing antibodies.Citation8 The minimum antibody titer needed for protection is not known but animal studies suggest that very low antibody titers (e.g., up to 100-fold lower than the threshold of detection of a standard pseudovirion-based neutralizing assay [PBNA]) may be protective.Citation8 This result is also relevant to cLIA (the immunoassay used in this study) since PBNA and cLIA are highly correlated and similarly sensitiveCitation9. Since no antibody threshold for protection has been defined, Phase II development of a multivalent HPV L1 VLP vaccine required a direct comparison with a licensed HPV vaccine to ensure the investigational vaccine and the licensed vaccine induce comparable antibody response to the HPV types already covered by the licensed vaccine.
Seven multivalent HPV L1 VLP vaccine candidates were tested for immunogenicity and safety in 3 Phase II studies. Study 1 investigated 3 dose formulations of an 8vHPV vaccine. All vaccine formulations were strongly immunogenic and induced anti-HPV6/11/16/18 responses that were non-inferior to responses induced by the licensed qHPV vaccine. Nonetheless, responses were numerically lower in all the 8vHPV vaccine groups, suggesting that addition of new types to the vaccine may negatively impact immunogenicity to the 4 types contained in the qHPV vaccine. Study 3 demonstrated that this negative effect was not overcome by administering qHPV vaccine and 5vHPV vaccine concurrently in different limbs. Study 2 results demonstrated that increasing the amount of antigen and adjuvant of 3 dose formulations of a 9vHPV vaccine increased immunogenicity, and the mid- and high-dose formulations of the 9vHPV vaccine resulted in levels of immunogenicity similar to those induced by the qHPV vaccine. Additionally, L1 VLPs for HPV31/33/45/52/58 were strongly immunogenic with all 3 9vHPV vaccine dose formulations tested.
The vaccine candidates were generally well tolerated, although an increase in the frequency of injection-site AEs compared with the licensed qHPV vaccine was noted. This result may reflect the higher amounts of VLP and adjuvant in the vaccine candidates than in the qHPV vaccine. However, this observation is unlikely to be of clinical significance as the differences between vaccination groups were modest and discontinuations due to AEs were rare in all groups. Overall, the results supported selection of the mid-dose 9vHPV vaccine as the best candidate for Phase III evaluation.
As noted in the methods section, subjects enrolled in Study 2 who received the mid-dose 9vHPV vaccine or qHPV vaccine continued in the Phase III study. Because the study used a seamless Phase II/III adaptive design, the study team remained blinded to treatment groups until the final efficacy, immunogenicity and safety analyses were conducted. For this reason, the complete Phase II findings could not be reported until Phase III analysis was completed. The approach used to conduct dose selection while keeping the study team blinded to treatment allocation has been reported.Citation10 In the Phase III study, the 9vHPV vaccine induced non-inferior anti-HPV6/11/16/18 responses compared with qHPV vaccine and was highly efficacious in preventing infection and disease caused by HPV31/33/45/52/58.Citation11
In summary, immunogenicity and safety were assessed in several multivalent HPV vaccine candidates in Phase II studies. A dose formulation of 9vHPV vaccine was selected in the Phase II (dose selection) portion of a seamless Phase II/III study. Phase II evaluation showed that the selected 9vHPV vaccine dose formulation (1) provided non-inferior antibody responses compared with the licensed qHPV vaccine with respect to the 4 HPV types covered by both vaccines; (2) was strongly immunogenic against 5 additional oncogenic HPV types not addressed by the currently licensed vaccines; and (3) was generally well tolerated. Phase II immunogenicity and safety findings were subsequently confirmed in the Phase III portion of the study. Moreover, during the Phase III study, the selected vaccine candidate was found to be highly efficacious in preventing persistent infection and disease associated with the vaccine HPV types.
Material and Methods
Studies and population
Seven vaccine candidates were evaluated in 3 Phase II studies that were conducted sequentially. Study 1 (Protocol V502-001, NCT00260039) represented an initial (or the first) evaluation of the possible candidates. Studies 2 (Protocol V503-001 Part A, NCT00543543) and 3 (Protocol V504-001, NCT00551187) were then conducted concurrently with the goal of selecting a vaccine dose formulation for use in Phase III studies. In all 3 studies the licensed qHPV vaccine was used as a control. Doses of antigens and adjuvant administered are summarized in .
All three studies were conducted in accordance with principles of Good Clinical Practice and were approved by the appropriate institutional review boards and regulatory agencies. Study participants were required to be generally healthy, and have no history of abnormal Pap test results, no more than 4 lifetime sexual partners and no previous abnormal cervical biopsy results. Enrollment exclusion criteria included pregnancy (determined by urine or serum β-human chorionic gonadotropin testing), known allergy to any vaccine component, thrombocytopenia, immunosuppression or prior immunosuppressive therapy, or previous receipt of an HPV vaccine.
Study 1 was a double-blind Phase II immunogenicity and safety study. The study was designed to evaluate the immunogenicity of one of 3 doses of an 8vHPV type 6/11/16/18/31/45/52/58 vaccine that contained the original qHPV types utilizing the same amount of VLP as the qHPV vaccine (20 μg, 40 μg, 40 μg, and 20 μg of HPV6, 11, 16, 18, respectively), plus additional VLPs of the HPV types 31/45/52/58 in varied protein concentrations. Six hundred and eighty young women 16 to 23 years of age were enrolled and equally randomized to one of 3 dose formulations of the 8vHPV vaccine or qHPV vaccine (control). The study was initiated on 06-Dec-2005 and was completed on 22-Aug-2007.
Study 2 was the Phase II portion of a double-blind dose ranging, immunogenicity, safety, and efficacy Phase II/III study. The study was designed to evaluate the immunogenicity of one of 3 dose formulations of a 9vHPV type 6/11/16/18/31/33/45/52/58 vaccine, with the amount of VLPs of both the original HPV types (6/11/16/18) and the additional HPV types (31/33/45/52/58) varied. One-thousand two hundred and forty-two young women 16 to 26 years of age were enrolled and equally randomized to one of 3 dose formulations of a 9-valent vaccine or qHPV vaccine (control). The study was initiated on 26-Sep-2007; subjects who received the selected dose formulation of 9vHPV vaccine or qHPV vaccine continued into Phase III evaluation;Citation10,11 efficacy and immunogenicity analyses for the Phase III part of the study were conducted based on a visit cut-off date of 10-Apr-2013.Citation11
Study 3 was a double-blind Phase II immunogenicity and safety study. The study was designed to compare the immunogenicity of qHPV vaccine given alone or concomitantly with a 5vHPV type 31/33/45/52/58 vaccine. Six-hundred and twenty three young women 16 to 26 years of age were enrolled and equally randomized to one of 2 groups: subjects in the experimental group were administered concomitantly qHPV vaccine and 5vHPV vaccine in opposite arms; subjects in the control group were administered qHPV vaccine and placebo, in opposite arms. The study was initiated on 04-Oct-2007 and was completed on 20-May-2009.
Subjects in Studies 1 and 3, and subjects in Study 2 who did not receive the 9vHPV dose formulation which was ultimately selected for Phase III studies, or the qHPV vaccine, were followed for 7 months when the study terminated. Subjects who received the selected dose formulation of 9vHPV vaccine or qHPV vaccine continued into Phase III evaluation for a total of at least 42 months.Citation11
Randomization and vaccine administration
Following informed consent and determination that all inclusion criteria and none of the exclusion criteria were met, eligible subjects received an allocation number and were randomized to a vaccination group. An Interactive Voice Response System (IVRS) was used to allocate study subjects and balance randomization between sites. The IVRS assigned the subject an allocation number from an allocation schedule. All vaccines were administered as a 3-dose regimen (at day 1, month 2, and month 6). All participants were required to be afebrile (oral temperature <37.8°C) within 24 hours before each injection. Participants were instructed to use effective contraception through month 7. All participants underwent pregnancy testing that was based on urine or serum analyses for β-human chorionic gonadotropin before each vaccination. Pregnant women were not vaccinated.
Assessment
All participants were assessed for immunogenicity for all vaccine HPV types at day 1 and month 7. In Studies 2 and 3, serum samples obtained at day 1 and months 3 and 7 were tested for anti-HPV6/11/16/18/31/33/45/52/58 by a 9-valent competitive Luminex immunoassay (HPV-9 cLIA).Citation12 Version 1 of the HPV-9 cLIA (representing the pre-validated assayCitation10) was used in these 2 studies. In Study 1, serum samples obtained at day 1 and month 7 were tested for anti-HPV6/11/16/18/31/45/52 by an 8-valent cLIA (HPV-8 cLIA), an assay similar to the HPV-9 cLIA but not testing for anti-HPV33. Although the HPV-8 cLIA and HPV-9 cLIA are based on the same principles, they are different assays; therefore, no numerical comparison can be made between the results of the 2 assays. Moreover, since the HPV-8 cLIA and HPV-9 cLIA antibody titers to each HPV type are determined using type-specific monoclonal antibodies,Citation12 it is not possible to make a direct comparison of assay results across HPV types.
Subjects also underwent PCR testing of cervical and external genital samples for detection of HPV DNA at day 1 and month 7. HPV PCR status was tested at day 1 for 14 HPV types, including the 9 vaccine types as well as 5 oncogenic HPV types which are not contained in the 9vHPV vaccine (HPV/35/39/51/56/59). HPV seropositivity or PCR-positivity at day 1 were not a reason for exclusion from the study. However, the results of the serology and PCR testing were part of the criteria to define the per-protocol analysis population.
All participants were observed for at least 30 minutes after each vaccination for any immediate reaction, with particular attention to any evidence of a hypersensitivity reaction. All subjects received a vaccination report card (VRC) at the day 1, month 2 and month 6 study vaccination visits. On the VRC, subjects recorded oral temperatures for 4 days following vaccination and injection-site and systemic adverse events (AEs) for 15 days following vaccination.
Statistical methods
The primary approach to the analyses of immunogenicity was per-protocol. Each vaccine component was analyzed separately. To be included in the PPI populations for HPV6 and HPV11, subjects had to be seronegative to both HPV6 and HPV11 at day 1 and PCR negative to HPV6 and HPV11 from day 1 through month 7. To be included in the PPI populations for the other vaccine HPV types, subjects were required to be seronegative at day 1 and PCR negative from day 1 through month 7 only for the HPV type being analyzed. In addition, subjects had to receive all 3 doses of the correct vaccine within a pre-established number of days and have at least 1 post-dose 3 serology result within a pre-established time. Given the high correlation between post-dose 2 and post-dose 3 antibody response,Citation13 analyses based on post-dose 2 anti-HPV response were considered predictive of the post-dose 3 response. Therefore, selection of a vaccine dose formulation for use in Phase III was based on interim immunogenicity analyses of post-dose 2 immunogenicity results from Study 2, which shortened the time to dose selection.Citation10
Within each study, the primary immunogenicity objective was to demonstrate that Month 7 GMTs for serum anti-HPV6/11/16/18 were non-inferior in subjects who received experimental vaccine compared to subjects in the control group. Successful demonstration of the primary immunogenicity non-inferiority hypothesis required that the lower bound of the 2-sided 95% CI of the GMT ratio (experimental arm/control arm) was greater than 0.5 for each of anti-HPV types 6/11/16/18. Separately for each anti-HPV type, the 95% CI of GMT ratio was derived from an ANOVA model with log-anti-HPV as the response and vaccination group as the fixed effect (Studies 2 and 3) or vaccination group and country as the fixed effect (Study 1). All three studies were powered for this non-inferiority objective.
For the purpose of dose selection in the interim Month 3 immunogenicity analysis in Study 2, immune response against each HPV type was summarized by vaccine group, and the GMT ratio (experimental arm / control arm) with associated 2-sided 95% CI was derived within each study. The estimates from these studies were also reviewed by an external committee independent of the project team.Citation10 For example, the committee was asked to pay particular attention to ensure that there was no evidence of reduction in immune response against HPV16 and HPV18 in the selected dose formulation group.
All subjects who received at least 1 study vaccination and had follow-up data were included in the analysis of safety. AEs were summarized as frequencies and percentages by participant group and type of AE.
Disclosure of Potential Conflicts of Interest
Alain Luxembourg, Roger Maansson, Erin Moeller, Michael Ritter, and Joshua Chen are current or former employees of Merck and Co., Inc. and may hold stock/stock options. Celine Bouchard reports honoraria for lectures and travel from GlaxoSmithKline and Merck and Co., Inc. Anna R. Giuliano reports receiving grants from Merck and Co., Inc. and membership in Merck and Co., Inc. advisory boards and speakers bureaus. Ole-Erik Iversen reports honoraria through his institution for the conduct of clinical trials and compensation from GlaxoSmithKline and Merck and Co., Inc. for performing vaccine clinical trials. Elmar A. Joura reports grants, personal fees and non-financial support from Merck and Co., Inc., grants, personal fees and non-financial support from Sanofi Pasteur MSD and GlaxoSmithKline and personal fees from Roche Diagnostics. Mary E. Penny reports receiving grant support from MSD and has received grant support from Sanofi Pasteur MSD, Glaxo and Novartis. Jaime A. Restrepo has nothing to disclose. Darron Brown has served on an Advisory Board at Merck and Co., Inc. and has lectured on the quadrivalent HPV vaccine (honoraria received from Merck and Co., Inc. are donated to charities). His laboratory has received research funding from Merck and Co., Inc. Indiana University and Merck and Co., Inc. have an agreement that pays the University, based on certain landmarks related to vaccine development. DB receives a portion of these funds as income. Josefina Romaguera reports participating in vaccine trials for Merck and Co., Inc., Inovio and Hoffmann La Roche Inc., and other clinical trials for AbbVie as faculty of the University of Puerto Rico, as well as being a speaker for Merck and Pfizer.
Acknowledgments
The authors thank Heather L. Sings and Karyn Davis (Merck and Co., Inc.) for assistance in the preparation of this manuscript.
Funding
Funding for this study was provided by Merck & Co., Inc., Kenilworth, NJ USA.
References
- Paavonen J. Human papillomavirus infection and the development of cervical cancer and related genital neoplasias. Int J Infect Dis 2007; 11 Suppl 2: S3-S9; PMID:18162244; http://dx.doi.org/10.1016/S1201-9712(07)60015-0
- Madkan VK, Cook-Norris RH, Steadman MC, Arora A, Mendoza N, Tyring SK. The oncogenic potential of human papillomaviruses: a review on the role of host genetics and environmental cofactors. Br J Dermatol 2007; 157: 228-41; PMID:17553059; http://dx.doi.org/10.1111/j.1365-2133.2007.07961.x
- Stamataki S, Nikolopoulos TP, Korres S, Felekis D, Tzangaroulakis A, Ferekidis E. Juvenile recurrent respiratory papillomatosis: still a mystery disease with difficult management. Head Neck 2007; 29: 155-62; PMID:17022088; http://dx.doi.org/10.1002/hed.20491
- Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, Vignat J, Ferlay J, Bray F, Plummer M, et al. Global burden of human papillomavirus and related diseases. Vaccine 2012; 30 Suppl 5: F12-3; PMID:23199955; http://dx.doi.org/10.1016/j.vaccine.2012.07.055
- Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 2012; 30 Suppl 5: F123-38; PMID:23199956; http://dx.doi.org/10.1016/j.vaccine.2012.04.108
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11: 1048-56; PMID:20952254; http://dx.doi.org/10.1016/S1470-2045(10)70230-8
- Serrano B, Alemany L, Tous S, Bruni L, Clifford GM, Weiss T, Bosch FX, de Sanjose S. Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect Agent Cancer 2012; 7: 38; PMID:23273245; http://dx.doi.org/10.1186/1750-9378-7-38
- Stanley M, Pinto LA, Trimble C. Human papillomavirus vaccines-immune responses. Vaccine 2012; 30 Suppl 5: F83-7; PMID:23199968; http://dx.doi.org/10.1016/j.vaccine.2012.04.106
- Brown D, Muller M, Sehr P, Pawlita M, Seitz H, Rubio I, Antonello J, Radley D, Roberts C, Saah A. Concordance assessment between a multiplexed competitive Luminex immunoassay, a multiplexed IgG Luminex immunoassay, and a pseudovirion-based neutralization assay for detection of human papillomavirus types 16 and 18. Vaccine 2014; 32: 5880-7; PMID:25148777; http://dx.doi.org/10.1016/j.vaccine.2014.08.004
- Chen J, Gesser R, Luxembourg A. A seamless Phase IIB/III adaptive outcome trial: design rationale and implementation challenges. Clinical Trials 2015; 12 1:84–90.
- Joura EA, Giuliano AR, Iversen O-E, Bouchard C, Mao C, Mehlsen J, Moreira ED Jr, Ngan Y, Petersen LK, Lazcano-Ponce E, Pitisuttithum P, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015; 372:711–23.
- Roberts C, Green T, Hess E, Matys K, Brown MJ, Haupt RM, Luxembourg A, Vuocolo S, Saah A, Antonello J. Development of a human papillomavirus competitive luminex immunoassay for 9 HPV types. Hum Vaccin Immunother 2014; 10; PMID:25424920; http://dx.doi.org/10.4161/hv.29205
- Villa LL, Ault K, Giuliano AR, Costa RLR, Petta CA, Andrade RP, Brown DR, Ferenczy A, Harper DM, Koutsky LA, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16 and 18. Vaccine 2006; 24: 5571-83; PMID:16753240; http://dx.doi.org/10.1016/j.vaccine.2006.04.068