Abstract
The WHO European Region has been declared polio-free since 2002. By 2010, inactivated polio vaccine (IPV) was the only polio vaccine in use in the EU/EEA for the primary vaccination of children. A systematic review of the literature on polio seroprevalence studies, complemented by the analysis of available vaccine coverage data, has been carried out with the aim of assessing the level of protection against polio in the European population. A total of 52 studies, with data from 14 out of the 31 EU/EEA countries, were included in the analysis. This systematic review shows that, overall, seroprevalence for PV1 and PV3 is high in most countries, although seroimmunity gaps have been detected in several birth cohorts. In particular, relatively low immunity status was found in some countries for individuals born in the 60's and 70's. Discrepancies between reported vaccination coverage and immunity levels have been also highlighted. Countries should make sure that their population is being vaccinated for polio to reduce the risk of local poliovirus transmission in case of importation. Moreover, assessing immunity status should be priority for those traveling to areas where wild polioviruses are still circulating.
Introduction
The WHO European Region has been declared polio-free since 2002.Citation1 As a consequence, those member states of the European Union (EU) and the European Economic Area (EEA) that still included oral polio vaccine (OPV) in the childhood immunization schedule progressively switched to an inactivated polio vaccine (IPV) schedule.Citation2 By 2010, IPV was the only polio vaccine in use in the EU/EEA for the primary vaccination of children, while OPV is still in use in Poland as a booster dose at 6 years of age.Citation3 This was in line with the WHO global strategy to limit the circulation of vaccine-derived poliovirus once the circulation of wild-type poliovirus (WPV) had been stopped.Citation2 Out of the 3 serotypes, only wild poliovirus 1 and 3 still circulate globally; the most recent wild poliovirus 3 detection was in November 2012, while poliovirus 1 is the predominant circulating wild strain.Citation4
The uncontrolled transmission of polio virus in Pakistan, as well as the increased international spread during the low season in 2014, led the Director-General of the WHO to declare, in May 2014, the international spread of WPV in 2014 a Public Health Emergency of International Concern (PHEIC), in accordance with the International Health Regulations (IHR).Citation5
The likelihood of WPV transmission and disease after importation into EU countries is considered low thanks to effective vaccination programmes and very good hygiene levels.Citation6 Nevertheless, the changed global situation together with the presence of pockets of under-immunized population groups provides grounds for questioning this assumption.
As shown in the past and, most recently, by the detection of wild poliovirus type 1 (WPV1) from sewage samples collected in May 2013 in Israel and in March 2014 in Brazil, importation of poliovirus to polio-free regions may happen at any time as long as poliovirus is circulating in the world.
Knowledge of the immunity status of the population is a necessary component for assessing the risk of WPV transmission after importation into EU. In order to describe the susceptibility to polio in the EU, we conducted a systematic review of the literature on polio seroprevalence studies and complemented that information by estimating the number of susceptible individuals using available vaccine coverage data.
Methods
Search strategy and selection criteria
PubMed®, Embase.com® and Cochrane Library® have been searched up to 31st May 2014 for studies on the seroepidemiology of polio. The concepts of polio and seroprevalence, serology, immunity and prevalence were combined. Free-text/natural vocabulary (i.e. keywords) and medical subject heading (MeSH and Emtree) terms were used. No language or geographical restrictions were applied in the search string. Reference lists of retrieved articles were also reviewed.
References retrieved were uploaded to Endnote X6 (Thomas Reuter, 2012. Endnote X6.0.1. Philadelphia). Three investigators (D.N., A.M., P.C.S.) systematically screened search results by title and abstract. Exclusion criteria were: studies performed in non-EU/EEA or European overseas countries and territories, not polio, not in humans, no seroprevalence, clinical trial, outbreak situation, missing information on age of study population, only data on immigrants and study performed before vaccination era. Full text articles of selected abstracts were retrieved and data extracted (D.N.) if they met the criteria. Discrepancies were resolved by consensus.
Data extraction and measurement of the outcome
Information on country, region, study population, study year, randomization, sample size, measurement tool, age, seroprevalence rates per serotype per age group and vaccine schedule was extracted. Data on infants less than one year were excluded due to potential confusion with maternal antibodies.
To measure immunity to polio, WHO recommends using a micro neutralization test with Sabin strains of poliovirus types 1 (PV1), 2 (PV2), and 3 (PV3) as challenge samples Citation7. In particular, the strains PV1-LSc/2ab, PV2-P712 and PV3-Leon as strains from which the Sabin oral vaccine were derived, and the strains PV1-Mahoney-Brunhilde, PV2-MEF-1 and PV3-Saukett as strains from which the Salk inactivated vaccine were derived.Citation8,9 The cut off titer recommended by the WHO to demonstrate poliovirus immunity in humans is ≥1:8,Citation10 however less conservative titres were also included (e.g. ≥1:2). We define high immunity when at least 90% of the population is immune to polio.Citation2
Data analysis
Seroprevalence by year of birth, starting from the onset of vaccination programmes in each country, has been calculated. To do so, age groups were recoded to birth cohorts by subtracting the age of the participants from the study year. When the study year was unknown, year of publication minus 1 was used. After recalculating the birth cohorts, the median (and range if more than one study was available) seroprevalence per decade was calculated by polio type and country. Some papers reported data from populations samples tested in different periods; in such cases the different evaluations are displayed as different studies (). Since the main goal of the study was to assess the risk for WPV reintroduction in Europe and wild type PV2 is no longer circulating,Citation11 only data for PV1 and PV3 are presented.
Table 1. Studies included in the systematic review (n = 52) and estimated median P1 (%) and P3 (%) seroprevelance by decade of birth
To obtain coverage data on 3 doses of polio vaccine the Centralized Information System for Infectious Diseases (CISID) has been used (data available from 1980).Citation12 Median coverage by decade and country has been calculated. Only countries for which we had data on seroprevalence were included. As vaccine effectiveness for 3 doses of either IPV or OPV is close to 100%,Citation13–15 vaccination coverage was considered a good proxy of seroprevalence, hence vaccine coverage data were not adjusted.
Results
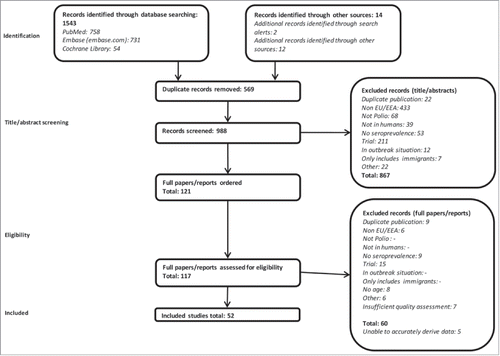
A total of 52 studies, with data from 14 out of the 31 EU/EEA countries, were included Citation16-67; these 14 countries account for around 80% of the EU/EEA population.Citation68The flow diagram of the study selection process can be found in .
All included studies used the (micro) neutralization test for determining immunity to poliovirus; 20 four studies used the cut off titer recommended by the WHO (≥1:8) and 6 used a more conservative cut off (5 ≥1:10, and one ≥1:16) (). Concerning the Sabin strains of poliovirus used as challenge samples, 4 studies used the 3 Sabin strains corresponding with OPV (PV1-LSc/2ab, PV2-P712, PV3-Leon); 15 studies used the 3 inactivated polio virus strains (PV1-Mahoney-Brunhilde, PV2-MEF-1, PV3-Saukett); 3 studies used a combination of these strains and for 30 studies it was unknown; 22 studies did not report any information on cut off titer.
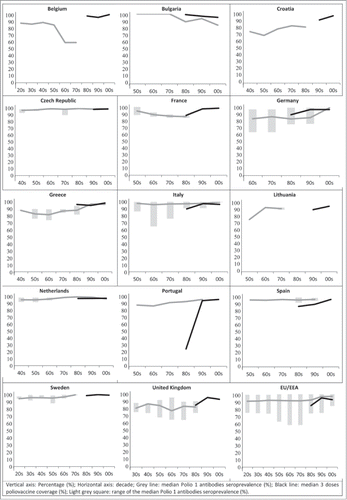
Seroprevalence for PV1 () in the studies ranged from 57.8% in Belgium to 100% in Bulgaria and the Czech Republic. Most of the studies show PV1 seroprevalence over 80% for all the time periods available. However, for individuals born in the sixties and seventies, some countries reported lower PV1 seroprevalence values (when several estimates were available, the lowest value has been reported) (Belgium 57.8% (60s); Germany 62.4% (60s); Italy 65% (60s) and UK 64.4% (70s)).
Figure 2. Median Polio 1 antibodies seroprevalence and 3 doses poliovaccine coverage by decade and EU/EEA Country. Vertical axis: Percentage (%); Horizontal axis: decade; Gray line: median Polio 1 antibodies seroprevalence (%); Black line: median 3 doses poliovaccine coverage (%); Light gray square: range of the median Polio 1 antibodies seroprevalence (%).
Concerning PV3 (), seroprevalence ranged from 61.4% in Belgium to 100% in Bulgaria and Czech Republic. Overall, seroprevalence for this serotype is over 80% in most of the studies. Also for PV3, some countries reported low seroprevalence values in individuals born in the sixties, seventies and eighties (Belgium 61.4% (60s); Germany 47.5% (80s); Italy 65.5% (60s–90s); Portugal 63.5% (80s) and UK 48.6% (60s)).
Figure 3. Median Polio 3 antibodies seroprevalence and 3 doses poliovaccine coverage by decade and EU/EEA Country. Vertical axis: Percentage (%); Horizontal axis: decade; Gray line: median Polio 3 antibodies seroprevalence (%); Black line: median 3 doses poliovaccine coverage (%); Light gray square: range of the median Polio 3 antibodies seroprevalence (%).
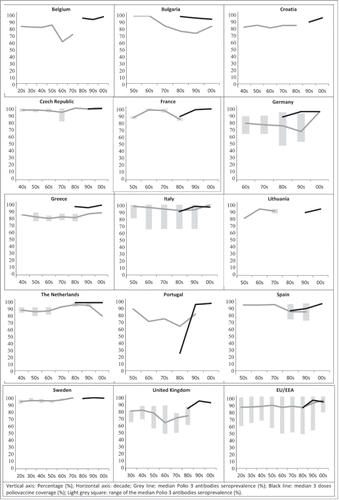
Data on polio vaccine coverage () ranged from 25% in the eighties in Portugal to 99.2% in the nineties in Sweden. Overall, reported vaccine coverage was very high, with all countries being over 90% after the nineties. In some countries like Germany, Italy and the UK, differences were found in the estimates produced by the different studies available (Table 2, ).
In most countries where both seroprevalence and coverage data was available for the last decades, values were similar. For PV1, there is a slight difference of around 10% in Bulgaria and Germany in the nineties (). For PV3, differences between both data sources can be seen in Bulgaria, Germany, Greece -both in the eighties and the ninetiesand for the last decade in the Netherlands (). Italy, Spain and Portugal reported vaccination coverage levels below the measured sero-immunity.
Discussion
This systematic review shows that, overall, seroprevalence for PV1 and PV3 is high in most countries and periods where data are available, although several birth cohorts showed seroimmunity gaps in specific populations. On average, Czech Republic, Italy, and Sweden showed immunity rates above 90% for almost all birth cohorts to all types of polio, while Belgium, Croatia, Greece, and the United Kingdom showed immunity rates below that threshold for almost all birth cohorts to all types of polio. On the other hand, the epidemiological evidence supported by good quality surveillance show, also in these countries, that general immunity levels have been sufficient to stop wild polioviruses circulation. France, Lithuania, The Netherlands and Spain showed some gaps over time in the immunization status of the population. Data from Germany, Italy and the UK showed that there were considerable differences in immunity within the country. This difference was especially visible in Germany as former East-Germany showed immunity rates 20–30% lower than former West-Germany. In Italy differences could be partially explained by differences in implementing vaccination programmes as the regional health authorities are in charge of developing their own vaccination strategies.Citation69
Relatively low immunity status was found in some countries for individuals born in the 60's and 70's. For the interpretation of these results, the different cut-offs used in the studies has to be considered. Additionally, vaccination programmes started at different points in time, with different vaccines and catch up strategies. Therefore it is very hard to assess the situation at the beginning of the polio vaccination campaigns. As an example, in some countries, like the former East-Germany, higher coverage levels were obtained only when IPV was replaced by OPV in the 60's. Assuming these populations have not been exposed to the virus other than through vaccination, currently the seroprevalence would be below 80% for these individuals, aged 35 to 55 years, even without considering any waning immunity effect. Such evidence should not be considered as implying on immediate epidemic risk, given the generally high levels of sanitation and hygiene in the EU, but should be taken into consideration especially for individual protection of those in that age group traveling in areas where wild polioviruses are still circulating.
Reported vaccination coverage for 3 doses of polio for the last 30 years is generally very high. However, in countries where both reported coverage and seroprevalence data were available, these results were not always supported with equally high seroprevalence levels. This is the case for Bulgaria, Germany, and Greece. These differences could be due either to low potency of the vaccine used (primary vaccine failure) or to difficulties in vaccination coverage assessment in the countries, which would lead to inaccurate data reported to CISID. Vaccination coverage assessment is essential to monitor the performance of immunization systems in order to evaluate the progress toward the achievement of goals for controlling and/or eliminating vaccine preventable diseases. Last but not least, it is important to mention that a good overall coverage in a region or country does not guarantee the absence of transmission should polio virus be introduced. Areas or groups with low coverage can still exist. Being able to identify these groups would help implementing specific immunization campaigns.
This study presents some limitations. Many studies in the systematic review used cut off titres different from the ones recommended by WHO. Using cut off titres below 1:8 (22 studies) could lead to an overestimation of the population's immunity to polio and, conversely, titres over 1:8 (6 studies) could lead to an underestimation of the population's immunity. Considering this, the overall estimate of seroprevalence in this review is more likely to be biased toward overestimation of the EU population's immunity. In addition, the current analysis did not take into consideration any decline of immunity over the time, since data are presented by birth cohort independently from when the seroprevalence was assessed. The sampling methods quality varied and was not optimal for some studies, so sampled subjects were not always representative of the country's population. Additionally, only 14 out of the 31 countries included in the EU/EEA are represented in the systematic review and as such the reported data may not represent the whole situation in the EU/EEA. On the other hand, these 14 countries account for around 80% of the EU/EEA population. Finally, no specific data on recognized vulnerable populations were reported in the studies, thus reported estimates are referred to the general population only.
Conclusions
The aim of the present review was to assess the immunological protection against WPV in the EU population, which is one of the components of the risk of WPV reintroduction in the European Region. Vaccine-derived polioviruses (VDVP) have not been taken into consideration and therefore PV1 and PV3 only have been presented as they are the only WPV circulating strains. Even though the immunity status of the EU/EEA population is high overall, this study points to some elements that could theoretically allow local transmission following introduction of poliovirus. In fact, immunological protection of EU/EEA population is patchy. Seroimmunity to polio was below the herd threshold in some countries, especially in relation to PV3. A gap in polio immunity in some EU/EEA countries for individuals currently aged 35 to 55 years was found. Additionally, discrepancies between vaccination coverage and seroprevalence levels in some countries are worth further investigation and clarification; reported immunization coverage in children and young adults may be overestimated in some countries, so leaving a higher number than might be assumed of susceptible in these young ages. Conversely it is important to underline that a population's immunity is only one determinant of poliovirus circulation in the community. Environmental factors and the overall hygiene level are crucial aspects for assessing the risk of polio re-introduction in the EU/EEA. The overall good levels of hygiene and sanitation in the EU/EEA countries represent an important risk mitigation factor. In conclusion, even in the presence of immunity gap, the overall protection of the EU/EEA population against the risk of wild polioviruses should be considered good.
Recommendations
Countries should make sure that their population is being vaccinated for polio to reduce the risk of poliovirus transmission in case of importation. Moreover, assessing immunity status should be priority for those traveling to areas where wild polioviruses are still circulating. One or more IPV booster doses, according to the national recommendations, should be considered in such cases. Increase in vaccination coverage should go hand to hand with proper vaccination monitoring. For some countries no seroprevalence studies were available (or not recent ones), therefore further research would be advised to get a better overview of the EU/EEA situation. Monitoring of hard to reach populations is also needed in order to assess the susceptibility of polio in these groups. Finally, it would be advised to comply with the WHO guidelines to enable more accurate comparison between studies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- World Health Organisation. Certification of poliomyelitis eradication 2002. Available from: http://www.euro.who.int/en/health-topics/communicable-diseases/poliomyelitis/publications/pre-2009/certification-of-poliomyelitis-eradication.
- World Heatlh Organisation & Global Polio Eradication Initiative. Polio eradication & endgame strategic plan 2013–2018. 2013.
- European Centre for Disease Prevention and Control. Vaccination schedule 2014. Available from: http://vaccine-schedule.ecdc.europa.eu/Pages/Scheduler.aspx.
- Polio Global Eradication Initiative. Polio. The Virus 2014. Available from: http://www.polioeradication.org/Polioandprevention/Thevirus.aspx
- World Health Organisation. WHO statement on the meeting of the International Health Regulations Emergency Committee concerning the international spread of wild poliovirus May 5th2014. Available from: http://www.who.int/mediacentre/news/statements/2014/polio-20140505/en/.
- European Centre for Disease Prevention and Control. Wild-type poliovirus 1 transmission in Israel – what is the risk to the EU/EEA? Risk Assessment 2013. Available from: http://ecdc.europa.eu/en/publications/Publications/polio-risk-assessment-transmission-in-Israel.pdf
- World Health Organisation. Guidelines for WHO/EPI collaborative studies on poliomyelitis: standard procedure for determining immunity to poliovirus using the microneutralization test 1993. Available from: http://apps.who.int/iris/handle/10665/70486.
- Verdijk P, Rots NY, van Oijen MG, Oberste MS, Boog CJ, Okayasu H, Sutter RW, Bakker WA. Safety and immunogenicity of inactivated poliovirus vaccine based on Sabin strains with and without aluminum hydroxide: a phase I trial in healthy adults. Vaccine 2013; 31(47):5531–6. Epub 2013/09/26. eng; PMID:24063976; http://dx.doi.org/10.1016/j.vaccine.2013.09.021
- Albrecht P, van Steenis G, van Wezel AL, Salk J. Standardization of poliovirus neutralizing antibody tests. Rev Infect Dis 1984; 6 Suppl 2: S540–4; PMID:6330853. Epub 1984/05/01. eng
- World Health Organisation. Polio laboratory manual 2004. Available from: http://whqlibdoc.who.int/hq/2004/WHO_IVB_04.10.pdf
- Adams P. Ending polio, one type at a time. Bull World Health Organ 2012; 90(7):482–3; PMID:22807591. Pubmed Central PMCID: 3397709
- World Health Organisation. Centralized information system for infectious diseases (CISID). Vaccination Coverage. Available from: http://data.euro.who.int/cisid/Default.aspx?TabID=347514.
- Roland W. Sutter VMC, Pedro Mas Lago. The role of routine polio immunization in the post-certification era. Bull World Health Organ 2004; 82:31–9; PMID:15106298
- McBean AM, Thoms ML, Albrecht O, Cuthie JC, Bernier R, and The Field Staff and Coordinating Committee. Serologic response to oral polio vaccine and enhanced-potency inactivated polio vaccines. A J Epidemiol. 1988; 128(3):615–28
- Modlin JF, Halsey NA, Thoms ML, Meschievitz CK, Patriarca PA. Humoral and mucosal immunity in infants induced by three sequential inactivated poliovirus vaccine-live attenuated oral poliovirus vaccine immunization schedules. Baltimore Area Polio Vaccine Study Group. J Infect Dis [Internet]. 1997; 175 Suppl 1:S228–34. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/036/CN-00141036/frame.html http://jid.oxfordjournals.org/content/175/Supplement_1/S228.full.pdf; http://dx.doi.org/10.1093/infdis/175.Supplement_1.S228
- Lamy ME, Cornu C, Desmyter J. Poliovirus antibodies in age groups: an assessment of obligatory vaccination in Belgium. Dev Biol Stand 1979; 43:207–13; PMID: 230111. Epub 1979/01/01. eng.
- Parmakova K, Korsun N, Kojouharova M, Georgieva D, Mladenova Z, Kurchatova A. Seroprevalence of poliovirus antibody in Bourgas Region, Bulgaria. Prob Infect Parasitic Dis. 2011; 39(1):15–6
- Borcic B, Kruzic V, Kaic B, Ljubin-Sternak S, Ljubicic M, Dobrovsak-Sourek V. Immunity of the Croatian population to poliomyelitis–a serosurvey. Acta medica Croatica : casopis Hravatske akademije medicinskih znanosti. 1998; 52(4–5):229–33; PMID:9988903
- Teply V, Sramova H, Skridlovska E, Strnad P. Immunity status of Czech population with regard to poliomyelitis in 1970–1975. J Hyg Epidemiol Microbiol Immunol 1977;21(3):315–23; PMID:564368
- Matyasova I, Rainetova P, Castkova J. 2001 serological survey in the Czech Republic–poliomyelitis. Cent Eur J Public Health 2003 Dec; 11 Suppl:S31–5; PMID:15080257. Epub 2004/04/15. eng
- Malvy D, Fuchs F, Dubois F, Roure C, Aymard M, Drucker J. A study of poliomyelitis related sero-immunity in a selected sample of the French population. Medecine et Maladies Infectieuses. 1996; 26(6–7):714–20; http://dx.doi.org/10.1016/S0399-077X(96)80102-8
- Diedrich S, Schreier E. State of immunity against polio in Germany: Polio serology surveillance 1993. Deutsche Medizinische Wochenschrift 1995; 120(8):239–44; PMID:7867480; http://dx.doi.org/10.1055/s-2008-1055339
- Diedrich S, Schreier E. The German Health Interview and Examination Survey for Children and Adolescents (KiGGS): State of immunity against poliomyelitis in German children. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2007; 50(5–6):771–4; PMID:17514462; http://dx.doi.org/10.1007/s00103-007-0239-1
- Grutzner L, Pichl H, Lehmann I, Lenz H. Poliomyelitis immunity in the federal republic of Germany in 1969. II. Zentralblatt für Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene Erste Abteilung Originale Reihe A: Medizinische Mikrobiologie und Parasitologie. 1971; 217(3):284–99; PMID:5572044
- Sauerbrei A, Groh A, Bischoff A, Prager J, Wutzler P. Antibodies against vaccine-preventable diseases in pregnant women and their offspring in the eastern part of Germany. Med Microbiol Immunol 2002 Mar; 190(4):167–72. Epub 2002/05/15. eng; PMID: 12005329; http://dx.doi.org/10.1007/s00430–001-0100-3
- Doerr HW, Glueck H, Esser I. Immunity against poliomyelitis in the German Federal Republic. Deutsche Medizinische Wochenschrift. 1979; 104(30):1065–7; PMID:467253; http://dx.doi.org/10.1055/s-0028-1129038
- Maass G, Doerr HW. Studies on the state of immunity against poliovirus types 1, 2 and 3 in the Federal Republic of Germany. Deutsche Medizinische Wochenschrift. 1986; 111(44):1670–6; http://dx.doi.org/10.1055/s-2008-1068690
- Maass G, Weber B, Doerr HW. Investigation into the immune status against poliovirus (5th Cooperative Study of the German Association for the Fight against Viral Diseases). Deutsche Medizinische Wochenschrift. 1991; 116(39):1457–62; PMID:1655377; http://dx.doi.org/10.1055/s-2008-1063772
- Stark K, Schonfeld C, Barg J, Molz B, Vornwald A, Bienzle U. Seroprevalence and determinants of diphtheria, tetanus and poliomyelitis antibodies among adults in Berlin, Germany. Vaccine. 1999; 17(7–8):844–50; PMID:10067690; http://dx.doi.org/10.1016/S0264-410X(98)00269-2
- Weber B, Rabenau H, Cinatl J, Maass G, Doerr HW. Quantitative detection of neutralizing antibodies against polioviruses and non-polio enteroviruses (NPEV) using an automated microneutralization assay: A seroepidemiologic survey. Zentralblatt fur Bakteriologie. 1994; 280(4):540–9; PMID:8061416; http://dx.doi.org/10.1016/S0934-8840(11)80515-3
- Wicker S, Rabenau HF, Gottschalk R, Doerr HW, Allwinn R. Seroprevalence of vaccine preventable and blood transmissible viral infections (measles, mumps, rubella, polio, HBV, HCV and HIV) in medical students. Med Microbiol Immunol. 2007; 196(3):145–50; PMID:17273881; http://dx.doi.org/10.1007/s00430-007-0036-3
- Franck S, Allwinn R, Rabenau HF, Doerr HW. Epidemiological analysis of immunity to poliovirus after termination of an era of vaccination with OPV in Germany: An analysis of the German Association against Viral Diseases (DVV). Zentralblatt fur Bakteriologie. 1999; 289(4):475–81; PMID:10603664; http://dx.doi.org/10.1016/S0934-8840(99)80086-3
- Kyriazopoulou-Dalaina V. Poliovirus antibody in Northern Greece. J Hyg 1986; 96(3):479–82; PMID:3016076; http://dx.doi.org/10.1017/S0022172400066274
- Frantzidou F, Diza E, Halkia D, Antoniadis A. A seroprevalence study of poliovirus antibody in the population of northern Greece. Clin Microbiol Infect. 2005; 11(1):68–71; PMID:15649308; http://dx.doi.org/10.1111/j.1469-0691.2004.00998.x
- Patti AM, Santi AL, Vulcano A, Casagni L, Lamberti A, De Stefano Caraffa D, Vellucci L, Fiore L, Fara GM. Surveillance of poliomyelitis in Italy: immunity status of population against polio and environmental circulation of Poliovirus. General illustration of the results. Annali di igiene : medicina preventiva e di comunità. 2002; 14(4 Suppl 5):1–57
- Santoro R, Lombardi F, Novello F. Serum antibodies to poliomyelitis in Italy. Bull World Health Organ 1984; 62(4):591–5; PMID:6333296
- Mastroeni I, Patti AM, Fabrizi A, Santi AL, Manduca AM, Vescia N, Squarcione S, Fara GM. Immunity status against poliomyelitis in persons 13–14 years old living in Rome. Vaccine 1997; 15(6–7):747–50; PMID:9178477; http://dx.doi.org/10.1016/S0264-410X(96)00208-3
- Volpi A, Ragona G, Biondi W. Seroimmunity to polioviruses in an urban population of Italy. Bulletin of the World Health Organization. 1976; 54(3):275–8; PMID:191208
- Tafuri S, Prato R, Martinelli D, Calvario A, Bozzi A, Labianca M, Patti A, Lopalco PL, Germinario C. Serological survey on immunity status against polioviruses in children and adolescents living in a border region, Apulia (Southern Italy). BMC Infect Dis 2008; 8:150; PMID:18218115
- Pianetti A, Salvaggio L, Biffi MR, Baffone W, Bruscolini F, De Donato S, Albano A. Antipoliomyelitis immunity status in a cohort of young men drafted into military service, residing in the suburban Milan area. Eur J Epidemiol 1997; 13(6):725–7; PMID:9324221; http://dx.doi.org/10.1023/A:1007331900645
- Pianetti A, Bruscolini F, Rocchi MBL, Baffone W, Salvaggio L, Albano A, Biffi MR. Poliomyelitis surveillance: Seroepidemiological research in a population cohort. Igiene Moderna 2003; 118(5):299–310
- Trivello R, Farisano G, Bonello C, Moschen ME, Baldo V, Majori S, Moretti G, Marin V, Piron L, Renzulli G. Immunity status to poliovirus in Veneto region (North-East Italy). A seroepidemiological survey. Annals of Clinical and Laboratory Science 1994; 24(6):542–7; PMID:7847782
- Majori S, Baldo V, Poli A, Riolfatti M, Alborino F, Bonello C, Frau S, Baldovin T, Dal Zotto A, Romano G, et al. Immunity to poliovirus among children and the elderly in north-east Italy. J Prevent Med Hyg 2006; 47(1):12–5; PMID:17061405
- Reali D, Carducci A, Ruschi MA. Serum antibodies to polioviruses in a Tuscan population, Italy. Eur J Epidemiol 1990; 6(3):309–12; PMID:2174794; http://dx.doi.org/10.1007/BF00150438
- Natoli D, Milici M, Brai M. Immunity to poliovirus in a junior high school population in Palermo. Annali Sclavo; rivista di microbiologia e di immunologia 1980; 22(4):633–9; PMID:6264871
- Veronesi L, Affanni P, Verrotti di Pianella C, Colucci ME, Tanzi ML. Immunity status against poliomyelitis in childbearing women in a province of northern Italy. A cross-sectional analysis. Annali di igiene : medicina preventiva e di comunità 2013; 25(5):427–33
- Albano A, Bruscolini F, Pianetti A. Antipoliomyelitic immunity twenty years after the introduction of the oral poliovaccine. Igiene Moderna 1986; 86(3):187–97
- Tanzi ML, Bracchi U, Bocelli V, Bombarda G, Zoni R, Bellelli E. [The immune status versus poliomyelitis in a sample of the population of the provinces of Cremona and Mantova]. Ann Ig 1991; 3(2):105–13; PMID: 1725590. Epub 1991/03/01. Stato immunitario verso la poliomielite in un campione di popolazione delle province di Cremona e di Mantova. ita
- Stagni G, Merletti L, De Rosa F. Specific antibody levels against poliovirus and degree of immune protection in the population of Umbria. Bollettino dell Istituto Dermatologico SGallicano. 1979; 58(3):210–5; PMID:229884
- Ribera G, Angelillo IF, Ricciardi G, Grasso GM, Villari P. Poliomyelitis immunity status in the centro-meridional Italy. Annali Di Igiene : Medicina Preventiva E Di Comunità 1995; 7(6):421–9
- Maiello A, Ossola O, Guidetti A, Zotti C, Moiraghi Ruggenini A. Poliomyelitis surveillance: seroepidemiologic study on a Piedmont population sample. Annali Dell'Istituto Superiore Di Sanità 1995; 31(3):317–22
- Andreassen Rix B, Zhobakas A, Wachmann CH, Bakasenas V, Ronne T. Immunity from diphtheria, tetanus, poliomyelitis, measles, mumps and rubella among adults in Lithuania. Scandinavian J Infect Dis 1994; 26(4):459–67; PMID:7984979; http://dx.doi.org/10.3109/00365549409008620
- Conyn-Van Spaendonck MAE, De Melker HE, Abbink F, Elzinga-Gholizadea N, Kimman TG, Van Loon T. Immunity to poliomyelitis in the Netherlands. Am J Epidemiol 2001; 153(3):207–14; PMID:11157405; http://dx.doi.org/10.1093/aje/153.3.207
- Van der Maas NA, Mollema L, Berbers GA, Van Rooijen DM, Van der Avoort HG, Conyn-Van Spaendonck MA, de Melker HE, van der Klis FR. Immunity against poliomyelitis in the Netherlands, assessed in 2006 to 2007: The importance of completing a vaccination series. Eurosurveillance 2014; 19(7); PMID:24576472; http://dx.doi.org/10.2807/1560-7917.ES2014.19.7.20705
- Rebelo de Andrade H PdM. Vírus da Poliomielite. In: Avaliação do programa nacional de vacinação e melhoria do seu custo-efectividade: 2° inquérito serológico nacional Portugal continental 2001-2002. Ministério da Saúde, Direcção-Geral da Saúde, Lisboa. 2004:159–78.
- Pachon I, Amela C, De Ory F. Age-specific seroprevalence of poliomyelitis, diphtheria and tetanus antibodies in Spain. Epidemiol Infect 2002; 129(3):535–41; PMID:12558336; http://dx.doi.org/; http://dx.doi.org/10.1017/S0950268802007781
- Plans Rubio P, Rabella Garcia N, Otegui Zabalo M, Espunes Vendrell J, Dominguez Garcia A, Plasencia Taradach A. [Evaluation of the immunity level achieved with the oral polio vaccine in schoolchildren aged 6 to 12 years of Catalonia (Spain)]. Med Clin (Barc) 2006; 127(16):612–4; Epub 2006/12/06. Evaluacion del grado de proteccion inmunitaria conseguida con la vacuna oral antipoliomielitica en la poblacion infantil de 6–12 anos de Cataluna. spa; PMID:17145026; http://dx.doi.org/10.1157/13094418
- Bottiger M, Zetterberg B, Salenstedt CR. Seroimmunity to poliomyelitis in Sweden after the use of inactivated poliovirus vaccine for 10 years. Bull World Health Organ. 1972; 46(2):141–9; PMID:4537478
- Svensson A, Bottiger M, Gustavsson O. Immunity in the Swedish population: Diphtheria, tetanus and poliomyelitis. Int J Epidemiol 1998; 27(5):909–15; PMID:9839752; http://dx.doi.org/10.1093/ije/27.5.909
- White PM, Green J. Prevalence of antibody to poliovirus in England and Wales 1984-6. Br Med J (Clin Ees ed). 1986;293(6555):1153–5. Pubmed Central PMCID: PMC1341857. Epub 1986/11/01. eng; PMID: 3021273; http://dx.doi.org/10.1136/bmj.293.6555.1153
- Codd AA, White E. Protection against poliomyelitis. Lancet. 1977; 2(8047):1078; PMID:72982; http://dx.doi.org/10.1016/S0140-6736(77)91913-4
- Roebuck M, Chamberlain R. Prevalence of antibodies to poliovirus in 1978 among subjects aged 0–88 years. Br Med J (Clin Res ed). 1982 6;284(6317):697–700; PMID: 6279225. Pubmed Central PMCID: PMC1496662. Epub 1982/03/06. eng.
- Mortimer PP, Cunningham P. Sero immunity to poliovirus in children and young women: England 1972–4. J Hyg 1975; 74(2):283–7; PMID:164503; http://dx.doi.org/10.1017/S0022172400024359
- Bainton D, Freeman M, Magrath DI. Immunity of children to diphtheria, tetanus, and poliomyelitis. Br Med J 1979; 1(6167):854–7; PMID:219933; http://dx.doi.org/10.1136/bmj.1.6167.854
- Joseph CA, Begg NT, Stanwell-Smith RE, Magrath DI. Antibody state to poliovirus in first year university students, 1984. Br Med J 1987; 295(6591):171–3; PMID:2820540; http://dx.doi.org/10.1136/bmj.295.6591.171
- Smith WC, Small RG, Dunkerley RA, Green DM. Herd immunity to poliovirus in Dundee. Health Bull 1982; 40(6):296–305; PMID:6298147
- Galbraith N, Fernandes R. Polioantibody titres in children aged 7–15 years in London. The Lancet 1969; 294(7624):792–3; PMID:4186037; http://dx.doi.org/10.1016/S0140-6736(69)90496-6
- Eurostat. Population on 1 January 2014. Available from: http://epp.eurostat.ec.europa.eu/portal/page/portal/population/data/main_tables. 2014.
- Alfonsi V DAF, Rota MC, Giambi C, Ranghiasci A, Iannazzo S, Regional coordinators for infectious diseases and vaccinations,. Immunisation registers in Italy: a patchwork of computerisation. Euro surveillance : bulletin européen sur les maladies transmissibles = European communicable disease bulletin [Internet]. 2012; (17(17):pii=20156). Available from: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20156