Since 1984, the European Union (EU) has been funding fundamental, applied or clinical research in all life science disciplines through its multiannual framework programmes for research and technological development (FP). The funding opportunities are fostered by different instruments () and range from cooperative projects involving researchers from multiple countries, disciplines and types of organizations (academic, industry, regulators, patients' organizations, etc.) to individual research actions, infrastructure development and training activities. It also includes dedicated instruments for small and medium-size enterprises (SMEs) as well as public-public and public-private partnerships. In this paper, we focus on the support to the vaccine and immunotherapy research performed during the 7th Framework Program (FP7, 2007–2013) and discuss the current and future opportunities of the new EU funding program for research and innovation, Horizon 2020 (2014–2020).Citation1
Table 1. FP7 and Horizon 2020 instruments for research funding
More than €148 million was allocated to 73 FP7 immunotherapy projects. Nineteen of those were collaborative (half of them included at least one clinical trial), amounting to €117 million. The remaining €31 million include individual grants from the European Research Council (ERC) and the Marie Sklodowska-Curie (MSC) training actions. The type of diseases targeted ranged from cancer, diabetes or allergies, to rare diseases. Typically, the projects developed new advanced technologies such as refined vectors for the production of genetically engineered cells, DNA and RNA-based vaccines, or new types of antibodies. Illustrative examples include the projects ATTACK (Adoptive engineered T-cell trials to achieve cancer killingCitation2) and Cell-PID (Advanced cell-based therapies for the treatment of primary immunodeficiencyCitation3).
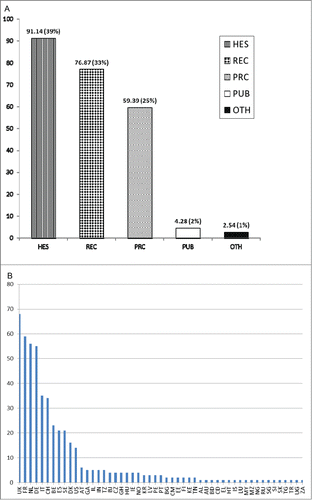
The project ADITEC (Advanced Immunization Technologies) is a significant example of a FP7 collaborative project in the vaccine field.Citation4 This 5-years high impact project, receiving a €30 million EU contribution, integrates all the required expertise in immunization research (adjuvants, vectors, antigens, delivery systems, animal models, clinical trials) to study human immune responses under conditions of health and disease. ADITEC gathers 43 partners (28 academic, 13 SMEs and 2 pharmaceutical companies) from 13 different countries including the USA. After only 3 years, 9 clinical trials have been conducted or are on-going, and the project has so far delivered more than 110 peer reviewed publications (more than 70 in 2014). ADITEC is part of a large portfolio of FP7 research projects developing vaccines for infectious diseases. Overall in FP7 investments in vaccine research and development reached on average €40 million per year, with a total EU contribution of more than €317 million for the period. The majority of the funds (€234 million) was allocated to 43 collaborative projects (a third of which were performing clinical trials) dealing with antimicrobial resistance (n = 5, €25.5 million), emerging epidemics (n = 10, €41.5 million), poverty-related diseases (malaria, tuberculosis and HIV/AIDS, n = 16, €82.7 million), neglected infectious diseases (n = 9, €42.4 million) and cross-infectious diseases combining more than one of the former categories (n = 3, €42.2 million). A sector analysis of the participants revealed that higher education, research organizations and private for-profit organizations are highly represented in this field () and that the United Kingdom, France, The Netherlands and Germany are the most successful applicants in this area (). The remaining funds (€83 million) related to other specific programmes such as the ERC and the MSC grants, and the Innovative Medicines Initiative (IMICitation5). Indeed, the European Commission has explored new ways in engaging and creating original public-private (IMI) and public-public (the European and Developing Countries Clinical Trials Partnership, EDCTPCitation6) partnerships in order to stimulate the development and the testing of new vaccines and medicinal products.
Figure 1. (A) Sum of EU contribution (in €million) per participants organization type (%) in FP7 collaborative vaccine projects for infectious diseases. Legend: HES, higher or secondary education; REC, research organization; PRC, private for profit (excluding education); PUB, public body; OTH, others. (B): Number of participants per country in 43 FP7 collaborative vaccine projects for infectious diseases.
Created in 2003 as a European response to the global health crisis caused by the 3 main poverty-related diseases HIV/AIDS, tuberculosis and malaria, the EDCTP aimed to accelerate the development of new or improved drugs, vaccines, microbicides and diagnostics against these 3 diseases, with a focus on clinical trials and capacity building in sub-Saharan Africa. Between 2003 and 2013, the EDCTP supported 26 research projects on vaccines, for a total of more than €64 million. With an EU contribution of €683 million for the next 10 y (2014–2023), complemented with a similar amount coming from the European Member States, for a total of almost €1.4 billion, the new phase of the program, EDCTP2, will enlarge its program to also include neglected infectious diseases, such as dengue, rabies, African trypanosomiasis or leishmaniases. All stages of clinical trials will be funded, though the main focus will remain on phase II and phase III trials.
IMI, created in 2009 and now in its second program (IMI2), is Europe's largest public-private partnership. This joint initiative between the European Commission and the European Federation of Pharmaceutical Industries and Associations (EFPIA) aims to promote the development of new, improved diagnostics and therapies for patients. IMI2, with a budget of €3.3 billion for the period 2014–2024, has just published its first calls for Horizon 2020. Vaccine research is one of the overarching priorities, as reflected by the recent launch of a €215 million call for proposals to boost research on Ebola.Citation7 Half of the funds for this call will come from Horizon 2020 and the other half from the pharmaceutical companies that are members of EFPIA. Eight projects dealing with vaccine development (VSV-Ebovac, Ebovac 1 and 2), vaccine manufacture capability (Eboman), deployment of and compliance with vaccination regimens (Ebodac) and rapid diagnostic tests (Mofina, Filodiag and Ebola Modrad) were funded.
Two previous projects on vaccines, BioVacSafe (Biomarkers for enhanced vaccine immunosafety) and Advance (Accelerated development of vaccine benefit-risk collaboration in Europe) have already been funded, for a cumulative EU contribution of over €22 million, with an additional €19 million coming from the private sector partners. While still in the preparatory phases, the next relevant projects in IMI will address zoonoses anticipation and preparedness, consistency approaches to quality control in vaccine manufacture, and pertussis vaccination.
Horizon 2020 is addressing the entire innovation cycle, from basic research to implementation in order to drive economic growth and to enhance job creation. Horizon 2020 has 3 main pillars dedicated to support Excellence in Science, Industrial Leadership and Societal Challenges. The first calls for proposals 2014–2015 in the societal challenge “Health, demographic change and wellbeing” were published in December 2013. Applicants were asked to focus on problem solving, and therefore vaccine or immunotherapy projects could find funding opportunities in various topics not specifically advertising vaccine or immunotherapy research as such. As an example, the topics PHC-16 “Tools and technologies for advanced therapies” and PHC-13 “New therapies for chronic non-communicable diseases” offered interesting opportunities. These calls are now closed but other opportunities will be published in the 2016–2017 program. The recent Ebola crisis led the EU to quickly mobilize €24.4 million from Horizon 2020 via an exceptional procedure, without a call for proposals.Citation8 Among the 5 successful proposals, one includes a trial of the most advanced vaccine against Ebola developed by GlaxoSmithKline and 2 proposals that deal with the passive administration of antibodies. All the projects started on November 1st, 2014.
Notably, Horizon 2020 is open to participation of entities based in the USA due to a mutual agreement between the European Commission and the NIH.Citation9 In addition to the collaborative projects of the third pillar, “societal challenges,” the first pillar of Horizon 2020 offers opportunities for frontier research science via its ERC grants,Citation10 for international training networks via MSC actionsCitation11 and for the development of international infrastructure platforms and key enabling technologies. The second pillar will ensure support to research and innovation performers, including significant, tailored support to SMEs for which a dedicated instrumentCitation12 has been developed.
In conclusion, Horizon 2020 offers an even broader scope of funding opportunities for research in the field of vaccines and immunotherapy for the period 2014–2020.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed. The views expressed in this publication are the sole responsibility of the authors and do not necessarily reflect the views of the European Commission. Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use which might be made of the following information.
References
- Home - Research Participant Portal - European Commission [Internet]. Brussels (BE): funding opportunities-horizon 2020 manual. 2015- [cited 2015 June 17]. Available at: http://ec.europa.eu/research/participants/portal/desktop/en/opportunities/h2020/index.html.
- Attack Cancer. Adoptive engineered T cell trials to achieve cancer killing [Internet]. 2013- [cited 2015 June 17]. Available at: http://www.attack-cancer.eu.
- Cell-PID Network. Advanced cell-based therapies for the treatment of primary immunodeficiency. [Internet] 2011- [cited 2015 June 17]. Available at: http://www.cell-pid.eu.
- ADITEC. Advanced immunization technologies. [Internet] 2014- [cited 2015 June 17]. Available at: http://www.aditecproject.eu.
- The Innovative Medicines Initiative. [Internet] 2010-[cited 2015 June 17]. Available at: http://www.imi.europa.eu.
- EDCTP: Home. The European & developing countries clinical trials partnership [Internet] 2014- [cited 2015 June 17]. Available at: http://www.edctp.org.
- The Innovative Medicines Initiative. [Internet] 2010-[cited 2015 June 17]. Available at: http://www.imi.europa.eu/content/ebola-programme.
- EDCTP: Home. The European & developing countries clinical trials partnership [Internet] 2014- [cited 2015 June 17]. Available at: http://www.edctp.org.
- Zerhouni EA, Potocnik J. European union and NIH collaborate (Letters). Science 2008; 322:1048; PMID:18974314; http://dx.doi.org/10.1126/science.1167667
- The European Research Council. Funding and grants. [Internet] 2014- [cited 2015 June 17] Available at: http://erc.europa.eu/funding-and-grants.
- Horizon 2020. Marie sklodowska-curie actions. [Internet] 2014- [cited 2015 June 17]. Available at: http://ec.europa.eu/programmes/horizon2020/en/h2020-section/marie-sk%C5%82odowska-curie-actions.
- Home - Research Participant Portal - European Commission [Internet]. Brussels (BE): funding opportunities-horizon 2020 manual. 2014- [cited 2015 June 17]. HORIZON 2020 DEDICATED SME INSTRUMENT. Available at: http://ec.europa.eu/research/participants/portal/desktop/en/opportunities/h2020/calls/h2020-smeinst-2-2014.html.
