Abstract
Pandemic outbreaks of influenza are caused by the emergence of a pathogenic and transmissible virus to which the human population is immunologically naïve. Recent outbreaks of highly pathogenic avian influenza (HPAI) of the H5N1 subtype are of particular concern because of the high mortality rate (60% case fatality rate) and novel subtype. In this study, we have engineered an influenza virus-like particle (VLP) that contains a synthetic, consensus-based HA molecule using a new methodology, computationally optimized broadly reactive antigen (COBRA). Three COBRA H5N1 HA proteins have been engineered based upon (1) human clade 2 H5N1 sequences, (2) human and avian clade 2 sequences, and (3) all H5N1 influenza sequences recorded between 2005-2008. Each hemagglutinin protein retained the ability to bind the appropriate receptors, as well as the ability to mediate particle fusion, following purification from a mammalian expression system. COBRA VLP vaccines were administered to mice and the humoral immune responses were compared to those induced by VLPs containing an HA derived from a primary viral isolate. Using a single vaccination (0.6 ug HA dose with an adjuvant) all animals vaccinated with COBRA clade 2 HA H5N1 VLPs had protective levels of HAI antibodies to a representative isolate from each subclade of clade 2, but lower titers against other clades. The addition of avian sequences from other clades expanded breadth of HAI antibodies to the divergent clades, but still not all of the 25 H5N1 viruses in the panel were recognized by antibodies elicited any one H5N1 COBRA VLP vaccine. Vaccination of mice with a cocktail of all 3 COBRA HA VLP vaccines, in a prime-boost regimen, elicited an average HAI titer greater than 1:40 against all 25 viruses. Collectively, our findings indicate that the elicited antibody response following VLP vaccination with all 3 COBRA HA vaccine simultaneously elicited a broadly-reactive set of antibodies that recognized H5N1 viruses from 11 H5N1 clades/subclades isolated over a 12-year span.
Keywords:
Introduction
Since its re-emergence in late 2004, outbreaks of highly pathogenic avian H5N1 influenza virus have been reported in markets and poultry farms throughout Southeast Asia.Citation1 The virus has spread via wild bird populations.Citation2 H5N1 influenza viruses, like many other viruses from pandemic subtypes, can cause severe morbidity and mortality in humans.Citation3,4 386 out of 650 people died between 2003-2014 from H5N1 influenza virus infection (WHO/GIP, January 2014) and the first case of H5N1 diagnosed in North America occurred in Canada in 2014.Citation5 H5N1 influenza continues to spread across the globe, however, viruses of this subtype cannot easily spread between humans. Laboratory generated H5N1 variants with amino acid mutations in the hemagglutinin (HA) amino acid allowing for aerosol transmission from infected to uninfected ferrets have been established.Citation6,7 Two H5N1 strains only need 2 additional mutations to create a human transmissible variant.Citation6 These findings raise serious concerns about the possible evolution of highly pathogenic H5N1 influenza viruses to become transmissible among people and result in a global pandemic.
An effective vaccine could protect the human population against an emerging, transmissible H5N1 influenza strain. However, one of the challenges to developing effective H5N1 influenza vaccines is the distinct viral lineages that result in antigenic diversity within the subtype. H5N1 viruses are separated into distinct clades based upon phylogenetic distance among the hemagglutinin (HA) genes.Citation1,8,9 There are 10 antigenic clades based upon HA gene sequences. The clades are geographically diverse and are evolving under unique pressures specific to each respective location.Citation10 Genetic diversity within clade 2 has resulted in distinct subclades and sub-subclades.Citation9 Despite high levels of HA protein sequence homology between clades (>90%), there is little receptor-blocking antibody cross-reactivity across clades and even within subclades.Citation9 Developing vaccines that are able to overcome the challenge of H5N1 antigenic diversity is a crucial step in pandemic preparedness.
In order to overcome these limitations, we reported a new methodology of antigen design using multiple rounds of consensus generation termed computationally optimized broadly reactive antigen (COBRA). This method was designed to address the diversity specifically within clade 2 and utilized global surveillance efforts to generate a vaccine with the potential to elicit increased breadth of antibody responses within this antigenically diverse clade. In this study, we generated 2 second-generation H5N1 COBRA HA antigens to expand the breadth of antibody responses against H5N1 influenza viruses from various clades. Each of the COBRA HA antigens were expressed on virus-like particles (VLPs) and purified as vaccine immunogens. These new versions included addition of avian sequences. One of the vaccines added avian Clade 2 HA sequences to the human Clade 2 sequences to yield a Human-Avian Clade 2 COBRA and the second version added avian and human sequences from all clades of H5N1 referred to as All H5N1 COBRA. Both were tested in pre-clinical animal models and compared to wild-type H5N1 HA antigens and our first generation H5N1 COBRA HA vaccine. Each COBRA HA elicited HAI activity against a subset of H5N1 primary viral isolates. However, only when all 3 vaccines were combined did the elicited antibody response recognize all 25 viruses from 11 different clades and subclades of H5N1.
Results
VLP characterization of the 3 H5N1 COBRA HA VLP vaccines
The design and characterization of the computationally optimized broadly reactive antigen (COBRA) has been described previously.Citation11-13 Primary consensus sequences within each subclade were then aligned and the most common amino acid was chosen resulting in secondary consensus sequences representing each subclade (). The secondary consensus sequences were aligned and the most common amino acid at each position was selected resulting in the final consensus sequence referred to as clade 2 COBRA HA (). Using a predictive structural model of the 3 COBRA H5N1 HA sequences, 3-dimensional trimerized HA proteins were designed (). Despite nearly identical predicted structures, the COBRA HA proteins had subtle differences in the major antigenic binding and receptor-binding sites. There were 11 amino acid positions that differed in at least one of the 3 H5N1 COBRA HA sequences (). Phylogenetic analysis of the COBRA HA with all human isolates of H5N1 HA proteins indicated that COBRA retained a clade 2-like sequence without being grouped specifically within any clade 2 subclade cluster (). The human/avian COBRA 2 HA was more centrally located on the tree and was close to isolates in clade 8. In contrast, the COBRA HA was generated using all sequences (All H5N1 COBRA) and was situated on the tree close to clades 5 and 9. Furthermore, a BLAST search using each of the COBRA HA sequences revealed that each sequence was unique sequences that has not been isolated from the environment (data not shown).
Figure 1. Schematic of the Design of the H5N1 COBRA HA proteins. A phylogenetic tree was inferred from hemagglutinin amino acid sequences using the maximum likelihood method and clade/sub-clade groupings were identified. The first generation, human COBRA-2 (upper left) was designed using sequences identified in clade 2 from human infections (between 2005-2007) and has previously been described (Giles et al. 2010. Vaccine). Primary consensus sequences were generated for each outbreak group. Secondary consensus sequences were then generated for each subclade using the primary sequences as inputs. The secondary consensus sequences were then aligned and the resulting consensus, designated COBRA, was generated. The second generation of H5N1 COBRA HA proteins were designed using clade 2 isolates from both human and avian infections (upper right) and using all HA H5N1 sequences from all clades (2005-2010) in the NCBI and GASIAD databases.
Figure 2. Schematic of 3 dimensional structure of trimerized H5N1 COBRA HA proteins. (A) Predicted structural model of the H5N1 COBRA HA sequences. HA sequences were downloaded encoded NCBI GenBank and GISAID to develop the COBRA HA sequences. Each COBRA HA structure presented was generated using the 3D-JIGSAW algorithm based onCitation33 and renderings were performed using MacPyMol. (B) Amino acid positions in major HA antigenic sites that differ between the 3 COBRA H5N1 HA sequences.
Figure 3. Phylogenetic diversity of H5N1 influenza. The unrooted phylogenetic tree was inferred from HA amino acid sequences derived from 28 representative isolates in various clades and subclades and also the COBRA HA using the maximum likelihood method. Clade/subclade clusters are identified on the right. Sequences were aligned with MUSCLE 3.7 software and the alignment was refined by Gblocks 0.91 b software. Phylogeny was determined using the maximum likelihood method with PhyML software. Trees were rendered using TreeDyn 198.3 software.Citation42 The NCBI accession numbers for the HA sequences used in phylogeny inference were obtained through the Influenza Virus Resource.Citation43
Challenge with highly pathogenic avian influenza (HPAI) H5N1 viruses
To determine the protective efficacy of each H5N1 COBRA vaccine, mice were vaccinated in different regimens at different doses and then challenged with highly pathogenic H5N1 viruses (). The first set of mice were vaccinated 2 times with a 3 μg dose (at week 0 and 4) and then were challenged (week 8) with a lethal dose (5 × 103 PFU) of one of 2 the wild-type H5N1 viruses representing both major antigenic clusters (a clade 1 and a clade 2.1 isolate). All COBRA VLP-vaccinated mice and mice vaccinated with WS/05 VLPs were protected from weight loss and death, while mock-vaccinated animals rapidly lost weight and reached experimental endpoints between 6-9 days post-infection (DPI) (). All COBRA VLP-vaccinated mice failed to develop any overt signs of disease, while mock-vaccinated mice developed visible illness and had high viral titers in the lungs 3 days post-infection ().
Figure 4. Highly pathogenic H5N1 influenza virus challenge of mice. BALB/c mice (5 mice/group) vaccinated at weeks 0 and 4 with each vaccine plus alum adjuvant were infected with 5 × 10e+6 PFU of the highly pathogenic clade 2.1 H5N1 virus A/Whooperswan/Mongolia/244/2005 (WS/05) or the clade 1 H5N1 virus A/Vietnam/1203/2004 (VN/04). Mice were monitored daily for weight loss (A and C) and viral lung titers on selected mice on day 3 post-infection (B and D). Statistical significance of the antibody titer data was determined using 2-way analysis of variance followed by the Bonferroni posttest to analyze differences between each vaccine group for each of the different antigens that were tested (multiparametric). Significance was defined as P < 0.05. Statistical analyses were performed with GraphPad Prism software.
A second set of mice were vaccinated with a single 3 μg dose of vaccine and challenged at week 8 with a lethal dose of A/Vietnam/1203/2004. Again, these mice had little or no weight loss, no clinical signs of disease, and no mortality (data not shown). However, the VN/04 virus was detected in the lungs of all mice (. On day 3, unvaccinated mice had an average viral titer in the lungs of 10e + 6 pfu. Mice vaccinated with human COBRA-2 VLP or WS/05 VLP had 1.5 logs lower viral titer than unvaccinated mice (10e + 4.5 pfu). Mice vaccinated with either of the second-generation COBRA vaccines had even lower viral titers between 10e + 2.5 to 10e + 3.5 pfu.
Figure 5. Viral lung titers in mice vaccinated with a single vaccination. BALB/c mice (5 mice/group) vaccinated one time with a 3μg dose with each vaccine plus alum adjuvant and then were infected with 5 × 10e+6 PFU with the clade 1 H5N1 virus A/Vietnam/1203/2004 (VN/04). Mice were monitored daily for weight loss (data not shown) and viral lung titers from selected mice on day 2 and 3 post-infection. Statistical significance of the antibody titer data was determined using 2-way analysis of variance followed by the Bonferroni posttest to analyze differences between each vaccine group for each of the different antigens that were tested (multiparametric). Significance was defined as P < 0.05. Statistical analyses were performed with GraphPad Prism software.
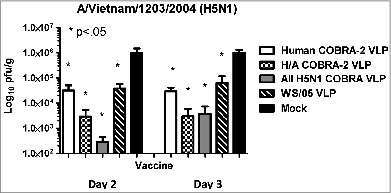
In order to differentiate the protective efficacy between the 3 vaccines, a third set of mice were vaccinated with a single half-log lower dose (0.6 μg) of each vaccine dose of each vaccine and challenged with either WS/05 or VN/04 viruses (). Once again, mock vaccinated mice rapidly lost weight to viral challenge and all mice succumbed to disease between days 6-8 post-infection. Mice vaccinated with Human COBRA-2, All H5N1, or WS/05 VLPs and challenged with VN/04 virus had 5-8% weight loss between days 5-6 post-infection and then recovered (). Mice vaccinated with the Human-Avian COBRA-2 VLPs had no weight loss and all mice survived infection. In contrast, mice challenged with the WS/05 virus suffered more severe weight loss regardless of vaccine used for immunization (). Mice vaccinated with the All H5N1 COBRA vaccine lost between 15-20% of their weight by day 6 with 40% of the mice dying from infection. Mice vaccinated with any of the other 3 vaccines lost on average 10% weight by day 6 following challenge with WS/05 virus.
Figure 6. Highly pathogenic H5N1 influenza virus challenge of mice. BALB/c mice (5 mice/group) vaccinated one time with a 0.6 ug dose with each vaccine plus alum adjuvant were infected with 5 × 10e + 6 PFU of WS/05 or VN/04. Mice were monitored daily for weight loss (A and B) and viral lung titers from selected mice on day 3 post-infection (C and D). Statistical significance of the antibody titer data was determined using 2-way analysis of variance followed by the Bonferroni posttest to analyze differences between each vaccine group for each of the different antigens that were tested (multiparametric). Significance was defined as P < 0.05. Statistical analyses were performed with GraphPad Prism software.
In contrast to mice that received higher doses of vaccine or more than one vaccination, there was little to no detectable HAI titers against VN/04, WS/05, or Indo/05 viruses (data not shown). The lack of HAI activity and the increased weight loss directly translated into higher viral titers in the lungs of mice 2 and 3 days p.i. (). At day 3 post-infection, mock-vaccinated mice had 10e + 6 pfu VN/04 viral titers in the lungs, which was similar to mice vaccinated with WS/05 VLP or the All H5N1 COBRA VLP vaccine (). Mice vaccinated with human COBRA-2 or human/avian COBRA-2 had ∼log less VN/04 viral titers, albeit these viral titers were significantly higher than mice vaccinated with 3 ug mice (P > 0.05). Vaccinated mice that were challenge with WS/05 virus had lower viral titers on day 2 and 3 post-infection than mice challenged with VN/04, with titers ranging from 10e + 4.5 to 10e + 5.5 pfu (). WS/05 viral titers on day 3 were significantly lower in mice vaccinated with human COBRA-2 or human/avian COBRA-2 compared to mock vaccinated mice ().
VLPs elicit antibody responses in vaccinated mice
Previously, we demonstrated that VLPs with the human COBRA-2 HA were more effective at eliciting antibodies that recognized a broader number of H5N1 isolates compared to VLPs with an HA from a wild-type clade 2.2 isolate.Citation11,14,15 However, antibodies elicited by the COBRA-2 VLP did not recognize all strains tested, particularly in clades 1 and 4. In order to expand the breadth of antibody recognition, we developed second-generation H5N1 COBRA HA proteins to include epitopes from both human and avian isolates representing all clades of H5N1. BALB/c mice (n = 15) were vaccinated twice at 4-week intervals via intramuscular injection with purified VLPs (3μg based upon HA content) with one of the 3 H5N1 COBRA HA vaccines. At day 35, serum was analyzed for antibody responses. All vaccinated mice had high-titer anti-HA antibodies that bound to recombinant HA derived from both clade 1 and various subclades of clade 2 (data now shown). Although all 3 COBRA HA VLP vaccines elicited similar IgG titers, COBRA-vaccinated animals had higher HAI antibody titers for all viruses tested (P > 0.001).
While both second generation COBRA HA proteins elicited antibodies that recognized similar numbers of H5N1 viruses compared to the first-generation human COBRA-2 vaccine (9-11 out of 16 viruses), there were some unique differences in virus recognition by each COBRA HA antigen. H5N1 viruses can be grouped into 3 antigenic clusters with clades 0, 1, 3, 4, 5, 6, 7.1, and 9 as Antigenic Cluster 1 and subclades 2.2.1, 2.1.3.2, 2.3.4, 2.4, 2.5, and 8 into Antigenic Cluster 2. Viruses in subclade 2.3.2 and 7.2 stand as individual groups.Citation16 The Human/Avian and All H5N1 COBRA HA antigens elicited antibodies with HAI activity against both clade 1 viruses (). In contrast, only the human COBRA-2 HA elicited antibodies that recognized the clade 7 isolate. All three H5N1 COBRA HA antigens elicited HAI activity against the clade 2 viruses, Indo/05, Tk/05, Eg/07, Hubei/10, and both second generation HA vaccines also elicited antibodies that recognized VN/04, Tk/11, and Bng/11 isolates. In general, HAI titers appeared lower against viral isolates from 2011 and 2012, regardless which COBRA HA antigen was used for vaccination (). The All H5N1 COBRA and Human COBRA-2 HA VLP vaccines elicited HAI activity against both clade 2.3.4 isolates. None of the vaccines elicited high HAI activity against the 2.3.2.1 cluster of viruses (). The wild-type WS/05 HA VLP vaccine elicited low HAI titer antibodies to viruses isolated between 2004-2007, but did not recognize the other viruses isolated between 2008-2012. Overall, there was only one virus, IN/12 (clade 2.1.3.2) that was not recognized by any of these COBRA HA VLP vaccines. Therefore, each individual COBRA HA VLP vaccine was able to elicit antibodies with a broader HAI activity against a larger number of H5N1 viruses from both antigenic clusters than VLP vaccines with a wild-type HA.
Table 1. HAI serum antibody titers from vaccinated mice against a panel of H5N1 isolates
A cocktail of H5N1 COBRA HA VLPs elicits protective antibody responses against H5N1 isolates
Since each of the 3 H5N1 COBRA HA proteins elicit HAI activity to different H5N1 viral strains, we decided to mix a cocktail of the 3 COBRA VLP vaccines together in order stimulate the broadest breadth of HAI activity against an expanded panel of 25 H5N1 isolates. Mice were vaccinated with 3 ug dose of each of the 3 H5N1 COBRA HA VLP vaccines intramuscularly at weeks 0 and 3. All mice vaccinated with the cocktail of H5N1 vaccines had HAI activity against the entire panel of 25 H5N1 viruses that were isolated from 2002-2014 (). These mice were protected against both weight loss and death following a lethal challenge using either VN/04 or WS/05 viruses and none of the COBRA VLP vaccinated mice had detectable viral titers (data not shown).
Discussion
In this study, we compared the immunogenicity and protective efficacy of 3 COBRA HA strategies proposed to increase breadth of antibody responses to a panel of H5N1 influenza viruses. The COBRA HA antigens used in these studies were designed specifically to address the diversity present within clade 2,Citation11 as well as across all clades of H5N1. Since we do not know which of the various strains from any clade or subclade of H5N1 circulating in bird populations may transform into a human transmissible influenza virus, designing vaccines to address all the potential strains from any clade of H5N1 is a prudent strategy. The Clade 2 specific COBRA HA proteins (COBRA-2 and Human/Avian COBRA-2) were generated using the genetically diverse clade 2 strains isolated. Clade 2 viruses are currently circulating widely in the Eastern Hemisphere. Clade 2 is divided into distinct subclades, including 2.1, 2.2, 2.3, 2.4, and 2.5, with some subclades being further divided into sub-subclades.Citation16 Furthermore, within clade 2, humans have been infected with isolates representing clades 2.1, 2.2, and 2.3, with the recent human infections in Egypt identified as clade 2.2Citation16 and clade 2.1.3.2 infections in Bangladesh and southeast Asia. To generate a COBRA HA VLP vaccine for all isolates in the H5N1 influenza virus family, HA sequences from isolates representing all 10 clades were used for the All H5N1 COBRA HA vaccine. All the 1525 input sequences used to generate any of the 3 H5N1 COBRA sequences were collected between 2005 and 2008. Since these COBRA sequences were designed from sequences representing 2005-2008 isolates, we tested the power of the COBRA methodology to detect viruses isolated both prior to 2005 and isolated as recently as 2014. Using sequences from viruses isolated between 2005-2008 allowed us to show the power of the COBRA approach to continue to recognize drifting isolates of H5N1 over the following years 2009-2014 without having to re-calculate the vaccine and re-produce a new vaccine to detect these later strains. We demonstrated that we can elicit HAI antibodies that recognize H5N1 HPAIV collected between 2002 and 2014.
Each of the 3 COBRA HA antigens elicited HAI antibodies that recognized a similar number of H5N1 viruses in our panel (9-11 out of the initial 17). These titers were maintained for 72 weeks and these were protected against both VN/04 and WS/05 challenge with little weight loss or signs of morbidity (data not shown). However, individually, each of the COBRA HA VLP vaccines recognized a different set of viruses in the panel, with many of the viruses being recognized by antibodies elicited by at least one COBRA HA.
Some of the most difficult strains in our panel to recognize were detected with the COBRA antisera are part of the newly emerging sub-subclade in Southeast Asia. Beginning in 2011, an area from Bangladesh to Indonesia saw the introduction of these isolates into poultry, with some accompanying human infections and deaths.Citation17,18 In 2007, clade 2.2 viruses were predominant in this region, but these viruses were replaced by the introduction of viruses from sub-subclades 2.3.2.1 and 2.3.4 in 2011. However, viruses in sub-subclade 2.3.2.1 are progressively replacing clade 2.2 and 2.3.4 viruses. In addition, there has been segment reassortment between H5N1 and H9N2 viruses circulating in Bangladesh, where H5N1 viruses are acquiring the PB1 gene from a H9N2 virus subtype.Citation17,19,20 Point mutations have accumulated in these isolates over the last 5 years with potential modification of receptor binding and antigenic sites. The addition of these mutations may explain why these viruses were the most difficult viruses to detect with the elicited COBRA ferret antisera. If viruses in this sub-subclade are acquiring segments from H9N2 and additional mutations in HA enhance binding and entry into human cells, a human transmissible H5N1 isolate could emerge. Similar to the newly emerged H7N9 viruses in China that have killed 157 out of 448 cases (35% mortality rate) in 2 waves since early 2013.Citation21 These H7N9 isolates contain gene segments from H9N2 with a viral phenotype that has low pathogenicity in poultry, but a high mortality rate in humans.Citation22-26 The continued mutations of viruses in sub subclade 2.3.2.1, along with reassortment with H9N2 viruses, could result in a similar H5N1 phenotype.
While our 3 H5N1 COBRA VLP vaccines did not recognize all the viruses in the panel, collectively the 3 vaccines elicited a polyclonal antibody response that recognized all viruses in the panel. Therefore, we speculated if all 3 COBRA HA VLP vaccines were mixed in a cocktail, that the elicited antibody responses would recognize all isolates in the H5N1 viruses. There are structural changes between HA proteins with a clade and between different clades of H5N1 that may expose different epitopes on the HA molecule. Individually, the 3 H5N1 COBRA HA VLP vaccines elicited antibodies that neutralized different sets of H5N1 viruses in the panel based upon similarities in the structure of their HA molecules. The human COBRA-2 HA VLP vaccine had HAI activity against all the clade 2.1 and 2.2 isolates except those isolated in 2011 and 2012. The COBRA-2 HA has a similar structure around the receptor binding domain and the region equivalent to the Sa region of H1N1 viruses with specific residues that appear common in our different H5N1 COBRA HA sequences used in this study (). All of the Clade 2 viruses isolated from 2005-2008 had similar antigenic structures. In contrast, the HA structures of the more recently isolated clade 2 viruses have a more closed receptor binding domain (RBD) that may indicate why COBRA-2 did not elicit antibodies that recognized this altered receptor binding domain.Citation27 Both second-generation H5N1 COBRA HA VLP vaccines elicited antibodies that had HAI activity against these viruses from 2011, but not 2012 and they did not have HAI activity against Eg/08. Several additional examples can be identified from the empirical HAI titers determined in this study and the predicted HA structure, but in any case, once mice had been vaccinated simultaneously with all 3 H5N1 COBRA HA VLP vaccines, all 25 viruses in the panel were recognized in the HAI assay ().
Table 2. Amino acid differences identified in the 5 antigenic sites on the H5N1 HA proteins
A recent report describes a heterologous prime-boost regimen with a consensus-like HA from a single H5N1 strain that can elicit antibody responses that cross-neutralize across clades. The elicited antibodies were directed to the HA head regionCitation28 indicating that cross-neutralizing antibodies to H5N1 isolates is possible. Unfortunately in our study, none of the 3 H5N1 COBRA VLP vaccines alone elicited HAI activity against the entire panel of 25 H5N1 viruses. And only when a cocktail of COBRA VLP vaccines was used did we achieve HAI against all strains tested, albeit some a minimum titers (). The COBRA VLP cocktail vaccine elicited HAI antibody activity against H5N1 isolates representing a 12-year period of collection. Eventually, it would be desirable to design a single COBRA HA VLP vaccine that elicits protective immune responses against as many H5N1 influenza viruses as possible over several years. A new technology is under development to engineer a “directed COBRA” strategy that could expand the breadth of HAI activity elicited by a single H5N1 COBRA HA and to expand the protective immune responses to other, more distant, strains of H5N1 influenza. This strategy will be the focus of a future study.
Lack of HAI activity from sera obtained from vaccinated animals against an H5N1 influenza virus does not always correlate with protection against that same H5N1 influenza virus when the animal is challenged.Citation29-31 However, when HAI activity is detected against a particular H5N1 influenza strain, pre-clinical animal models are protected against challenge. Therefore, one of the limitations of our study is the lack of HAI activity against a strain in our panel does not mean the COBRA HA protein did not elicit protective immune responses. Future studies will be necessary to determine whether the COBRA HA-induced antibody responses are sufficient for protection against a panel of H5N1 high-path avian influenza viruses.
The data presented in this report demonstrates that a cocktail of COBRA H5N1 HA proteins, presented to the immune system on a single VLP platform, can elicit cross-clade HAI activity against H5N1 viruses from different clades over a 12-year span of time. Each of these COBRA HA antigens most likely elicits different sets of antibodies that bind to different antigenic regions of the HA molecule, but when administered together as a cocktail, they elicit antibodies that recognize divergent HA head epitopes that synergistically neutralize viral infection.
Materials and Methods
Antigen construction and synthesis
Influenza A HA nucleotide sequences isolated from human H5N1 infections were downloaded from the NCBI Influenza Virus Resource database.Citation11 Nucleotide sequences were translated into protein sequences using the standard genetic code. Full-length sequences from H5N1 viral infections isolated from avian and human sources between 2004 to 2008 were used in this analysis. For each round of consensus generation, multiple alignment analysis was applied and the consensus sequence was generated using AlignX (Vector NTI). The final amino acid sequence, termed computationally optimized broadly reactive antigen (COBRA), was reverse translated and optimized for expression in mammalian cells, including codon usage and RNA optimization (GeneArt; Regensburg, Germany). Three H5N1 HA constructs were synthesized and inserted into the pTR600 expression vector, as previously described.Citation32 We designed COBRA HA antigens to represent (1) human clade 2 isolates, (2) human and avian clade 2 isolates, and (3) all clades of H5N1. Unique hemagglutinin (HA) sequences (525) were downloaded from the NCBI Influenza Virus Resource (IVR) sequence database.Citation11 The sequences were first grouped into phylogenetic subclades. HA amino acid sequences for each individual outbreak group were aligned and the most common amino acid at each position was determined resulting in primary consensus sequences representing each outbreak group within each subclade (). Each COBRA HA structure was generated using the 3D-JIGSAW algorithmCitation33,34 and renderings were performed using MacPyMol (). A phylogenetic tree was inferred from hemagglutinin amino acid sequences using the maximum likelihood method and clade/sub-clade groupings were identified using Jalview (Dundee, UK) ().
In vitro expression
Human embryonic kidney (HEK) 293T cells (1 × 106) were transiently transfected with 3 μg DNA expressing each COBRA or wild-type HA gene cassette. Cells were incubated for 72 h at 37°C and then lysed with 1% Triton-X 100 and clarified supernatant harvested following centrifugation. Cell lysates were then electrophoresed on a 10% SDS-PAGE gel and transferred to a PVDF membrane. The blot was probed with pooled mouse antisera from infections with 6:2 reassortant H5N1 viruses expressing HA derived from either A/Vietnam/1203/2004 or A/Whooper Swan/244/2005. HA-antibody complexes were then detected using goat anti-mouse IgG HRP (Southern Biotech; Birmingham, AL, USA). HRP activity was detected using chemiluminescent substrate (Pierce Biotechnology; Rockford IL, USA) and exposed to X-ray film (ThermoFisher; Pittsburgh, PA, USA).
Functional characterization
To determine receptor-binding characteristics, virus-like particles (VLPs) containing COBRA HA proteins were purified from the supernatants of mammalian cell lines as previously described.Citation35 HEK 293T cells were transiently transfected with plasmids expressing HIV Gag, COBRA HA and neuraminidase (NA, A/Thailand/1(KAN-1)/2004) and incubated for 72 h at 37°C. Supernatants were collected and VLPs were purified via ultracentrifugation (100,000 × g through 20% glycerol, weight per volume) for 4 h at 4°C. The pellets were subsequently resuspended in phosphate buffered saline PBS, pH 7.2 and stored at −80°C until use. Protein concentration was determined by Micro BCATM Protein Assay Reagent Kit (Pierce Biotechnology, Rockford, IL, USA). COBRA HA VLPs were prepared in various amounts as measured by total HA protein and each individual preparation was 2-fold serially diluted in v-bottom microtiter plates. An equal volume of 1% horse erythrocytes (RBC) (Lampire; Pipersville, PA, USA) in PBS was added to the diluted VLPs and incubated for 60 minutes at room temperature. The HA titer was determined by the reciprocal dilution of the last well which contained agglutinated RBC.
Vaccine preparation
HEK 293T cells were transiently transfected with plasmids expressing M1 (A/Puerto Rico/8/1934, optimized for expression in mammalian cells), NA (A/Thailand/1(KAN-1)/2004, optimized for expression in mammalian cells) and COBRA HA and incubated for 72 h at 37°C. Supernatants were collected and cell debris removed by low speed centrifugation followed by vacuum filtration through a 0.22 μm sterile filter. VLPs were purified via ultracentrifugation (100,000 × g through 20% glycerol, weight per volume) for 4 h at 4C. The pellets were subsequently resuspended in PBS pH 7.2 and stored in single use aliquots at −80C until use. Total protein concentration was determined by Micro BCATM Protein Assay Reagent Kit (Pierce Biotechnology, Rockford, IL, USA).
HA specific content was determined by western blot and densitometry. Purified recombinant COBRA HA and purified VLPs were prepared in standard total protein amounts and were electrophoresed on a 10% SDS-PAGE gel and transferred to a PVDF membrane. The blot was probed with mouse polyclonal antisera pooled from mice infected with 6:2 reassortant H5N1 viruses with the surface glycoproteins derived from either A/Vietnam/1203/2004 or A/Whooper Swan/244/2005 and the HA-antibody complexes were detected using a goat anti-mouse IgG conjugated to horse radish peroxidase (HRP) (Southern Biotech; Birmingham, AL, USA). HRP was detected by chemiluminescent substrate (Pierce Biotechnology; Rockford IL, USA) and exposed to X-ray film (ThermoFisher; Pittsburgh, PA, USA). Density of bands was determined using ImageJ software (NIH). Density of recombinant HA bands were used to calculate a standard curve and the density of the purified VLPs was interpolated using the results from the recombinant HA. Experiments were performed in triplicate and multiple exposure times were analyzed for all iterations.
Mouse studies
BALB/c mice (Mus musculis, females, 6–8 weeks) were purchased from Harlan Sprague Dawley, (Indianapolis, IN, USA) and housed in microisolator units and allowed free access to food and water and were cared for under USDA guidelines for laboratory animals. Mice (15 mice per group) were vaccinated with purified VLPs (3.0μg) based upon HA content from the densitometry assay, via intramuscular injection at week 0 and then boosted with the same dose at week 4. Some mice were administered a cocktail of H5N1 COBRA VLP vaccines (1.0μg dose of each of the 3 vaccines). Vaccines at each dose were formulated with Imject® alum adjuvant (Imject® Alum, Pierce Biotechnology; Rockford, IL, USA) according to the manufacturer's protocol or vehicle alone. Twenty-eight days after each vaccination, blood was collected from anesthetized mice via the retro-orbital plexus and transferred to a microfuge tube. Tubes were centrifuged and sera was removed and frozen at −20 ± 5°C.
Four weeks after final vaccination, mice were challenged intranasally with 5 × 10Citation3 plaque forming units (PFU) of the highly pathogenic H5N1 virus A/Whooper Swan/Mongolia/244/2005 (Clade 2.2) or A/VietNam/1203/2004 (Clade 1) in a volume of 50μl. After infection, mice were monitored daily for weight loss, disease signs and death for 14 days after infection. Individual body weights and death were recorded for each group on each day after inoculation. Experimental endpoint was defined as >20% weight loss or display of neurological disease such as hind limb paralysis. And day 2 and 3, 5 mice were humanely sacrificed on lungs collected for determination of viral titers. All H5N1 influenza virus studies were performed under high-containment biosafety level 3 enhanced conditions (BSL3+). All procedures were in accordance with the NRC Guide for the Care and Use of Laboratory Animals, the Animal Welfare Act, and the CDC/NIH Biosafety in Microbiological and Biomedical Laboratories.
ELISA assay
The ELISA assay was used to assess total antibody titer and IgG isotype titer to the HA. High binding, 96-well polystyrene plates (Costar; Lowell, MA, USA) were coated overnight with 50 ng/well of recombinant HA. Coating antigens were derived from the following representative viral isolates: A/Vietnam/1203/2004 (clade 1), A/Indonesia/5/2005 (clade 2.1), A/Whooper Swan/244/2005 (clade 2.2) and A/Anhui/1/2005 (clade 2.3). Plates were blocked with 5% milk diluted in PBS with 0.05% Tween 20 (blocking buffer). Serum samples were diluted in blocking buffer and added to plates. Serum was 2-fold serially diluted and allowed to incubate for 1 hour at room temperature. Plates were washed and HRP-conjugated polyclonal goat anti-murine IgG were diluted in blocking buffer and added to plates. Plates were incubated for 1 h at room temperature, washed and HRP activity detected with TMB substrate (Sigma-Aldrich; St. Louis, MO, USA). Plates were incubated in the dark for 15 minutes and then the reaction was stopped with 2N H2SO4. Optical densities at a wavelength of 450 nm (OD450) were read by a spectrophotometer (BioTek; Winooski, VT, USA) and end point dilution titers were determined. End point titers were determined as the reciprocal dilution of the last well, which had an OD450 above the mean OD450 plus 2 standard deviations of naïve animal sera.
Hemagglutination inhibition (HAI) assay
The HAI assay was used to assess functional antibodies to the HA able to inhibit agglutination of horse erythrocytes. The protocol was adapted from the CDC laboratory-based influenza surveillance manual.Citation36 To inactivate non-specific inhibitors, sera were treated with receptor destroying enzyme (RDE; Denka Seiken, Co., Japan) prior to being tested.Citation32,37-40 Briefly, 3 parts RDE was added to one part sera and incubated overnight at 37°C. RDE was inactivated by incubation at 56°C for ∼30 min. RDE treated sera was 2-fold serially diluted in v-bottom microtiter plates. An equal volume of each H5N1 virus, adjusted to approximately 8 HAU/50 μl, was added to each well. The plates were covered and incubated at room temperature for 20 min followed by the addition of 1% horse erythrocytes (HRBC) (Lampire Biologicals, Pipersville, PA, USA) in PBS. Red blood cells were stored at 4°C and used within 72 h of preparation. The plates were mixed by agitation, covered, and the RBCs were allowed to settle for 1 h at room temperature.Citation41 The HAI titer was determined by the reciprocal dilution of the last well that contained non-agglutinated RBC. Positive and negative serum controls were included for each plate. All mice were negative (HAI ≤1:10) for pre-existing antibodies to currently circulating human influenza viruses prior to vaccination.
Plaque assay
Madin-Darby Canine Kidney (MDCK) cells were plated (5 × 105) in each well of a 6-well plate. Samples were diluted (final dilution factors of 100 to 10−6) and overlayed onto the cells in 100 μl of DMEM supplemented with penicillin-streptomycin and incubated for 1 hr. Samples were removed, cells were washed twice and media was replaced with 2 ml of L15 medium plus 0.8% agarose (Cambrex; East Rutherford, NJ, USA) and incubated for 72 h at 37°C with 5% CO2. Agarose was removed and discarded. Cells were fixed with 10% buffered formalin, and then stained with 1% crystal violet for 15 min. Following thorough washing in dH2O to remove excess crystal violet, plates were allowed to dry, plaques counted, and the plaque forming units (PFU)/ml were calculated.
Statistical analysis
Statistical significance of the antibody data was determined using a 2-way analysis of variance (ANOVA) with Bonferroni's post-test to analyze differences between each vaccine group for the different test antigens (multiparametric). Differences in weight loss, sickness score, and viral titers were analyzed by 2-way ANOVA, followed by Bonferroni's post-test for each vaccine group at multiple time points. Significance was defined as P < 0.05. Statistical analyses were done using GraphPad Prism software.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Tim Alefantis, Mark Parrington, Harry Kleanthous, and Greg Kirchenbaum for helpful discussions and review. In addition, we thank Ruben Donis and Richard Webby for providing the H5N1 reassortant viruses. We would like to thank R. Sodnomdarjaa and the Mongolian State Central Veterinary Laboratory for providing permission to use the A/Whooper Swan/Mongolia/244/2005 influenza virus.
Funding
This work was supported by NIH/NIAID award U01AI077771, a Sponsored Research Agreement from Sanofi-Pasteur, and the Vaccine and Gene Therapy Institute of Florida. KYJL served as an intern from the University of British Columbia Science Co-op Program.
References
- Webster RG, Govorkova EA. H5N1 influenza–continuing evolution and spread. N Engl J Med 2006; 355(21):2174-7; PMID:17124014; http://dx.doi.org/10.1056/NEJMp068205
- Wibawa H, Henning J, Wong F, Selleck P, Junaidi A, Bingham J, Daniels P, Meers J. A molecular and antigenic survey of H5N1 highly pathogenic avian influenza virus isolates from smallholder duck farms in Central Java, Indonesia during 2007-2008. Virol J 2011; 8:425; PMID:21896207; http://dx.doi.org/10.1186/1743-422X-8-425
- Nguyen DC, Uyeki TM, Jadhao S, Maines T, Shaw M, Matsuoka Y, Smith C, Rowe T, Lu X, Hall H, et al. Isolation and characterization of avian influenza viruses, including highly pathogenic H5N1, from poultry in live bird markets in Hanoi, Vietnam, in 2001. J Virol 2005; 79(7):4201-12; PMID:15767421; http://dx.doi.org/10.1128/JVI.79.7.4201-4212.2005
- Wallace RG, Hodac H, Lathrop RH, Fitch WM. A statistical phylogeography of influenza A H5N1. Proc Natl Acad Sci U S A 2007; 104(11):4473-8; PMID:17360548; http://dx.doi.org/10.1073/pnas.0700435104
- Pabbaraju K, Tellier R, Wong S, Li Y, Bastien N, Tang JW, Drews SJ, Jang Y, Davis CT, Fonseca K, Tipples GA. Full-genome analysis of avian influenza A(H5N1) virus from a human, North America, 2013. Emerg Infect Dis 2014; 20(5):887-91; PMID:24755439
- Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 2012; 336(6088):1534-41; PMID:22723413; http://dx.doi.org/10.1126/science.1213362
- Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, Zhong G, Hanson A, Katsura H, Watanabe S, et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 2012; 486(7403):420-8; PMID:22722205
- Ducatez MF, Cai Z, Peiris M, Guan Y, Ye Z, Wan XF, Webby RJ. Extent of antigenic cross-reactivity among highly pathogenic H5N1 influenza viruses. J Clin Microbiol 2011; 49(10):3531-6; PMID:21832017; http://dx.doi.org/10.1128/JCM.01279-11
- Organization WH, Antigenic and genetic characteristics of H5N1 viruses and candidate H5N1 vaccine viruses developed for potential use as human vaccines.http://www.who.int/csr/disease/avian_influenza/guidelines/H5VaccineVirusUpdate20080214.pdf, 2008
- Finnefrock AC, Liu X, Opalka DW, Shiver JW, Casimiro DR, Condra JH. HIV type 1 vaccines for worldwide use: predicting in-clade and cross-clade breadth of immune responses. AIDS Res Hum Retroviruses 2007; 23(10):1283-92; PMID:17961117; http://dx.doi.org/10.1089/aid.2007.0098
- Giles BM, Ross TM. Development of a computationally optimized broadly reactive (COBRA) hemagglutinin for elicitation of protective antibodies against multiple clades of H5N1. Vaccine 2011; 29:3043-54; PMID:21320540; http://dx.doi.org/10.1016/j.vaccine.2011.01.100
- Giles BM, Ross TM. Computationally optimized antigens to overcome influenza viral diversity. Expert Rev Vaccines 2012; 11(3):267-9; PMID:22380818; http://dx.doi.org/10.1586/erv.12.3
- Kirchenbaum GA, Ross TM. Eliciting broadly protective antibody responses against influenza. Curr Opin Immunol 2014; 28C:1-76
- Giles BM, Bissel SJ, Dealmeida DR, Wiley CA, Ross TM. Antibody breadth and protective efficacy is increased by vaccination with computationally optimized hemagglutinin but not with polyvalent hemagglutinin base H5N1 VLP vaccines. Clin Vacc Immunol 2012; 19(2):128-39; PMID:22190399; http://dx.doi.org/10.1128/CVI.05533-11
- Giles BM, Crevar CJ, Carter DM, Bissel SJ, Schultz-Cherry S, Wiley CA, Ross TM. Computationally-optimized hemagglutinin expressed on a virus-like particle vaccine elicits broadly-reactive antibodies that protect non-human primates from H5N1 clade 2 influenza infection. J Inf Dis 2012; 205(10):1562-70; http://dx.doi.org/10.1093/infdis/jis232
- Zhou F, Wang G, Buchy P, Cai Z, Chen H, Chen Z, Cheng G, Wan XF, Deubel V, Zhou P. A triclade DNA vaccine designed on the basis of a comprehensive serologic study elicits neutralizing antibody responses against all clades and subclades of highly pathogenic avian influenza H5N1 viruses. J Virol 2012; 86(12):6970-8; PMID:22496212; http://dx.doi.org/10.1128/JVI.06930-11
- Haque ME, Giasuddin M, Chowdhury EH, Islam MR. Molecular evolution of H5N1 highly pathogenic avian influenza viruses in Bangladesh between 2007 and 2012. Avian Pathol 2014; 43(2):183-94; PMID:24689433; http://dx.doi.org/10.1080/03079457.2014.898244
- Osmani MG, Ward MP, Giasuddin M, Islam MR, Kalam A. The spread of highly pathogenic avian influenza (subtype H5N1) clades in Bangladesh 2010 and 2011. Prev Vet Med 2014; 114(1): 21-7; PMID:24485276; http://dx.doi.org/10.1016/j.prevetmed.2014.01.010
- Parvin R, Heenemann K, Halami MY, Chowdhury EH, Islam MR, Vahlenkamp TW. Full-genome analysis of avian influenza virus H9N2 from Bangladesh reveals internal gene reassortments with two distinct highly pathogenic avian influenza viruses. Arch Virol 2014; 159(7):1651-61; PMID:24420161
- Monne I, Fusaro A, Nelson MI, Bonfanti L, Mulatti P, Hughes J, Murcia PR, Schivo A, Valastro V, Moreno A, et al. Emergence of a highly pathogenic avian influenza virus from a low-pathogenic progenitor. J Virol 2014; 88(8):4375-88; PMID:24501401; http://dx.doi.org/10.1128/JVI.03181-13
- Policy C. f.I.D.R.a., Influenza Update. http://www.cidrap.umn.edu 2014
- Feng Y, Mao H, Xu C, Jiang J, Chen Y, Yan J, Gao J, Li Z, Xia S, Lu Y. Origin and characteristics of internal genes affect infectivity of the novel avian-origin influenza A (H7N9) virus. PLoS One 2013; 8(11):e81136; PMID:24278391; http://dx.doi.org/10.1371/journal.pone.0081136
- Wu A, Su C, Wang D, Peng Y, Liu M, Hua S, Li T, Gao GF, Tang H, Chen J, et al. Sequential reassortments underlie diverse influenza H7N9 genotypes in China. Cell Host Microbe 2013; 14(4):446-52; PMID:24055604; http://dx.doi.org/10.1016/j.chom.2013.09.001
- Lam TT, Wang J, Shen Y, Zhou B, Duan L, Cheung CL, Ma C, Lycett SJ, Leung CY, Chen X, et al. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature 2013; 502(7470):241-4; PMID:23965623; http://dx.doi.org/10.1038/nature12515
- Liu D, Shi W, Shi Y, Wang D, Xiao H, Li W, Bi Y, Wu Y, Li X, Yan J, et al. Origin and diversity of novel avian influenza A H7N9 viruses causing human infection: phylogenetic, structural, and coalescent analyses. Lancet 2013; 381(9881):1926-32; PMID:23643111; http://dx.doi.org/10.1016/S0140-6736(13)60938-1
- Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med 2013; 368(20):1888-97; PMID:23577628; http://dx.doi.org/10.1056/NEJMoa1304459
- Yang ZY, Wei CJ, Kong WP, Wu L, Xu L, Smith DF, Nabel GJ. Immunization by avian H5 influenza hemagglutinin mutants with altered receptor binding specificity. Science 2007; 317(5839):825-8; PMID:17690300; http://dx.doi.org/10.1126/science.1135165
- Wang G, Zhou F, Buchy P, Zuo T, Hu H, Liu J, Song Y, Ding H, Tsai C, Chen Z, et al. DNA prime and virus-like particle boost from a single H5N1 strain elicits broadly neutralizing antibody responses against head region of H5 hemagglutinin. J Infect Dis 2014; 209(5):676-85; PMID:23911711; http://dx.doi.org/10.1093/infdis/jit414
- Bright RA, Carter DM, Crevar CJ, Toapanta FR, Steckbeck JD, Cole KS, Kumar NM, Pushko P, Smith G, Tumpey TM, et al. Cross-clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an influenza virus-like particle. PLoS One 2008; 3(1):e1501; http://dx.doi.org/10.1371/journal.pone.0001501
- Giles BM, Bissel SJ, Dealmeida DR, Wiley CA, Ross TM. Antibody breadth and protective efficacy are increased by vaccination with computationally optimized hemagglutinin but not with polyvalent hemagglutinin-based H5N1 virus-like particle vaccines. Clin Vaccine Immunol 2012; 19(2):128-39; PMID:22190399; http://dx.doi.org/10.1128/CVI.05533-11
- Talaat KR, Luke CJ, Khurana S, Manischewitz J, King LR, McMahon BA, Karron RA, Lewis KD, Qin J, Follmann DA. A live attenuated influenza A(H5N1) vaccine induces long-term immunity in the absence of a primary antibody response. J Infect Dis 2014; 209(12):1860-9; PMID:24604819; http://dx.doi.org/10.1093/infdis/jiu123
- Ross TM, Xu Y, Bright RA, Robinson HL. C3d enhancement of antibodies to hemagglutinin accelerates protection against influenza virus challenge. Nat Immunol 2000; 1(2):127-31; PMID:11248804; http://dx.doi.org/10.1038/77802
- Soding J. Protein homology detection by HMM-HMM comparison. Bioinformatics 2005; 21(7):951-60; PMID:15531603; http://dx.doi.org/10.1093/bioinformatics/bti125
- Soding J, Biegert A, Lupas AN. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res 2005; 33(Web Server issue):W244-8; PMID:15980461; http://dx.doi.org/10.1093/nar/gki408
- Giles BM, Ross TM. A computationally optimized broadly reactive antigen (COBRA) based H5N1 VLP vaccine elicits broadly reactive antibodies in mice and ferrets. Vaccine 2011; 29(16):3043-54; PMID:21320540; http://dx.doi.org/10.1016/j.vaccine.2011.01.100
- Gillim-Ross L, Subbarao K. Emerging respiratory viruses: challenges and vaccine strategies. Clin Microbiol Rev 2006; 19(4):614-36; PMID:17041137; http://dx.doi.org/10.1128/CMR.00005-06
- Bright RA, Medina MJ, Xu X, Perez-Oronoz G, Wallis TR, Davis XM, Povinelli L, Cox NJ, Klimov A. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet 2005; 366(9492):1175-81; PMID:16198766; http://dx.doi.org/10.1016/S0140-6736(05)67338-2
- Bright RA, Ross TM, Subbarao K, Robinson HL, Katz JM. Impact of glycosylation on the immunogenicity of a DNA-based influenza H5 HA vaccine. Virology 2003; 308(2):270-8; PMID:12706077; http://dx.doi.org/10.1016/S0042-6822(03)00008-4
- Bright RA, Shay DK, Shu B, Cox NJ, Klimov AI. Adamantane resistance among influenza A viruses isolated early during the 2005-2006 influenza season in the United States. JAMA 2006; 295(8):891-4; PMID:16456087; http://dx.doi.org/10.1001/jama.295.8.joc60020
- Mitchell JA, Green TD, Bright RA, Ross TM. Induction of heterosubtypic immunity to influenza A virus using a DNA vaccine expressing hemagglutinin-C3d fusion proteins. Vaccine 2003; 21(9-10):902-14; PMID:12547601; http://dx.doi.org/10.1016/S0264-410X(02)00539-X
- Askonas B, McMichael A, Webster RG. The immune response to influenza viruses and the problem of protection against infection. in Basic and Applied Influenza Research, B. AS, Editor. 1982, CRC Press. p. 159-88
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 2008; 36(Web Server issue):W465-9; PMID:18424797; http://dx.doi.org/10.1093/nar/gkn180
- Bao Y, Bolotov P, Dernovoy D, Kiryutin B, Zaslavsky L, Tatusova T, Ostell J, Lipman D. The influenza virus resource at the National Center for Biotechnology Information. J Virol 2008; 82(2):596-601; PMID:17942553; http://dx.doi.org/10.1128/JVI.02005-07
