Abstract
Pain on vaccine injection and subsequent site reactions of pain and swelling may influence confidence in vaccines and their uptake. This study aimed to identify factors associated with reported pain on injection and reactogenicity following administration of a strain specific meningococcal B outer membrane vesicle vaccine. A retrospective analysis of data was conducted from a phase II single center randomized observer-blind study that evaluated the safety, reactogenicity and immunogenicity of this vaccine in 2 cohorts of healthy 8 to 12 y old children. Vaccine administration technique was observed by an unblinded team member and the vaccine administrator instructed on standardized administration. Participants kept a daily diary to record local reactions (erythema, induration and swelling) and pain for 7 d following receipt of the vaccine. Explanatory variables were cohort, vaccine, age, gender, ethnicity, body mass index, atopic history, history of frequent infections, history of drug reactions, pain on injection, vaccinator, school population socioeconomic status, serum bactericidal antibody titer against the vaccine strain NZ98/254, and total IgG. Univariate and multivariable analyses were conducted using ordinal logistic regression for factors relating to pain on injection and reactogenicity. Perceived pain on injection was related to vaccine formulation, vaccine administrator and ethnicity. Reactogenicity outcomes varied with ethnicity and vaccine administrator. Maintaining community and parental confidence in vaccine safety without drawing attention to differences between individuals and groups is likely to become increasingly difficult. Vaccine administration technique alone has the potential to significantly reduce pain experienced on injection and local vaccine reactions.
Introduction
Factors that may influence vaccine coverage includes fear of injections, pain on injection and subsequent local reaction to the vaccination.Citation1-3 While many fears about vaccines are clearly rooted in myth,Citation4 others appear to be based on personal experiences and the experiences of others.Citation3 These include the fear of needles (needle phobia) and fear of experiencing pain.Citation3,5 Fear of the pain of injection has been shown to be a factor associated with the acceptability of vaccines for both adults and childrenCitation2,3 and distress with injections has been found to be positively correlated with a recent bad experience with a needle or reaction at the injection site.Citation5
Parents have articulated their desire for limiting vaccine injections due to the pain and distress of the procedure and the sequelae.Citation6 Reducing reactions to vaccines such as tenderness, pain and swelling associated with vaccination could assist in improving both confidence and vaccine coverage. Known factors influencing reactogenicity include the characteristics, administration technique and administration site of the vaccine.Citation7,8
This study aimed to identify factors associated with reported pain on injection and reactogenicity following administration of a strain specific meningococcal B outer membrane vesicle vaccine (MeNZB™).
Results
There were 615 participants in the school-based clinical trials of whom 608 received at least one dose of vaccine. There were 554 children assigned to receive the MeNZB™ vaccine and 61 receiving the Norwegian (not included in this study). A total of 547 children received at least one dose of MeNZB. Participants were children aged 8 – 12 y of European/Caucasian (39%), indigenous Maori (28%), Pacific Island (27%), Asian (4%) and ‘Other’ ethnicity (2%). Because of low numbers of ‘other’, these were grouped with NZ European for analysis. In NZ ‘Other’ are a very small heterogeneous group considered to have similar risks for some infectious diseases to European (). Pain at the time of injection and reactogenicity outcomes by severity are reported in .
Table 1. Demographics of trial participants
Factors associated with variations in injection pain
Pain at the time of injection relates to the injection process, not reactogenicity. Perceived pain on injection was related to vaccine formulation, vaccine administrator (moderate to severe pain ranged between 29% and 52%), and ethnicity. Maori and Pacific ethnicities were associated with less perceived pain than other groups and NZ European associated with the highest perceived pain on injection. These variables all remained significant in the multivariable analysis presented in . Body Mass Index (BMI) was not related to pain on injection.
Table 2. Odds Ratios for variables associated with the reporting of pain on injection following multivariable analysis
Factors associated with variations in reactogenicity
Initial univariate analyses showed no evidence of an association of health history, gender, age, socioeconomic status, baseline bactericidal antibody titer or IgG with any of the outcome variables measuring reported reactogenicity so these variables were not included in the multivariable analyses.
Pacific ethnicity was associated with lower reporting of local reactions and NZ European the highest (). Vaccine administrator significantly affected all reactogenicity outcomes. The results of the multivariable analysis are presented in .
Figure 1. Summary of ethnicity and reactogenicity results from univariable analysis Injection site pain P = 0.0001, Erythema P = <0.0001, Swelling P = 0.07, Induration P = 0.4, Pain on injection P = 0.03.
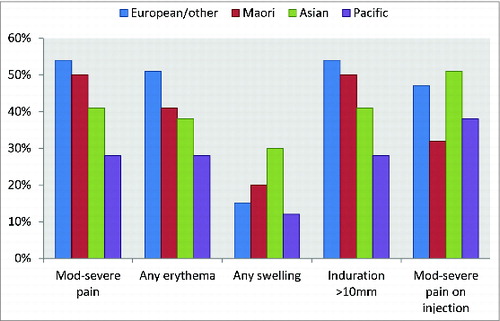
Table 3. Odds Ratios for variables associated with the reporting of pain following injection, erythema, swelling and induration following dose one of NZ meningococcal B vaccine following multivariable analysis
Table 4. Summary of pain on injection and reactogenicity with severity for all doses
Those with lower BMI reported more pain. In case this result was being caused by a linear relation not being an appropriate assumption we categorized the values to 4 categorical variables representing ‘underweight’, ‘normal weight’, ‘overweight’ and ‘obese’ and the same inverse relationship still held. Because the direction of the relationship of BMI with pain was not what had been expected a separate analysis was run including the interaction of BMI with vaccine administrator to see if the effect was being caused by the behavior of certain vaccinators. This interaction was significant (p = 0.02). Examination of the effect of BMI for each vaccine administrator found the effect was in the direction of that seen combined in all but one administrator (vaccinator 6).
Discussion
This study aimed to explore a range of potential variables that may be associated with an increased likelihood of the occurrence of reported common local reactions following a vaccine known to be relatively reactogenic.Citation9-12 We found ethnicity and vaccine administrator to be significantly associated with both perceived pain on injection and reported injection site reactions.
Ethnicity
Research exploring ethnic and racial differences in pain perception is limited and has focused on African Americans and Caucasians.Citation13 Higher reporting of injection site pain and fussiness following pertussis vaccination has been reported for black infants compared with white infants.Citation14 There is no literature that reports evaluation of comparative pain perception in the Maori and Pacific ethnic groups participating in this study. In NZ priority coding is used for ethnic group so if a person declares Maori or Pacific descent this will be the primary ethnic code, even if the ancestry is distant. While many Maori also have Caucasian (or ‘other’) ancestry this is less common among Pacific Islanders. We found Pacific, but not Maori, ethnicity to be significantly associated with reduced reporting of pain and local erythema compared with NZ European. There is a perception that a darker skin color masks any redness, possibly reducing the reporting of erythema. However, induration and swelling are less subjective and can be felt and measured.
Vaccinator
There have been a range of injection administration techniques associated with reactogenicityCitation8 and we have assumed vaccine administrator is an indirect proxy for injection technique. Despite all administrators being instructed on injection technique, there was likely to be considerable variation with some reverting to their own familiar or preferred technique. This is likely to be a variable in vaccine trials in general and certainly a variable outside of clinical trials. Our findings indicate that reactogenicity profile of the vaccine varied significantly between individual vaccine administrators. The technique chosen for the trials was that described in the NZ Immunisation Handbook at the timeCitation15 which recommended a 60–70 degree angle of injection with controlled release. The study procedures also included the practice of aspirating. Current evidence shows that the 60–70 degree angle can result in greater reactogenicity and that aspiration may make the injection more painful.Citation16 In addition it is possible that individual vaccine administrators tended to select shorter needles or more included to a particular gauge. The recommendations they were given were a choice of 23–25 G × 16 mm or 25 mm.Citation8 Although administration technique appears important, the key determinants cannot be established from this study and selection of needle may also play a part.
The independent inverse association of pain following injection and BMI (larger children reported less pain) was surprising as the reverse was expected based on the assumption that subcutaneous administration was more likely to occur in larger children. Previous studies have suggested that larger infants and young children are more likely to have local reactions from acellular pertussis vaccines, possibly due to inadvertent subcutaneous administration.Citation8 A possible reason for this unexpected result may be because the vaccine administrators had concerns about injecting too deeply in a slim child, risking hitting bone and as a consequence inadvertently injected subcutaneously, alternatively it may have been due to needle selection. This inverse relationship was seen in those vaccinated by all but one of the 7 administrators in the study, implying that it is modifiable.
Vaccine formulation
While the site of vaccine manufacture could be expected to account for differences in reactogenicity we did not find any significant association. However the effect on perceived pain on injection suggests that there may have been differences in formulation.
Strengths of study
The MeNZB™ data set was obtained under rigorous clinical trial conditions These data have been extensively published for this and other age groups.Citation9-11,17
Limitations of study
One of the limitations of the MeNZB™ vaccine data is the way ethnicity data were requested. The demographic questionnaire did not seek ethnicity information in a way that was consistent with Statistics NZ nor the Ministry of Health. While the population of NZ is largely made up from people of European, Maori, Pacific and Asian descent, the ethnicity question (as required by the vaccine manufacturer) asked for Asian, Black, Caucasian and Hispanic first followed by Pacific Islander, Maori and Other. Another difference in this data collection is that the MeNZB™ case report form only allowed for a single choice rather than as many as applied for participants of mixed ethnicity.
Implications for practice, policy and further research
A primary implication from these findings is that an individual vaccine administrator or vaccine technique has the ability to significantly influence the reactogenicity profile of a vaccine during a clinical trial from modestly reactogenic to highly reactogenic. The spectrum of proportion of vaccinees experiencing pain on injection and local reactions varied widely between vaccine administrators and likely impacts on injection experience for the vaccinee. While the MeNZB™ vaccine is no longer in use, the formulation included the outer membrane vesicle (B:4:P1.7b,4; NZ98/254), a highly immunonogenic and reactogenic component of the new meningococcal Group B vaccine Bexsero recently licensed in Europe, Canada and Australia.Citation18
Maintaining community and parental confidence in vaccine safety without drawing attention to differences between individuals and groups is likely to become increasingly difficult. This study confirms that there are extrinsic factors both non-modifiable (ethnicity) and modifiable (vaccine administrator) associated with reported vaccine reactogenicity.
Vaccine administration technique alone has the potential to significantly reduce pain experienced on injection and local vaccine reactions. Further determining which aspects make this difference will provide valuable evidence for practice.
Patients and Methods
This was a retrospective analysis of data from a phase II single center (South Auckland, New Zealand) randomized observer-blind study that evaluated the safety, reactogenicity and immunogenicity of the MeNZB™ vaccine in 2 cohorts of healthy 8 to 12 y old children.Citation9 The New Zealand (NZ) Standing Committee on Therapeutic Trials approved the vaccines for use in this trial which was conducted in accordance with the Declaration of Helsinki guidelines on good clinical practice and local regulations for clinical trials including prior approval from the Auckland Regional appropriate Ethics Committee.Citation17 This study used first dose data from participants who received vaccine based on the NZ epidemic strain (MeNZB™). Approval to conduct this retrospective analysis was granted by the Northern × Regional Ethics Committee NTX/07/02/CPD.
Population
The eligible study population was children aged 8–12 y of age attending schools in South Auckland. Forty two schools were invited to participate in recruitment, with 39 schools accepting.Citation9
Exclusion criteria for children participating in the trial were those whose parents were unwilling or unable to give informed consent; previous receipt of a meningococcal B vaccine; history of infection or close contact with N.meningitidis; having recently received other vaccines; having a contraindication to receipt of the vaccine; fever within 3 days; recent antibiotic treatment or blood products or the presence or suspected significant presence of a chronic disease.Citation9
Vaccines
Manufacture of the NZ vaccine (later licensed as MeNZB™) was based on the Norwegian parent vaccine MenBvac (B:15:P1.7,16; 44/76) with the substitution of the NZ epidemic strain (B:4:P1.7b,4; NZ98/254). The NZ strain was isolated from a single case and prepared by fermented growth in a synthetic medium. The outer membrane vesicles were extracted using the detergent deoxycholate, separated by centrifugation and then adsorbed to aluminum hydroxide.Citation10
Each 0.5 mL vaccine dose contained 25 μg total protein and 1.65 mg aluminum hydroxide. Children from cohort A received MeNZB™ vaccine manufactured in a single batch by the Norwegian Institute of Public Health (NIPH), Oslo, Norway or the Norwegian parent vaccine. Children from cohort B received MeNZB™ vaccine manufactured by Novartis, Siena, Italy or the NIPH with a single batch supplied by each manufacturer. Thus, there were 3 vaccine batches from 2 manufacturing sites. Data for participants who received the Norwegian parent vaccine, MenBvac (control group in cohort A), were not included in this analysis because the vaccine formulation differed.
Vaccine administration
Vaccine technique was attempted to be standardized. An unblinded team member and vaccinator were instructed that the vaccines were to be administered intramuscularly in the deltoid of the non-dominant arm.Citation9,15 The technique used was described in the trial's Standard Operating Procedures as administration using a 60–70 degree angle. Once in the muscle the plunger was to be withdrawn slightly (aspiration) and the vaccine injected at a slow even rate. The needle was to be removed quickly and pressure applied to the injection site. Seven of the 8 study vaccinators administered dose one. There was no systematic documentation of variant administrations.
Reactogenicity monitoring
Participants kept a daily diary to record local reactions, signs and symptoms for 7 d following receipt of the vaccine. A standardized diary card along with a tape measure and digital thermometer were provided. Each parent was instructed how assess, measure, and record tenderness, erythema, swelling and induration at injection site. When measuring visible reactions they were asked to measure the widest dimension of any local reaction. Swelling was described as a visible raised area of skin where the injection was given. Induration was described as a lump that could be felt under the skin where the injection was given but could not be seen. For both studies the diary cards also had an illustration of each of the reactogenicity variables. For the cohort B, pictures of different possible reactions were shown to parents and used to show them how to do the measurements. A custom-made rag doll was used to illustrate the difference between these 2 variables. The first set of reactogenicity recordings were performed by trial nurses at 30 minutes post injection. Families were contacted by telephone at 24–48 hours post vaccination and the diary cards were collected from the students on Day 8 and the information recorded checked. Where information on the diary cards was missing or inconsistent, parents were contacted to clarify.
Outcome variables
Injection site erythema, induration and swelling were recorded in millimeters, and pain on injection and pain following injection scored from 0 to 3 for nil, mild, moderate and severe. Mild pain was defined as pain with no limitation of normal daily activities; moderate as causing some limitation of normal daily activities; and severe as resulting in an inability to perform normal daily activities. Local reactions occurred on the day of injection and generally continued for no more than 3 d with the peak usually on the day of injection. The highest score for the 7 day period was used for analysis. Pain on Injection was recorded by the vaccinator at the time of injection prior to the participant being escorted to the waiting area.
Explanatory variables
Cohort, vaccine (which included vaccine manufacturer and batch), age, gender, ethnicity, body mass index (BMI), atopic history, history of frequent infections (defined as history of frequent antibiotic use), history of drug reactions, pain on injection (also an outcome variable), vaccine administrator, school decile (a proxy measure of socioeconomic status of the school attendees), serum bactericidal antibody (SBA) titer against the vaccine strain NZ98/254, and total IgG as measured by ELISA.
Data analysis
Analyses were performed using SAS v9.1 statistical software and SPSS PASW version 18. Measures of pain and the continuous variables erythema, swelling and induration were initially dichotomized into present or absent (or in the case of pain 0–1 and 2–3) and associations with vaccine, ethnicity, gender, atopy, health history, age, level of socioeconomic deprivation and vaccine administrator examined using cross tabulations and differences tested using the Chi Square statistic. Correlations between baseline SBA, IgG and BMI and the local reactogenicity outcomes pain, erythema, induration and swelling at dose one were conducted using Spearman's rank correlations. Outcomes were used to inform a multivariable analysis.
Multivariable analysis was conducted using ordinal logistic regression. Explanatory variables were those that were independently significant in the univariate analyses: vaccine formulation, BMI, ethnicity, and vaccine administrator with each of the outcomes pain on injection, pain following injection, erythema, induration and swelling. Pain, induration and erythema are reported as ordinal outcomes 0–3, and swelling as binary outcomes (present or absent) as the distribution did not satisfy the assumptions required for ordinal logistic regression.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Harrington PM, Woodman C, Shannon WF. Vaccine, yes; injection, no: maternal responses to the introduction of Haemophilus influenzae type b (Hib) vaccine. Br J Gen Pract 1999; 49:901-2; PMID:10818657
- Kwan TT, Chan KK, Yip AM, Tam KF, Cheung AN, Young PM, Lee PW, Ngan HY. Barriers and facilitators to human papillomavirus vaccination among Chinese adolescent girls in Hong Kong: a qualitative-quantitative study. Sex Transm Infect 2008; 84:227-32; PMID:18256106; http://dx.doi.org/10.1136/sti.2007.029363
- Nir Y, Paz A, Sabo E, Potasman I. Fear of injections in young adults: prevalence and associations. Am J Trop Med Hyg 2003; 68:341-4; PMID:12685642
- Francois G, Duclos P, Margolis H, Lavanchy D, Siegrist CA, Meheus A, Lambert PH, Emiroglu N, Badur S, Van Damme P. Vaccine safety controversies and the future of vaccination programs. Pediatr Infect Dis J 2005; 24:953-61; PMID:16282928; http://dx.doi.org/10.1097/01.inf.0000183853.16113.a6
- Jacobson RM, Swan A, Adegbenro A, Ludington SL, Wollan PC, Poland GA. Making vaccines more acceptable – methods to prevent and minimize pain and other common adverse events associated with vaccines. Vaccine 2001; 19:2418-27; PMID:11257372; http://dx.doi.org/10.1016/S0264-410X(00)00466-7
- Meyerhoff AS, Weniger BG, Jacobs RJ. Economic value to parents of reducing the pain and emotional distress of childhood vaccine injections. Pediatr Infect Dis J 2001; 20:S57-62; PMID:11704725; http://dx.doi.org/10.1097/00006454-200111001-00009
- Cook IF. Evidence based route of administration of vaccines. Hum Vaccine 2008; 4:67-73; PMID:17881890; http://dx.doi.org/10.4161/hv.4.1.4747
- Petousis-Harris H. Vaccine injection technique and reactogenicity–evidence for practice. Vaccine 2008; 26:6299-304; PMID:18804137
- Hosking J, Rasanathan K, Mow FC, Jackson C, Martin D, O'Hallahan J, Oster P, Ypma E, Reid S, Aaberge I, et al. Immunogenicity, reactogenicity, and safety of a P1.7b,4 strain-specific serogroup B meningococcal vaccine given to preteens. Clin Vaccine Immunol 2007; 14:1393-9; PMID:17898183; http://dx.doi.org/10.1128/CVI.00167-07
- Thornton V, Lennon D, Rasanathan K, O'Hallahan J, Oster P, Stewart J, Tilman S, Aaberge I, Feiring B, Nokleby H, et al. Safety and immunogenicity of New Zealand strain meningococcal serogroup B OMV vaccine in healthy adults: beginning of epidemic control. Vaccine 2006; 24:1395-400; PMID:16242221; http://dx.doi.org/10.1016/j.vaccine.2005.09.043
- Wong S, Lennon D, Jackson C, Stewart J, Reid S, Crengle S, Tilman S, Aaberge I, O'Hallahan J, Oster P, et al. New Zealand epidemic strain meningococcal B outer membrane vesicle vaccine in children aged 16–24 months. Pediatr Infect Dis J 2007; 26:345-50; PMID:17414400; http://dx.doi.org/10.1097/01.inf.0000258697.05341.2c
- Jackson C, Lennon DR, Sotutu VT, Yan J, Stewart JM, Reid S, Crengle S, Oster P, Ypma E, Aaberge I, et al. Phase II meningococcal B vesicle vaccine trial in New Zealand infants. Arch Dis Child 2009; 94:745-51; PMID:18838420; http://dx.doi.org/10.1136/adc.2007.132571
- Rahim-Williams B, Riley JL, Williams AKK, Fillingim RB. A quantitative review of ethnic group differences in experimental pain response: do biology, psychology, and culture matter? Pain Med 2012; 13:522-40; PMID:22390201; http://dx.doi.org/10.1111/j.1526-4637.2012.01336.x
- Christy C, Pichichero ME, Reed GF, Decker MD, Anderson EL, Rennels MB, Englund JA, Edwards KM, Steinhoff MC. Effect of gender, race, and parental education on immunogenicity and reported reactogenicity of acellular and whole-cell pertussis vaccines. Pediatrics 1995; 96:584-7; PMID:7659481
- Ministry of Health. Immunisation Handbook 2002. Wellington: Ministry of Health; 2002.
- Greenberg DP, Doemland M, Bettinger JA, Scheifele DW, Halperin SA, Waters V, Kandola K, for the II. Epidemiology of pertussis and haemophilus influenzae type b disease in Canada with exclusive use of a diphtheria-tetanus-acellular pertussis-inactivated poliovirus-haemophilus influenzae type b pediatric combination vaccine and an adolescent-adult tetanus-diphtheria-acellular pertussis vaccine: implications for disease prevention in the United States. Pediatr Infect Dis J 2009; 28:521-8; PMID:19436236; http://dx.doi.org/10.1097/INF.0b013e318199d2fc
- Oster P, Lennon D, O'Hallahan J, Mulholland K, Reid S, Martin D. MeNZB(TM): a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine 2005; 23:2191-6; PMID:15755593; http://dx.doi.org/10.1016/j.vaccine.2005.01.063
- Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, Evans A, Telford KL, Ypma E, Toneatto D, et al. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis 2010; 51:1127-37; PMID:20954968; http://dx.doi.org/10.1086/656741
