Abstract
The introduction of targeted therapies like the tyrosine kinase (TKI) and mammalian target of rapamycin (mTOR) inhibitors has improved patients´ survival in general. Nevertheless the prognosis remains limited. Therapies with a new mode of action are urgently warranted, especially those who would provoke long-term responders or long-lasting complete remissions as observed with unspecific immunotherapy with the cytokines interleukin-2 and interferon-α. In the recent years a deeper understanding of the underlying immunology of T cell activation led to the development of checkpoint inhibitors, which are mainly monocloncal antibodies and which enhances the presence of the co-stimulatory signals needed for T cell activation or priming. This review discusses the clinical data and ongoing studies available for the inhibition of the PD-1 (CD279) and CTLA-4 (CD152) axis in mRCC. In addition, potential future immunological targets are discussed. This approach of T-cell activation or re-activation by immunological checkpoint inhibition holds the inherent promise to directly affect the tumor cell and thereby to potentially cure a subset of patients with mRCC.
Introduction
Metastatic disease in renal cell carcinoma (mRCC) is present in about 20 to 30% of patients at the time of initial diagnosis. Another third will develop metastatic disease later on. In general, if this tumor stage is present, the prognosis was poor and mRCC was regarded as a therapy-refractory disease. The discovery that interleukin-2 is a T cell stimulating cytokine paved the way to the first effective therapies in metastatic RCC. The cytokines Il-2 and IFN-α alone or in combination with 5-Fluouracil significantly improved the survival of mRCC patients. The cytokines, applied in different dose regimes and termed unspecific immunotherapy, led to a remarkable clinical benefit in terms of disease stabilization or remission in up to 30% of patients.Citation1,2 Despite some long lasting responses or complete remissions the majority of patients had a survival benefit of only some months.Citation1,3 Therefore cytokine-based immunotherapy is currently replaced by the targeted therapy of mammalian target of rapamycin (mTOR) and tyrosine kinase inhibitors (TKI) for the treatment of mRCC patients. These agents improved median overall survival up to 30 months.Citation4,5 In addition, the multiplicity of substances allows for a second-line therapy and potential subsequent therapies.Citation6,7
Unspecific immunotherapy did not only lead to the activation of the immune system to target the cancer cells. Adverse immune effects like increased frequencies of regulatory T cells (Tregs) and decreased frequencies of circulating myeloid and plasmocytoid dendritic cells were reported in cytokine treated mRCC patients, which may explain the limitations of this therapy.Citation8,9 These facts and a missing well-defined mode of action with a T-cell response not specifically directed against the RCC tumor cells were the major drawbacks of this unspecific stimulation of the immune system in the recent years.
Currently, in RCC a transformation from the ancient unspecific therapy with cytokines to rather specific approaches, which directly target the renal cell cancer cell and the tumor microenvironment is observed.Citation10 One of the underlying principles in specific immunotherapy is that tumors express antigens the so called tumor-associated antigens (TAAs) that are recognized by (cytolytic) T lymphocytes (CTLs) derived from the tumor-bearing patient.Citation11 The described approaches of active immunotherapy have in common that TAAs shall activate naïve T cells, which then target the tumor. Several randomized immunotherapy trials have been reported and are on its ways in the adjuvant or metastatic setting. For example, AGS-003 (Argos Therapeutic, NC, USA) is a dendritic cell based (DC) vaccine based on individual tumor mRNA combined with synthetic CD40L RNA.Citation12 Vitespen (Oncophage®; Antigenics Inc., MA, USA) is an autologous tumor derived heat shock protein Gp96 preparation.Citation13 Reniale® (Liponova, Hanover, Germany) is an autologous DC vaccine and IMA901 (Immatics, Tübingen, Germany) is a synthetic peptide vaccine.Citation14,15 The results of these trials are promising, but none of the vaccines has gained general market status in Europe or the US. Currently, phase III studies are ongoing for AGS-003 and IMA901 for a further evaluation.Citation16,17 Unfortunately, the immune system can be controlled and edited by local or systemic environments to prevent an effective T cell activation at checkpoints of T cell activation.
Immunosurveillance and immunoediting
The hypothesis of immunosurveillance and the concept of immunoediting both describe the biological – immunological approach of cancer development.Citation18–20 The original concept of the immunosurveillance hypothesis formulated by Sir Macfarlane Burnet and Lewis Thomas postulates that small accumulations of tumor cells develop within the body. These tumor cells provoke an effective cellular immune reaction, which protects from neoplastic disease and leads to the regression of the tumor with no signs of clinical existence.Citation18,20 Later on this hypothesis was re-formulated to the concept of immunoediting, which consists of 3 phases, elimination, equilibrium and tumor escape. The elimination corresponds to the immunosurveillance. In the equilibrium the immune system allows the selections and promotion of different tumor cells with the capacity to survive the immune attacks. In the escape phase the immunologically sculptured tumor leads to an uncontrolled outgrowth of tumor cells within the immunocompetent host and the tumor becomes clinically apparent.Citation19 Thereby the tumor has different mechanisms to render itself “invisible” to the host´s immune system and to silence immune cells.Citation21 Theses tumor-driven mechanisms of immune suppression includes the downregulation of HLA molecules and/or tumor associated antigens (TAA), which leads to a decreased immunogenicity or the induction of suppressive cytokines like IL-10 or TGF-β.Citation22 These cytokines can lead to recruitment of regulatory T cells (Tregs), myeloid derived suppressor cells (MDCS) or tumor-associated M2 macrophages which can act as immunosuppressors.Citation21,23,24
Therefore, agents called immunomodulators or checkpoint inhibitors, which counteract tumor-induced immunosuppression are an external editing of the immune system to potentially shift tumor growth from the escape phase to the equilibrium state or toward tumor elimination.
Checkpoints and the regulation of the immune system
Activated T cells eliminate pathogens and tumor cells, but an exaggerated activation provokes autoimmune disorders. To understand the biology of checkpoint inhibition, it is of importance to figure out the control of T cells with its activating and inhibitory co-receptors, which are major regulators of T cell response and mediate tolerance to “self.”Citation25,26
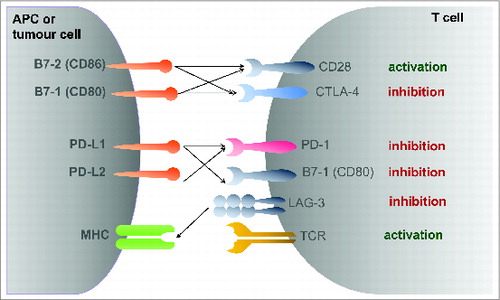
In order to prime, e.g. to activate naïve T cells the antigen-presenting cell (DC/APC) must present the TAA in the correct major histocompatibility complex molecule (´signal one´) in the presence of a co-stimulus, e.g., the expression of CD80 (B7–1) and CD86 (B7–2).Citation25 A high expression of such co-stimulatory molecules is achieved by the activation of APCs. If the co-stimulatory signal is not present, the DC cells might present the antigen on to the CD8+ T cell as a tolerogenic signal, which is also mediated through PD-1 (CD279) and CTLA-4 (CD152) ().Citation27,28 This phenomenon termed peripheral tolerance is influenced by the antigen-specific tolerogenic role of DCs.Citation22,27,28
CD152 – CTLA-4
The discovery that the B7-family members CD80/86 act as ligands to CD28 provided the molecular basis for the additional co-stimulatory signal needed for T cell activation.Citation29 This view of T cell co-stimulation had to be adopted with the discovery of cytotoxic T-lymphocyte antigen-4 (CTLA-4, CD152), which inhibits CD80/86, but binds with a much higher affinity than CD28.Citation30 Antibody binding experiments demonstrated that CTLA-4 ligation blocks CD28-dependent T cell activation.Citation31 Here, crosslinking of anti-CTLA-4 antibodies inhibited IL-2 production. In this context, it is thought that CTLA-4 prevents an exaggerated T cell activation if the co-stimulatory activation of CD80/86 to CD28 is provided.Citation32 For example, CTLA-4 deficient mice develop massive lymphoproliferation and fatal multiorgan tissue destruction as an expression of autoimmunitiy.Citation33 On the other hand, in preclinical models CTLA-4 blockade augments anti-tumor immunitiy and prolonged survival in a mice colon carcinoma model.Citation34
In the clinic, ipilimumab (Yervoy, Bristol-Myers Squibb, New York City, NY, USA) and tremelimumab (Pfizer, New York City, NY, USA) are 2 humanized antibodies, which binds to CTLA-4 and thereby re-activate T cell effector function (). So far, only ipilimumab is approved for the treatment of metastatic melanoma.Citation35 Data from 2 large phase III trials are available for Ipilimumab. In the first trial patients with unresectable stage III or IV melanoma who had progressed after initial first-line therapy were randomized in a 3:1:1 fashion to receive ipilimumab (3 mg/kg b.w.) plus the glycoprotein 100 (gp100) peptide vaccine vs. ipilumumab alone vs. gp100 peptide vaccine alone. Overall survival was improved to 10.0 months vs. 6.4 months in the ipilimumab + gp100 combination arm compared to gp100 alone. Patients who received ipilimumab alone demonstrated an overall survival of 10.1 months.Citation35 In the second trial ipilimumab (10 mg/kg) was administered to previously untreated patients with metastatic melanoma in combination with dacarbazine (850 mg/m2) compared to dacarbazine vs. placebo. In the ipilimumab plus dacarbazine group overall survival was significantly improved by 2.1 months with higher survival rates at 2 and 3 (20.8% vs. 12.2%) years follow-up.Citation36
Table 1. Overview of available checkpoint modulators for cancer immunotherapy
The second CTLA-4 monocloncal antibody tremelimumab failed to demonstrate a significant prolongation in survival in a large phase III study of metastatic melanoma patients.Citation37
CTLA-4 – Ipilimumab phase II study
In RCC the results of one phase II trial are available.Citation38 In the first cohort 21 metatastic RCC patients were treated with ipilimumab at a loading dose of 3 mg/kg and then with 1 mg/kg every 3 weeks (n = 21). All patients had previous IL-2 treatment (). Three (14%) of theses patients had a grade III toxicity of enteritis presenting with diarrhea. One of the 21 patients, the one with diarrhea, had a partial response. In the second cohort 40 patients received 3 mg/kg of ipilimumab every 3 weeks. 26 patients had a previous IL-2 therapy.Citation38 43% of patients had a severe immune-mediated toxicity (14 grade III, 2 grade IV and 1 grade V), with enteritis (n = 13) and hypophysitis (n = 1). One patient with adrenal metastasis showed primary adrenal insufficiency and one patient had aseptic meningitis. Of the patients with enteritis 3 had a colonic perforation and one underwent colectomy for bleeding.Citation38 Among the 40 patients there were 5 partial responses at 7, 8, 12, 17 and 21 months´ duration. The association between autoimmune toxicity and objective tumor regression was significant; there was no patients responding to therapy who had no autoimmune event.Citation38
Table 2. Published clinical trials of checkpoint modulators for mRCC as discussed in the review
CTLA-4 – Tremelimumab in combination with sunitinib phase I study
In a phase I study 28 patients, who had received ≤1 previous systemic treatment, received tremelimumab (6 mg/kg, 10 mg/kg, or 15 mg/kg) intravenously once every 12 weeks and oral sunitinib (50 mg daily for 4 weeks then 2 weeks off or 37.5 mg daily as a continuous dose) in a 3+3 dose escalation scheme.Citation39 The primary objective was drug safety. Dose limiting toxicities were observed in 2 of 5 patients receiving 50 mg sunitinib and tremelimumab 6 mg/kg, in 3 of 6 patients receiving tremelimumab 15 mg/kg plus continuously sunitinib at 37.5 mg and in 3 of 7 patients in the expansion cohort of tremelimumab 10 mg/kg plus sunitinib 37.5 mg daily. There was one sudden death in the tremelimumab 10 mg/kg plus sunitinib group. The onset of rapid acute renal failure was the most common DLT. Due to these results a further investigation of tremelimumab doses greater than 6 mg/kg plus sunitinib 37.5 mg daily was not recommended.Citation39
PD-1/PD-L1 axis
Programmed death 1 (PD-1) is a key immune checkpoint receptor expressed by activated T cells, where it terminates immune responses upon antigen stimulation to avoid massive immune destruction. The two ligands PD-L1 (B7-H1) and PD-L2 (B7-DC) expressed in peripheral and tumor tissue bind to the PD-1 receptor on the T cell surface.Citation40 Here, PD-L1 seems to be selectively expressed by tumor cells and cells in the tumor microenvironment in response to inflammatory stimuli, where it can inhibit cytokine production and the cytolytic activity of PD-1 positive, tumor-infiltrating CD4+ and CD8+ T cells.Citation40-42 PD-1 directed antibodies inhibit the interaction between PD-1 receptor and its ligands, while anti-PD-L1 antibodies block interactions between PD-L1 and both PD-1 and CD80.Citation43,44
The interesting point in the disruption of the PD-1/PD-L1 axis is whether there will be a long-lasting anti-tumor response and a tumor control after the cessation of the blockade. This would reflect a persistent anti-tumor immunity with the generation of an effective immunologic memory for an ongoing tumor control. Several antibodies have been used in early clinical trials that either target PD-1 or PD-L1.Citation45 Subsequently, the results of antibodies administered in RCC patients are discussed, mainly based on the published trial results and results of clinical trial, which were presented at the annual meeting of the American society of clinical oncology (ASCO) in 2014.
PD-1 antibody
BMS-936558 also named nivolumab is a fully humanized anti-PD-1 antibody (Bristol-Myers Squibb, New York City, New York) that has shown anti-tumor activity in 296 patients with solid cancers.Citation44 Nivolumab was given in a starting dose of 1 mg/kg and then expanded to 3 and 10 mg/kg in cohorts of 3 to 6 patients, among theses 33 had mRCC at doses of 1 and 10 mg/kg. The objective response rates were 4 of 17 patients (24%) treated with a dose of 1.0 mg/kg and 5 of 16 (31%) treated with a dose of 10.0 mg/kg. Five out of 8 patients, who started treatment one year before analysis, had a response longer than one year. Stable disease, defined as stability longer than 24 weeks, was observed in an additional 9 (27%) patients. Of all 296 patients treated 14% had a grade 3 or 4 drug-related adverse event (AE) with diarrhea (11%), rash (12%), pruritus (9%), pneumonitis (3%) and increased levels of alanine (4%) or aspartate aminotransferase (3%) or thyroid-stimulating hormone (3%). Endocrine disorders of hypophysitis (2%) and hyperthyroidism (1%) were rare.
Based on theses results a large phase III trial of 822 patients with metastatic or advanced RCC was initiated (CheckMate 025).Citation46 RCC patients with 1 or 2 prior anti-angiogenic therapies were randomized to receive either nivolumab (3 mg/kg every 2 weeks) vs. everolimus (10 mg p.o daily). Primary study aim is overall survival. The study has terminated recruitment and is currently under follow-up. Final data collection for primary outcome measure is announced for February 2016.Citation46
In advanced treatment–refractory melanoma (n = 107, 62% had at least 2 prior therapies) nivolumab treated patients demonstrated an overall survival of 16.8 months with one and 2 y survival rates of 62% and 43%. Nivolumab doses ranged between 0.1 and 10 mg/kg, including a dose expansion cohort.Citation47 In a phase III study (CheckMate 066) previously untreated metastatic melanoma patients without a BRAF mutation were randomized 1:1 to nivolumab (3 mg/kg every 2 weeks) vs. dacarbazine (1000 mg/m2 every 3 weeks). Progression-free survival was significantly prolonged in the nivolumab group (5.1 vs. 2.1 months) and the overall survival rate at one year was 72.9% in the nivolumab arm compared to 42.1 % in the dacarbazine arm, while median OS was not reached in the nivolumab arm so far.Citation48
These improvements obtained with nivolumab in the treatment of melanoma patients underline the expectations, which are anticipated from the ongoing RCC trials.
PD-1 antibody – dose escalation
In the primary study of nivolumab in solid cancer no dose –toxicity relationship was observed with 0.3 to 10 mg/kg.Citation44 At ASCO 2014 a dose ranging phase II study of nivolumab was presented, which assessed the primary aim of a dose response-relationships of nivolumab in pretreated mRCC patients (at least 1 prior antiangiogenetic therapy; n = 168).Citation49 Patients were randomized 1:1:1 to 0.3, 2 and 10 mg/kg nivolumab i.v. every three weeks with treatment until progression or intolerable toxicity. Patients received a median of 6 (0.3 mg/kg) to 8 (2 and 10 mg/kg) doses of nivolumab. Reasons for drug discontinuation were predominately progressive disease in 81%, 74% and 69% of patients and drug-related toxicity in 2%, 9% and 7% of patients in the 0.3, 2 and 10 mg/kg arms. Treatment related adverse evens of any grade III or IV were 5% (0.3 mg/kg), 17% (2 mg/kg) and 13% (10 mg/kg) in the respective group. Objective response rate was not different between the groups (20–22%) and most patients responding had the response within their first 3 months. Interestingly, complete responses were rare with a rate of 0–2%. Progression-free survival was similar in all groups with 2.7 months, 4.0 months and 4.2 months for the 0.3, 2 and 10 mg/kg groups (p = 0.9). In addition, median overall survival ranged between 18.2 months (0.3 mg/kg), 25.5 months (2 mg/kg) and 24.7 months (10 mg/kg), which is quite a promising result for second and third-line treated patients.
PD-1 antibody in combination with VEGF-TKI
Data of a phase II study of nivolumab in combination with the TKI sunitinib or pazopanib in mRCC patients was presented at this years ASCO annual meeting as an abstract.Citation50 Nivolumab dose (every 3 weeks) was escalated from 2 mg/kg to 5 mg/kg, sunitinib (S + N; n = 33) and pazopanib (P + N, n = 20) given in the recommended dosage in previously treated patients with the primary aim to assess overall tolerability and safety. In the sunitinib arm nivolumab was escalated from 2 mg/kg (n = 7) to 5 mg/kg (n = 7) and then expanded in another 19 patients who were treatment naive. In the pazopanib arm 10 patients were treated with 2 mg/kg and no further escalation as the maximum tolerated dose was reached. Severe adverse events grade III or IV occurred in 82% of the S+N arm and in 70% of the P+N2 arm, including TKI class related AEs like hypertension, diarrhea, liver enzyme elevations and fatigue. Immune associated AE of pneumonitis and endocrinopathy were rare, but renal and hepatic AE were higher than anticipated for VEGF-TKI or nivolumab monotherapy. Treatment was discontinued due to AE in 12 (36,4%, S+N arm) and 5 (25%, P+N2 arm) patients. No treatment related death occurred. Efficacy was higher in the combinations than anticipated for the single agents. At the first assessment at 6 weeks 41.2% (7/17) and 55.6% (5/9) responded, 30% (n = 10) and 35% (n = 7) had a stable disease in the S+N and the P+N2 arm. Ongoing responders were observed in 58.8% (10/17, S+N) and 33.3% (3/9, P+N) of patients. Interestingly, time to response was relatively short with most patients responding within 6 to 12 weeks and 5 patients responded following the discontinuation of the therapy.Citation50
PD-1 antibody in combination with ipilimumab
Another phase I study by Hammers et al. was presented as an abstract at the ASCO meeting 2014.Citation51 Patients were treated with combinations of nivolumab and ipilimumab 4 times in 3 weeks intervals followed by continuous treatment of nivolumab 3 mg/kg every 3 weeks. Combination therapy was either nivolumab 3 mg/kg plus ipilimumab 1 mg/kg (N3 + I1, n = 21) vs. nivolumab 1 mg/kg plus ipilimumab 3 mg/kg (N1+I3, n = 23). About 80% of patients had received a previous systemic treatment. Treatment related AE led to the discontinuation of therapy in 2 (9.5%) and 6 (26.1%) of N3+I1 and N1+I3 treated patients. Treatment related adverse events grade III and IV were higher in the N1+I3 arm (60.9% vs. 28.6%) with gastrointestinal disorders and hepatic dysfunction. According to the authors grade III or IV AE were manageable with established guidelines. Responses were higher in the combination than reported for each monosubstance of nivolumab and ipilimumab in RCC. Progression-free survival was 64% and 64% in the N3+I1 and N1+I3 arm. Median duration of response was 31.1 weeks (4.1 to 32.1+) in the N3+I1 arm and not reached in the N1+I3 arm (12.1+ to 35.1+). Objective response was observed in 9 of 21 (43%) and 10 of 23 (43%) and stable disease in 5 (24%) and 8 (35%) patients for N3+I1 and N1+I3. Most responses occurred within 6 to 12 weeks and 2 of the N3+I3 and 4 of the N1+I3 responders exhibited ongoing responses.Citation50
The encouraging results of this phase I study led to the initiation of a phase III study in mRCC patients as a first-line treatment with the combination of nivolumab 3 mg/kg with ipilimumab 1 mg/kg intravenously every 3 weeks for 4 doses and then nivolumab 3 mg/kg solutions intravenously every 2 weeks compared to sunitinib monotherapy (CheckMate 214). Approximately 1070 patients are intended to be enrolled.Citation52
PD-L1 antibody
The second antibody that targets the PD-1 receptor-ligand axis is BMS-936559 a fully humanized PD-L1 specific antibody (Bristol-Myers Squibb, New York City, New York).Citation43 PD-L1 antibody has been evaluated in 207 patients with advanced solid tumors, among 17 patients with mRCC treated at a dosage of 10 mg/kg in 6 weeks cycles on days 1, 15, and 29 of each cycle. Two of the 17 patients showed an objective response with duration of 4 and 17 months; 7 patients had stable disease at the 24-week follow-up. Most events were low grade, with treatment-related grade 3 or 4 events in 19 of 207 (9%). The most common drug-related adverse events were fatigue, infusion reactions, diarrhea, arthralgia, rash, nausea, pruritus, and headache. Potential immune-related adverse events were observed in 81 of 207 patients (39%); the included hypothyroidism, hepatitis, and one case each of sarcoidosis, endophthalmitis, diabetes mellitus, and myasthenia gravis.Citation43
Future perspective
Immunotherapy of metastatic RCC has evolved from the rather unspecific approaches of the cytokine area to specific immunotherapy. Currently, one of the main foci in specific immunotherapy is the use of checkpoint inhibitors. Here, targeting the PD-1/PD-L1 axis is the most heavily investigated area and is regarded as the lead axis. Among the discussed antibodies, for which clinical results are available, several other PD-1 antibodies like pembrolizmab (MK-3475, Merck Sharp & Dohme Corp., Whitehouse Station, NJ, USA.Citation53), AMP-224 (AmpIimmune Inc., Gaithersburg, MD, USACitation54), Pidilizumab (CT-011, Curetech, Yavne, IsraelCitation55) and PD-L1 antibodies like MEDI4736 (MedImmune/AstraZeneca, London, U.KCitation56), MPDL3280A (Genentech/Roche, Basel, SwitzerlandCitation57), and MSB0010718C (MerckSerono, Darmstadt, GermanyCitation58) are in different stages of preclinical and clinical development in phase I/II studies of various cancer entities ().Citation45
Checkpoint inhibition with nivolumab and ipilimumab activates or inhibits co-stimulatory signals in T cell activation. This new mode of action demonstrated a prolonged overall survival for melanoma patients and it is anticipated that this will also be achieved in mRCC patients. Nevertheless this “manipulation” of the immune system also leads to new probably class specific immune related adverse events which might be dose limiting. As observed so far, theses immune related AE lead to autoimmune reactions like hypothyroidism, hepatic dysfunction or hepatitis, sarcoidosis, endophthalmitis, gastrointestinal dysfunction like enteritis or myasthenia gravis. Especially the combination of ipilimumab and nivolumab in the dose of I3 + N1 seemed to be dose limiting.Citation50
Immunological checkpoints can inhibit or activate T cells. These leads to the speculation if immunomodulation should target co-stimulatory molecules, which inhibit or the activate T cells. Based on the clinical results available from the 2 lead substances nivolumab and ipilimumab, especially in melanoma, it seems to be more beneficial to target the inhibitory axis, in terms of efficacy and reduced side effects.
Checkpoint inhibition is not limited to the CTLA-4 and the PD-1/PD-L1 axis. Different monoclonal antibodies, which activate or inhibit T cell function, are under development. Among the target molecules antibodies against the tumor necrosis family receptors (TNFR) with OX40 (CD134), 4–1BB (CD137), GITR (glucocorticoid-induced TNFR-related protein/ tumor necrosis factor receptor superfamily member 18) and CD40 are available and warrant further evaluation in RCC.Citation45 The future will show if any antibody directed checkpoint inhibition would become a paradigm changing therapy in mRCC.
Not only co-stimulatory signals are the target of T cell activation therapies, but also the direct agonize of the MHC class II receptor. IMP321 is a LAG-3 fusion protein, which agonizes MHC class II–driven dendritic cell activation. IMP321 was evaluated in a dose escalation phase I study in mRCC patients.Citation59 In this study IMP321 induced both sustained CD8 T-cell activation and an increase in the percentage of long-lived effector-memory CD8 T cells. The safety of IMP321 was excellent, as no clinical significant local or systemic treatment-related adverse events were recorded; 10% of patients experienced grade I local reactions. In the 21 patients treated no objective response occurred, but in patients with higher doses PFS was significantly better compared to lower doses.Citation59 These interesting results warrant further investigation in combination studies, not only with standard anti-angiogenetic therapy.
Although checkpoint inhibitors are attributed to the treatment modality of specific immunotherapy it has to be stressed that only the mode of action is specific, e.g. the target receptor is well defined. On the other hand, the provoked effector functions by the respective checkpoint modulation are not well defined. It is not known against which respective tumor-associated antigens the T cells will be activated and in which strength the anti-tumor immune response is provoked. For the future, these facts warrant that checkpoint modulators are not only be used as a stand-alone drug, but rather in combination with a vaccination specific approach in which the immune response is direct against specific TAA and the strength of the response can be measured.
Disclosure of Potential Conflicts of Interest
AS is subinvestigator, JB and PJG are principal investigator of the nivolumab CheckMate25 clinical trial. PJG is investigator of the nivolumab Checkmate214 study. JB, AS and PJG have received honoraria for advisory role from BMS.
Funding
JB is supported by a grant by the Deutsche Forschungsgemeinschaft (DFG, SFB 685 C5).
References
- Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, Seipp CA, Rogers-Freezer L, Morton KE, White DE, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol 2003; 21(16):3127–32; PMID:12915604; http://dx.doi.org/10.1200/JCO.2003.02.122
- Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, Patard JJ, Mulders PF, Sinescu IC; European Association of Urology Guideline Group. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol 2010; 58(3):398–406; PMID:20633979; http://dx.doi.org/10.1016/j.eururo.2010.06.032
- Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol 2004; 171(3):1071–6; PMID:14767273; http://dx.doi.org/10.1097/01.ju.0000110610.61545.ae
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009; 27(22):3584–90; PMID:19487381; http://dx.doi.org/10.1200/JCO.2008.20.1293
- Motzer RJ, Hutson TE, McCann L, Deen K, Choueiri TK. Overall survival in renal-cell carcinoma with pazopanib versus sunitinib. N Engl J Med 2014; 370(18):1769–70; PMID:24785224; http://dx.doi.org/10.1056/NEJMc1400731
- Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME, Rusakov IG, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011; 378(9807):1931–9; PMID:22056247; http://dx.doi.org/10.1016/S0140-6736(11)61613-9
- Kruck S, Bedke J, Kuczyk MA, Merseburger AS. Second-line systemic therapy for the treatment of metastatic renal cell cancer. Exp Rev Antican Ther 2012; 12(6):777–85; PMID:22716494; http://dx.doi.org/10.1586/era.12.43
- van der Vliet HJ, Koon HB, Yue SC, Uzunparmak B, Seery V, Gavin MA, Rudensky AY, Atkins MB, Balk SP, Exley MA. Effects of the administration of high-dose interleukin-2 on immunoregulatory cell subsets in patients with advanced melanoma and renal cell cancer. Clin Cancer Res 2007; 13(7):2100–08; PMID:17404092; http://dx.doi.org/10.1158/1078-0432.CCR-06-1662
- Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood 2006; 107(6):2409–14; PMID:16304057; http://dx.doi.org/10.1182/blood-2005-06-2399
- Bedke J, Stenzl A. Immunotherapeutic strategies for the treatment of renal cell carcinoma: where are we now? Exp Rev Antican Ther 2013; 13(12):1399–408; PMID:24215158; http://dx.doi.org/10.1586/14737140.2013.856761
- van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Sciencel 1991; 254(5038):1643–7; PMID:1840703; http://dx.doi.org/10.1126/science.1840703
- Amin A, Dudek A, Logan T, Lance RS, Holzbeierlein JM, Master VA, Kumar Pal S, Knox JJ, Karsh LI, Plessinger D, et al. Prolonged survival with personalized immunotherapy (AGS-003) in combination with sunitinib in unfavorable risk metastatic RCC (mRCC). J Clin Oncol 2013; 31((suppl 6); abstr 357)
- Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, Mulders P, Zielinski H, Hoos A, Teofilovici F, et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet 2008; 372(9633):145–54; PMID:18602688; http://dx.doi.org/10.1016/S0140-6736(08)60697-2
- Jocham D, Richter A, Hoffmann L, Iwig K, Fahlenkamp D, Zakrzewski G, Schmitt E, Dannenberg T, Lehmacher W, von Wietersheim J, et al. Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal-cell carcinoma after radical nephrectomy: phase III, randomised controlled trial. Lancet 2004; 363(9409):594–9; PMID:14987883; http://dx.doi.org/10.1016/S0140-6736(04)15590-6
- Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med 2012; 18(8), 1254–61; PMID:22842478; http://dx.doi.org/10.1038/nm.2883
- IMA901 in Patients Receiving Sunitinib for Advanced/Metastatic Renal Cell Carcinoma. http://clinicaltrials.gov/ct2/show/NCT01265901).
- Phase 3 Trial of Autologous Dendritic Cell Immunotherapy (AGS-003) Plus Standard Treatment of Advanced Renal Cell Carcinoma (RCC) (ADAPT). http://clinicaltrials.gov/show/NCT01582672).
- Burnet M. Cancer; a biological approach. I. The processes of control. Br Med J 1957; 1(5022):779–86; PMID:13404306; http://dx.doi.org/10.1136/bmj.1.5022.779
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immun 2002; 3(11):991–8; PMID:12407406; http://dx.doi.org/10.1038/ni1102-991
- Thomas L. “Discussion” Cellular and Humoral Aspects of the Hypersensitive States. Lawrence HS (ed.), New York: Taylor & Francis; 1957:529–33.
- Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol 2010; 22(2):231–7; PMID:20144856; http://dx.doi.org/10.1016/j.coi.2010.01.009
- Bedke J, Gouttefangeas C, Singh-Jasuja H, Stevanovic S, Behnes CL, Stenzl A. Targeted therapy in renal cell carcinoma: moving from molecular agents to specific immunotherapy. World J Urol 2014; 32(1); 31–8; PMID:23404195; http://dx.doi.org/10.1007/s00345-013-1033-3
- Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest 2007; 117(5):1167–74; PMID:17476346; http://dx.doi.org/10.1172/JCI31202
- Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kübler H, Yancey D, Dahm P, Vieweg J. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res 2008; 14(24):8270–8; PMID:19088044; http://dx.doi.org/10.1158/1078-0432.CCR-08-0165
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Ann Rev Immunol 2005; 23:515–48; PMID:15771580; http://dx.doi.org/10.1146/annurev.immunol.23.021704.115611
- Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Ann Rev Immunol 2001; 19:225–52; PMID:11244036; http://dx.doi.org/10.1146/annurev.immunol.19.1.225
- Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol 2005; 6(3):280–6; PMID:15685176; http://dx.doi.org/10.1038/ni1165
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Ann Rev Immunol 2003; 21:685–711; PMID:12615891; http://dx.doi.org/10.1146/annurev.immunol.21.120601.141040
- Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med 1991; 173(3):721–30; PMID:1847722; http://dx.doi.org/10.1084/jem.173.3.721
- Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med 1991; 174(3), 561–9; PMID:1714933; http://dx.doi.org/10.1084/jem.174.3.561
- Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med 1996; 183(6):2541–50; PMID:8676075; http://dx.doi.org/10.1084/jem.183.6.2541
- Sansom DM, Manzotti CN, Zheng Y. What's the difference between CD80 and CD86? Trends Immunol 2003; 24(6):314–9; PMID:12810107; http://dx.doi.org/10.1016/S1471-4906(03)00111-X
- Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995; 3(5):541–7; PMID:7584144; http://dx.doi.org/10.1016/1074-7613(95)90125-6
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996; 271(5256):1734–6; PMID:8596936; http://dx.doi.org/10.1126/science.271.5256.1734
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363(8):711–23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466
- Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364(26):2517–26; PMID:21639810; http://dx.doi.org/10.1056/NEJMoa1104621
- Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, Garbe C, Gogas H, Schachter J, Linette G, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol 2013; 31(5):616–22; PMID:23295794; http://dx.doi.org/10.1200/JCO.2012.44.6112
- Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, Levy C, Allen T, Mavroukakis S, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunothera 2007; 30(8):825–30; PMID:18049334; http://dx.doi.org/10.1097/CJI.0b013e318156e47e
- Rini BI, Stein M, Shannon P, Eddy S, Tyler A, Stephenson JJ Jr, Catlett L, Huang B, Healey D, Gordon M. Phase 1 dose-escalation trial of tremelimumab plus sunitinib in patients with metastatic renal cell carcinoma. Cancer 2011; 117(4):758–67; PMID:20922784; http://dx.doi.org/10.1002/cncr.25639
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8(8):793–800; PMID:12091876
- Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 2003; 9(5):562–7; PMID:12704383; http://dx.doi.org/10.1038/nm863
- Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002; 99(19):12293–7; PMID:12218188; http://dx.doi.org/10.1073/pnas.192461099
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366(26):2455–65; PMID:22658128; http://dx.doi.org/10.1056/NEJMoa1200694
- Topalian SL, Hodi FS, Brahmer JR, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366(26):2443–54; PMID:22658127; http://dx.doi.org/10.1056/NEJMoa1200690
- Aranda F, Vacchelli E, Eggermont A, Galon J, Fridman WH, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Immunostimulatory monoclonal antibodies in cancer therapy. Oncoimmunology 2014; 3(1):e27297; PMID:24701370; http://dx.doi.org/10.4161/onci.27297
- Study of BMS-936558 vs. Everolimus in Pre-Treated Advanced Or Metastatic Clear-cell RCC. http://clinicaltrials.gov/ct2/show/NCT01668784).
- Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32(10):1020–30; PMID:24590637; http://dx.doi.org/10.1200/JCO.2013.53.0105
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N Engl J Med (2015); 372(4):320-30. doi: 10.1056/NEJMoa1412082
- Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel T, Harrison MR, Vaishampayan UN, Drabkin HA, George S, Logan TF, et al. Nivolumab for metastatic renal cell carcinoma (mRCC): Results of a randomized, dose-ranging phase II trial. J Clin Oncol 2014; 32:5s, abstr 5009
- Amin A, Plimack ER, Infante JR, Ernstoff MS, Rini BI, McDermott DF, Knox JJ, Pal KS, Voss MH, Sharma P, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC). J Clin Oncol 2014; 32:5s, abstr 5010
- Hammers HJ, Plimack ER, Infante JR, Ernstoff MS, Rini BI, McDermott DF, Razak ARA, Pal KS, Voss MH, Sharma P, et al. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC). J Clin Oncol 2014; 32:5s, abstr 4504
- Nivolumab Combined With Ipilimumab Versus Sunitinib in Previously Untreated Advanced or Metastatic Renal Cell Carcinoma (CheckMate 214). http://clinicaltrials.gov/ct2/show/NCT02231749).
- Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014; 384(9948):1109–17; PMID:25034862; http://dx.doi.org/10.1016/S0140-6736(14)60958-2
- Infante JR, Powderly JD, Burris HA, Kittaneh M, Houston Grice J, Smothers JF, Brett S, Fleming ME, May R, Marshall S, et al. Clinical and pharmacodynamic (PD) results of a phase I trial with AMP-224 (B7-DC Fc) that binds to the PD-1 receptor. J Clin Oncol 2013; 31((suppl 6); abstr 3044)
- Armand P, Nagler A, Weller EA, Devine SM, Avigan DE, Chen YB, Kaminski MS, Holland HK, Winter JN, Mason JR, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol 2013; 31(33):4199–4206; PMID:24127452; http://dx.doi.org/10.1200/JCO.2012.48.3685
- Lutzky J, Antonia SJ, Blake-Haskins A, Li X, Robbins PB, Shalabi AM, Vasselli J, Ibrahim RA, Khleif S, Segal NH. A phase 1 study of MEDI4736, an anti–PD-L1 antibody, in patients with advanced solid tumors. J Clin Oncol 2014; 32:5s
- Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515(7528):558–62; PMID:25428503; http://dx.doi.org/10.1038/nature13904
- Herry CR, O'Sullivan Coyne GH, Madan RA, Schlom J, von Heydebreck A, Cuillerot JM, Sabzevari H, Gulley JL. Phase I open-label, multiple ascending dose trial of MSB0010718C, an anti-PD-L1 monoclonal antibody, in advanced solid malignancies. J Clin Oncol 2014; 32:5s, abstr 3064.
- Brignone C, Escudier B, Grygar C, Marcu M, Triebel F. A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma. Clin Cancer Res 2009; 15(19):6225–6231; PMID:19755389; http://dx.doi.org/10.1158/1078-0432.CCR-09-0068