Abstract
The induction of a balanced immune response targeting the major structural proteins, Gag and Env of HIV, is important for the development of an efficacious vaccine. The use of DNA plasmids expressing different antigens offers the opportunity to test in a controlled manner the influence of different vaccine components on the magnitude and distribution of the vaccine-induced cellular and humoral immune responses. Here, we show that increasing amounts of env DNA results in greatly enhanced Env antibody titers without significantly affecting the levels of anti-Env cellular immune responses. Co-immunization with Env protein further increased antibody levels, indicating that vaccination with DNA only is not sufficient for eliciting maximal humoral responses against Env. In contrast, under high env:gag DNA plasmid ratio, the development of Gag cellular responses was significantly reduced by either SIV or HIV Env, whereas Gag humoral responses were not affected. Our data indicate that a balanced ratio of the 2 key HIV/SIV vaccine components, Gag and Env, is important to avoid immunological interference and to achieve both maximal humoral responses against Env to prevent virus acquisition and maximal cytotoxic T cell responses against Gag to prevent virus spread.
Abbreviations
| IM | = | intramuscular |
| EP | = | electroporation |
| IFN-γ | = | interferon gamma |
| bAb | = | binding antibody |
Introduction
An ideal anti-HIV vaccine should induce effective humoral immunity that disseminate into mucosal sites and is able to prevent infection, and as a second line of defense, multifunctional cytotoxic T cells able to destroy infected cells. We, and others have been working on the development of DNA-based vaccine regimens, which represent an attractive vaccine platform due to its simplicity, versatility and relative ease of manufacturing. To maximize the efficacy of DNA as vaccine platform several components need to be considered including the use of plasmid backbone optimized for efficient replication in bacteria, potent promoters (i.e., human CMV, simian CMV) and polyadenylation signals (i.e., BGH, SV40) and the use of RNA/codon optimized inserts taking into consideration also optimization of the trafficking of the proteins (reviewed Refs. Citation1-3). Many labs found that inclusion of cytokine or chemokine DNAs as immunological adjuvants augment the vaccine-induced immune responses. In particular, the use of IL-12 was shown to increase the levels of antigen-specific cytotoxic T cells and humoral responses in mice, macaques as well as in the HVTN 080 human immunogenicity trial,Citation4-14 demonstrating translation from this basic observation from mice to macaques to humans. In addition to optimizing the vaccine components, different DNA delivery systems have been tested including intramuscular (IM) injection with needle and syringe, IM delivery followed by in vivo electroporation (IM/EP); skin or intradermal electroporation, gene gun or biojector, liposome delivery with Vaxfectin®, and Dermavir (reviewed Refs. Citation1-3,15,16 and referenced therein).
In this report, we used the IM/EP delivery of HIV and SIV DNA in rhesus macaques with 6 different vaccine regimens to maximize the induction of humoral and cellular immune responses. We show that the ratio of env to gag DNA in the vaccine mixture is critical for the balanced induction of humoral and cellular immune responses.
Results
Comparison of SIV and HIV DNA vaccine platforms
We have performed several vaccine studies in macaques using mixtures consisting of SIV gag and SIV or HIV env DNA that also included DNA expressing rhesus macaque IL-12 (rmIL-12) as molecular adjuvant. The DNAs were administered via the intramuscular (IM) injection followed by in vivo electroporation. The molecular ratio of env:gag DNAs varied from 1:1 to 3:1, in an effort to enhance the magnitude of the Env-specific humoral responses. In this report, we present a cross-study comparison of immune responses from macaques receiving the same SIV gag DNA combined with either SIV env DNA (groups 1–3) or with HIV env DNA (groups 4–6), as described in .
Table 1. Vaccination overview
The animals of group 1 (N = 16) received SIV env and SIV gag DNA at a 1:1 ratio (0.5 mg and 0.5 mg, respectively). The animals of group 2 (N = 4) and group 3 (N = 16) received SIV env and SIV gag DNA at a 3:1 DNA ratio (3 mg and 1 mg, respectively). The animals of group 3 were also co-immunized with purified adjuvanted SIV Env protein at the same site. Similar to these studies, we compared DNA combinations that included HIV env and SIV gag at different molecular ratios. Group 4 animals (N = 6) received HIV env and SIV gag DNA at a 1:1 DNA ratio (1 mg and 1 mg, respectively). Group 5 (N = 9) and group 6 (N = 25) animals received HIV env and SIV gag DNA at a 3:1 DNA ratio (3 mg and 1 mg, respectively). Similar to group 3, the animals of group 6 were co-immunized with purified adjuvanted protein (HIV-1 gp120). We analyzed humoral () and cellular () responses 2 weeks after the 2nd vaccination and compared the immune responses induced by the different vaccine regimens.
Figure 1. Binding antibody titers to SIV Gag and SIV or HIV Env among the different vaccine groups; (A-D). Endpoint bAb titers (log) to SIVmac251 gp120 Env (A), p27gag (B and D) and HIV IIIB gp120 Env (C) were measured 2 weeks after the 2nd vaccination. Asterisks designate statistically significant differences between groups (*** P< 0.001 and ****P < 0.0001) using the non-parametric 2-tailed t-test (Mann-Whitney). Median values are indicated.
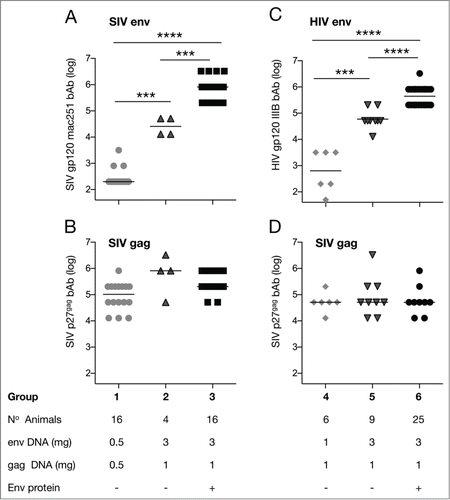
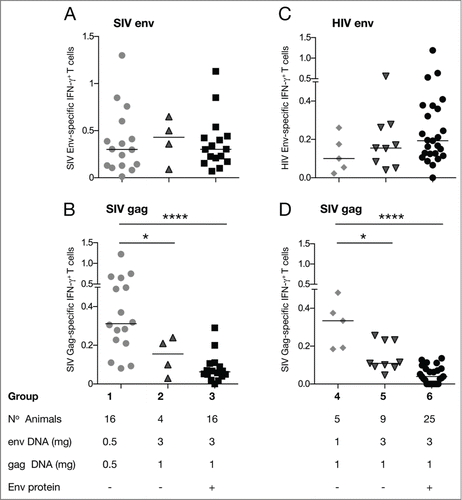
Figure 2. Cellular immune responses in the different vaccine groups. Antigen-specific T cell responses were measured in PBMC 2 weeks after the 2nd vaccination. PBMC were stimulated with peptide pools covering SIV gp160 mac239 (A), SIV p39gag (B and D) or HIV gp120 PTE (C). The frequency of the antigen-specific T cells producing IFN-γ is shown. Asterisks designate statistically significant differences between groups (*P < 0.05, ***P < 0.001 and ****P < 0.0001) using the non-parametric 2-tailed t-test (Mann-Whitney). Median values are indicated. Note that only 5 animals from group 4 (panels C and D) were analyzed for cellular responses.
Augmentation of Env antibody titers by increasing env DNA dose
To compare the humoral responses (), we measured binding antibody (bAb) titers to SIVmac251 Env and to SIV p27gag by standard ELISA (, respectively). We found that increasing the amount of SIV env DNA in the vaccine led to a significant augmentation of the bAb titers to SIV mac251 Env (group 1, mean titer 2.5 log and group 2, mean titer 4.4 log; ). These data demonstrate that the 0.5 mg dose did not maximize bAb responses and that increasing the env DNA dose achieved significantly higher bAb levels. We also compared the responses to those obtained after inclusion of Env protein in the vaccine (group 3). In this co-immunization protocol the adjuvanted protein is administered into the same muscle following the DNA delivery. Inclusion of gp120 Env protein led to a further significant increase in humoral responses (group 3, mean titer ˜6 log). These data show that the env DNA-only vaccine, even including a higher dose of env DNA, did not induce maximal bAb responses. Inclusion of gp120 protein was necessary to maximize the development of humoral responses. We previously reported that inclusion of protein using a molecular env:gag DNA ratio of 1:1 resulted in great increase of Env humoral responses.Citation17-19
We also compared the SIV p27gag humoral responses and found no differences in bAb titers among the different vaccine regimens (range of titers ˜5–6 log among the 3 groups; ) and showed that the development of Gag bAb was not affected by the increased amounts of env DNA or the inclusion of Env protein in the vaccine.
Similar observations were made using a vaccine that included SIV gag DNA and HIV env DNA (). Increasing the molecular ratio of env:gag DNA to 3:1 led to significant augmentation of HIV IIIB Env bAb responses (group 4 mean titer ∼3.5 log; group 5 mean titer 5.3 log; ). Thus, like the SIV env plus SIV gag DNA vaccine, we found significant augmentation of the Env bAb titers upon increasing the env DNA amount in the vaccine. Inclusion of purified SIV Env protein in the vaccine further elevated the Env bAb titer significantly (group 6; mean titer ˜6.5 log) (). Thus, for both the SIV and HIV DNA-only vaccine regimens the magnitude of the Env humoral responses could be increased using higher amounts of env DNA. Even under these conditions, both vaccines failed to reach maximal bAb levels, which were achieved however upon inclusion of Env protein in the co-immunization protocol. As noted for the SIV env-SIV gag vaccine groups, similar levels of Gag humoral responses were induced by these vaccine regimens (mean titer of ˜5 log among the 6 groups) ().
In summary, we concluded that the Env-specific humoral responses induced by the DNA vaccine platform can be improved by increasing the env DNA dose for both SIV and HIV resulting in significantly higher Env humoral responses without affecting the humoral responses to Gag.
High molecular ratio of env to gag DNA impaired the development of SIV Gag-specific T cell responses
Next, we analyzed the cellular responses in these vaccine groups (). We found that increasing the dose of SIV or HIV Env DNA did not affect the levels of Env-specific IFN-γ+ T cell immune responses (, respectively). The groups that received SIV Env DNA showed a median response of 0.3–0.43% IFN-γ+ T cells (), while the HIV env group showed a median response of 0.1–0.19 % IFN-γ+ T cells. These data demonstrate that the 0.5 mg env DNA dose induced the highest cellular responses, albeit this dose was suboptimal to inducing maximal humoral responses (, respectively).
Unexpectedly, we found that using a higher dose of SIV env DNA led to significant, ˜4-fold, reduction in Gag-specific IFN-γ+ T cell levels (; group 1, mean ˜0.4%; group 2, ˜0.1% of Gag-specific T cells). We noted that the response measured from animals of group 3 was further slightly reduced upon inclusion of adjuvanted protein (compare group 2 and group 3). The animals enrolled in these studies have diverse MHC haplotypes and there was no apparent association found between MHC and the observed vaccine responses. Thus, these data indicated that SIV Env potently suppressed the development of Gag-specific T cell immunity while not affecting the Gag humoral responses.
To further investigate this dichotomy in humoral versus cellular responses to Gag and Env and to understand whether this is restricted to SIV Env, we also analyzed the cellular immune responses in groups 4 to 6, which received a combination of the same SIV gag DNA and HIV env DNA. A higher dose of HIV env DNA also led to ˜2-fold reduction in Gag-specific T cell levels () comparing groups 4 and 5, although similar levels of Gag humoral responses were found (). Upon co-immunization with HIV Env protein (group 6) a further reduction in Gag-specific T cells was noticed ().
In conclusion, a vaccine consisting of high molecular ratio of HIV or SIV env DNA to gag DNA is responsible for the potent negative effect on the development of Gag-specific cellular immune responses. These data suggest a dominant immunological interference of the SIV as well as the HIV Env epitopes resulting in the suppression of the development of robust Gag-specific cellular responses, while not affecting the development of humoral responses.
Discussion
In this report, we compared SIV/HIV DNA vaccine mixtures containing different molecular ratios of env and gag DNA with the goal to maximize the induction of humoral and cellular immunity to Env and Gag in rhesus macaques. In this cross-study comparison, we found that increasing the env DNA dose significantly contributed toward maximizing Env humoral responses. We also noted that a vaccine combining high dose of env DNA and Env protein, for both SIV and HIV, led to further increase in Env antibody titers, suggesting that DNA only does not fully maximize such responses. We previously reported that inclusion of protein in the vaccine also had the advantage of better mucosal dissemination of the humoral responses,Citation17,19 which could also be the result of higher antibody responses. On the other hand, no changes in the levels of Env-specific cellular responses were noted under these conditions, suggesting that the responses reached with the 0.5 mg DNA dose could not be further increased using a 3 mg dose. Unexpectedly, we found that a higher Env DNA dose led to a significant reduction in Gag-specific cellular responses, while not affecting the levels of the Gag humoral responses. These findings could also explain the failure to detect Gag cellular responses in a clinical trial, where a 3:1 ratio of env:gag DNA was used as vaccine primeCitation20,21 and it is possible that this contributed to the efficacy failure of the phase 2 trial.Citation22 The great loss of cellular responses to Gag is of concern because several lines of evidence support an important role of these responses in control of viremia. We had reported that Gag cellular responses significantly contributed to the control of SIVsmE660 upon challenge of vaccinated macaques.Citation19 Similarly, several studies in long-term non-progressors demonstrated unequivocally an association of Gag cellular immune responses and control of HIV.Citation23-31 Immune interference of Env and Gag has been previously reported in DNA vaccinated mice. Toapanta et alCitation32 reported that HIV Env but not SIV or EIAV Env interfered with the development of HIV Gag responses. On the other hand, Bockl and WagnerCitation33 reported that co-delivery of HIV env with HIV gag DNA led to largely abrogated Gag-specific T cell responses and that this effect was linked to the H-2(d) T cell epitope V11V in Env. Both studies share the overall conclusion that there is an immunological interference between Env and Gag in Balb/c mice. Bockl and WagnerCitation33 further observed that changing the ratio of the gag and env DNA in the vaccine mixture and the spatial or temporal separation of the Gag and Env antigens in the vaccination induced more balanced T cell responses. Overall, this study in mice is in good agreement with our data from DNA vaccinated macaques. Together these findings show that there is strong immunological interference between Env and Gag epitopes, probably linked to the needed association of peptide and MHC class I, which may explain why the interference does not affect humoral responses.
Similar to the Env interference in the development of Gag cellular immune responses, we have previously reported that there is also immunological interference among epitopes within Gag, with the less conserved epitopes suppressing the responses to the conserved epitopes.Citation34-36 A vaccine strategy priming with conserved epitopes followed by full-length Gag boost was shown to induce robust cellular and humoral responses to the conserved epitopes. This approach altered the immunodominance hierarchy, magnitude and breadth of cellular responses targeting Gag epitopes. In addition to playing a role in controlling viremia in SIV infected macaques and in HIV infected persons,Citation19,23-31 GagPol-specific CD4+ T-cells were also shown to increase the development of Env-specific humoral response in mice, suggesting a cross-talk between immune responses and intrastructural help for Env-specific B cells responses.Citation37 Taken together these results indicate that the balanced ratio of the vaccine components is critical for the induction of optimal cellular and humoral immune responses to Gag and Env. The use of DNA as vaccine platform offers the flexibility to adjust vaccine components to maximize their efficacy.
Materials and Methods
Animals
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The Advanced BioScience Laboratories, Inc.. Institutional Animal Care and Use Committee (OLAW assurance number A3467-01 and USDA Certificate number 51-R-0059) approved the animal protocol. Indian rhesus macaques were housed and handled in accordance with the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care International at the Advanced BioScience Laboratories, Inc.. (Rockville, MD). Some of the animals were housed at the NIH facility (Bldg 14). The animals were vaccinated as part of other studies (AUP417,Citation19 AUP490,Citation38,39 AUP522, and AUP469). The data presented in this work were not previously reported.
Vaccine
SIV gag DNA vectors included p57gag (plasmid 206S) and the MCP3p39gag (plasmid 209S). The SIV env DNAs expressed gp160 [group 1, 2 and 4 animals of group 3Citation19,38,39] or gp160 and gp120 (12 animals in group 3). The HIV env DNAs expressed clade B and clade C gp160 and gp140 (group 4 and 5) and clade B gp120 (group 5). For group 6, the plasmids expressed gp160 and gp120 (N = 9) or only gp120 (N = 16) and no difference in gp120 PTE-specific T cell responses was found among these animals
All HIV and SIV plasmids express RNA/codon-optimized SIV and HIV genes from the CMV promoter using the vector CMVkan.Citation40 The SIV gag and SIV env expressing plasmids were described elsewhere.Citation38,41,42 Optimized rhesus IL-12 DNACitation6 was included as immunological vaccine adjuvant. Endotoxin-free DNAs (Qiagen) were prepared according to the manufacturer's protocol. HEK293 grown SIV or HIV Env proteins were lectin-column purified and 100 μg Env protein adjuvanted with 10 μg EM-005Citation17,18 was included in some of the vaccines (groups 3 and 6).
Vaccination
The macaques received SIV gag DNA and SIV or HIV env DNA as indicated in . All DNA vaccine mixtures contained 0.1 μg of macaque IL-12 DNA and were formulated in 0.6 ml of sterile water (Hospira, Inc.., Lake Forest, IL). The DNA vaccine mixtures were delivered via intramuscular (IM) injection at 2 different sites (0.3 ml each site) followed by in vivo electroporation using the Elgen 1000 device (Inovio, Pharmaceuticals, Inc., Blue Bell, PA) as reported previously.Citation43 Blood was collected 2 weeks after the 2nd vaccination. SIV/HIV-specific cellular immune responses were measured in PBMC and humoral responses were measured in the plasma.
Cellular responses
Cellular immune responses were measured by intracellular cytokine staining assay using cryopreserved PBMCs stimulated with peptide pools spanning SIV mac239 (gp160), SIVmac239 gag (p39gag) and using the most frequent potential HIV T cell epitopes (PTE) Env pool (Cat #11551, AIDS Research and Reference Reagent Program, Germantown, MD) using 15-mer peptides overlapping by 11 AA (final concentration of 1 µg/ml). Immunostaining and flow cytometric analysis was performed as described.Citation19,39 For all the analyzed vaccinated animals a PBMC sample cultured without peptide stimulation was included as negative control. The frequency of IFN-γ+ T cells is reported after subtracting the background value obtained in the absence of peptides from that of peptide-stimulated samples. Samples were considered positive when the frequency of IFN-γ+ T cells in the peptide-stimulated samples was at least 2-fold higher than the frequency obtained in the unstimulated medium-only control sample.
Humoral responses
Binding antibodies to SIVmac251 p27gag, SIVmac251 Env and HIV IIIB Env were monitored on 4-fold serial dilutions of plasma samples by standard ELISAs (Advanced Bioscience Laboratory, Rockville, MD). The mean optical absorbance (A450) plus 3 standard deviations obtained from non-immune rhesus macaque plasma samples were applied as cut-off for the assays.
Disclosure of Potential Conflicts of Interest
GNP and BKF are inventors on US Government-owned patents and patent applications related to DNA vaccines and gene expression optimization. SR is a founder and shareholder in Immune Design Corp., which has certain rights to the adjuvant used in this study under license agreement with IDRI. NYS is a full time employee of Inovio Pharmaceuticals and as such receives compensation in the form of salary and stock options. There are no further patents, products in development or marketed products to declare.
Acknowledgments
We thank D Weiss, J Treece, I. Kalisz and the staff at Advanced Bioscience Laboratory for excellent support, J. Bear and B. Chowdhury for technical support, and T. Jones for editorial assistance. The following reagent was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 PTE Env Peptides (Cat #11551).
Funding
This work was supported in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health (NCI/NIH) (BKF, GNP), and Bill and Melinda Gates Foundation Grant #42387 (SGR).
References
- Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol 2011; 23:421-9; PMID:21530212; http://dx.doi.org/10.1016/j.coi.2011.03.008
- Flingai S, Czerwonko M, Goodman J, Kudchodkar SB, Muthumani K, Weiner DB. Synthetic DNA vaccines: improved vaccine potency by electroporation and co-delivered genetic adjuvants. Front Immunol 2013; 4:354; PMID:24204366; http://dx.doi.org/10.3389/fimmu.2013.00354
- Felber BK, Valentin A, Rosati M, Bergamaschi C, Pavlakis GN. HIV DNA vaccine: stepwise improvements make a difference. Vaccines 2014; 2:354-79; http://dx.doi.org/10.3390/vaccines2020354
- Hirao LA, Wu L, Khan AS, Hokey DA, Yan J, Dai A, Betts MR, Draghia-Akli R, Weiner DB. Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques. Vaccine 2008; 26:3112-20; PMID:18430495; http://dx.doi.org/10.1016/j.vaccine.2008.02.036
- Winstone N, Wilson AJ, Morrow G, Boggiano C, Chiuchiolo MJ, Lopez M, Kemelman M, Ginsberg AA, Mullen K, Coleman JW, et al. Enhanced control of pathogenic SIVmac239 replication in macaques immunized with a plasmid IL12 and a DNA prime, viral vector boost vaccine regimen. J Virol 2011; 85:9578-87; PMID:21734035; http://dx.doi.org/10.1128/JVI.05060-11
- Jalah R, Patel V, Kulkarni V, Rosati M, Alicea C, Ganneru B, von Gegerfelt A, Huang W, Guan Y, Broderick KE, et al. IL-12 DNA as molecular vaccine adjuvant increases the cytotoxic T cell responses and breadth of humoral immune responses in SIV DNA vaccinated macaques. Hum Vaccin Immunother 2012; 8:1620-9; PMID:22894956; http://dx.doi.org/10.4161/hv.21407
- Boyer JD, Robinson TM, Kutzler MA, Parkinson R, Calarota SA, Sidhu MK, Muthumani K, Lewis M, Pavlakis G, Felber B, et al. SIV DNA vaccine co-administered with IL-12 expression plasmid enhances CD8 SIV cellular immune responses in cynomolgus macaques. J Med Primatol 2005; 34:262-70; PMID:16128921; http://dx.doi.org/10.1111/j.1600-0684.2005.00124.x
- Chattergoon MA, Saulino V, Shames JP, Stein J, Montaner LJ, Weiner DB. Co-immunization with plasmid IL-12 generates a strong T-cell memory response in mice. Vaccine 2004; 22:1744-50; PMID:15068858; http://dx.doi.org/10.1016/j.vaccine.2004.01.036
- Chong SY, Egan MA, Kutzler MA, Megati S, Masood A, Roopchard V, Garcia-Hand D, Montefiori DC, Quiroz J, Rosati M, et al. Comparative ability of plasmid IL-12 and IL-15 to enhance cellular and humoral immune responses elicited by a SIVgag plasmid DNA vaccine and alter disease progression following SHIV(89.6P) challenge in rhesus macaques. Vaccine 2007; 25:4967-82; PMID:17335943; http://dx.doi.org/10.1016/j.vaccine.2006.11.070
- Egan MA, Chong SY, Megati S, Montefiori DC, Rose NF, Boyer JD, Sidhu MK, Quiroz J, Rosati M, Schadeck EB, et al. Priming with plasmid DNAs expressing interleukin-12 and simian immunodeficiency virus gag enhances the immunogenicity and efficacy of an experimental AIDS vaccine based on recombinant vesicular stomatitis virus. AIDS Res Hum Retroviruses 2005; 21:629-43; PMID:16060834; http://dx.doi.org/10.1089/aid.2005.21.629
- Kalams SA, Parker S, Jin X, Elizaga M, Metch B, Wang M, Hural J, Lubeck M, Eldridge J, Cardinali M, et al. Safety and immunogenicity of an HIV-1 gag DNA vaccine with or without IL-12 and/or IL-15 plasmid cytokine adjuvant in healthy, HIV-1 uninfected adults. PLoS One 2012; 7:e29231; PMID:22242162
- Kalams SA, Parker SD, Elizaga M, Metch B, Edupuganti S, Hural J, De Rosa S, Carter DK, Rybczyk K, Frank I, et al. Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery. J Infect Dis 2013; 208:818-29; PMID:23840043; http://dx.doi.org/10.1093/infdis/jit236
- Robinson TM, Sidhu MK, Pavlakis GN, Felber BK, Silvera P, Lewis MG, Eldridge J, Weiner DB, Boyer JD. Macaques co-immunized with SIVgag/pol-HIVenv and IL-12 plasmid have increased cellular responses. J Med Primatol 2007; 36:276-84; PMID:17669216; http://dx.doi.org/10.1111/j.1600-0684.2007.00245.x
- Schadeck EB, Sidhu M, Egan MA, Chong SY, Piacente P, Masood A, Garcia-Hand D, Cappello S, Roopchand V, Megati S, et al. A dose sparing effect by plasmid encoded IL-12 adjuvant on a SIVgag-plasmid DNA vaccine in rhesus macaques. Vaccine 2006; 24:4677-87; PMID:16288822; http://dx.doi.org/10.1016/j.vaccine.2005.10.035
- Hutnick NA, Myles DJ, Bian CB, Muthumani K, Weiner DB. Selected approaches for increasing HIV DNA vaccine immunogenicity in vivo. Curr Opin Virol 2012; 1:233-40; PMID:22440782; http://dx.doi.org/10.1016/j.coviro.2011.08.003
- Bodles-Brakhop AM, Heller R, Draghia-Akli R. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: current clinical developments. Mol Ther 2009; 17:585-92; PMID:19223870; http://dx.doi.org/10.1038/mt.2009.5
- Jalah R, Kulkarni V, Patel V, Rosati M, Alicea C, Bear J, Yu L, Guan Y, Shen X, Tomaras GD, et al. DNA and protein co-immunization improves the magnitude and longevity of humoral immune responses in rhesus macaques. PLoS One 2014; 9:e91550; PMID:24626482; http://dx.doi.org/10.1371/journal.pone.0091550
- Li J, Valentin A, Kulkarni V, Rosati M, Beach RK, Alicea C, Hannaman D, Reed SG, Felber BK, Pavlakis GN. HIV/SIV DNA vaccine combined with protein in a Co-immunization protocol elicits highest humoral responses to envelope in mice and macaques. Vaccine 2013; 31:3747-55 ; PMID:23624057; http://dx.doi.org/10.1016/j.vaccine.2013.04.037
- Patel V, Jalah R, Kulkarni V, Valentin A, Rosati M, Alicea C, von Gegerfelt A, Huang W, Guan Y, Keele BF, et al. DNA and virus particle vaccination protects against acquisition and confers control of viremia upon heterologous SIV challenge. Proc Natl Acad Sci U S A 2013; 110:2975-80; PMID:23359688; http://dx.doi.org/10.1073/pnas.1215393110
- De Rosa SC, Thomas EP, Bui J, Huang Y, deCamp A, Morgan C, Kalams SA, Tomaras GD, Akondy R, Ahmed R, et al. HIV-DNA priming alters T cell responses to HIV-adenovirus vaccine even when responses to DNA are undetectable. J Immunol 2011; 187:3391-401; PMID:21844392; http://dx.doi.org/10.4049/jimmunol.1101421
- Koup RA, Roederer M, Lamoreaux L, Fischer J, Novik L, Nason MC, Larkin BD, Enama ME, Ledgerwood JE, Bailer RT, Mascola JR, et al. Priming immunization with DNA augments immunogenicity of recombinant adenoviral vectors for both HIV-1 specific antibody and T-cell responses. PloS One 2010; 5; e9015; PMID:20126394; http://dx.doi.org/10.1371/journal.pone.0009015
- Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, Grove D, Koblin BA, Buchbinder SP, Keefer MC, Tomaras GD, et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med 2013; 369:2083-92; PMID:24099601; http://dx.doi.org/10.1056/NEJMoa1310566
- Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med 2007; 13:46-53; PMID:17173051; http://dx.doi.org/10.1038/nm1520
- Masemola A, Mashishi T, Khoury G, Mohube P, Mokgotho P, Vardas E, Colvin M, Zijenah L, Katzenstein D, Musonda R, et al. Hierarchical targeting of subtype C human immunodeficiency virus type 1 proteins by CD8+ T cells: correlation with viral load. J Virol 2004; 78:3233-43; PMID:15016844; http://dx.doi.org/10.1128/JVI.78.7.3233-3243.2004
- Mothe B, Ibarrondo J, Llano A, Brander C. Virological, immune and host genetics markers in the control of HIV infection. Dis Markers 2009; 27:105-20; PMID:19893207; http://dx.doi.org/10.1155/2009/360362
- Zuniga R, Lucchetti A, Galvan P, Sanchez S, Sanchez C, Hernandez A, Sanchez H, Frahm N, Linde CH, Hewitt HS, et al. Relative dominance of Gag p24-specific cytotoxic T lymphocytes is associated with human immunodeficiency virus control. J Virol 2006; 80:3122-5; PMID:16501126; http://dx.doi.org/10.1128/JVI.80.6.3122-3125.2006
- Rolland M, Heckerman D, Deng W, Rousseau CM, Coovadia H, Bishop K, Goulder PJ, Walker BD, Brander C, Mullins JI. Broad and Gag-biased HIV-1 epitope repertoires are associated with lower viral loads. PLoS One 2008; 3:e1424; PMID:18183304; http://dx.doi.org/10.1371/journal.pone.0001424
- Mothe B, Llano A, Ibarrondo J, Daniels M, Miranda C, Zamarreno J, Bach V, Zuniga R, Pérez-Álvarez S, Berger CT, et al. Definition of the viral targets of protective HIV-1-specific T cell responses. J Transl Med 2011; 9:208; PMID:22152067; http://dx.doi.org/10.1186/1479-5876-9-208
- Mothe B, Llano A, Ibarrondo J, Zamarreno J, Schiaulini M, Miranda C, Ruiz-Riol M, Berger CT, Herrero MJ, Palou E, et al. CTL responses of high functional avidity and broad variant cross-reactivity are associated with HIV control. PLoS One 2012; 7:e29717; PMID:22238642; http://dx.doi.org/10.1371/journal.pone.0029717
- Schneidewind A, Brumme ZL, Brumme CJ, Power KA, Reyor LL, O'Sullivan K, Gladden A, Hempel U, Kuntzen T, Wang YE, et al. Transmission and long-term stability of compensated CD8 escape mutations. J Virol 2009; 83:3993-7; PMID:19091871; http://dx.doi.org/10.1128/JVI.01108-08
- Honeyborne I, Prendergast A, Pereyra F, Leslie A, Crawford H, Payne R, Reddy S, Bishop K, Moodley E, Nair K, et al. Control of human immunodeficiency virus type 1 is associated with HLA-B*13 and targeting of multiple gag-specific CD8+ T-cell epitopes. J Virol 2007; 81:3667-72; PMID:17251285; http://dx.doi.org/10.1128/JVI.02689-06
- Toapanta FR, Craigo JK, Montelaro RC, Ross TM. Reduction of anti-HIV-1 Gag immune responses during co-immunization: immune interference by the HIV-1 envelope. Curr HIV Res 2007; 5:199-209; PMID:17346134; http://dx.doi.org/10.2174/157016207780077057
- Bockl K, Wild J, Bredl S, Kindsmuller K, Kostler J, Wagner R. Altering an artificial gagpolnef polyprotein and mode of ENV Co-administration affects the immunogenicity of a clade C HIV DNA vaccine. PLoS One 2012; 7:e34723; PMID:22509350; http://dx.doi.org/10.1371/journal.pone.0034723
- Kulkarni V, Rosati M, Valentin A, Ganneru B, Singh AK, Yan J, Rolland M, Alicea C, Beach RK, Zhang GM, et al. HIV-1 p24gag derived conserved element DNA vaccine increases the breadth of immune response in mice. PLos One 2013; 8:e60245; PMID:23555935; http://dx.doi.org/10.1371/journal.pone.0060245
- Kulkarni V, Valentin A, Rosati M, Alicea C, Singh AK, Jalah R, Broderick KE, Sardesai NY, Le Gall S, Mothe B, et al. Altered immunodominance hierarchy and increased T-cell breadth upon HIV-1 conserved element DNA vaccination in macaques. PLoS One 2014; 9:e86254; PMID:24465991; http://dx.doi.org/10.1371/journal.pone.0086254
- Kulkarni V, Valentin A, Rosati M, Rolland M, Mullins JI, Pavlakis GN, Felber BK. HIV-1 conserved elements p24CE DNA vaccine induces humoral immune responses with broad epitope recognition in macaques. PLoS One 2014; 9:e111085; PMID:25338098; http://dx.doi.org/10.1371/journal.pone.0111085
- Nabi G, Genannt Bonsmann MS, Tenbusch M, Gardt O, Barouch DH, Temchura V, Uberla K. GagPol-specific CD4(+) T-cells increase the antibody response to Env by intrastructural help. Retrovirology 2013; 10:117; PMID:24156704; http://dx.doi.org/10.1186/1742-4690-10-117
- Vargas-Inchaustegui DA, Tuero I, Mohanram V, Musich T, Pegu P, Valentin A, Sui Y, Rosati M, Bear J, Venzon DJ, et al. Humoral immunity induced by mucosal and/or systemic SIV-specific vaccine platforms suggest novel combinatorial approaches for enhancing responses. Clin Immunol 2014; 153:308-22; PMID:24907411; http://dx.doi.org/10.1016/j.clim.2014.05.008
- Valentin A, McKinnon K, Li J, Rosati M, Kulkarni V, Pilkington GR, Bear J, Alicea C, Vargas-Inchaustegui DA, Jean Patterson L, et al. Comparative analysis of SIV-specific cellular immune responses induced by different vaccine platforms in rhesus macaques. Clin Immunol 2014; 55:91-107; PMID:25229164; http://dx.doi.org/10.1016/j.clim.2014.09.005
- Rosati M, von Gegerfelt A, Roth P, Alicea C, Valentin A, Robert-Guroff M, Venzon D, Montefiori DC, Markham P, Felber BK, et al. DNA vaccines expressing different forms of simian immunodeficiency virus antigens decrease viremia upon SIVmac251 challenge. J Virol 2005; 79:8480-92; PMID:15956591; http://dx.doi.org/10.1128/JVI.79.13.8480-8492.2005
- Rosati M, Bergamaschi C, Valentin A, Kulkarni V, Jalah R, Patel V, von Gegerfelt AS, Montefiori DC, Venzon DJ, Khan AS, et al. DNA vaccination in rhesus macaques induces potent immune responses and decreases acute and chronic viremia after SIVmac251 challenge. Proc Natl Acad Sci U S A 2009; 06:15831-6; PMID:19717425; http://dx.doi.org/10.1073/pnas.0902628106
- Kulkarni V, Rosati M, Bear J, Pilkington GR, Jalah R, Bergamaschi C, Singh AK, Alicea C, Chowdhury B, Zhang GM, et al. Comparison of intradermal and intramuscular delivery of SIV Env DNA by in vivo electroporation in macaques. Hum Vaccin Immunother 2013; 9:2081-94; PMID:23811579; http://dx.doi.org/10.4161/hv.25473
- Rosati M, Valentin A, Jalah R, Patel V, von Gegerfelt A, Bergamaschi C, Alicea C, Weiss D, Treece J, Pal R, et al. Increased immune responses in rhesus macaques by DNA vaccination combined with electroporation. Vaccine 2008; 26:5223-9; PMID:18468743; http://dx.doi.org/10.1016/j.vaccine.2008.03.090
