?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Human enterovirus 71 (EV71) has been associated with outbreaks of hand-foot-and-mouth disease (HFMD) in China. Susceptibility to EV71 is associated with age, but few studies have been accomplished to measure such a relationship. A better understanding of the connection between susceptibility and age is necessary to develop strategies for control of HFMD. In 2010, a survey of an epidemic of EV71 was conducted in a northern city of Jiangsu Province in China. Samples were tested serologically to identify the EV71 neutralizing antibody. Two different mathematical models have now been employed to describe how this antibody varied with age, and parameters in the model were estimated from survey data. Both models depicted the variations in EV71-neutralizing antibody. Seroprevalence was high for neonates but decreased to near zero at 5 months of age. Subsequently, the EV71 antibody levels increased and then remained stable after about 36 months. For models 1 and 2, values for the coefficient of determination (R2) were 0.9458 and 0.9576, and values for root mean square error (RMSE) were 0.0755 and 0.0752, respectively. Model 2, formulated from the characteristics of development of the immune system, was more reliable than model 1, formulated from survey data, because the impact of the survey on the structure of the model was removed. Moreover, model 2 provided the possibility to define the parameters in a biological sense.
Introduction
Hand-foot-and-mouth disease (HFMD), a common illness caused by various enteric viruses, is more likely to occur in infants and young children. Most of the symptoms are mild, but there can be painful vesicular lesions on the hands, feet, mouth, and tongue. Some patients have infection episodes, including pulmonary edema, myocarditis, and aseptic meningitis but, even if the disease progresses, few of these lead to death.Citation1,2 Since 2008, large-scale outbreaks of HFMD have occurred in many regions of China and have resulted in severe public health problems.Citation3,4
Human enterovirus 71 (EV71), coxsackievirus A16 (CoxA16), and some types of ECHO virus are causative agents of this disease. HFMD caused by CoxA16 usually results in a mild, self-limiting disease. However, patients infected by EV71 may have serious complications, and the disease may be fatal.Citation5 EV71 is considered to be the dominant pathogen during HFMD epidemics within the Southeast and East Asian regions, including China.Citation6,7
Although the pathogenesis, clinical features, and molecular biology of EV71 have been investigated,Citation8-12 no vaccine or effective drug against EV71 is available. Thus, epidemiological surveillance of HFMD and its pathogens is necessary for early warning of HFMD outbreaks so that their impact can be reduced.Citation13
The distribution of the EV71 antibody among children has been measured, but it is difficult to evaluate the impact of an HFMD epidemic. Quantitative analyses have been rarely accomplished, although such a description of changes in EV71 antibodies in children is essential to develop, for HFMD, control policies based on predictive mathematical models.
In epidemiological studies, mathematical models are used to infer the values of parameters or behaviors that cannot be evaluated experimentally or are too expensive to be implemented.Citation14 A main use of epidemic models is to predict, from a few cases, the scale of the outbreak, thus allowing preventive actions to be taken.Citation15 For theoretical models of epidemic HFMD, differences of susceptibility for children of different ages should be considered.Citation16,17 This has rarely been taken into account, however, due to the lack of a factor adequately representing susceptibility.
In this report, 2 different mathematical models were used to describe the relationship between prevalence of EV71 antibody and age. With data from 420 samples collected in a city in the northern part of the Jiangsu province of China, changes in the presence of EV71 neutralizing antibody were analyzed, and mathematical formulas were derived, providing quantitative methods to analyze the variation of the susceptibility to EV71 with age. The two models allow a better consideration of susceptibility to EV71 among children of different ages and permit application into simulations of transmission and control of HFMD. Further, development of the models provides a reference for further collection of data.
Results
EV71 positive rate
Evaluated were samples from 20 newborns and 400 children with ages ranging from 1 month to 15 y ().
Table 1. EV71 positive rate of 420 subjects
Results for Model 1
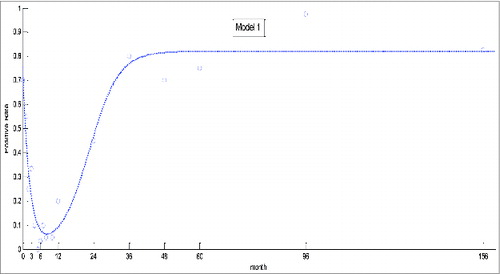
Estimated results of coefficients are shown in . Coefficients of determination (R2) and root mean square errors (RMSE) were 0.9458 and 0.0755, respectively. Overall, model 1 was statistically significant, as was the test for each coefficient.
Table 2. Fitting coefficients and statistical test of model 1
The predicted curve generated from model 1 was consistent with the actual change of positive rate, although it underestimated the actual rate at 12 months (). Thus, as the immune systems of the children developed, the presence of antibody increased more quickly than predicted by the model.
Results for model 2
The analytical solution to EquationEq. (1)(1)
(1) of model 1 is
. Due to the setting up of the initial time, the solution (EquationEq. (2)
(2)
(2) ) is
Theoretical changes in the positive rate generated by model 2, which combined the results of EquationEqs. (1)(1)
(1) and Equation(2)
(2)
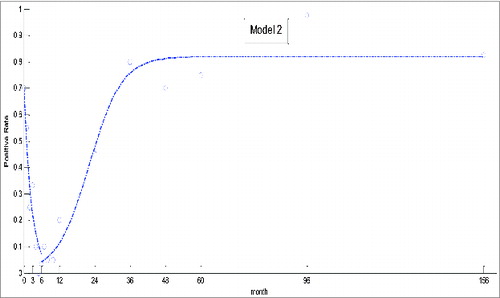
(2) , are depicted in . In contrast to Model 1, results from this dynamic model were more consistent with the actual changes. R2 and RMSE values for model 2 were 0.9576 and 0.0752, respectively.
Discussion
The presence of EV71 neutralizing antibody is closely related with age.Citation2,19 As demonstrated here, EV71 antibody decreased between 0 to 4 months, remained at a low level until 10 months, and increased to a maximum at 36 months. The overall trends were consistent with those for another investigation conducted in the same region,Citation24 although, in our study, the seroprevalence between 4 and 10 months fluctuated somewhat. For neonates, the positive rate was higher than that for other areas. This may be due to the presence of maternal antibody, for there is a strong correlation of neutralizing antibody in prenatal women and neonates.Citation2,19,24 Moreover, the low level of EV71 neutralizing antibody in children between 5 and 6 months of age showed that they were more likely to be infected, indicating the optimal time for vaccination. Nevertheless, application of these findings into strategies to improve the efficiency of control of HFMD remains to be determined.
For various epidemics, mathematical models have guided the development of control policies. A variety of models have been formulated, analyzed, and applied to infectious diseases.Citation27 The theoretical models provided scientific guidance, facilitating experimental investigations and the collection and analysis of epidemiological data.Citation14,20 For the study of HFMD epidemics caused by EV71, mathematical models have been developed to predict the occurrence of outbreaks. Most research has involved ordinary differential equations (ODE) to formulate a compartmental model. Some children were moved between compartments, and ODE was used to describe such changes. An indicator, such as basic reproduction numbers, was estimated from models to evaluate the effectiveness of disease control.Citation21,22 However, some factors affecting HFMD epidemics at a population level, such as the difference in immunological response at different ages were usually neglected; the relationship between susceptibility to EV71 and age of children was rarely analyzed quantitatively. In this report, 2 models, originating from survey data and from development of the immune system, were used to explore variations between positive rates and age.
For model 1, parameters were estimated to minimize deviations between the theoretical curve and reported data. The Levenberg-Marquardt algorithm was applied to estimate parameters, and an optimal solution derived by least squares was obtained. Therefore, the structure of the fitting equation was relevant. For the present report, the structure of the fitting equation was determined based on the observed changes in trends provided by an epidemiological survey. Thus, results generated from model 1 were more likely to be affected by the results of investigation. Biased survey results may result in a biased model. Moreover, although they reflected actual changes, the parameters in model 1 are difficult to be explained biologically.
For model 2, a dynamic model, the process was different. First, the original equation was formulated based on the characteristics of development of the immune system. Hence, the form of the model was not relevant. Second, parameters in the model were estimated from data derived in an epidemiological survey. Results from the dynamic model would be more reliable because the impact of the survey on the structure on model was eliminated. Model 2 also provides an explanation of parameters from a biological view. According to the results of model 2, parameter k was the decreasing rate of EV71 antibody caused by decay of innate immunity, and was the increasing rate owing to development of the immune system of the children. The positive rate was high in initial stages. As maternal immunity disappeared, the positive rate increased, then remained stable as immunity to EV71 developed.
Both of the models approximately described the changes of EV71 neutralizing antibody and derived quantitative formulas. Model 2 focused on variation caused by the individual's immune system and was more appropriate than model 1 for application and interpretation.
The observed positive rate for children older than 2 y old were not sufficiently close to curves generated by the models, and variations of EV71 antibody among older children were higher. The interval for sampling was longer for older children; seroprevalence was tested only in 6 age groups for children over 12 months of age. Therefore, more age groups should be employed for older children in further studies.
Meanwhile, the results below one year of age fluctuated slightly at the 2 sides of curve. The deviation of model curve with actual survey was more likely to be caused by overlook of feeding pattern. Little research was carried out to explore the impact of feeding pattern on infection of HFMD. One report published in Chinese indicated that breast-feeding for 3–6 months reduced risk of infection of HFMD.Citation25 As a limitation of this report, the difference of feeding pattern was not considered at the stage of investigation due to low exclusive breastfeeding rate. The unbalanced distribution of breast-feeding and milk-feeding among age groups less than one year may result in unstable fluctuation of positive rate. Group based on feeding pattern and verification by external data will be considered in further study.
In the last decade, human EV71 has caused outbreaks of HFMD in mainland China, resulting in thousands of fatal cases.Citation6 The major protective mechanism against EV71 is cell-mediated immunity. Since humoral immunity with neutralizing antibodies is necessary for protection against EV71 infections,Citation25,26 seroepidemiological surveys of EV71 have been conducted in various areas of China.Citation23 Our research, however, was the first to establish a dynamic model of susceptibility to EV71 in relation to age. The results presented provide a better understanding of differences in immunological response to EV71 from a population level and a better evaluation of interventions that consider differences in immunity.
Materials and Methods
Study site and sample
The current survey was conducted in a rural county located in the northern part of Jiangsu, a province of eastern China. There was a higher HFMD prevalence relative to nearby areas, and, since 2008, several outbreaks of HFMD have occurred in this county. In 2009, the incidence reached 127.8 per 100 000.Citation18 Stratified random sampling was used to recruit participants into 18 groups determined in advance. Since August 2010, 420 children, with 210 each of 2 groups, were recruited from 2 townships of the county. All samples were tested serologically to identify the EV71 antibody. The study was approved by the Ethics Committee of Nanjing Medical University. Legal guardians of all participants provided signed informed consent.
Measurement of EV71 neutralizing antibody
EV71 serum neutralizing antibodies were measured by the micro cytopathic effect method. In 96-well microplates, serum samples were diluted at ratios starting at 1:8. They were inactivated at 56° for 30 min, neutralized with 100 CCID 50/50 μl of human EV71 virus at 37° for 2 h. A suspension of rhabdomyosarcoma (RD) cells was added at a concentration of 2 × 10Citation5 cells/ml, 100 μl/well, and the preparations were cultivated in a 35° CO2 incubator for 7 days, at which time the serum neutralizing antibodies were measured by their cytopathic effect.
Modeling
After the time of birth, the rate of EV71 infection fell to near zero at 5 months. Afterwards, the rate increased and then became stable at about 36 weeks. Therefore, the changing pattern of EV71 antibody was probably a combination of exponential decrease and S-shaped logistic growth.
Model 1 was based on survey data. In the first step of the modeling, average age was allocated to correspond to the positive rate between the last 2 age groups such that a one-to-one relationship between antibody level and age group was established.
In detail, let be children's positive rate of EV71 antibody at time
. Based on the observed trend, a mathematical model
was employed to describe the change in EV71 antibody over time. The model combined the effect of exponential decay with S-shaped growth. The parameters in model 1 were estimated by a Levenberg-Marquardt algorithm based on nonlinear least squares.
Model 2 was based on development of the immune system. Due to the immature immune system of newborns, innate immunity from mothers provides protection against disease. Thus, the reduction of EV71 antibody before 6 months is chiefly caused by the loss of innate immunity. Afterwards, the immune systems of the children develop, leading to an increase in EV71 antibody until a plateau is reached.Citation19
To consider the impact of EV71 antibody of immune system, suppose that is the positive rate of EV71 antibody resulting from innate immunity and that
is positive rate due to the development of the individual's own immune system at time t.
and
satisfy that
(1)
(1) and
(2)
(2) EquationEq. (1)
(1)
(1) describes the reduction of EV71 antibody, and
denotes the rate of decay. EquationEq. (2)
(2)
(2) describes the change of EV71 antibody based on the child's own immune system.
is the changing rate of antibody at the initial stage of the development.
denotes the final, stable level of the positive rate.
The two models were solved by Matlab 8.0 (R2012b).
Model solving
The analytical solution to EquationEq. (1)(1)
(1) is
where
denotes the initial value of the equation, i.e., the positive rate of neonates. Obtained by the survey, its value was 0.7. To estimate the unknown parameter,
, suppose that
is the retention time for children before EV71 antibody turned to be negative. The distribution of
, a random variable, is
Therefore, the mathematical expectation of is
.Citation20 In this report,
represents the average retention time of maternal antibody by babies. By use of data from birth to 6 months, it was estimated to be
, therefore,
. Thus, the solution to EquationEq. (1)
(1)
(1) is
.
The analytical solution to EquationEq. (2)(2)
(2) was
, and the positive rate of EV71 antibody remained stable at 82%. Therefore,
was replaced with 0.82, and the solution of EquationEq. (2)
(2)
(2) was estimated to be
where
and
are undetermined constants. To estimate C, the positive rate at the sixth month was regarded as the initial value, and
represented the changing rate of EV71 antibody at the stage at which the immune system started to develop. Using the initial value of
when
, C was estimated to be 23.6. Another parameter,
, was determined by the points of the inflection, i.e.,
20. The increment of the positive rate decelerated after 3 y of age. Therefore,
, and
. The final solution of EquationEq. (2)
(2)
(2) is
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors wish to thank Donald L. Hill (University of Alabama at Birmingham, USA) for editing.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81273184].
References
- Ang LW, Koh BK, Chan KP, Chua LT, James L, Goh KT. Epidemiology and control of hand, foot and mouth disease in Singapore, 2001–2007. Ann Acad Med Singapore 2009; 38:106-12; PMID:19271036
- Chen K, Chang H, Wang S, Cheng Y, Yang J. Epidemiologic features of hand–foot–mouth disease and herpangina caused by enterovirus 71 in Taiwan, 1998–2005. Pediatrics 2007; 120:244-52; PMID:17606591; http://dx.doi.org/10.1542/peds.2006-3331
- Yang F, Ren L, Xiong Z, Li J, Xiao Y, Zhao R, He Y, Bu G, Zhou S, Wang J, Qi J. Enterovirus 71 outbreak in the People's Republic of China in 2008. J Clin Microbiol 2009; 47:2351-52; PMID:19439545; http://dx.doi.org/10.1128/JCM.00563-09
- Li W, Teng G, Tong H, Jiao Y, Zhang T, Chen H, Wu H. Study on risk factors for severe hand, foot and mouth disease in China. PLoS One 2014; 9:e87603; PMID:24489943; http://dx.doi.org/10.1371/journal.pone.0087603
- Chen F, Li J, Liu T, Wen G, Xiang W. Clinical and neuroimaging features of enterovirus71 related acute flaccid paralysis in patients with hand-foot-mouth disease. Asian Pac J Trop Med 2013; 6:68-72; PMID:23317889; http://dx.doi.org/10.1016/S1995-7645(12)60203-X
- Li W, Yi L, Su J, Lu J, Ke C, Zeng H, Guan D, Ma C, Zhang W, Xiao H, et al. Seroprevalence of human enterovirus 71 and coxsackievirus A16 in Guangdong, China, in pre-and post-2010 HFMD epidemic period. PLoS One 2013; 8:e80515; PMID:24324604; http://dx.doi.org/10.1371/journal.pone.0080515
- Wu Y, Yeo A, Phoon MC, Tan EL, Poh CL, Quak SH, Chow VT. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: The role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis 2010; 14:1076-81; http://dx.doi.org/10.1016/j.ijid.2010.07.006
- Tan EL, Chow VT, Quak SH, Yeo WC, Poh CL. Development of multiplex real-time hybridization probe reverse transcriptase polymerase chain reaction for specific detection and differentiation of enterovirus 71 and coxsackievirus A16. Diagn Microbiol Infect Dis 2008; 61:294-301; PMID:18394844; http://dx.doi.org/10.1016/j.diagmicrobio.2008.02.009
- Tu PV, Thao NT, Perera D, Huu TK, Tien NT, Thuong TC, How OM, Cardosa MJ, McMinn PC. Epidemiologic and virologic investigation of hand, foot, and mouth disease, Southern Viet Nam, 2005. Emerg Infect Dis 2007; 13:1733-41; PMID:18217559; http://dx.doi.org/10.3201/eid1307.060999
- Ooi MHl, Solomon T, Podin Y, Mohan A, Akin W, Yusuf MA, del Sel S, Kontol KM, Lai BF, Clear D, et al. Evaluation of different clinical sample types in diagnosis of human enterovirus 71-associated hand-foot-and-mouth disease. J Clin Microbiol 2007; 45:1858-66; PMID:17446325; http://dx.doi.org/10.1128/JCM.01394-06
- Zhang Y, Liu H, Wang L, Yang F, Hu Y, Ren X, Li G, Yang Y, Sun S, Li Y, et al. Comparative study of the cytokine/chemokine response in children with differing disease severity in enterovirus 71-induced hand, foot, and mouth disease. Plos One 2013; 8:e67430; PMID:23840697; http://dx.doi.org/10.1371/journal.pone.0067430
- Zhang Y, Yang E, Pu J, Liu L, Che Y Wang J1, Liao Y1, Wang L1, Ding D1, Zhao T, et al. The gene expression profile of peripheral blood mononuclear cells from EV71-infected Rhesus infants and the significance in viral pathogenesis. Plos One 2014; 9:1-10
- Cao Z, Zeng D, Wang Q, Zheng X, Wang F. An epidemiological analysis of the Beijing 2008 Hand-Foot-Mouth epidemic. Chinese Sci Bull 2010; 55:764-72
- Ferguson NM, Donnelly CA, Anderson RM. Transmission intensity and impact of control policies on the foot and mouth epidemic in Great Britain. Nature 2001; 413:542-48; PMID:11586365; http://dx.doi.org/10.1038/35097116
- Keeling MJ, Rohani P. Modelling Infectious Diseases in Humans and Animals. NJ: Princeton University Press, 2007.
- Feng H, Duan G, Zhang R, Zhang W. Time series analysis of hand-foot-mouth disease hospitalization in Zhengzhou: establishment of forecasting models using climate variables as predictors. Plos One 2014; 9:e87916. 17; PMID:24498221; http://dx.doi.org/10.1371/journal.pone.0087916
- Hu M, Li Z, Wang J, Jia L, Liao Y, Lai S, Guo Y, Zhao D, Yang W. Determinants of the incidence of hand, foot and mouth disease in China using geographically weighted regression models. PLoS One 2012; 7:e38978; PMID:22723913; http://dx.doi.org/10.1371/journal.pone.0038978
- Ding Q, Zhu F, Li L, Li D, Liu Y. Serum epidemiology survey of HEV 71 among Children in Ganyu County. Jiangsu J Prev Med 2011; 22:4-6.
- Ooi EE, Phoon MC, Ishak B, Chan SH. Seroepidemiology of human enterovirus 71, Singapore. Emerg Infect Dis. 2002; 8:995-97; PMID:12194783; http://dx.doi.org/10.3201/eid0809.010397
- Hethcote HW. The mathematics of infectious diseases. SIAM Rev 2010; 42:599-653; http://dx.doi.org/10.1137/S0036144500371907
- Ma E, Wong S, Wong C, Chuang SK, Tsang T. Effects of public health interventions in reducing transmission of hand, foot, and mouth disease. Pediatr Infect Dis J. 2011; 30:432-35; PMID:21343840; http://dx.doi.org/10.1097/INF.0b013e3182127782
- Ma E, Fung C, Yip SH, Wong C, Chuang SK, Tsang T. Estimation of the basic reproduction number of enterovirus 71 and coxsackievirus A16 in hand, foot, and mouth disease outbreaks. Pediatr Infect Dis J. 2011; 30:675-79; PMID:21326133; http://dx.doi.org/10.1097/INF.0b013e3182116e95
- Wang Y, Feng Z, Yang Y, Self S, Gao Y, Wakefield J, Zhang J, Wang L, Chen X, Yao L, et al. Hand, foot, and mouth disease in China: patterns of spread and transmissibility. Epidemiology 2011; 22:781-92; PMID:21968769; http://dx.doi.org/10.1097/EDE.0b013e318231d67a
- Ji H, Li L, Liu Y, Ge H, Wang X, Hu J, Wu B, Fu J, Zhang Z, Chen X. et al. Seroepidemiology of human enterovirus71 and coxsackievirusA16 in Jiangsu province, China. Virol J. 2012; 9:248; PMID:23102275; http://dx.doi.org/10.1186/1743-422X-9-248
- Yang C, Deng C, Wan J, Zhu L, Leng Q. Neutralizing antibody response in the patients with hand, foot and mouth disease to enterovirus 71 and its clinical implications. Virol J. 2011, 8:306; PMID:21679417; http://dx.doi.org/10.1186/1743-422X-8-306
- Foo DG, Alonso S, Chow VT, Poh CL. Passive protection against lethal enterovirus 71 infection in newborn mice by neutralizing antibodies elicited by a synthetic peptide. Microbes Infect. 2007, 9:1299-306; PMID:17890123; http://dx.doi.org/10.1016/j.micinf.2007.06.002
- Hethcote HW. The mathematics of infectious diseases. SIAM Review. 2000, 42:599-653; http://dx.doi.org/10.1137/S0036144500371907
- Zhang W, Li L, Meng F, Li J, Ji H, Dong Y, Pan H, Chu K, Xu K, Zhu F. Risk factors of Hand-foot-mouth disease. ACTA UNIVERSITATIS MEDICINALIS NANJING. 2012, 4:495-99