Abstract
This phase III, randomized, open-label, multicenter study (NCT01027845) conducted in Japan assessed the immunogenicity, safety, and reactogenicity of 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV, given intramuscularly) co-administered with diphtheria-tetanus-acellular pertussis vaccine (DTPa, given subcutaneously). Infants (N=360 ) were randomized (2:1) to receive either PHiD-CV and DTPa (PHiD-CV group) or DTPa alone (control group) as 3-dose primary vaccination (3–4–5 months of age) and booster vaccination (17–19 months of age). Immune responses were measured before and one month after primary/booster vaccination and adverse events (AEs) were recorded. Post-primary immune responses were non-inferior to those in pivotal/efficacy European or Latin American pneumococcal protein D-conjugate vaccine studies. For each PHiD-CV serotype, at least 92.6% of infants post-primary vaccination and at least 97.7% of children post-booster had pneumococcal antibody concentrations ≥0.2 μg/ml, and at least 95.4% post-primary and at least 98.1% post-booster had opsonophagocytic activity (OPA) titers ≥8 . Geometric mean antibody concentrations and OPA titers (except OPA titer for 6B) were higher post-booster than post-priming for each serotype. All PHiD-CV-vaccinated children had anti-protein D antibody concentrations ≥100 EL.U/ml one month post-primary/booster vaccination and all were seroprotected/seropositive against each DTPa antigen. Redness and irritability were the most common solicited AEs in both groups. Incidences of unsolicited AEs were comparable between groups. Serious AEs were reported for 47 children (28 in PHiD-CV group); none were assessed as vaccine-related. In conclusion, PHiD-CV induced robust immune responses and was well tolerated when co-administered with DTPa in a 3-dose priming plus booster regimen to Japanese children.
Abbreviations:
- AE, adverse event
- AOM, acute otitis media
- ATP, according-to-protocol
- CAP, community-acquired pneumonia
- CI, confidence interval
- COMPAS, Clinical Otitis Media and PneumoniA Study
- DTPa, diphtheria-tetanus-acellular pertussis
- ELISA, enzyme-linked immunosorbent assay
- GMC, geometric mean concentration
- GMT, geometric mean titer
- HBV, hepatitis B virus
- Hib, Haemophilus influenzae type b
- IPD, invasive pneumococcal disease
- NTHi, nontypeable Haemophilus influenzae
- OPA, opsonophagocytic activity
- PCV, pneumococcal conjugate vaccine
- PHiD-CV, 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine
- POET, Pneumococcal Otitis Efficacy Trial
- SAE, serious adverse event
- SAS, Statistical Analysis System
- SDD, SAS Drug and Development
- 7vCRM, 7-valent pneumococcal CRM-conjugate vaccine
- WHO, World Health Organization
Introduction
Streptococcus pneumoniae causes a spectrum of diseases in young children.Citation1 In Japan, the incidence of invasive pneumococcal disease (IPD) in 2 surveys conducted in 2000–2010 and 2007–2011 was 41–63 per 100,000 children aged under 5 yCitation2,3 The most common serotypes causing IPD were 6B, 23F, 19F, and 14,Citation2-6 and these serotypes also contributed to a high proportion of those isolated from pediatric cases of pneumoniaCitation7,8 and acute otitis media (AOM)Citation9,10 in Japan. Since 2010, when the 7-valent pneumococcal CRM-conjugate vaccine (7vCRM; Prevenar/Prevnar™, Pfizer Inc.., New York, USA) was introduced, proportions of IPD cases caused by serotypes 14 and 19F have decreased, those caused by serotypes 6B and 23F have changed little, and proportions caused by serotype 19A and non-vaccine serotypes 15A and 22F have increased.Citation6 In 2013, the 13-valent CRM-conjugate vaccine (Prevenar/Prevnar 13™) was licensed in Japan.Citation11
Nontypeable Haemophilus influenzae (NTHi) is another common cause of recurrent AOM and lower respiratory diseases in Japanese children.Citation12,13 The 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV; Synflorix, GlaxoSmithKline, Rixensart, Belgium) contains serotypes 1, 4, 5, 6B, 7F, 9V, 14, and 23F conjugated individually to NTHi protein D, with serotypes 18C and 19F conjugated to tetanus toxoid and diphtheria toxoid, respectively.Citation14 The efficacy and effectiveness of PHiD-CV against IPD, community-acquired pneumonia (CAP), and AOM, and of an 11-valent precursor formulation (11Pn-PD) against AOM,Citation15 has been demonstrated in randomized trials.Citation16,17
In Japan, there are limited data on the safety of vaccine co-administration and intramuscular injection since both practices have been uncommon. Pediatric vaccines are usually administered subcutaneously, although intramuscular injection has become more popular recently.Citation18 In this non-inferiority study of Japanese infants, post-primary immune responses following intramuscular administration of PHiD-CV were compared to those in a pivotal immunologic study conducted in EuropeCitation19 as well as in European and Latin American pneumococcal protein D-conjugate vaccines efficacy studies.Citation17,20 Also, the immunogenicity and safety of PHiD-CV and diphtheria-tetanus-acellular pertussis vaccine (DTPa), when co-administered as 3-dose primary vaccination in infancy followed by a booster dose in the second year of life, were evaluated and compared with the control group (DTPa alone).
Results
Study population
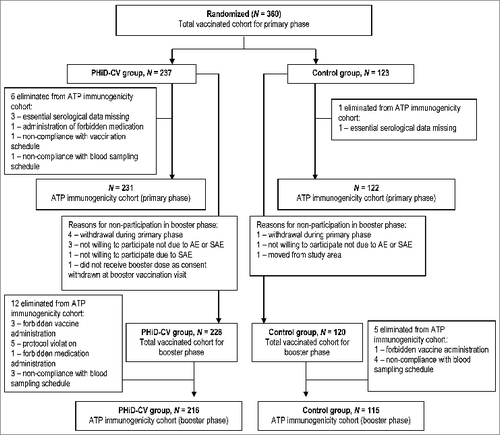
A total of 360 healthy infants were enrolled. The number of children in each group, reasons for elimination from the according-to-protocol (ATP) immunogenicity cohorts, and reasons for withdrawal are provided in . The demographic characteristics of both groups were comparable. The proportion of children who received Haemophilus influenzae type b (Hib) vaccine was comparable between groups and no children received hepatitis B virus (HBV) vaccination ().
Table 1. Demographic characteristics (ATP cohorts for immunogenicity)
Figure 1. Trial profile. Withdrawals from the study: Primary phase, PHiD-CV group; allergic reaction to the study vaccines of grade 1 intensity (one child), SAE (Kawasaki's disease, one child), simultaneous participation in another clinical trial (one child), sudden infant death syndrome (one child). Primary phase, control group: move from the study area (one child). Booster phase, PHiD-CV group: consent withdrawal not due to an AE (one child), move from the study area (one child).
Immunogenicity
In the comparison of post-priming immunogenicity results from this study to immune responses elicited by PHiD-CV in a pivotal European non-inferiority study,Citation19 the 2-sided 95% confidence interval (CI) upper limits for the antibody geometric mean concentration (GMC) ratios were below the protocol-defined limit of 2 for each of the 10 vaccine pneumococcal serotypes (). This indicated that the primary confirmatory objective of non-inferiority was reached. The secondary objectives of non-inferiority of immune responses measured by opsonophagocytic activity (OPA) titers to those elicited by 11Pn-PD in the European Pneumococcal Otitis Efficacy Trial (POET)Citation20 and by PHiD-CV in the Latin American Clinical Otitis Media and PneumoniA Study (COMPAS)Citation17 were also met ().
Table 2. 22F-ELISA antibody geometric mean concentration (GMC) ratios between pivotal immunologic non-inferiority PHiD-CV study in Europe and PHiD-CV study in Japan one month after the third vaccine dose (ATP cohort for immunogenicity)
Table 3. Opsonophagocytic activity (OPA) geometric mean titer (GMT) ratios between 11Pn-PD/acute otitis media efficacy study in Europe (POET) or PHiD-CV/pneumococcal diseases efficacy study in Latin America (COMPAS) and PHiD-CV study in Japan one month after the third vaccine dose (ATP cohort for immunogenicity)
In the PHiD-CV group, for each of the 10 vaccine pneumococcal serotypes, at least 92.6% of infants had antibody concentrations ≥0.2 μg/ml and at least 95.4% had OPA titers ≥8 one month after primary vaccination (). This descriptive analysis of immunogenicity showed robust increases in antibody GMCs and OPA geometric mean titers (GMTs) from pre-booster values after PHiD-CV booster vaccination (). For each of the 10 vaccine pneumococcal serotypes, at least 97.7% of infants had antibody concentrations ≥0.2 μg/ml and at least 98.1% had OPA titers ≥8 post-booster (). Antibody GMCs and OPA GMTs were higher post-booster than post-priming for each serotype, apart from the OPA titer for serotype 6B ().
Table 4. 22F-ELISA antibody and opsonophagocytic activity (OPA) seropositivity rates for individual pneumococcal serotypes following vaccination with PHiD-CV co-administered with DTPa. Pre-vaccination and post-priming data are for ATP immunogenicity cohort for primary vaccination. Pre-booster and post-booster data are for ATP immunogenicity cohort for booster vaccination
Figure 2. 22F-ELISA antibody geometric mean concentrations (GMCs) or opsonophagocytic activity (OPA) geometric mean titers (GMTs), with 95% confidence intervals, against individual pneumococcal serotypes before and one month after vaccination with PHiD-CV co-administered with DTPa (logarithmic scale, ATP cohorts for immunogenicity). Pre-vacc, before the first dose (at approximately 3 months of age); Post-priming, one month after 3-dose priming (at approximately 6 months of age); Pre-booster, before booster dose (17 to 19 months of age); Post-booster, one month after booster dose (18 to 20 months of age).
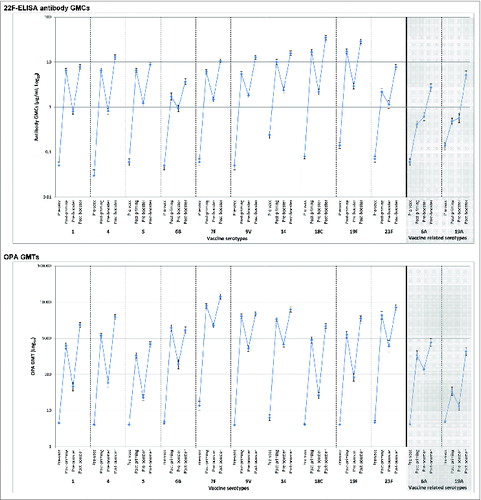
In the assessment of cross-reactive antibodies against vaccine-related serotypes 6A and 19A, after primary vaccination, at least 70.0% of children in the PHiD-CV group had antibody concentrations ≥0.2 μg/ml (), while these percentages were 0% and 4.1%, respectively, in the control group (Table S1). Post-booster, for each of these serotypes, at least 95.3% of children in the PHiD-CV group had antibody concentrations ≥0.2 μg/ml (). For each of these serotypes, percentages of children with OPA titers ≥8 were high in the PHiD-CV group after primary (≥61.5%) and booster (≥89.6%) vaccination. In the control group, for each serotype, less than 6% of children had OPA titers ≥8 after primary vaccination (Table S1). All children in the PHiD-CV group had anti-protein D antibody GMCs ≥100 EL.U/ml at both time points, while in the control group the percentages were less than 40% (Table S2).
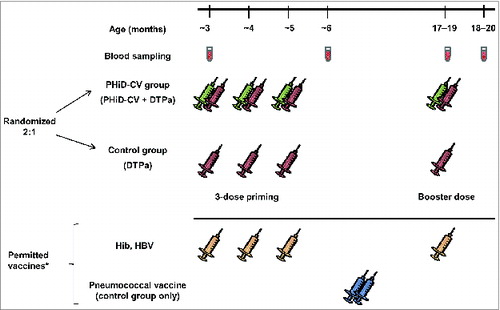
One month after primary and booster vaccination, all children in both groups were seropositive/seroprotected against each of the DTPa antigens (Table S3). All children who received 4 doses of Hib vaccine had seroprotective anti-PRP antibody concentrations ≥1 μg/ml one month after the fourth dose (Table S4). To ensure pneumococcal conjugate vaccine (PCV) administration was not prevented in the control group, vaccination with 7vCRM was permitted between primary and booster vaccination (), but its dose schedule was not specified. Consequent variations in the interval between vaccination and blood sampling did not allow immune responses against 7vCRM to be considered.
Figure 3. Study design. *Children in the control group were allowed catch-up vaccination with 7vCRM (2 doses administered between the second blood sampling time point and 7 d before the DTPa booster dose). Children in both groups were allowed to receive Haemophilus influenzae type b (Hib) and hepatitis b virus (HBV) vaccines concomitantly with the study vaccines. Administration of Bacille Calmette-Guérin, oral polio, measles-rubella, varicella and mumps vaccines was allowed, according to local recommendations, up to 28 d before or at least 7 d after DTPa or PHiD-CV administration.
Reactogenicity and safety
In the descriptive assessment of reactogenicity, almost all of the solicited local symptoms and most of the solicited general symptoms were reported within the first 4 d after each dose (Table S5). During the 4-day and 8-day post-vaccination periods, redness and irritability were the most frequent solicited local and general symptoms in both groups (Table S5, ).
Table 5. Incidence of solicited local symptoms at each injection site and solicited general symptoms within 8 d (days 0–7) after each vaccine dose (total vaccinated cohorts)
Solicited local symptoms of any intensity were reported with similar incidences at the PHiD-CV and DTPa injection sites in the PHiD-CV group, except for incidences after the first dose, which were higher at the PHiD-CV injection site (). In the control group, incidences at the DTPa injection site were consistent with those reported at the DTPa site in the PHiD-CV group (). Incidences of grade 3 redness or grade 3 swelling after each primary dose and grade 3 pain post-booster tended to be higher at the PHiD-CV injection site than at the DTPa injection site in both groups. After booster vaccination, large swelling reactions were reported in 26 children (11.4%) at the PHiD-CV injection site, 21 (9.2%) at the DTPa injection site in the PHiD-CV group, and 8 (6.7%) in the control group. All but 2 (DTPa injection site in PHiD-CV group) were local or diffuse swelling reactions not involving adjacent joints and all except 2 resolved without sequelae within 6 d (one reaction at PHiD-CV injection site lasted 33 days; one reaction at DTPa site in PHiD-CV group was 'resolving' at study end).
Solicited general symptoms had similar incidences in both groups, except for fever after the booster dose, which had a higher incidence in the PHiD-CV group (). Post-booster, the incidence of grade 3 fever was 2.6% in the PHiD-CV group, although only one report (temperature 40.6°C on day 7) was considered related to vaccination.
Incidences of unsolicited adverse events (AEs) were within the same range in both groups after primary and booster vaccination (Table S6) and the most common events reflected the childhood diseases normally observed in young children. The most frequent unsolicited AE related to vaccination in both groups was injection site induration after primary and booster vaccination.
During the entire 15-month study period, serious AEs (SAEs) were reported in 28 children in the PHiD-CV group and 19 in the control group. A 19-week-old child in the PHiD-CV group died due to sudden infant death syndrome 9 d after the second vaccine dose. None of the SAEs were considered vaccine-related.
Discussion
The primary objective of this non-inferiority study was to compare the immunogenicity of PHiD-CV after 3-dose priming of Japanese infants to that induced by PHiD-CV in an earlier pivotal European study.Citation19 Higher antibody responses were induced in Japanese infants against each of the 10 vaccine pneumococcal serotypes. Moreover, descriptive analysis results indicate that PHiD-CV when given intramuscularly as a 3-dose primary series followed by a booster dose in co-administration with subcutaneous DTPa was immunogenic for all vaccine serotypes and NTHi protein D. This vaccine regimen was also generally well tolerated in Japanese infants and toddlers.
Other non-inferiority objectives of the study were to compare post-primary immunogenicity in the present study with immune responses observed in POET, which assessed the efficacy of 11Pn-PD against AOM in European children,Citation15 and COMPAS, which assessed the efficacy of PHiD-CV against IPD, CAP, and AOM in Latin American children.Citation17 Post-priming OPA responses, a measure of the functionality of vaccine-induced antibodies, in Japanese children were non-inferior to those observed in POET and COMPAS. This measure is considered to be predictive of vaccine protective potential against pneumococcal disease: OPA titers ≥8 correlated with 11Pn-PD efficacy against AOMCitation20 and were a better predictor of IPD protection with 7vCRM than the 22F-inhibition enzyme-linked immunosorbent assay (22F-ELISA).Citation21 Therefore, although there is no established correlate of PCV protection and standardization of OPA assays is ongoing,Citation22 transposability of efficacy results based on OPA immune responses is widely accepted.Citation23,24 In COMPAS, PHiD-CV showed efficacy of 26% against World Health Organization (WHO)-defined consolidated CAP and 22% against likely-bacterial CAP, 67% against vaccine serotype AOM, 65% against any IPD, and 100% against vaccine serotype IPD.Citation17 The same effectiveness against vaccine serotype IPD was reported in a randomized controlled study of PHiD-CV in Finland.Citation16 Furthermore, post-marketing surveillance data suggest decreases in IPD, pneumonia, and AOM incidences after the introduction of PHiD-CV in Brazil, Chile, Colombia, Kenya, Finland, Iceland, the Netherlands, Canada, and New Zealand.Citation25-38 In POET, vaccine efficacy (58%) was shown for AOM caused by 11 vaccine serotypes combined (10 serotypes in common with PHiD-CV plus serotype 3), with robust results for serotypes 6B, 14, 19F, and 23F.Citation15
The validity of comparing 11Pn-PD and PHiD-CV could be questioned because of differences in vaccine formulation.Citation14 However, the efficacy of PHiD-CV against AOM episodes caused by vaccine serotypes in the COMPAS study (67%)Citation17 was similar to that observed with 11Pn-PD in POETCitation15 and in the Finnish Otitis Media trial of 2 7-valent PCVs (7vCRM and an investigational OMPC conjugate vaccine).Citation39 This suggests that, if carriers have differential effects on vaccine efficacy against AOM, they are masked by other factors. Moreover, 11Pn-PD and PHiD-CV induce similar immune responses against vaccine pneumococcal serotypes and protein D carrier protein.Citation40 Other immunogenicity studies have shown non-inferiority between vaccines with different serotype valencies and containing different types or quantities of carrier protein, such as comparisons of PHiD-CV and 7vCRMCitation19 and 7vCRM and the 13-valent CRM-conjugate vaccine.Citation41 These observations suggest the comparison of immune responses elicited by PHiD-CV to those elicited by 11Pn-PD is valid despite differences in vaccine formulation.
In the non-inferiority analyses of the present study, there was a trend for higher antibody GMCs or OPA GMTs in Japanese children. This was consistent with the findings of a review of pneumococcal antibody and functional OPA responses in 12 infant studies of PHiD-CV,Citation42 in which there was a general trend for higher post-primary antibody responses in Asian and Latin American children in comparison to European children, regardless of primary vaccination schedule. Genetic and social factors, differences in epidemiological patterns of pneumococcal disease and colonization, and transmission of maternal antibodies can have an influence on immune responses as well as differences in vaccination schedulesCitation43 and co-administered vaccines. However, despite these differences across regions, PHiD-CV showed similar effectiveness or efficacy against vaccine serotype IPD in a European setting (in Finland with a 3–4–5 primary vaccination schedule)Citation16 and in Latin America (in COMPAS with a 2–4–6 schedule).Citation17,42 It should also be noted that the importance of differences in immune responses may be minimized once herd immunity is established via high coverage in vaccination programs,Citation43,44 although herd immunity has not been documented in all settings with high vaccination coverage.
As well as immune responses against vaccine serotypes, there is interest in cross-protective immunity induced by PCVs to vaccine-related serotypes. Serotypes 6A and 19A, related to vaccine serotypes 6B and 19F included in 7vCRM and PHiD-CV, account for approximately 4–8% and 2–6% of pneumococcal isolates, respectively, in Japanese children with IPD,Citation2,4,5 although one study found 22% of strains isolated from bacteremic cases between 2008 and 2010 were serotype 6A.Citation3 Evidence from studies conducted in various countries shows that antibodies induced by 7vCRM against serotype 6B have the ability to cross-protect against 6A disease, but antibodies induced by the 19F antigen provide limited cross-protection against 19A disease.Citation45 Also, functional antibodies are induced with 7vCRM against both serotypes but to a much lesser extent against serotype 19A.Citation46 This may explain why the proportion of IPD cases caused by serotype 19A did not diminish after the introduction of 7vCRM in JapanCitation6 and other countries.Citation47 Comparative studies of PHiD-CV and 7vCRM have shown consistently higher levels of functional antibodies against serotype 19A in PHiD-CV recipients than in 7vCRM-immunized children.Citation45 In our study, at least 90% of children had antibody concentrations ≥0.2 μg/ml and OPA titers ≥8 against both serotype 6A and serotype 19A after booster vaccination and these percentages tended to be higher than in previous PHiD-CV studies.Citation14
All children had seroprotective/seropositive immune responses against each of the DTPa antigens one month after primary and booster vaccination. Also, all children who received 4 doses of Hib vaccine had seroprotective anti-PRP antibody concentrations. Therefore, in line with previous reports,Citation48 our study results did not show any signal of negative interference on the immune response to the co-administered vaccine antigens.
Intramuscular injection is generally recommended for adjuvant-containing vaccines because subcutaneous or intradermal administration can cause local irritation, induration, skin discoloration, inflammation, and granuloma formation.Citation49 In Japan, intramuscular administration is increasingly common, and is recommended for some vaccines by the Japan Pediatric Society.Citation18 As in previous studies,Citation50 intramuscular administration of PHiD-CV was generally well tolerated. Although the present study was not designed to compare the intramuscular route to other administration routes of PHiD-CV (note that no group received subcutaneous PHiD-CV), the profile of solicited local and general symptoms was consistent with that reported with subcutaneously-administered 13-valent CRM-conjugate vaccine in young Japanese children.Citation11 Nearly all solicited local symptoms and the majority of solicited general symptoms appeared within the first 4 d after vaccination. Incidences of solicited local symptoms tended to be higher after booster vaccination than after priming, which was consistent with previously-reported incidences after PHiD-CV booster dose.Citation50 After primary vaccination, the incidence of pain tended to be higher at the PHiD-CV intramuscular injection site than at the DTPa subcutaneous injection site in both groups, while incidences of redness and swelling of any intensity (after dose 1) were similar. After booster vaccination, incidences of all solicited local symptoms were within the same ranges at the PHiD-CV and DTPa injection sites.
Incidences of large swelling reactions at both injection sites were higher than those reported in an analysis of previous PHiD-CV randomized controlled studies (0.6% at PHiD-CV injection site, 1.3% at DTPa injection site).Citation50 In a safety monitoring study of 7vCRM administered subcutaneously to Japanese children, the incidence of injection site edema extending into the forearm was <0.1% with single or co-administration.Citation51 In our study, all but 2 large swelling reactions (diameter >50 mm) were local or diffuse, not involving adjacent joints. Following the booster dose of subcutaneously-administered 13-valent CRM-conjugate vaccine in Japanese toddlers, incidences of severe (diameter >70 mm) and moderate (25–70 mm) swelling were 2.3% and 36.4%, respectively.Citation11 Previously, 51% of Japanese children showed mild swelling following a booster dose of DTPa Kaketsuken after first immunization during the first year of life.Citation52
Potential limitations of this study included its open design, which might have introduced bias toward increased reporting of AEs in the PHiD-CV group since parents and investigators were aware that the children had received a new vaccine in addition to the antigens received by children in the control group. There were differences in primary vaccination schedule and geographical location among the studies that might have influenced immune responses. However, the vaccination schedules included in the non-inferiority analyses were aligned with universal mass vaccination schedules used in Japan. Also, in all studies, infants received 3-dose priming with pneumococcal protein D-conjugate vaccine and it could be considered a strength of the study that non-inferiority was demonstrated despite differences in priming schedule and study location.
In conclusion, the immune response induced by PHiD-CV 3-dose priming was at least as good as in European and Latin American studies, which provided evidence of protective efficacy against IPD, pneumonia, or AOM. A full vaccination course of PHiD-CV administered intramuscularly to Japanese children was immunogenic for all 10 vaccine pneumococcal serotypes and NTHi protein D and had an acceptable safety and reactogenicity profile. These results suggest that PHiD-CV has the potential to provide efficacy against pneumococcal diseases in Japanese children.
Methods
Study design and participants
This was a randomized, open-label, controlled study (ClinicalTrials.gov, NCT01027845) conducted in 16 centers (listed in Table S7) in Japan according to the Declaration of Helsinki and Good Clinical Practice guidelines between December 2009 and September 2011. The protocol was approved by each study center's institutional review board. Written informed consent was obtained from a parent/legal guardian.
Healthy infants were enrolled by pediatricians during well-baby/hospital clinics and randomized (2:1) to receive either PHiD-CV co-administered with DTPa (DPT Kaketsuken SyringeTM, Kaketsuken, Kumamoto, Japan) (PHiD-CV group) or DTPa alone (control group) as 3-dose primary vaccination at 3, 4, and 5 months of age and booster vaccination at 17–19 months of age (). Vaccine formulations are described in Table S8. A randomization list was generated by GlaxoSmithKline using MATEX (Statistical Analysis System [SAS®] program) and treatment allocation performed via an internet-based system.
PHiD-CV was administered intramuscularly and DTPa subcutaneously using standard vaccination techniques. Concomitant administration of Hib and HBV vaccines, and catch-up vaccination with 7vCRM in the control group between primary and booster vaccination, were permitted outside of the study protocol ().
The primary objective was to compare the immunogenicity of PHiD-CV in healthy Japanese children, one month after the third priming dose, to immune responses elicited by PHiD-CV in a European PHiD-CV pivotal non-inferiority study.Citation19 Comparison of immunogenicity in Japanese children to immune responses elicited by 11Pn-PD in the European POETCitation20 and to PHiD-CV immunogenicity in the Latin American COMPASCitation17 studies were evaluated as secondary objectives. Immunogenicity of PHiD-CV and DTPa when co-administered and the safety and reactogenicity of PHiD-CV and DTPa co-administration were also assessed.
Immunogenicity assessment
Blood sampling time points are shown in . Samples were stored at –20°C until analysis at GlaxoSmithKline Vaccines' laboratory in Rixensart, Belgium, or (for OPA testing) at SGS Lab Simon SA, Wavre, Belgium.
Serum anti-pneumococcal, serotype-specific IgG antibodies were measured against vaccine serotypes and vaccine-related serotypes 6A and 19A using GlaxoSmithKline's 22F-ELISA with a cut-off value of 0.05 μg/ml, as described before.Citation53,54 Antibody concentration of 0.2 μg/ml measured by 22F-ELISA corresponds to 0.35 μg/ml with the WHO reference laboratory non-inhibition assay.Citation55 OPA was measured using pneumococcal killing assay in HL60 phagocyte cells, as described previously,Citation56 with a cut-off titer of 8.Citation57 Antibodies against NTHi protein D were measured by ELISA (cut-off, 100 EL.U/ml). Immune responses to DTPa and Hib vaccine antigens were analyzed according to standard techniques described previously.Citation48
Safety assessment
Local (pain, redness, swelling at the injection site) and general (fever [axillary temperature ≥37.5°C], drowsiness, irritability, loss of appetite) symptoms were actively solicited during an 8-day post-vaccination period. An 8-day period was used instead of the more common 4-day assessmentCitation50 on the request of the Pharmaceuticals and Medical Devices Agency in Japan.
The intensity of each solicited AE was graded on a scale from 1 to 3. Pain at the injection site was considered to have a grade 3 intensity if the child cried when the limb was moved/was spontaneously painful, redness and swelling at the injection site if the diameter was >30 mm, and fever if axillary temperature was >39.5°C. Grade 3 irritability/fussiness was recorded if the child cried and could not be comforted/prevented normal activity, and grade 3 loss of appetite if the child did not eat at all. Grade 3 intensity for all other AEs was defined as preventing normal everyday activity and/or causing parents/guardians to seek medical advice. Large swelling reactions (swelling with a diameter >50 mm, noticeable diffuse swelling, or noticeable increase of limb circumference) were solicited after the booster vaccine dose.
Other AEs were recorded within a 31-day post-vaccination follow-up period and SAEs (any medical event resulting in death, life-threatening event, or event causing disability, or requiring hospitalization or prolongation of hospitalization) were recorded during the entire study period. All solicited local symptoms were considered causally related to vaccination. For other AEs, assessment of causal relationship to vaccination was based on the investigator's clinical judgment.
Statistical analysis
To obtain 200 evaluable children, it was planned to enroll 240 children in the PHiD-CV group to provide ≥98% or 85% power (under equal mean or 1.2-fold decrease in antibody GMC, respectively) to show immunological non-inferiority of PHiD-CV versus PHiD-CV in the pivotal European study.Citation19 Non-inferiority was to be demonstrated if the upper limit of the 2-sided 95% CI on the antibody GMC ratios (GMCs from PHiD-CV group of European study over GMCs from PHiD-CV group of current study) was below a limit of 2-fold for all 10 vaccine pneumococcal serotypes one month after the third primary vaccine dose.
Non-inferiority to immunogenicity observed in POETCitation20 or COMPASCitation17 efficacy studies was to be demonstrated if the upper limit of the 2-sided 95% CI on the OPA GMT ratios (GMTs from 11Pn-PD/PHiD-CV group from POET or COMPAS over GMTs from PHiD-CV group of current study) was below a limit of 2.5-fold for all 10 vaccine serotypes one month post-primary vaccination.
Immunogenicity analyses were performed on ATP immunogenicity cohorts of the primary vaccination phase and booster vaccination phase, defined as vaccinated subjects who complied with all protocol-defined procedures and for whom antibody assay results were available. Antibody GMCs and OPA GMTs were calculated with 95% CIs and seropositivity/seroprotection rates for each serotype or antigen were calculated with exact 95% CIs. Analyses of safety were performed on the total vaccinated cohorts. Solicited AEs were analyzed for 4-day and 8-day follow-up periods. Incidences of AEs were calculated with exact 95% CIs. The immunogenicity assessment of PHiD-CV and DTPa when co-administered and the reactogenicity and safety assessments were descriptive analyses. Non-overlapping 95% CIs indicate potential differences between study groups and time points; however these comparisons should be interpreted with caution since there was no adjustment for multiple comparisons. The statistical analyses were performed using SDD (SAS® Drug and Development) web portal version 3.5 and SAS® version 9.2.
Disclosure of Potential Conflicts of Interest
SI, NK, HK, YT, MM, AI, and TO received grants from the GlaxoSmithKline group of companies as principal investigators during the conduct of the study. SI, NK, HK, YT, MM, and TO received support for travel to meetings for the study from the GlaxoSmithKline group of companies. NK and SI were also granted personal fees, consulting fees, or honoraria for consulting and/or participation in advisory board meetings from the GlaxoSmithKline group of companies. Outside the submitted work, HK received payment for lectures, including service on speaker's bureaus from Pfizer and MSD, and payment for manuscript preparation from Pfizer. MM obtained grants related to a clinical study from Pfizer and SI received grants and payment from the GlaxoSmithKline group of companies, Pfizer and MSD for lectures including services on speaker's bureaus and/or payment for manuscript preparation. AS, DB, FS, JRG, NF, and TS are employees of GlaxoSmithKline group of companies. DB, JRG, and TS own restricted shares/stock options of the GlaxoSmithKline group of companies.
Supplemental Materials
Supplemental data for this article can be accessed on the http://www.tandfonline.com/khvi publisher's website.
Authors’ Contributions
The study was designed by JRG, TS, AS, NF, and DB. Centers and/or investigators were recruited by SI, TS, and AS. AI, HK, YT, NK, MM, TO, and SI contributed to the collection and assembly of data. The analysis was performed or supervised by FS, NF, JRG, and DB, and interpretation of results by SI, DB, JRG, TS, FS, NF, and AS. FS and NF provided statistical expertise. Each author made a significant contribution to the study and to the development of this manuscript, approved this final submitted version, and agrees with submission.
Trademark Statement
Synflorix is a trademark of the GlaxoSmithKline group of companies. DPT Kaketsuken Syringe is a trademark of Kaketsuken. Prevenar/Prevnar are trademarks of Pfizer Inc.
supplementary_material.docx
Download MS Word (47.8 KB)Acknowledgments
The authors would like to thank the children and their families for participating in this trial and, for contributing in many ways to this study, the investigators, study nurses, and other staff members at the study sites, in particular: Miho Ohshima, investigator at Sapporo Tokushukai Hospital; Osamu Komiyama, investigator at Tokyo Medical Center; Hiroshi Ichiseki, investigator at Shonan Atsugi Hospital; Go Yamamoto and Tokutaro Mukae, investigators at Shonan Kamakura General Hospital; Takashi Higashide, investigator at HugHug Kids Clinic; Katsuhiko Yoshii, investigator at Cibune Clinic; Shigeru Mori, investigator at Momotaro Clinic; Masanori Ikeda, investigator at Fukuyama Medical Center; Tomoyuki Miyamoto, investigator at Yokosuka General Hospital Uwamachi; Nobuko Yokoyama, investigator at Chuden Hospital; Mikihiro Aoki, investigator at Nagasaki Medical Center; and Tetsuo Taguchi, investigator at Niigata Prefectural Hospital.
The authors also thank, from GlaxoSmithKline Vaccines, Makoto Ono and Atsushi Maruyama, and Sophie Ledant for local and global study management; Ann Dhoest (freelance for GlaxoSmithKline Vaccines) and Kristel Vercauteren (XPE Pharma and Science for GlaxoSmithKline Vaccines) for protocol and study report writing; Thomas Déplanque (XPE Pharma & Science for GlaxoSmithKline Vaccines) for manuscript coordination and Joanne Knowles (independent medical writer, on behalf of GlaxoSmithKline Vaccines) for initial drafting of the manuscript and incorporation of comments received from the authors.
Funding
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also took responsibility for all costs associated with the development and publishing of the present manuscript.
References
- O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009; 374:893-902; PMID:19748398; http://dx.doi.org/10.1016/S0140-6736(09)61204-6
- Sakata H. Invasive Streptococcus pneumoniae infections in children in Kamikawa and Soya subprefecture, Hokkaido, Japan, 2000-2010, before the introduction of the 7-valent pneumococcal conjugate vaccine. J Infect Chemother 2011; 17:799-802; PMID:21701961; http://dx.doi.org/10.1007/s10156-011-0264-8
- Nakamura R, Togashi T. Population-based incidence of invasive Haemophilus influenzae and pneumococcal diseases before the introduction of vaccines in Japan. Pediatr Infect Dis J 2013; 32:1394-6; PMID:23804122; http://dx.doi.org/10.1097/INF.0b013e3182a14971
- Chiba N, Morozumi M, Sunaoshi K, Takahashi S, Takano M, Komori T, Sunakawa K, Ubukata K. Serotype and antibiotic resistance of isolates from patients with invasive pneumococcal disease in Japan. Epidemiol Infect 2010; 138:61-8; PMID:19538821; http://dx.doi.org/10.1017/S0950268809990239
- Sakai F, Chiba N, Ono A, Yamagata MS, Ubukata K, Sunakawa K, Takahashi T. Molecular epidemiologic characteristics of Streptococcus pneumoniae isolates from children with meningitis in Japan from 2007 through 2009. J Infect Chemother 2011; 17:334-40; PMID:21161561; http://dx.doi.org/10.1007/s10156-010-0180-3
- Chiba N, Morozumi M, Shouji M, Wajima T, Iwata S, Sunakawa K, Ubukata K. Rapid decrease of 7-valent conjugate vaccine coverage for invasive pneumococcal diseases in pediatric patients in Japan. Microb Drug Resist 2013; 19:308-15; PMID:23480525; http://dx.doi.org/10.1089/mdr.2012.0180
- Chiba N, Kobayashi R, Hasegawa K, Morozumi M, Nakayama E, Tajima T, Iwata S, Ubukata K. Antibiotic susceptibility according to genotype of penicillin-binding protein and macrolide resistance genes, and serotype of Streptococcus pneumoniae isolates from community-acquired pneumonia in children. J Antimicrob Chemother 2005; 56:756-60; PMID:16131518; http://dx.doi.org/10.1093/jac/dki302
- Tanaka J, Ishiwada N, Wada A, Chang B, Hishiki H, Kurosaki T, Kohno Y. Incidence of childhood pneumonia and serotype and sequence-type distribution in Streptococcus pneumoniae isolates in Japan. Epidemiol Infect 2012; 140:1111-21; PMID:21875450; http://dx.doi.org/10.1017/S0950268811001592
- Hotomi M, Billal DS, Kamide Y, Kanesada K, Uno Y, Kudo F, Ito M, Kakehata S, Sugita R, Ogami M, et al. Serotype distribution and penicillin resistance of Streptococcus pneumoniae isolates from middle ear fluids of pediatric patients with acute otitis media in Japan. J Clin Microbiol 2008; 46:3808-10; PMID:18832131; http://dx.doi.org/10.1128/JCM.01782-08
- Otsuka T, Kitami O, Kondo K, Ota H, Oshima S, Tsuchiya A, Shirai T, Fujii K, Nakamure M, Shoji Y, et al. Incidence survey of acute otitis media in children in Sado Island, Japan - Sado Otitis Media Study (SADOMS). PLoS One 2013; 8:e68711; PMID:23844235; http://dx.doi.org/10.1371/journal.pone.0068711
- Togashi T, Yamaji M, Thompson A, Giardina PC, Aizawa M, Patterson S, Gruber WC, Scott DA. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in healthy infants in Japan. Pediatr Infect Dis J 2013; 32:984-9; PMID:23538524; http://dx.doi.org/10.1097/INF.0b013e318293007e
- Sunakawa K, Takeuchi Y, Iwata S. Nontypeable Haemophilus influenzae (NTHi) epidemiology. Kansenshogaku Zasshi 2011; 85:227-37; PMID:21706841
- Takakura M, Fukuda Y, Nomura N, Mitsuyama J, Yamaoka K, Asano Y, Sawamura H, Katsuragawa K, Hashido H, Matsukawa Y, et al. Antibacterial susceptibility surveillance of Haemophilus influenzae isolated from pediatric patients in Gifu and Aichi prefectures (2009-2010). Jpn J Antibiot 2012; 65:305-21; PMID:23383433
- Prymula R, Schuerman L. Ten-valent pneumococcal nontypeable Haemophilus influenzae PD conjugate vaccine: Synflorix. Expert Rev Vaccines 2009; 8:1479-500; PMID:19863240; http://dx.doi.org/10.1586/erv.09.113
- Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, Kohl I, Lommel P, Poolman J, Prieels JP, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 2006; 367:740-8; PMID:16517274; http://dx.doi.org/10.1016/S0140-6736(06)68304-9
- Palmu AA, Jokinen J, Borys D, Nieminen H, Ruokokoski E, Siira L, Puumalainen T, Lommel P, Hezareh M, Moreira M, et al. Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against invasive pneumococcal disease: a cluster randomised trial. Lancet 2013; 381:214-22; PMID:23158882
- Tregnaghi MW, Sáez-Llorens X, Lopez P, Abate H, Smith E, Posleman A, Calvo A, Wong D, Cortes-Barbosa C, Ceballos A, et al. Efficacy of Pneumococcal Nontypable Haemophilus influenzae Protein D Conjugate Vaccine (PHiD-CV) in Young Latin American Children: A Double-Blind Randomized Controlled Trial. PLoS Med 2014; 11:(6): e1001657; PMID:24892763
- Japan Pediatric Society. Demanding paper regarding change of description of intramuscular administration of inactivated vaccines in package inserts. 2011. Available from: http://www.jpeds.or.jp/uploads/files/saisin_1106273.pdf.
- Vesikari T, Wysocki J, Chevallier B, Karvonen A, Czajka H, Arsene JP, Lommel P, Dieussaert I, Schuerman L. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr Infect Dis J 2009; 28:S66-S76; PMID:19325449
- Schuerman L, Prymula R, Henckaerts I, Poolman J. ELISA IgG concentrations and opsonophagocytic activity following pneumococcal protein D conjugate vaccination and relationship to efficacy against acute otitis media. Vaccine 2007; 25:1962-8; PMID:17258357
- Schuerman L, Wysocki J, Tejedor JC, Knuf M, Kim KH, Poolman J. Prediction of pneumococcal conjugate vaccine effectiveness against invasive pneumococcal disease using opsonophagocytic activity and antibody concentrations determined by enzyme-linked immunosorbent assay with 22F adsorption. Clin Vaccine Immunol 2011; 18:2161-7; PMID:21994351
- Song JY, Moseley MA, Burton RL, Nahm MH. Pneumococcal vaccine and opsonic pneumococcal antibody. J Infect Chemother 2013; 19:412-25; PMID:23657429
- Feavers I, Goldblatt D, Griffiths E, Nahm M, Zhou T. WHO workshop on standardization of pneumococcal opsonophagocytic assay, 25-26 January 2007. Geneva, Switzerland: World Health Organization; 2007.
- European Medicines Agency. Synflorix. European Public Assessment Report. 2013. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000973/WC500054346.pdf.
- Domingues CM, Verani JR, Montenegro Renoiner EI, de Cunto Brandileone MC, Flannery B, de Oliveira LH, Santos JB, de Moraes JC; Brazilian Pneumococcal Conjugate Vaccine Effectiveness Study Group. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study. Lancet Respir Med 2014; 2:464-71; PMID:24726406
- Sartori AL, Minamisava R, Afonso ET, Antunes JLF, Bierrenbach AL, Morais-Neto OL, Toscano CM, Andrade AL. Reduction in all-cause otitis-related outpatient visits in children after PCV10 introduction in Brazil [Abstract ISPPD-0244]. pneumonia 2014; 3:240.
- Minamisava R, Sgambatti S, Morais-Neto OL, Cristo EB, Escalante JJC, Bierrenbach AL, Andrade AL. Impact of PCV10 introduction on pneumonia mortality rates in Brazil: a time series analysis [Abstract ISPPD-0556]. pneumonia 2014; 3:248.
- Afonso ET, Minamisava R, Bierrenbach AL, Escalante JJ, Alencar AP, Domingues CM, Morais-Neto OL, Toscano CM, Andrade AL. Effect of 10-valent pneumococcal vaccine on pneumonia among children, Brazil. Emerg Infect Dis 2013; 19:589-97; PMID:23628462
- Pan American Health Organization. SIREVA II Regional Report. 2012. Available from: http://www.paho.org/hq/index.php?option=com_content&view=category&layout=blog&id=3609&Itemid=3953.
- Scott JAG, Hammitt LL, Bwanaali T, Morpeth SC, Makumi A, Mburu J, Otiende M, Moisi JC, Karani A, Mlamba A, et al. The impact of introducing 10-valent pneumococcal conjugate vaccine in Kenya on invasive pneumococcal disease among children under 5 years. Poster session presented at: 8th International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD-8); 2012 Mar 11-15; Iguaçu Falls, Brazil.
- Rinta-Kokko H, Auranen K, Palmu A, Nohynek H, Nuorti P, Siira L, Toropainen M, Virtanen M, Jokinen J. Effectiveness of 10-valent pneumococcal conjugate vaccine (PCV10) against invasive pneumococcal disease (IPD) during ongoing national vaccination programme (NVP) in Finland [Abstract ISPPD-0560]. pneumonia 2014; 3:140-1.
- Jokinen J, Rinta-Kokko H, Siira L, Palmu A, Virtanen MJ, Nohynek H, Toropainen M, Nuorti P. Impact of 10-valent pneumococcal conjugate vaccine (PCV10) on invasive pneumococcal disease (IPD) among vaccine-eligible children in Finland. Paper presented at: 8th World Congress of the World Society for Pediatric Infectious Diseases (WSPID); 2013 Nov 19-22; Cape Town, South Africa.
- Erlendsdóttir H, Haraldsson A, Hrafnkelsson B, Kristinsson KG. An early reduction of invasive pneumococcal infections after PCV-10 immunisation [Abstract ISPPD-0390]. pneumonia 2014; 3:176.
- Sigursson S, Kristinsson KG, Erlendsdóttir H, Hrafnkelsson B, Haraldsson A. Acute otitis media and pneumonia in young children in Iceland: an early reduction of incidence after PCV-10 immunization [Abstract ISPPD-0185]. pneumonia 2014; 3:170-1.
- Knol MJ, Sanders EAM, Vlaminckx B, de Melker HE, Van der Ende E. Incidence of invasive pneumococcal disease in the Netherlands after introduction of 7-valent and 10-valent pneumococcal conjugate vaccination [Abstract ISPPD-0261]. pneumonia 2014; 3:175.
- De Wals P, Lefebvre B, Markowski F, Deceuninck G, Defay F, Douville-Fradet M, Landry M. Impact of 2+1 pneumococcal conjugate vaccine program in the province of Quebec, Canada. Vaccine 2014; 32:1501-6; PMID:24486346
- Deceuninck G, De Wals P. Effectiveness of three pneumococcal conjugate vaccines (PCVs) to prevent invasive pneumococcal disease (IPD) in Quebec, Canada [Abstract ISPPD-0333]. pneumonia 2014; 3:163.
- Institute of Environmental Science & Research Limited. Invasive pneumococcal disease reports. 2011-2013. Available at: https://surv.esr.cri.nz/surveillance/IPD.php.
- Fletcher MA, Fritzell B. Pneumococcal conjugate vaccines and otitis media: an appraisal of the clinical trials. Int J Otolaryngol 2012; 2012:312935; PMID:22701486
- Schuerman L, Borys D, Hoet B, Forsgren A, Prymula R. Prevention of otitis media: now a reality? Vaccine. 2009; 27:5748-54; PMID:19666154
- Jefferies JM, Macdonald E, Faust SN, Clarke SC. Thirteen-valent pneumococcal conjugate vaccine (PCV13). Hum Vaccin 2011; 7:1012-8; PMID:21941097
- Borys D, Vesikari T, Tregnaghi MW, Sáez-Llorens X, Iwata S, Lommel P, Moreira M, Ruiz-Guiñazú J. Population variability of the immune response following primary vaccination with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D-conjugate vaccine (PHiD-CV) [Abstract ISPPD-0404]. pneumonia 2014; 3:95-6.
- Deloria Knoll M, Park DE, Johnson TS, Chandir S, Nonyane BA, Conklin L, Fleming-Dutra KE, Loo JD, Goldblatt D, Whitney CG, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on immunogenicity. Pediatr Infect Dis J 2014; 33 Suppl 2:S119-29; PMID:24336054
- Spijkerman J, Veenhoven RH, Wijmenga-Monsuur AJ, Elberse KE, van Gageldonk PG, Knol MJ, de Melker HE, Sanders EA, Schouls LM, Berbers GA. Immunogenicity of 13-valent pneumococcal conjugate vaccine administered according to 4 different primary immunization schedules in infants: a randomized clinical trial. JAMA 2013; 310:930-7; PMID:24002279
- Hausdorff WP, Hoet B, Schuerman L. Do pneumococcal conjugate vaccines provide any cross-protection against serotype 19A? BMC Pediatr 2010; 10:4; PMID:20122261
- Grant LR, O'Brien SE, Burbidge P, Haston M, Zancolli M, Cowell L, Johnson M, Weatherholtz RC, Reid R, Santosham M, et al. Comparative immunogenicity of 7 and 13-valent pneumococcal conjugate vaccines and the development of functional antibodies to cross-reactive serotypes. PLoS One 2013; 8:e74906; PMID:24086394
- Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011; 378:1962-73; PMID:21492929
- Knuf M, Szenborn L, Moro M, Petit C, Bermal N, Bernard L, Dieussaert I, Schuerman L. Immunogenicity of routinely used childhood vaccines when coadministered with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV). Pediatr Infect Dis J 2009; 28:S97-S108; PMID:19325452; http://dx.doi.org/10.1097/INF.0b013e318199f61b
- Centers for Disease Control and Prevention. General recommendations on immunization – recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1-64.
- Chevallier B, Vesikari T, Brzostek J, Knuf M, Bermal N, Aristegui J, Borys D, Cleerbout J, Lommel P, Schuerman L. Safety and reactogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with routine childhood vaccines. Pediatr Infect Dis J 2009; 28:S109-18; PMID:19325447; http://dx.doi.org/10.1097/INF.0b013e318199f62d
- Nishi J, Tokuda K, Imuta N, Minami T, Kawano Y. Prospective safety monitoring of Haemophilus influenzae type b and heptavalent pneumococcal conjugate vaccines in Kagoshima, Japan. Jpn J Infect Dis 2013; 66:235-7; PMID:23698486; http://dx.doi.org/10.7883/yoken.66.235
- Kimura M, Kuno-Sakai H, Kamiya H, Ueda K, Isomura S, Koike M, Kato T, Ozaki T, Hirose M, Egami T. Immunogenicity and reactogenicity of the component acellular pertussis vaccine produced by a combination of column purified pertussis toxin and filamentous haemagglutinin. Acta Paediatr Jpn 1995; 37:562-74; PMID:8533580; http://dx.doi.org/10.1111/j.1442-200X.1995.tb03378.x
- Concepcion NF, Frasch CE. Pneumococcal type 22F polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol 2001; 8:266-72; PMID:11238206
- Henckaerts I, Goldblatt D, Ashton L, Poolman J. Critical differences between pneumococcal polysaccharide enzyme-linked immunosorbent assays with and without 22F inhibition at low antibody concentrations in pediatric sera. Clin Vaccine Immunol 2006; 13:356-60; PMID:16522777; http://dx.doi.org/10.1128/CVI.13.3.356-360.2006
- Poolman JT, Frasch CE, Kayhty H, Lestrate P, Madhi SA, Henckaerts I. Evaluation of pneumococcal polysaccharide immunoassays using a 22F adsorption step with serum samples from infants vaccinated with conjugate vaccines. Clin Vaccine Immunol 2010; 17:134-42; PMID:19889940; http://dx.doi.org/10.1128/CVI.00289-09
- Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, Keyserling HL, Carlone GM. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol 1997; 4:415-22; PMID:9220157
- Henckaerts I, Durant N, De Grave D, Schuerman L, Poolman J. Validation of a routine opsonophagocytosis assay to predict invasive pneumococcal disease efficacy of conjugate vaccine in children. Vaccine 2007; 25:2518-27; PMID:17034907; http://dx.doi.org/10.1016/j.vaccine.2006.09.029
