Abstract
Influenza is one of the most important infectious diseases in humans. The best way to prevent severe illness caused by influenza infection is vaccination. Cell culture-derived influenza vaccines are being considered in addition to the widely used egg-based system in order to support the increasing seasonal demand and to be prepared in case of a pandemic. Cell culture based systems offer increased safety, capacity, and flexibility with reduced downstream processing relative to embryonated eggs. We have previously reported a chick embryo cell line, termed PBS-12SF, that supports replication of human and avian influenza A viruses to high titers (>107 PFU/ml) without the need for exogenous proteases or serum proteins. Viral infections in cells are limited by the Interferon (IFN) response typified by production of type I IFNs that bind to the IFNα/β receptor and activate an antiviral state. In this study, we investigated how neutralizing the interferon (IFN) response in PBS-12SF cells, via shRNA-mediated knock-down of IFNAR1 mRNA expression, affects influenza virus production. We were successful in knocking down ∼90% of IFNAR1 protein expression by this method, resulting in a significant decrease in the response to recombinant chIFNα stimulation in PBS-12SF cells as shown by a reduction in expression of interferon-responsive genes when compared to control cells. Additionally; IFNAR1-knock-down cells displayed enhanced viral HA production and released more virus into cell culture supernatants than parental PBS-12SF cells.
List of Abbreviations
| AIV | = | Avian Influenza Virus |
| BSA | = | Bovine Serum Albumin |
| BSL2 | = | Biosafety Level 2 |
| CDC | = | Center for Disease Control |
| cDNA | = | cDNA |
| CEK | = | Chick Embryo Kidney cell line |
| chIFNα | = | Chicken Interferon α |
| HA | = | Hemagglutination |
| IFIH1 | = | Interferon-Induced Helicase C domain-containing 1 |
| IFN | = | Interferon |
| IFNAR | = | Interferon α/β, receptor |
| IFNα | = | Interferon α |
| IFNβ | = | Interferon β |
| IRF3 | = | Interferon Response Factor 3 |
| ISG | = | Interferon-Stimulated Gene |
| JAK/STAT | = | Janus Kinase/Signal Transducer Activation of Transcription |
| MDA5 | = | Melanoma Differentiation-Associated gene 5 |
| MDCK | = | Madin-Darby Canine Kidney cell line |
| MSU | = | Michigan State University |
| Mx1 | = | Myxovirus (influenza virus) resistance 1 gene |
| NS1 | = | Non-Structural protein 1 |
| OAS | = | 2′-5′, Oligoadenylate Synthetase |
| PBS | = | Phosphate-Buffered Saline |
| PBS-12SF | = | Second generation of PBS-1 cell line, serum-free |
| PFU | = | Plaque Forming Unit |
| PKR | = | RNA-regulated Protein Kinase |
| PVDF | = | Polyvinylidene Difluoride |
| qPCR | = | Quantitative Real-time Reverse-Transcriptase Polymerase Chain Reaction |
| RNA | = | Ribonucleic Acid |
| shRNA | = | Short Hairpin Ribonucleic Acid |
| Vero | = | African green monkey kidney cell line |
| WHO | = | World Health Organization |
Introduction
Influenza is still among the most common causes of human respiratory illness. The World Health Organization (WHO) estimates that 5%–10% of the world's adults and 20%–30% of its children are infected with influenza virus in a typical epidemic season, resulting in about 3–5 million hospitalizations and 250,000–500,000 deaths.Citation1 During the last century, several pandemics have occurred infecting large portions of the human population and causing high levels of mortality. The best way to prevent severe illness caused by influenza infection is vaccination.Citation1 New cell culture-based vaccine production systems under development promise to be more expandable and flexible than current egg-based systems.Citation2,3 Currently, the Flucelvax® vaccine is produced using Madin-Darby Canine Kidney (MDCK) cells and is available to adults 18 y of age and older.Citation4 Continuous cell lines such as the MDCK, African green monkey kidney (Vero), and PER.C6 lines have all been shown to be susceptible to influenza A and B infection, yielding high enough titers for vaccine production with relative ease of growth in serum-free culture media as well as adapted to growth in suspension in the case of MDCK and PER.C6.Citation2 We have previously reported a novel non-tumorigenic avian cell line, termed PBS-12SF, which was adapted to grow in serum-free conditions and is able to release titers of human and reassortant H5N1 influenza strains higher than other commonly used cell lines, ie., primary chick embryo kidney (CEK), MDCK, and cells without exogenous proteases.Citation5,6 Importantly, PBS-12SF cells express sialic acid receptors for both human and avian influenza viruses.Citation5
During viral infection, one of the first signaling mechanisms to be activated by the cell is the innate immune response typified by production of interferons (IFN).Citation7 Type I IFNs, such as interferon α and interferon β (IFNα/IFNβ) play an essential role in the innate immune response against influenza viruses.Citation8,9 In mammals, IFNα/β bind to IFN α-β receptor (IFNAR) on the cell surface and induce an antiviral state characterized by up-regulation of several IFN-stimulated genes (ISGs), such as 2′-5′ oligoadenylate synthetase (OAS), myxovirus (influenza virus) resistance 1 (Mx1), melanoma differentiation-associated gene 5 (MDA5), RNA-regulated protein kinase (PKR), interferon response factor 3 (IRF3), among others. Several avian homologs of JAK/STAT and IFN-stimulated proteins have been identified, such as chOAS, Mx, interferon-induced helicase C domain-containing 1 (IFIH1) or chMDA5, PKR and cIRF3.Citation6,10-15
The IFN-α/β receptor (IFNAR) is a heterodimeric receptor composed of 2 subunits, the IFNAR1 and IFNAR2. Both are required to activate downstream JAK/STAT signaling pathways and for activation of the antiviral state.Citation7,16 Absence of α/β interferon receptor increases influenza viral replication in mouse embryonic fibroblasts, suggesting that IFN-α/β receptor provides protection against viral replication.Citation17 To determine if a deficiency in interferon signaling would enhance yields of influenza virus in PBS-12SF cells, we transiently suppressed expression of the IFNAR1 subunit via RNA interference. We evaluated mRNA abundance of IFN-stimulated genes in IFNAR1 knock-down cells and production of a human H1N1 influenza viral strain. We found that reduced IFN response in PBS-12SF cells due to knock-down of the IFNAR1 subunit enhanced production of a human influenza virus and correlated with decreased expression of known IFN responsive genes.
Results
Chicken IFN α increases the mRNA abundance of IFN-stimulated genes in PBS-12SF cells
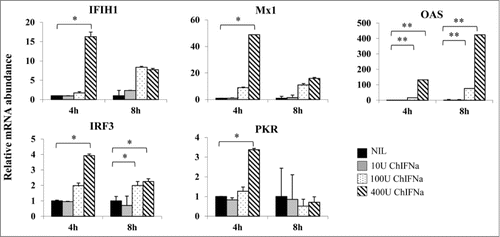
To determine if PBS-12SF cells responded to stimulation with chicken IFN α (chIFNα), the IFN response of 5 common IFN-stimulated genes was studied after incubation with increasing concentrations of chIFNα for 4 or 8 h. RNA was isolated, and qPCR was used to determine the mRNA abundance of the following genes: IFIH1, Mx1, OAS, IRF3, and PKR. All genes had an overall significant response (P < 0.05) to chIFNα treatment after 4 h with 400 U of chIFNα (). OAS had a higher response to chIFNα at 4 h, and its response continued increasing after 8 h. In contrast, Mx1, IRF3 and PKR mRNA abundance increased at 4 h, then decreased after 8 h. No significant differences were observed when 10 U of chIFNα was used (). From these results, it appeared that PBS-12SF cells indeed respond to IFNα and that OAS, Mx1, IFIH1, IRF3 and PKR can be used as molecular tags to study the IFN response in PBS-12SF cells.
Figure 1. Induction of antiviral gene expression in PBS-12SF cells by stimulation with different amounts of recombinant chicken IFNα (chIFNa). Total RNA was isolated from PBS-12SF cells after 4 and 8 h induction with 0, 10, 100 and 400U chIFNa. Cells were harvested at 4 and 8 h post induction. RNA was isolated for synthesis of cDNA. Abundance of various mRNAs was assessed using 30ng of cDNA by quantitative real-time RT-PCR (qPCR) analysis with SYBR Green. Genes studied included: IFIH1, Mx1, OAS, IRF3, and PKR. β-actin was used to normalize relative expression, and data was analyzed by the 2ΔΔCt method.Citation20 Mean values and standard deviations of 2 independent experiments are shown. Significance was calculated by Student's t test (*, P ≤ 0.05 and **, P ≤ 0.01).
Human influenza and avian-human influenza A reassortant virus induce IFNβ and activate the antiviral state in PBS-12SF cells
Once it was established that PBS-12SF cells were responsive to IFNα stimulation, we next determined whether influenza virus infection would stimulate the IFNα/β response in PBS-12SF cells. To accomplish this, cells were infected with a human A/NewCaledonia/20/1999 H1N1 or an avian-human reassortant VNH5N1-PR8/CDC-RG influenza strain. IFNα and IFNβ mRNA abundance was quantified by qPCR using specific primers () at 24, 48 and 72 h post-infection. We observed a robust IFN response to influenza virus infection in PBS-12SF cells, with particularly strong induction of IFNβ mRNA (). Although the reassortant VNH5N1 strain has been shown to successfully infect PBS-12SF cells,Citation5 its action on both interferon-α and -β mRNA abundance was not as marked as that of the H1N1 strain when compared to uninfected cells. Interestingly, upregulation of both interferons when compared to uninfected cells appeared to occur most dramatically at 24 h and again at 72 h, with a reduction or absence of this up-regulation at 48 h. When IFNα/IFNβ are produced by the cells, they bind to IFNAR on the cell surface and induce an antiviral state characterized by production of several antiviral proteins, such as OAS, Mx1, and IFIH1, among others, limiting viral replication. Based on that, the relative mRNA abundance of 3 chicken IFN-stimulated genes: OASL, Mx1 and IFIH1 was determined. We found that all 3 genes were significantly overexpressed in influenza infected cells when compared to uninfected control cells (), with H1N1-infected cells again showing a more dramatic response than H5N1-infected cells.
Figure 2. INF response and antiviral gene expression in PBS-12SF cells following infection with 2 influenza A viruses. Cells were infected with the indicated virus at an MOI of 0.1. Cells were harvested at 24 and 48 h post-infection, and RNA was isolated for synthesis of cDNA. Relative mRNA (mRNA) abundance was determined by qPCR using specific primers (see ) for IFNα, IFNβ, OAS, IFIH1, and Mx1 genes. Data was analyzed by the 2ΔΔCt methodCitation20 using β-actin as an endogenous control. Mean values and standard deviations of 3 independent experiments are shown. Significance was calculated by Student's t test (*, P ≤ 0.05 and **, P ≤ 0.01).
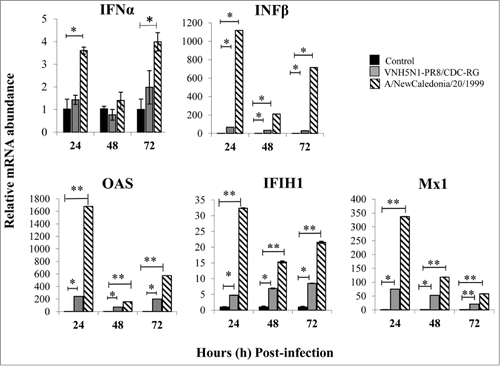
Table 1. Primers used for qPCR and shRNA sequences used for knocking down expression of IFNAR1
Knock-down of IFNAR1 down-regulates IFN-stimulated genes in PBS-12SF cells
Given that PBS-12SF cells demonstrated a robust response to IFNα and that influenza virus infection of these cells induced expression of IFNα and IFNβ, we reasoned that knock-down of IFNα/β receptor should reduce expression of IFN responsive genes. To neutralize the IFN response in PBS-12SF cells, we knocked-down IFNAR1 by using short hairpin RNA (shRNA) mediated RNA interference. To study the expression of the IFNAR1 protein in IFNAR1-shRNA expressing cells, a polyclonal anti-chicken IFNAR1 antibody was produced. Peptide-competition assays were performed to identify the specific band corresponding to chicken IFNAR1 on Western blots. A strong band of around 60 kDa was competed out with increasing amounts of peptide (), indicating that this was the specific band of chicken IFNAR1 protein from PBS-12SF lysates. Western blot and imaging densitometry analyses of the 3 IFNAR1-shRNA expressing cells showed a decrease in the expression of IFNAR1 protein when compared to control cells (). A second band of unknown identity was observed at ∼120 kD although displayed no effects of peptide competition.
Figure 3. Lentiviral-mediated shRNA silencing of IFNAR1 reduces the mRNA abundance of IFN stimulated genes (ISG) in PBS-12SF cells. (A) Characterization of IFNAR1 antibody by peptide-competition assay. PBS-12SF cellular lysates were loaded on a 12% acrylamide gel and transferred to a PDVF membrane for detection by Western blot. Competition was performed by pre-incubating rabbit polyclonal chicken IFNAR1 antibody with increasing amounts (0, 0.1, 0.5, 1 (not shown) or 10 μg) of the immunizing peptide prior to use in Western blotting. A band at approximately 60 kDa was competed out with increasing amounts of peptide (rectangular box). An additional band at approximately 120 kDa was not competed, suggesting this was a non-specific binding. (B) IFNAR1 expression was knocked down in PBS-12SF cells using 3 shRNAs against IFNAR1. Representative Western blot analyses for IFNAR1 in lysates derived from parental PBS-12SF cells and lentiviral-transduced PBS-12SF cells was performed. β-actin was used as a loading control. (C) The mean intensity of the IFNAR1 protein band in (B) expressed as a ratio of the intensity of β-actin. The results shown are the mean of 2 separate experiments. (D) IFNAR1 knockdown reduces the mRNA abundance of OAS, IRF3 and IFIH1 after 4 hours induction with 400U chIFNα. Mean values and standard deviations of 3 independent experiments are shown. Significance was calculated by Student's t test (*, P ≤ 0.05 and **, P ≤ 0.01).
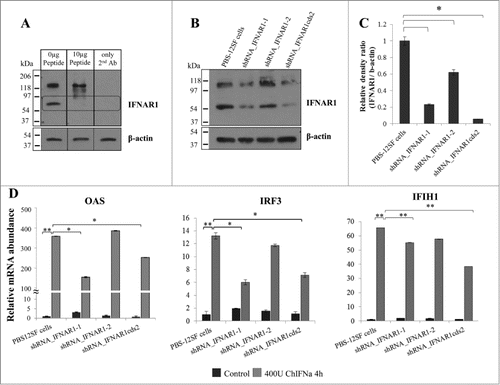
To study the IFN response in IFNAR1-shRNA expressing cells, cells were stimulated with chIFNα. Unstimulated cells of each type served as negative controls. PBS-12SF cells without shRNA were used as positive controls for chIFNα stimulation. The mRNA abundance of the IFN-stimulated genes: OAS, IRF3 and IFIH1 was determined by qPCR assay. We found that the relative abundance of OAS mRNA in shRNA_IFNAR1–1 and shRNA_IFNAR1cds2 cells was significantly lower (P < 0.05) than that observed in parental PBS-12SF cells stimulated with chIFNα (). Moreover, chIFNα induced IRF3 mRNA abundance was 2-fold lower in shRNA_IFNAR1–1 and shRNA_IFNAR1cds2 cells relative to parental PBS-12SF cells. Although IFIH1 gene expression had a downward trend in the 3 IFNAR1-shRNA expressing cells when compared to parental cells, these differences were not statistically significant (). The IFNAR1 protein was knocked down by 90% in shRNA_IFNAR1cds2 cells, and the response to chIFNα stimulation was significantly decreased when compared to the parental cell line (). However, there was a response to chIFNα in all IFNAR1-shRNA expressing cells, likely due to residual IFNAR1 expression.
Influenza virus production is greater in IFNAR1-knock-down PBS-12SF cells
We then determined whether the decreased response to chIFNα in IFNAR1 knock-down PBS-12SF cells had an effect on influenza virus production. Virus production in H1N1-infected cells was indirectly measured by hemaglutination (HA) assay in cell culture supernatants at 48 h P.I. The HA assay showed that IFNAR1-knock-down cells produced at least 2-fold more virus than parental PBS-12SF cells (). Additionally, the supernatant from shRNA_ IFNAR1cds2 contained the highest concentration of HA compared to other cells. We next analyzed levels of virus released into the supernatant by determining the copy number of the viral hemagglutinin gene at 48 h P.I. by qPCR. Supernatants from IFNAR1-knock-down PBS-12SF cells contained more influenza virus compared with supernatants from parental PBS-12SF cells (). Indeed, supernatants from shRNA_IFNAR1cds2 cells contained up to 2-logs (100-fold) more hemagglutinin gene copies than parental PBS-12SF cells.
Figure 4. Comparison of the mRNA abundance of hemaglutinin gene in H1N1-infected parental PBS-12SF cells and PBS-12SF cells transduced with IFNAR1-shRNA lentiviruses. Cells were infected at a MOI of 0.1 for 48 h, and supernatants were collected. Viral RNA was purified and qPCR was performed to detect the viral hemaglutinin cDNA. The hemaglutinin gene copy number per 140 µL of culture supernatant is represented in . Mean values and standard deviations of 3 independent experiments are shown. Significance was calculated by Student's t test (*, P ≤ 0.05).
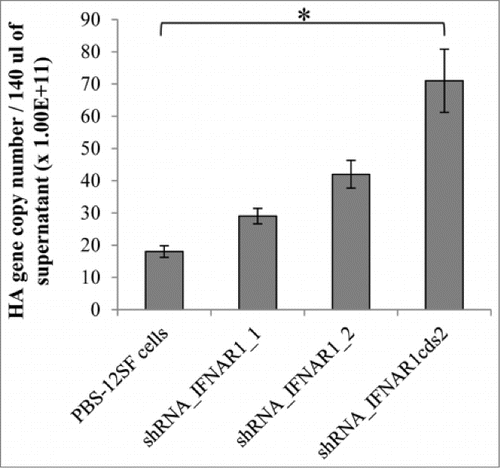
Table 2. Hemagglutination capability was compared in the human influenza virus strain A/NewCaledonia/20/1999 H1N1-infected PBS-12SF and PBS-12SF-IFNAR1-shRNA expressing cells. Cells were infected with influenza at a MOI of 0.1 Supernatants were collected at 48h post-infection, and viral titer measurements were determined
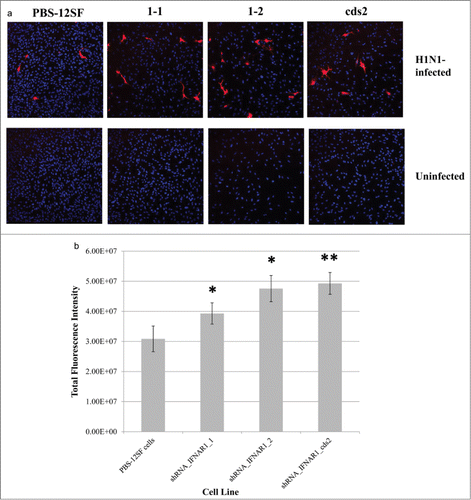
Immunostaining of Influenza A H1N1 nucleoprotein (NP) yielded significantly higher average fluorescence intensity in all 3 lines of PBS-12SF cells with expression of IFNAR1 knocked down than the parental PBS-12SF cell line when infected with influenza A/NewCaledonia/20/1999 for 24 h (). The shRNA_IFNAR1cds2 cell line exhibited the greatest average intensity, consistent with hemaglutinin gene copy number and HA assay results. There was no non-specific staining in the uninfected cell cultures (). Other controls included infected and uninfected cells incubated with primary antibody only or without antibodies. There was no non-specific staining in any of these other controls (data not shown). These results further demonstrate that influenza production is greater in IFNAR1 shRNA PBS-12SF cell lines.
Figure 5. Immunofluorescence of H1N1-infected parental PBS-12SF cells and PBS-12SF cells transduced with 3 separate IFNAR1 shRNA expressing lentiviral vectors. (A) Parental PBS-12SF cells and IFNAR1-shRNA cells were stained with DAPI (blue) and anti-Influenza A H1N1 nucleoprotein (red) as described in Materials and Methods. Images are representative of 10 fields from infected cells and 3 fields from uninfected cells. Imaging was performed at 20× with an Olympus FluoView FV1000 confocal laser scanning microscope. (B) Relative fluorescence in PBS-12SF cells and in PBS-12SF cells transduced with 3 IFNAR1 shRNA expressing lentiviral vectors. Fluorescence values are sums of regions of interests drawn around red-fluorescing cells in 10 fields from each infected slide chamber depicted in (C). An * indicates P < 0.05 and ** indicates P < 0.01.
Discussion
Influenza is one of the most significant infectious diseases in humans, and there has been an increased demand for seasonal influenza vaccine over the past 25 y. During the last 2009 H1N1 pandemic, the traditional egg-based vaccine production system was unable to provide sufficient vaccine in a timely manner because of limitations in both capacity and egg supply. The use of continuous cell lines for vaccine production is advantageous because of reduced downstream processing, reduced production space, and ease of expansion during high seasonal demand or in the event of an emerging rapidly spreading pandemic.
We have previously demonstrated that the immortalized non-tumorigenic chick embryo cell line, termed PBS-12SF, supports replication of unmodified recent isolates of human and avian influenza A viruses to high titers (>107 PFU/ml) without exogenous proteases or serum proteins.Citation5,6 These 2 characteristics are highly desirable because they reduce the risk of contamination as well as simplify downstream purification processes.Citation18 Nevertheless, immunocytochemistry analysis of PBS-12SF cells infected with influenza A virus revealed that ∼40% of cells remained uninfected in any production cycle (data not shown). It is well known that the first cellular defense against viral infection is the interferon (IFN) response, typified by production of interferon α and β (IFNα/IFNβ). In this report, we demonstrated that PBS-12SF cells robustly respond to recombinant chIFNα by upregulation of IFN response genes. In addition, PBS-12SF cells responded to infection with human H1N1 and avian-human reassortant H5N1 influenza strains with enhanced expression of IFNα and IFNβ despite the ability of influenza viral proteins such as non-structural protein 1 (NS1) to disrupt antiviral interferon signaling by multiple mechanisms.Citation19 There was also a concomitant upregulation of IFN-stimulated genes following influenza virus infection of PBS-12SF cells (). We reasoned that the robust IFN response observed in PBS-12SF cells may reduce influenza virus production within infected cells and protect uninfected cells in the culture from viral infection, thereby limiting viral replication. In support of this, Jiang et al. showed that pretreatment with chicken IFNα before AIV infection significantly reduced viral replication in chicken-origin lung cells, demonstrating the important role of IFNα in controlling viral replication in chickens.Citation8 IFNα/IFNβ bind to the IFNα/β receptor (IFNα/βR) on the cell surface and induce an antiviral state by up-regulating genes with direct and indirect antiviral functions. In this report, we neutralized the IFN response in PBS-12SF cells by knocking down expression of the IFNAR1 subunit of the IFNα/βR and examined the effect of dramatically reduced IFNAR1 expression on the response to recombinant chIFNα and on influenza viral production.
All knockdown cell lines exhibited an increase in dividing time when compared to the parental cell line. The shRNA_IFNAR1–1 and 1–2 lines took approximately 1.5 times longer than the PBS-12SF line to double, while the shRNA_IFNAR1cds2 line grew the slowest, taking approximately twice as long. Concomitantly, the cds2 line's IFNAR1 protein was knocked-down to the greatest degree (See ). Knockdown cell lines showed greater cytopathic effect (greater number of floating cells/less dense monolayer, “bubbly” appearance, lysed cells) during H1N1 infections when observed visually. No other morphological changes between the parental line and the knockdown lines were observed.
Our data clearly demonstrated that knocking down expression of the IFNAR1 subunit from the IFNα/β receptor had a significant effect on expression of IFN-stimulated genes in the PBS-12SF cell line (). Because approximately 20% of the IFNAR1 subunit was still present in the knock-down cells, some response to chIFNα was still observed. Accordingly, with lower expression of IFN-stimulated genes in the IFNAR1 knock-down cells, influenza H1N1 production was higher in all IFNAR1 shRNA-PBS-12SF cell cultures as determined by HA assay of cell culture supernatants, by the copy number of the hemagglutinin gene using qPCR, and by immunofluorescent staining. This suggests that the anti-viral mechanisms controlled by IFN are limited in the IFNAR1 knock-down PBS-12SF cells, allowing more viral particles to be released to the cell culture supernatant. Recently, Goodman et al, observed increased influenza viral replication in mouse embryonic fibroblasts lacking the IFNα/β receptor,Citation17 supporting the importance of the IFNα/β receptor in controlling viral replication. To our knowledge, this is the first report demonstrating that a decrease in abundance of the IFNAR1 subunit of the IFNα/β receptor, affects the IFN response and increases viral production in immortalized chick derived cells.
In summary, our results demonstrate that neutralizing the IFN response in PBS-12SF cell improved the production of influenza virus and offer a viable alternative system for the production of both avian and human influenza virus vaccines.
Material and Methods
Cells
Chick embryo cell line PBS-12SF was derived from parental PBS-1 cells. PBS-1 cells were initially derived from the CHCC-OU2 line as a more rapidly dividing subpopulation susceptible to a wide range of viruses, including influenza.Citation5,6 PBS-1 cells were adapted to animal product-free growth through sequential replacement of complete media with certified animal product-free medium as described.Citation5 Cells were grown and maintained in OptiPRO serum-free medium supplemented with 20 mM/mL GlutaMAX-1, 100 IU/ml of penicillin, 100 µg/mL of streptomycin and 2.5 µg/mL fungizone (Gibco, Life Technologies), also called complete media, at 37°C in a 5% CO2 incubator. For continued passages in culture, PBS-12SF cells are removed from plates using a synthetic trypsin (TrypLE™ Express,1X, Gibco®), counted using a hemacytometer and re-plated to approximately 60% confluency.
Viruses and virus infection
The following virus strains were used: a human influenza virus strain A/NewCaledonia/20/1999 obtained from ATCC and a reassortant vaccine virus VNH5N1-PR8-CDC-RG, which was kindly provided by Dr. Ruben O. Donis from the Center for Disease Control and Prevention (CDC). All infections were performed using multiplicity of infection (MOI) of 0.1 (one virus per 10 cells), and were performed in biosafety level 2 (BSL2) facilities.
Chemicals and antibodies
The immunization peptide was selected by ProSci, Inc.. from the chicken IFNAR1 sequence using a proprietary algorithm to find regions that maximize hydrophilicity, antigenicity, and surface probability but excluded regions that did not contain turns or that contain glycosylation sites. The selected sequence began at amino acid 150 of the chicken IFNAR1 protein with an N-terminal cysteine for conjugation to KLH (Residue 150: C-KINISPPEANQVRK). The polyclonal rabbit-derived IFNAR1-specific antibody was also produced by Prosci Inc.. Recombinant chicken Interferon α (chIFNa) was purchased from AbD Serotec.
Reverse transcriptase quantitative real-time PCR
Cells were lysed to isolate RNA using PerfectPure RNA cell culture kits (5 Prime). Culture supernatants were used to isolate viral RNA (QIAamp Viral RNA Mini Kit, QIAGEN). cDNA (cDNA) was synthesized using oligo-dT or influenza hemmaglutinin specific primers (). Quantitative real-time PCR (qPCR) was used to determine the mRNA (mRNA) abundance of IFNα, INFβ, and the IFN-stimulated genes: OAS, Mx1, IFIH1, PKR, and IRF3 (see sequence of primers in ). The specificity of all primers was verified using NCBI BLAST. Data from qPCR was analyzed by the 2ΔΔCt method.Citation20 β-actin was utilized as the endogenous control gene, and its expression was unchanged across treatments (data not shown). The copy number of influenza hemmaglutinin gene was determined by using specific HA primers () and compared to a standard curve derived using a cloned HA gene. Viral RNA was purified from the supernatant, and 10 µL of RNA was used to synthesize cDNA. Three µL was used for the qPCR.
RNA interference–mediated silencing
Interferon α receptor 1 (IFNAR1) knock-down was achieved by transducing cells with lentiviral particles containing IFNAR1-short hairpin RNA (shRNA)-expressing plasmids. Three short RNA sequences, termed IFNAR1–1, IFNAR1–2, IFNAR1cds2, which are specific and complementary to the IFNAR1 mRNA, were commercially designed, synthesized, and inserted into expression plasmids (Sigma Aldrich). IFNAR1-shRNA-containing plasmids were then co-transfected with lentiviral packaging and envelope plasmids to produce lentiviral particles. Lentiviral particles were designed and produced by Sigma-Aldrich. ShRNA sequences are detailed in . PBS-12SF cells were plated in 96 well plates at 104 cells/well. After 24 h, cells were transduced with various multiplicities of infection (MOI) 1, 2.5, 5, 10 and 20, in order to determine the optimal ratio. After 48 h, transduced cells were selected by using 400µg/mL of geneticin (G418).
Peptide competition assay and Western blot analyses
Cellular lysates were collected using RIPA buffer (Cell Signaling Technology) supplemented with protease inhibitors. Proteins (80 µg/lane) were loaded on a 12% acrylamide gel and transferred to a polyvinylidene difluoride (PVDF) membrane for detection by Western blot. The peptide-competition assay was performed by pre-incubating chicken IFNAR1 antibody with increasing amounts (0, 0.1, 0.5, 1 or 10 μg) of the immunizing peptide prior to use in Western blots. Equal loading was monitored by detection of β-actin. Detection of bound secondary antibody was performed by using ECL Super Signal West Pico chemiluminescent substrate (Thermo Scientific). Densitometry analyses of Western blots were performed using a BioRad Model GS-700 imaging densitometer and Multi-Analyst®/PC Software.
Hemagglutination assay
The HA assay, an indirect measure of number of viral particles, was performed by 2-fold serial dilution of 100 µL of culture supernatant with PBS in V-bottom plates. An equal volume (100 µL) of 0.5% chicken red blood cells (Innovative Research Inc..) was then added to each well. The plates were incubated at room temperature for 1h, and the virus HA titers were compared visually. Results are presented as hemagglutination units (HAU) per mL of reaction.
Immunostaining and imaging
Cells (parental PBS-12SF, shRNA_IFNAR1 knock-down lines 1–1, 1–2, and cds2) were seeded at a density of 1.8 × 105 cells/chamber in BioCoat fibronectin-coated tissue culture slides (Becton Dickinson Biosciences) and allowed to grow for 48 h. Media was replaced with conditioned complete media at 24 h. Cells were then either infected with A/New Caledonia/20/1999 at MOI 0.1 or provided fresh media. At 24 h post-infection, media was removed, and cells were washed with 1 × PBS then fixed with 4% formaldehyde for 10 min. Fixative was removed and cells washed with 1 × PBS. Cells were then permeabilized with 0.5% Triton for 30 s and washed 3 times with 1 × PBS. Blocking was performed with fresh 3% bovine serum albumin (BSA) for 30 min. Blocking buffer was then replaced with a mouse anti-influenza A nucleoprotein (NP) primary antibody (Hytest) at a dilution of 1:500 and incubated for 60 min. Chambers were washed 3 times with 1 × PBS and incubated with secondary antibody (Vector Laboratories DyLight anti-mouse IgG 594) at 1:1000 a dilution for 30 min in the dark. Chambers were washed 3 times with 1 × PBS and then incubated with NucBlue DAPI stain (Molecular Probes) for 5 min. Coverslips were mounted using Vectashield HardSet Mounting Medium (Vector Laboratories). Imaging was performed at 20× using an Olympus FluoView FV1000 confocal laser scanning microscope. Ten fields were collected from each infected sample and 3 fields were collected from each uninfected sample. Regions of interests were drawn around red-fluorescing cells and fluorescence intensities exported as .csv files. Sums of the regions of interest for each field were averaged across fields. Red:blue fluorescence intensity ratios were examined to normalize data to cell number with identical results and significance as exhibited in (data not shown).
Statistics
Student's t-test was used to identify differences between data sets. Data on graphs are reported as mean ±SEM (standard error of the mean). P values <0.05 were considered to be significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors wish to thank Ms. Sue Sipkovsky for excellent technical support. We also extend our gratitude for support to Mr. Thomas Herlache of the MSU Technologies office and Dr. Don Anderson of the MSU Office of the Vice President for Research.
Funding
We acknowledge funding through the MSU Targeted Support for Technology Development program.
References
- World Health Organization (WHO). Fact sheet 211. 2014
- Genzel Y, Reichl U. Continuous cell lines as a production system for influenza vaccines. Expert Rev Vaccines 2009; 8:1681-92; PMID:19943763; http://dx.doi.org/10.1586/erv.09.128
- Vlecken DH, Pelgrim RP, Ruminski S, Bakker WA, van der Pol LA. Comparison of initial feasibility of host cell lines for viral vaccine production. J Virol Methods 2013; 193:28-41; PMID:23684847; http://dx.doi.org/10.1016/j.jviromet.2013.04.020
- Center for Disease Control (CDC). 2012
- Coussens PM, Smith KA, Weber PS, Colvin CJ. Immortalized chick embryo cell line adapted to serum-free growth conditions and capable of replicating human and reassortant H5N1 influenza strains for vaccine production. Vaccine 2011; 29:8661-8; PMID:21911025; http://dx.doi.org/10.1016/j.vaccine.2011.08.122
- Smith KA, Colvin CJ, Weber PS, Spatz SJ, Coussens PM. High titer growth of human and avian influenza viruses in an immortalized chick embryo cell line without the need for exogenous proteases. Vaccine 2008; 26:3778-82; PMID:18524432; http://dx.doi.org/10.1016/j.vaccine.2008.04.048
- Uze G, Schreiber G, Piehler J, Pellegrini S. The receptor of the type I interferon family. Curr Top Microbiol Immunol 2007; 316:71-95; PMID:17969444
- Jiang H, Yang H, Kapczynski DR. Chicken interferon α pretreatment reduces virus replication of pandemic H1N1 and H5N9 avian influenza viruses in lung cell cultures from different avian species. Virol J 2011; 8:447; PMID:21939525; http://dx.doi.org/10.1186/1743-422X-8-447
- Koerner I, Kochs G, Kalinke U, Weiss S, Staeheli P. Protective role of β interferon in host defense against influenza A virus. J Virol 2007; 81:2025-30; PMID:17151098; http://dx.doi.org/10.1128/JVI.01718-06
- Grant CE, Vasa MZ, Deeley RG. cIRF-3, a new member of the interferon regulatory factor (IRF) family that is rapidly and transiently induced by dsRNA. Nucleic Acids Res 1995; 23:2137-46; PMID:7541908; http://dx.doi.org/10.1093/nar/23.12.2137
- Ko JH, Asano A, Kon Y, Watanabe T, Agui T. Characterization of the chicken PKR: polymorphism of the gene and antiviral activity against vesicular stomatitis virus. Jpn J Vet Res 2004; 51:123-33; PMID:15070037
- Liniger M, Summerfield A, Zimmer G, McCullough KC, Ruggli N. Chicken cells sense influenza A virus infection through MDA5 and CARDIF signaling involving LGP2. J Virol 2012; 86:705-17; PMID:22072756; http://dx.doi.org/10.1128/JVI.00742-11
- Potts JD, Kornacker S, Beebe DC. Activation of the Jak-STAT-signaling pathway in embryonic lens cells. Dev Biol 1998; 204:277-92; PMID:9851859; http://dx.doi.org/10.1006/dbio.1998.9077
- Schumacher B, Bernasconi D, Schultz U, Staeheli P. The chicken Mx promoter contains an ISRE motif and confers interferon inducibility to a reporter gene in chick and monkey cells. Virology 1994; 203:144-8; PMID:7518167; http://dx.doi.org/10.1006/viro.1994.1464
- Tatsumi R, Hamada K, Sekiya S, Wakamatsu M, Namikawa T, Mizutani M, Sokawa Y. 2′,5′-oligoadenylate synthetase gene in chicken: gene structure, distribution of alleles and their expression. Biochim Biophys Acta 2000; 1494:263-8; PMID:11121584; http://dx.doi.org/10.1016/S0167-4781(00)00174-3
- de Weerd NA, Nguyen T. The interferons and their receptors–distribution and regulation. Immunol Cell Biol 2012; 90:483-91; PMID:22410872; http://dx.doi.org/10.1038/icb.2012.9
- Goodman AG, Zeng H, Proll SC, Peng X, Cilloniz C, Carter VS, Korth MJ, Tumpey TM, Katze MG. The α/β interferon receptor provides protection against influenza virus replication but is dispensable for inflammatory response signaling. J Virol 2010; 84:2027-37; PMID:19939913; http://dx.doi.org/10.1128/JVI.01595-09
- Genzel Y, Fischer M, Reichl U. Serum-free influenza virus production avoiding washing steps and medium exchange in large-scale microcarrier culture. Vaccine 2006; 24:3261-72; PMID:16472544; http://dx.doi.org/10.1016/j.vaccine.2006.01.019
- Jia D, Rahbar R, Chan RW, Lee SM, Chan MC, Wang BX, Baker DP, Sun B, Peiris JS, Nicholls JM, et al. Influenza virus non-structural protein 1 (NS1) disrupts interferon signaling. PloS one 2010; 5:e13927; PMID:21085662; http://dx.doi.org/10.1371/journal.pone.0013927
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402-8; PMID:11846609; http://dx.doi.org/10.1006/meth.2001.1262
