Abstract
Options for managing herpes zoster (HZ)-related pain and complications have limited effectiveness, making HZ prevention through vaccination an important strategy. Limited data are available on HZ vaccine effectiveness against confirmed HZ and manifestations of HZ among vaccinated persons. We conducted a matched case-control study to assess HZ vaccine effectiveness for prevention of HZ and other HZ-related outcomes and a cohort study of persons with HZ to compare HZ-related outcomes by vaccination status. Cases were identified through active surveillance among persons age ≥60 years with HZ onset and health-care encounters during 2010-2011 in Southeastern Minnesota. Controls were age- and sex-matched to cases. Data were collected by medical record review and from participants via interviews and daily pain diaries. 266 HZ case-patients and 362 matched controls were enrolled in the vaccine effectiveness studies and 303 case-patients in the cohort study of HZ characteristics by vaccination status. Vaccination was associated with 54% (95% CI:32%-69%) reduction in HZ incidence, 58% (95% CI:31%-75%) reduction in HZ prodromal symptoms, and 70% (95% CI:33%-87%) reduction in medically-attended prodrome. HZ vaccine was statistically significant effective at preventing postherpetic neuralgia (PHN) measured at 30 d after rash onset, 61% (95% CI: 22%-80%). Among persons who developed HZ, no differences were found by vaccination status in severity or duration of HZ pain after rash onset. In this population-based study, HZ vaccination was associated with >50% reduction in HZ, HZ prodrome, and medically-attended prodrome.
Introduction
Herpes zoster (HZ), a localized painful cutaneous rash, is caused by reactivation of latent varicella-zoster virus and occurs most commonly after age 50 years. Almost 1 in 3 persons will develop HZ during their lifetime.Citation1 HZ significantly impacts on health, quality of life and medical costs;Citation1-3 the impacts increase with age.Citation2,4-6 The primary burden of HZ is pain.Citation7,8 HZ pain typically occurs acutely but can persist after the rash resolves (postherpertic neuralgia [PHN]); 8% to 27% of HZ patients report pain lasting at least 3 months following rash onset.Citation9 One often-overlooked component of acute HZ is the pain that occurs before rash onset (prodromal pain) which often leads to substantial anxiety for patients and extensive diagnostic evaluations.Citation1,7,8
Options for managing HZ-related pain and complications have limited effectiveness,Citation7 making HZ prevention through vaccination an important strategy.Citation10,11 An HZ vaccine is available and recommended in the United States for use in immune competent adults age ≥60 years.Citation12 The HZ vaccine prelicensure clinical trial found a 51% reduction in HZ and 67% reduction in PHN (pain ≥90 d after rash onset), among vaccinated adults aged ≥60 years.Citation13
Clinical trials usually yield higher vaccine performance estimates (efficacy) because they are conducted under ideal conditions therefore evaluation of vaccine performance in conditions of routine clinical practice (effectiveness) is needed.Citation14 To date, only 2 estimates of HZ vaccine effectiveness have been published.Citation15,16 Both studies used electronic-billing data without medical record confirmation which can result in misclassification of both exposure (vaccination status) and outcome (occurrence of HZ).Citation1,17 Furthermore, neither provided clinical information on HZ characteristics among vaccinated persons, precluding a complete description of the vaccine's impact. We evaluate HZ vaccine performance in a community setting using medical record review to confirm HZ vaccination and case status and compare the characteristics of HZ by vaccination status.
Results
Vaccine effectiveness
A total of 266 case-patients age ≥60 years with confirmed HZ and vaccination status and 362 matched controls were included in the HZ vaccine effectiveness study (). More than half of the participants were female and age ≥70 years. Case-patients were more likely than controls to be immunocompromised at the time of HZ diagnosis/index date. Controls were more likely than case-patients to have received HZ vaccine (46% vs. 28%, P < 0.0001). Characteristics of participants in the HZ-related outcome studies were similar (Tables S1W-S5W).
Table 1. Characteristics of participants in case-control study of Herpes Zoster (HZ) vaccine effectiveness
Overall, HZ vaccine effectiveness for preventing HZ was 54% (95% CI: 32%-69%) after adjusting for age, sex, immunocompromised status, asthma, and arthritis, with 67% effectiveness when vaccination occurred before age 70 years and 38% with vaccination at age ≥70 years. In sub-analyses, HZ vaccination was associated with a 58% reduction in HZ prodromal symptoms, a 70% reduction in medically-attended prodrome and reductions of 61%, 69% and 55% in PHN measured at 30, 60 and 90 d after rash onset, respectively. For some estimates, confidence intervals are wide, reflecting small number of participants in the respective sub-analysis ().
Table 2. Vaccine effectiveness against Herpes Zoster (HZ) overall and by age at vaccination and against other HZ-related outcomes
HZ characteristics by vaccination status
Of the 303 case-patients in the cohort study of HZ characteristics by vaccination status, 79 (26%) were vaccinated. Unvaccinated case-patients were younger than vaccinated case-patients (Table S6W). Antiviral medications were prescribed within 3 d of rash onset for 82% of vaccinated and unvaccinated case-patients.
Of 290 case-patients with information regarding symptoms before rash onset, 202 (70%) reported pain or discomfort before rash onset. In a multivariable analysis that included age, sex, vaccination status, and immunocompromised status, risk of and median duration of prodromal symptoms and the proportion who sought medical care for prodromal symptoms were lower in vaccinated case-patients, with the difference approaching statistical significance (). Severity of prodromal symptoms was significantly milder in vaccinated case-patients (median, 5.0 vs. 2.5, respectively, p = 0.02).
Table 3. Herpes Zoster (HZ) prodrome characteristics and non-pain complications by vaccination status
There were no differences in the dermatomal distribution of HZ (including ophthalmic, 14% of unvaccinated vs. 12% of vaccinated patients) or in rates of complications by vaccination status (). No hospitalizations or disseminated disease occurred.
Pain diaries were submitted by 261 (86%) case-patients; they had similar characteristics to patients enrolled in the cohort study (Table S7W). We used multiple analytic approaches to compare HZ pain severity and duration as a function of vaccination status among HZ case-patients, and found no differences (, ) with one exception: lower pain scores for vaccinated case-patients age 60-69 years at 30 d after rash onset compared with same age unvaccinated case-patients ().
Figure 1. Comparison of pain scores over time by vaccination status: All participants (A) and by age group (B). * P < 0.05 for the difference in pain scores. Pain scores estimated using growth curve analyses. Participants had a median of 76 d of pain diary entries (range: 1, 98, IQR: 65, 82) with no significant differences in first or last day of diary entry or number of pain diary entries by vaccination status (Wilcoxon rank sum test). The median day of the first recorded pain score was day 12 (range: 0, 41, IQR: 8, 19) and the median day for the last diary entry was day 91 (range: 12, 117, IQR: 87, 93).
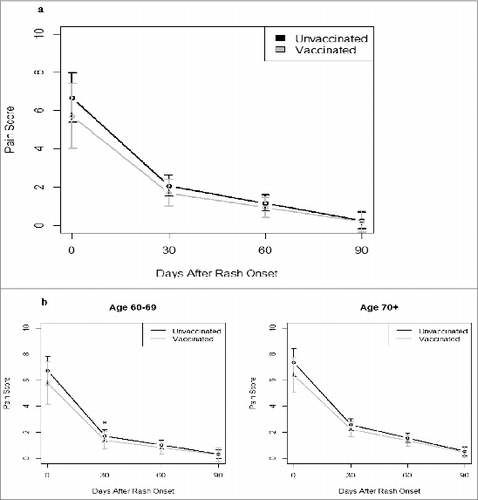
Figure 2. Duration of clinically significant Herpes Zoster pain* by vaccination status (adjusted for age, sex, and immunocompromised status). *Pain cessation defined as the first day the smoothed pain score decreased to 2 or less. Four participants (2 vaccinated, 2 unvaccinated) whose first pain score was not recorded until after day 30 and indicated pain cessation were excluded from this analysis. The proportional hazards model showed that at any time after herpes zoster (HZ) onset, pain cessation was as likely in vaccinated patients as in unvaccinated patients controlling for sex, age, and immunocompromised status (adjusted hazard ratio=1.28, 95% CI 0.95-1.73). Kaplan Meier curves (unadjusted) show that the median duration of clinically significant HZ pain was 25 d after rash onset (95% CI: 20-31 d) for vaccinated patients and 29 d after rash onset (95% CI: 26-33 d) for unvaccinated patients. The effect of vaccination on pain cessation did not differ by age group. When pain was defined as any score >0, the median duration of pain increased to 45 d after rash onset (95% CI: 32-71) for vaccinated patients and 48 d (95% CI: 44-54 d) for unvaccinated patients; however the effect of vaccination status on pain cessation was similar.
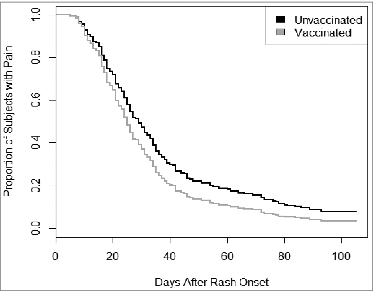
Table 4. Pain scores and percent of case-patients experiencing herpes zoster-associated pain at 30, 60 and 90 d after rash onset by vaccination status (adjusted for age group, sex and immunocompromised status)
Discussion
This is the first post-licensure community-based study to fully characterize HZ vaccine impact and use medical record review to confirm both acute HZ occurrence and vaccination. Our main finding was that the vaccine was 54% effective at preventing HZ among persons age ≥60 years an average of 3 years following vaccination. These results are consistent with those of the clinical trial (51% efficacy) and the 2 prior post-licensure effectiveness studies (48% and 55%).Citation13,15,16 Consistent results among studies with different designs conducted in different populations provide reassurance regarding the vaccine performance during routine clinical practice. A reduction of about 50% in HZ incidence can translate to large reduction in the burden of the disease given the approximately half million HZ cases that occur annually in the US among adults age ≥60 years;Citation1 this benefit is likely to increase as the American population ages.
We are the first to report that vaccination reduces the frequency and severity of prodromal pain and discomfort. Fewer HZ cases in vaccinated persons were accompanied by prodrome, and when the prodrome occurred, it was less severe than among unvaccinated case-patients. Furthermore, vaccinated case-patients were 70% less likely to seek medical care for HZ prodrome. These findings demonstrate an important benefit of vaccination because acute unexplained pain can cause substantial anxiety and often leads to extensive measures to diagnose and treat that unexplained pain.Citation1,7,8
Our study is the largest post-licensure population-based study to date to assess the effectiveness of HZ vaccine and its impact on characteristics of disease in vaccinated persons. We therefore conducted secondary analyses regarding vaccine performance among a variety of patient sub-groups, for prevention of less common outcomes, and incremental benefits (i.e., given HZ, how do its clinical characteristics and painful sequelae vary by vaccination status) even though we did not expect to be powered to derive precise estimates for all these objectives. Nonetheless, our findings are instructive.
Whereas our results suggested that the vaccine provided greater protection among participants vaccinated at a younger age, the age-related differences were not significant. Published data provide conflicting results on this question. The clinical trials of HZ vaccine showed lower efficacy among adults vaccinated at older ages (≥70 years vs. 60-69 years vs. 50-59 years)Citation13,18 suggesting an age effect, but a large post-licensure study showed similar effectiveness among adults vaccinated at age ≥60 years.Citation15 While most adults appear to receive medical care for acute HZ,Citation19 it is plausible that these differing results relate to health seeking practices which may vary by disease severity and individual- or community-level factors.
The prelicensure clinical trial showed that HZ vaccine was effective at preventing PHN; even more effective than at preventing HZ.Citation13 Our results are consistent with those findings, although they only reached statistical significance for pain lasting 30 d. The importance of the findings regarding prevention of PHN derives from the fact that the main health and non-health burdens associated with HZ are due to PHN, which significantly decreases quality of life by affecting mobility, self-care, usual activities, or social lifeCitation20,21 whereas available pain medications and neuropathic treatments are only modestly effective for controlling PHN pain.Citation7
To fully understand the impact of HZ vaccine, besides determining the proportion of HZ or PHN cases prevented, it is important to understand the characteristics and sequelae of HZ that occurs in vaccinated persons compared with unvaccinated. However, detecting incremental differences in subjective outcomes like pain is challenging. We did not find a statistically significant difference by vaccination status in either duration or severity of pain among case-patients with HZ. The prelicensure clinical trial, with >38,000 subjects, could not provide statistically significant evidence that persons with HZ were less likely to progress to PHN if they were vaccinated than if they were not.Citation22 However, taken together, the results of our vaccine effectiveness and cohort studies suggest that HZ vaccine is at least as effective at preventing PHN as it is at preventing HZ, and disease among vaccine recipients is somewhat milder.
Aside from the sample size considerations noted above, our study has several limitations. Our study community is atypical, being predominantly Caucasian, with many working in the health sector, and few barriers to seeking and receiving care, including preventive services (vaccination). Indeed, the HZ vaccination rates were higher than published national figures (14%-16% for 2010–2011).Citation23 Whereas this raises questions regarding generalizability, these same characteristics assured good HZ case finding, and reduced opportunity for ascertainment bias. One source of ascertainment bias could have been less health-care seeking for milder breakthrough HZ; we believe this was unlikely, and to the extent this did occur, it would have overestimated vaccine effectiveness but underestimated the benefit of the vaccine on modifying clinical presentation. We used non-HZ controls to calculate vaccine effectiveness against other HZ-related outcomes. Ideally, controls for these sub-analyses should have been drawn from a population regardless of occurrence of HZ but because the 2-year period prevalence of HZ is about 2% in Olmsted County,Citation1 the potential for bias from this approach is very small. Recall bias was possible regarding prodromal pain with retrospective collection of data. We analyzed data for participants recruited within 14 and within 21 d of onset and prodrome feature results were not different than those presented. Diary data for some participants was incomplete; half of the participants began recording pain on day 12 or later after rash onset therefore, pain scores and proportion of participants with pain near rash onset should be interpreted with caution. Criteria used for data smoothing and definition of pain cessation were subjective. We did sensitivity analyses with a wider and narrower bandwidth, used last day the smoothed pain score dropped to levels of ≤ 2 or ≤ 0.5 as criteria for pain cessation, controlled for antiviral use and results by vaccination status were robust to different definitions.
In conclusion, this is the largest post-licensure population-based study to date to comprehensively assess HZ vaccine including prevention of HZ and its sequelae, and changes in HZ clinical features among vaccinated patients. Vaccination was associated with a 54% reduction in HZ incidence, a 58% reduction in HZ prodromal symptoms, and a 70% reduction in medically-attended prodrome. The vaccine also had beneficial effects at reducing prolonged HZ-associated pain. The burden of HZ is substantial, and HZ vaccination can play an important preventive role.
Methods
Study design
We conducted a matched case-control study to assess HZ vaccine effectiveness for prevention of HZ and other HZ-related outcomes and a cohort study of persons with HZ to compare HZ-related outcomes among those vaccinated and those who remained unvaccinated.
HZ cases
For all analyses, HZ was identified as part of an active surveillance project conducted in 3 contiguous counties in southeastern Minnesota: Olmsted, Winona and Dodge, with a combined population of 220,000 residents. Cases were identified among persons who had health-care encounters for HZ with onset of HZ between January 1, 2010 and October 12, 2011.
Cases from Olmsted and Dodge Counties were identified based on ICD-9 codes of 053 using the resources of the Rochester Epidemiology Project (REP). REP identifies cases, usually within 72 hours of diagnosis, and uses billing data to link the diagnostic codes with visit dates, basic demographic, and procedure information for persons visiting clinics and hospital associated with the Olmsted Medical Center, the Mayo Clinic, and the solo physician practice in Rochester, MN, the Rochester Family Medical Clinic.Citation24 Cases from Winona County were identified by weekly contact with the major health-care facility in the county, the outpatient Winona Clinic and associated Hospital.
Data were collected by medical record review and from case-patients via 1) 3 telephone interviews conducted within 7 d, at about 21 d, and at approximately 100 d following diagnosis, and 2) pain diaries completed daily from time of study enrollment until either there was no HZ pain or 90 d following rash onset, whichever came first.
All cases included in the analyses were confirmed by medical record documentation of a physician diagnosis of either acute HZ or acute HZ complication accompanied by a description of an acute onset of dermatomal pain and vesicular rash not attributed to other causes. Healthcare providers were aware of the vaccination status of study participants.
Controls
We used REP to select controls for HZ case-patients. Potential controls were residents of the same county as the case-patient, who sought non-HZ-related medical care at the same clinic and at the closest time before the rash onset date in the matching case. They were matched to case-patients by date of birth (within 1 year) and sex. Potential controls whose medical records showed evidence of HZ within 3 years before the rash onset date in the matching case were excluded. To achieve at least 1:1 matching, 4 potential controls were invited to participate; all who consented were enrolled. Data on controls were collected by medical record review and one telephone interview which was conducted within 21 d of the rash onset date in the matching case.
Vaccine effectiveness studies
We assessed vaccine effectiveness for prevention of HZ, HZ prodrome, medically-attended prodrome, and PHN at 30, 60 and 90 d after rash onset. HZ case-patients were persons with acute HZ, aged ≥60 years (the age for which HZ vaccine is recommended), and who lived in Olmsted County (for whom we could use REP to select controls). Therefore, case-patients included in the HZ vaccine effectiveness study are a subset of those identified through active surveillance and included in the cohort study; they were selected based on county of residence because of the available approach to identify and match controls. Case-patients with HZ prodrome, with medically-attended prodrome, and with PHN were subsets of the HZ case-patients included in the HZ vaccine effectiveness study. HZ prodrome was defined as self-reported pain or discomfort before rash onset. Medically-attended prodrome was defined as a self-reported health-care visit before rash onset because of pain or discomfort. PHN was based on self-reported intensity of pain in the daily diaries (methods described below). The vaccine effectiveness studies against HZ prodrome, medically-attended prodrome, and PHN included the case-patients with the outcome of interest and their matched non-HZ controls.
Characteristics of HZ study
HZ case-patients in the cohort consisted of persons with HZ aged ≥60 years who lived in any of the 3 study counties. To assess HZ-related pain, we used the Zoster Brief Pain Inventory (ZBPI)Citation25 during the telephone interviews and selected questions from ZPBI in the daily pain diaries. ZBPI uses an 11-point Likert scale (0 [no pain] to 10 [worst pain possible]) to rate pain.
Analysis
We performed descriptive analyses of demographic, health characteristics, vaccination status, and clinical features of HZ. For all HZ-related outcomes, the index date for the control was the rash onset date in the matching case. To test for differences, Cochrane-Mantel-Haenszel test (for case-control analyses), and chi square test or logistic regression (for cohort analysis) were used for proportions and Wilcoxon rank sum test for means; p-values ≤ 0.05 were considered statistically significant.
Immune status was assessed using medical record data. Immunocompromised were patients who at the time of HZ onset/index date had: hematological malignancies, haematopoietic or solid organ transplant in the 12 months before HZ onset/index date, AIDS, or treatments associated with immunocompromise (chemotherapy, radiation therapy, systemic immune-suppressive therapy). Chronic systemic use of ≥10mg of prednisone/day was considered immunosuppressive.
Vaccination status was based on medical record data. When data on HZ vaccination was not found in the medical record or a participant reported vaccination not confirmed by medical record review, information from the Southeast Minnesota immunization registry was considered the gold standard. Only persons who had received HZ vaccine >30 d before HZ onset/index date were considered to be vaccinated.
For all HZ outcomes, vaccine effectiveness was calculated as 1– the respective matched odds ratio (OR). To calculate matched OR we used conditional logistic regression for matched pairs and controlled for age, sex, immunocompromised status, and comorbid conditions statistically significantly different between case-patients and controls (asthma and arthritis) in bivariable analysis. Analyses were performed in SAS (version 9.3, SAS Institute Inc.., Cary, NC).
For pain analyses we used responses to the questions on “worst [shingles] pain in the last 24 hours” from the first interview and “worst shingles pain today” from the daily pain diary. To describe the evolution of pain over time we used longitudinal linear mixed effects models, also called growth curve analyses.Citation26 Pain scores decreased rapidly within the first 30 d after rash onset, then more slowly over the remaining period. Therefore, we modeled the evolution of pain scores over time using piecewise linear splines with one knot at day 30. This approach fit the observed data qualitatively and is consistent with other studies indicating 30 d as a transition point in the resolution of HZ-associated pain.Citation27,28 We report pain scores and 95% confidence intervals (CI) at days 0, 30, 60, and 90 after rash onset by vaccination status and controlling for age, sex, and immunocompromised status.
To estimate the duration of pain after rash onset we used survival analysis. For the main analysis we evaluated duration of clinically significant pain, previously defined as a pain score of ≥3 because this level of pain has been found to be associated with functional disruption.Citation13,25 We reduced the day-to-day variation in reported pain scores using a Gaussian kernel smootherCitation29 with a 7-d bandwidth and defined pain cessation as the first day the smoothed pain score decreased to ≤2. We also evaluated duration of any pain, with pain cessation defined as the first day the smoothed pain score decreased to ≤0.5. We calculated time to pain cessation and median duration of pain for vaccinated and unvaccinated case-patients using Kaplan-Meier curves. We used proportional hazards regression to generate survival curves and estimate the proportion of subjects with pain at 30, 60, and 90 d by vaccination status adjusted for age, sex, and immunocompromised status and to calculate hazard ratios.
Analysis of pain scores was performed with R software (version 3.0.1, R Foundation for Statistical Computing, Vienna, Austria). Linear mixed models were fit using the R package “nlme.”Citation30
Human subjects
The study was approved by the Institutional Review Boards at Olmsted Medical Center, Mayo Clinic, and the Centers for Disease Control and Prevention, Atlanta, GA. Consent was obtained from all participants; additionally, only persons with previous consent to participate in REP (>93% of all Olmsted County residents) were eligible to be controls.
Disclosure of Potential Conflicts of Interest
B. P. Y. has served in the Advisory committees related to herpes zoster vaccines for both Merck and GSK and has received research grants from Merck. All other co-authors have no conflicts of interest to declare.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Supplementary Material
Download MS Word (51.4 KB)Acknowledgments
We express our appreciation to staff at Olmsted Medical Center for conducting interviews, staff at the Rochester Epidemiology Project for identifying and matching controls, all participants for responding to interview questions and providing daily diaries and Jessica Allen, CDC, for editorial assistance.
Funding
This study was funded by the Centers for Disease Control and Prevention with funds from the American Recovery and Reconstruction Act of 2009. Additionally, the study used the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007; 82:1341–9; PMID:17976353; http://dx.doi.org/10.4065/82.11.1341
- Yawn BP, Itzler RF, Wollan PC, Pellissier JM, Sy LS, Saddier P. Health care utilization and cost burden of herpes zoster in a community population. Mayo Clin Proc 2009; 84:787–94; PMID:19720776; http://dx.doi.org/10.4065/84.9.787
- Lydick E, Epstein RS, Himmelberger D, White CJ. Herpes zoster and quality of life: a self-limited disease with severe impact. Neurology 1995; 45:S52–3; PMID:8545021; http://dx.doi.org/10.1212/WNL.45.12_Suppl_8.S52
- Di Legami V, Gianino MM, Ciofi degli Atti M, Massari M, Migliardi A, Tomba GS, Zotti C; Zoster Study Group. Epidemiology and costs of herpes zoster: background data to estimate the impact of vaccination. Vaccine 2007; 25:7598–604; PMID:17889410; http://dx.doi.org/10.1016/j.vaccine.2007.07.049
- Pellissier JM, Brisson M, Levin MJ. Evaluation of the cost-effectiveness in the United States of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Vaccine 2007; 25:8326–37; PMID:17980938; http://dx.doi.org/10.1016/j.vaccine.2007.09.066
- Stein AN, Britt H, Harrison C, Conway EL, Cunningham A, Macintyre CR. Herpes zoster burden of illness and health care resource utilisation in the Australian population aged 50 years and older. Vaccine 2009; 27:520–9; PMID:19027048; http://dx.doi.org/10.1016/j.vaccine.2008.11.012
- Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ, Backonja M, Betts RF, Gershon AA, Haanpaa ML, McKendrick MW, et al. Recommendations for the management of herpes zoster. Clin Infect Dis 2007; 44(Suppl 1):S1–26; PMID:17143845; http://dx.doi.org/10.1086/510206
- Schmader K. Herpes zoster in the elderly: issues related to geriatrics. Clin Infect Dis 1999; 28:736–9; PMID:10825029; http://dx.doi.org/10.1086/515205
- Drolet M, Oxman MN, Levin MJ, Schmader KE, Johnson RW, Patrick D, Mansi JA, Brisson M. Vaccination against herpes zoster in developed countries: state of the evidence. Hum Vaccin Immunother 2013; 9:1177–84; PMID:23324598; http://dx.doi.org/10.4161/hv.23491
- Edmunds WJ, Brisson M, Rose JD. The epidemiology of herpes zoster and potential cost-effectiveness of vaccination in England and Wales. Vaccine 2001; 19:3076–90; PMID:11312002; http://dx.doi.org/10.1016/S0264-410X(01)00044-5
- Oxman MN. Immunization to reduce the frequency and severity of herpes zoster and its complications. Neurology 1995; 45:S41–6; PMID:8545018; http://dx.doi.org/10.1212/WNL.45.12_Suppl_8.S41
- Harpaz R, Ortega-Sanchez IR, Seward JF. Advisory Committee on Immunization Practices Centers for Disease C, Prevention. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57:1–30; quiz CE2-4; PMID:18528318
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–84; PMID:15930418; http://dx.doi.org/10.1056/NEJMoa051016
- Orenstein WA, Bernier RH, Hinman AR. Assessing vaccine efficacy in the field. Further observations. Epidemiol Rev 1988; 10:212–41; PMID:3066628
- Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA 2011; 305:160–6; PMID:21224457; http://dx.doi.org/10.1001/jama.2010.1983
- Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med 2013; 10:e1001420; PMID:23585738; http://dx.doi.org/10.1371/journal.pmed.1001420
- Yawn BP, Wollan P, St Sauver J. Comparing shingles incidence and complication rates from medical record review and administrative database estimates: how close are they? Am J Epidemiol 2011; 174:1054–61; PMID:21920944; http://dx.doi.org/10.1093/aje/kwr206
- Schmader KE, Levin MJ, Gnann JW, Jr., McNeil SA, Vesikari T, Betts RF, Keay S, Stek JE, Bundick ND, Su SC, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50–59 years. Clin Infect Dis 2012; 54:922–8; PMID:22291101; http://dx.doi.org/10.1093/cid/cir970
- Lu PJ, Euler GL, Jumaan AO, Harpaz R. Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: uptake of the first new vaccine to target seniors. Vaccine 2009; 27:882–7; PMID:19071175; http://dx.doi.org/10.1016/j.vaccine.2008.11.077
- Schmader KE, Sloane R, Pieper C, Coplan PM, Nikas A, Saddier P, Chan IS, Choo P, Levin MJ, Johnson G, et al. The impact of acute herpes zoster pain and discomfort on functional status and quality of life in older adults. Clin J Pain 2007; 23:490–6; PMID:17575488; http://dx.doi.org/10.1097/AJP.0b013e318065b6c9
- Katz J, Cooper EM, Walther RR, Sweeney EW, Dworkin RH. Acute pain in herpes zoster and its impact on health-related quality of life. Clin Infect Dis 2004; 39:342–8; PMID:15307000; http://dx.doi.org/10.1086/421942
- Food and Drug Administration. ZOSTAVAX: Statistical Review and Evaluation. 2005. http://wwwfdagov/ohrms/dockets/ac/05/briefing/5-4198B2_2pdf pages 7–9, accessed 16 June 2014.
- Williams WW, Lu PJ, O'Halloran A, Bridges CB, Pilishvili T, Hales CM, Markowitz LE; Centers for Disease Control and Prevention (CDC). Noninfluenza vaccination coverage among adults - United States, 2012. MMWR Morb Mortal Wkly Rep 2014; 63:95–102; PMID:24500288
- St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Pankratz JJ, Brue SM, Rocca WA. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012; 41:1614–24; PMID:23159830; http://dx.doi.org/10.1093/ije/dys195
- Coplan PM, Schmader K, Nikas A, Chan IS, Choo P, Levin MJ, Johnson G, Bauer M, Williams HM, Kaplan KM, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain 2004; 5:344–56; PMID:15336639; http://dx.doi.org/10.1016/j.jpain.2004.06.001
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2002; 2 ed. Thousand Oaks, CA: Taylor & Francis; 2002.
- Dworkin RH, Gnann JW, Jr., Oaklander AL, Raja SN, Schmader KE, Whitley RJ. Diagnosis and assessment of pain associated with herpes zoster and postherpetic neuralgia. J Pain 2008; 9:S37–44; PMID:18166464; http://dx.doi.org/10.1016/j.jpain.2007.10.008
- Drolet M, Brisson M, Levin MJ, Schmader KE, Oxman MN, Johnson RW, Camden S, Mansi JA. A prospective study of the herpes zoster severity of illness. Clin J Pain 2010; 26:656–66; PMID:20842005
- Nadaraya EA. On estimating regression. Theor Probab Appl 1964; 9:141–2; http://dx.doi.org/10.1137/1109020
- Pinheiro J, Bates D, DebRoy S, Sarkar D and R Core Team (2014). Linear and Nonlinear Mixed Effects Models. R package version. 3:1–117, http://CRAN.R-project.org/package=nlme.
