Abstract
In 1999, the UK introduced meningococcal serogroup C conjugate (MCC) vaccination at 2, 3, 4 months of age with a single dose for children 1–18 y In 2006, the schedule was refined to a 2 dose priming schedule with a booster in the second year of life. In 2013, the number of priming doses was reduced to a single priming dose, the booster maintained at 12 months of age and an adolescent booster dose introduced. The paper presents the evidence supporting the reduction in the number of priming doses. A UK study provided evidence for reducing the priming doses of MCC-TT together with the positive correlation of lower quantity of antigen and serum bactericidal antibody (SBA) levels post-primary but a higher magnitude of the booster response. Another UK study, demonstrated one dose of MCC-TT or MCC-CRM197 at 3 months gave comparable responses to 2 doses (SBA titres ≥8) both post-primary vaccination and post-booster Hib/MCC-TT at 12 months. However, the magnitude of the SBA GMT was higher in the MCC-TT primed post-booster. A single priming dose of MCC-TT (at 4 or 6 months) compared to 2 doses (2 and 4 months) gave higher SBA titres in all groups, post-primary and post-booster at 12–13 months, with the highest SBA responses observed in the 4 month single dose group. A study in Malta, comparing one dose of MCC-TT or MCC-CRM197 at (3 months) versus 2 doses of MCC-CRM197 (3 and 4 months), showed a high proportion (>84.72%) of subjects achieving SBA titres ≥8 following a single dose. These studies show that a single-dose priming MCC vaccination in infancy is sufficient.
Background
Neisseria meningitidis is a major cause of invasive bacterial infection worldwide. Efforts to control meningococcal infections have been aimed at the development of effective vaccines and subsequent implementation in appropriate immunisation schedules. Meningococcal serogroup C conjugate (MCC) vaccines were licensed in Europe in 1999, and have been used widely across Europe with many different vaccine schedules.Citation1 For those countries with high incidence of serogroup C (MenC) disease in the young, the optimal protection of children below 2 y of age is under continuous evaluation. Initially, a 3 dose priming schedule was thought to be required for adequate immune responses in infancy, however clinical trials conducted following the licensure of these vaccines have showed that a reduction in the number of priming doses to provide adequate protection. New vaccines are continually added to national immunisation schedules, and the need to maximise protection with as few doses as possible, has led to review of the available evidence and additional clinical trials to compare the immunogenicity profiles of different vaccine schedules. This paper presents the evidence for a single MCC dose infant priming schedule with the experience of the UK as an example.
Introduction of MCC Vaccines in the UK
The UK was the first country to introduce MCC vaccines in 1999, with an accelerated 3 dose primary immunisation schedule at 2, 3, and 4 months of age.Citation2 A catch-up campaign was also introduced vaccinating those up to the age of 18 years, which was later extended to those up to the age of 24 y. Infants aged 5 to 11 months of age were vaccinated with 2 doses and those aged 1year and above received 1 dose. The catch-up campaign started with those thought to be of increased risk of meningococcal disease, adolescents aged 15–17 y and the catch-up campaign vaccination was completed by late 2000. Three MCC vaccines were available in the UK, 2 conjugated to cross reacting material of diphtheria toxin, CRM197, Meningitec® (now marketed by Neuron Biotech) and Menjugate ® (now marketed by Novartis Vaccines) and one conjugated to tetanus toxoid, NeisVac-C® (Baxter Bioscience).
A comprehensive surveillance program was put in place to monitor the impact because these vaccines were introduced without direct evidence of efficacy. This surveillance showed there to be a strong impact on the overall disease incidence, with a dramatic decline in the vaccinated populations but also indirect effect in the unvaccinated population. Vaccination history of MenC cases was obtained of those cases which occurred between July 2001 and June 2002 and compared to cases reported in 1998–1999. Overall in the age groups targeted for vaccination, a reduction of 67% in the attack rate occurred.Citation3 Indirect effects of the vaccine campaign were supported by data from large carriage studies undertaken within the UK, before and after the introduction of MCC vaccines. At 1 y following the introduction of MCC vaccines, a 66% reduction in carriage of MenC meningococci was reported.Citation4
Three Dose Infant Schedule
For infants immunised with 3 doses at 2, 3 and 4 months of age, high antibody concentrations are present 1 month after the third dose but antibody levels decline rapidly during the first year of life.Citation5 A 3 dose course in infancy has been shown to provide high short term protection, with vaccine effectiveness at 97% within a year of vaccination which significantly falls to 68% more than a year after vaccination.Citation6 The pre-implementation trial of Richmond et al. (1999) showed there to be little difference between 2 and 3 doses of MCC in UK infants, with, the serum bactericidal antibody (SBA) geometric mean titres (GMTs) one month following 2 doses administered at 2 and 3 months of age to be 766.3 (95%CI 500–1174) compared to 1001 (95%CI 702–1455) one month following a third dose at 4 months of age. At both time points, 98% of subjects achieved an SBA titre of ≥8, the titer considered to be protective.Citation7 A rapid decline in antibody levels following the 3 infant doses to the second year of life was observed, however immunological memory was demonstrated with amamnestic responses seen following polysaccharide challenge.Citation5,8 The observed decline in vaccine effectiveness more than 12 months post-vaccination is consistent with measured declining SBA levels.
Study of SBA responses in vaccine failures showed that those vaccinated with MCC had an antibody response which was consistent with an anamnestic response suggesting that MenC disease occurred despite the MCC vaccine priming for immune memory. A significantly higher SBA response was observed in those who had been vaccinated compared to MenC cases in those who were not vaccinated, with SBA GMTs of 3690 (1167–11693) in the vaccinated compared to 1164 (389–3388) in the un-vaccinated. The fold difference of these SBA GMTs was significant following adjustment for age at onset and interval to blood sample. These data demonstrated that immune memory was not rapid enough for protection and that circulating antibody is important for protection against MenC disease.Citation9 The post-licensure surveillance data generated in the UK has enabled vaccine efficacy estimates to be calculated. To establish a population correlate of protection, the titre obtained by majority of vaccinated group is compared to titre obtained by majority of unvaccinated group can be compared with the vaccine efficacy in a specific age group. Campbell et al. reportedCitation6 an SBA titre of ≥8 was found to give a strong correlation with vaccine efficacy and led to the establishment of an SBA titre of ≥8 as a population based correlate. Hence, data are compared in this review using both the percentage of subjects with SBA titres ≥8 and the SBA GMTs. Individual protection against meningococcal disease is known to rely upon the circulating antibody, and given that SBA GMTs are known to decline over time after both the primary series and booster vaccination, it therefore may be advantageous to have a higher initial SBA GMT to provide longer individual protection.
Change to a 2 Dose Infant Priming Schedule Followed by a Booster
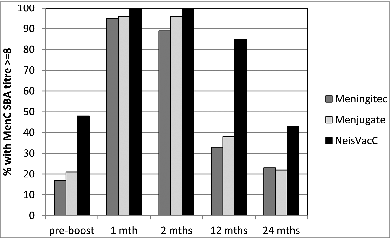
In order to maintain elevated circulating antibody levels, in September 2006, the UK immunisation schedule was modified to reduce the number of doses administered in infancy from 3 to 2 doses (administered at 3 and 4 months of age) and the inclusion of a booster vaccination at 12 months of age (2+1 schedule). This booster vaccine was chosen to be a MenC/Hib-TT conjugate, Menitorix® (GSK). This change in the immunisation schedule, to include a booster dose in toddlers had the expectation that the booster dose would provide extended individual protection. A trial conducted in the UK in which infants vaccinated with 2 doses of one of 3 available MCC vaccines in infancy followed by a booster vaccine at 12 months of age were followed for 2 y.Citation10 shows the percentage of subjects with SBA titres ≥8 prior to the 12 month booster, 1 month, 12 months and 24 months post-booster with subjects stratified by the priming MCC vaccine. Those subjects primed with NeisVac-C® had the highest percentage of subjects with SBA titres ≥8 at pre-booster. One month following boosting with Menitorix®, a high percentage of subjects in all 3 MCC groups had SBA titres ≥8, however this was greatest in those primed with NeisVac-C. At 12 months post-booster, a superior response in those primed with NeisVac-C® was still evident with a significantly higher percentage of subjects with SBA titres ≥8. Two years following boosting, a further decline in the percentage of subjects with SBA titres ≥8 was observed, however this percentage remained highest in those vaccinated with NeisVac-C®. Following two dose infant priming and boosting at 12 months, the relatively poor antibody persistence was surprising and demonstrated that the 2+1 schedule was not providing long term direct protection.
Figure 1. Proportion of subjects with SBA titres ≥8 by primary MCC vaccine (2 doses) and time since MenC/Hib-TT booster (Data from ref. 10Citation).
Seroprevalence Data
Despite the success of the UK MCC program in controlling MenC disease, there is evidence that individual protection in young children is short lived. Serological surveillance has been utilised for considerable time within the UK to inform vaccine policy. Assessment of the population levels of immunity in England to MenC, using measurements of specific functional antibody levels in age-stratified sera 10 y following MCC introduction has been performed.Citation11,12 Ishola and colleagues, compared the data collected in 2009 to previous seroprevalence data collected prior to vaccine introduction (1996–99) and following vaccine introduction (2000–2004). From these data we are able to determine if the booster has had a beneficial effect. Samples collected from those aged <6 months to 4 y in the 2009 survey would have been vaccinated under the 2+1 schedule and protective levels in these age groups were similar to the previous survey of samples collected in 2000–2004 when those within this age group would have received 3 doses in infancy. The secondary peak observed in the adolescent age groups in the 2000–2004 survey (those vaccinated with one dose at primary school age during the catch-up campaign) has moved into the older age groups in the 2009 survey. The antibody levels of those aged 5–9 y and 10–14 y in the 2009 survey are lower than those of the previous survey, although higher than those of the pre-vaccine era. These findings suggest that protective antibody levels have declined markedly in all immunised cohorts since the time of vaccination. The proportions of subjects with seroprotective antibody levels remains much higher in those eligible for catch-up vaccine at ≥5 years than those eligible between 1–4 y of age. These data provide evidence to suggest that the extended routine infant immunisation schedule, 2+1schedule, has not had any substantial impact on sustaining seroprotection. The incidence of MenC disease in the UK is currently low and modeling studies have suggested that indirect protection from the catch-up campaign is likely to persist for some time, however, the proportion of the childhood population without individual protection is increasing each year.
Evidence Supporting a Single Infant Priming Dose
In 2013, a change to the UK immunisation schedule was made reducing the number of infant doses to a single dose of MCC administered at 3 months of age. This change was instigated following evidence from clinical trials which showed that a single dose of MCC provided sufficient immunity in infancy until the booster dose of Hib/MenC at 12 months of age. Southern et al.Citation13 showed that following a single dose of at 2 months of age of 2 of the 3 MCC vaccines (NeisVac-C® or Menjugate®), a high percentage of subjects had SBA titres ≥8 (97% and 80%, for NeisVac-C® and Menjugate®, respectively). These data led to the exploration of a single dose of MCC at 3 months of age, which is thought to be an immunologically less demanding age.Citation14 UK infants were immunised at 3 months of age with NeisVac-C® or Menjugate® along with concomitant Pediacel® (Sanofi Pasteur) and 7-valent pneumococcal conjugate vaccine (Prevenar, Pfizer). Results of this trial showed at one month following the priming dose (at 4 months of age) the SBA GMT was significantly higher in the group who received NeisVac-C®, with GMTs of 95.8 (66.4–138.2) in those vaccinated with Menjugate® compared to 223.3 (162.9–306.1) following NeisVac-C® vaccination. At 2 months following the priming dose, antibody levels had declined in both vaccine groups, with GMTs no longer significantly different ().
Figure 2. MenC SBA GMTs (95% CI) by primary MCC vaccine (single dose) and following MenC/Hib-TT booster (Data from ref. 14Citation).
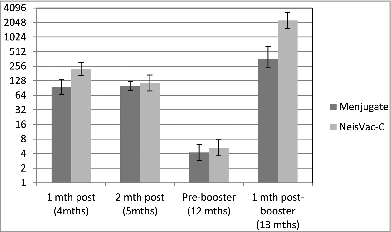
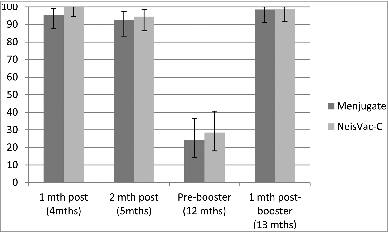
Antibody persistence was similar following either vaccine, with a further decline by pre-booster (12 months of age). However, the response one month following Menitorix® boosting was different, with those who were primed with NeisVac-C® having a significantly higher GMT, 2251 (1535.3–300.3) verses a GMT of 339.1 (235.4–538.1) in the Menjugate® group (). The difference between the 2 vaccine groups was not reflected in the proportion of subjects with SBA titres ≥8, as shown in . One month following vaccination, there was no significant difference between the 2 vaccine groups with 100% of those vaccinated with NeisVac-C® and 95.5% of those vaccinated with Menjugate® having SBA titres ≥8, which remained high 2 months following vaccination (94.4% and 92.5%, respectively). Over the following months, a decline in both groups was observed followed by an increase post booster, with similar proportions in both groups. This trial did not include a control group of those vaccinated with 2 doses in infancy, however comparison of the GMTs obtained in the trial of Findlow et al.Citation14 to those of Southern et al.,Citation13 show that the reduction of the number of priming doses from 2 to one does not impact on the magnitude of the SBA GMT prior to boosting or that following Menitorix boosting.
Figure 3. Proportion of subjects with SBA titre ≥8 by primary MCC vaccine (single dose) and following MenC/Hib-TT booster (Data from ref. 14Citation).
Further evidence supporting a single infant MCC dose comes from the trial reported by Poellabauer et al.Citation15 A multi-center randomized trial conducted in Spain and Poland, vaccinated infants either with a single dose of NeisVac-C® at 4 or 6 months and compared the responses to those vaccinated at 2 and 4 months. All subjects were boosted with the same vaccine at 12–13 months of age. The proportion of subjects with SBA titres ≥8 one month after the completion of primary vaccination was similar between the three groups, with over 99% achieving this titer. The SBA GMT was not surprisingly greatest in those who received 2 doses, with a GMT of 624.1 (95%CI 568.2–685.5) compared to 372.1 (95%CI 339.4–408.1) and 401.8(95%CI 361.6–446.5) for the 4 month and 6 month groups, respectively. Non-inferiority of single dose priming to a 2 dose priming schedule was shown. Following boosting, a lower SBA GMT was observed in the 2 dose priming group, with a GMT of 1538.0 (95%CI 1381.1–1712.6) compared to GMTs of 2472.1 (95%CI 2226.3–2745.0) and 1874.8 (95%CI 1684.4–2086.6) in the 4 month and 6 month groups. Lower SBA GMTs in the 2 dose priming group is consistent with data reported by Richmond et al.,Citation5 and Borrow et al.,Citation16 showing the quantity of antigen administered during priming correlates positively with post-primary SBA levels but negatively with the magnitude of the booster response. The reduced response in the 2 dose group may also be attributed to protein carrier-induced epitope suppression, with a dose dependent suppression of the antigen response.
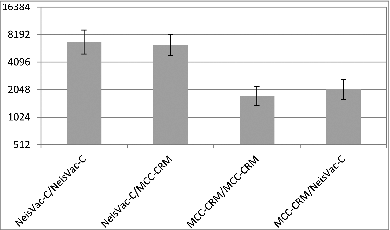
Currently the Menjugate® and NeisVac-C® are used interchangeably in the immunisation schedule, however there is evidence that NeisVac-C is more immunogenic and in that the responses to a booster vaccine (regardless of carrier protein) is better in those primed with NeisVac-C®.Citation17 Children vaccinated with 2 doses NeisVac-C® at 2 and 4 months or 3 doses of Meningitec® at 2, 4 and 6 months were randomized to receive a booster dose of Meningitec® or NeisVac-C®. Following boosting, the SBA GMT was higher in those who were primed with NeisVac-C® than Meningitec®, 6520 (95% CI 5359–7932) vs. 1903 (95% CI 1600–2262). The vaccine used for boosting did not affect the SBA GMT (), suggesting that the superior response is not due to the use of the same carrier protein in the priming and booster vaccine but due to a superior priming response to NeisVac-C®.
Figure 4. SBA GMTs 1 month following second dose of MCC at 14–18 months of age following MCC priming (Data from ref. 17Citation).
Additional data from a trial conducted in the UK and Malta supports the use of a single priming dose.Citation18,19 Infants were vaccinated with a single dose of either Menjugate® or NeisVac-C® at 3 months of age, compared to 2 doses of Menjugate® at 3 and 4 months of age. Blood samples collected at 5 months of age, showed the SBA GMT to be significantly higher in the 2 dose Menjugate® group (GMT of 618.3 (95%CI 538.3–710.1), compared to 53 (95%CI 39.9–70.5) and 155.4 (95%CI 112.3–213.8) in those who received one dose of Menjugate® or NeisVac-C®, respectively. However, the proportion of subjects with SBA titres ≥8 were the same in the 2 dose Menjugate® group and single dose NeisVac-C® group, with 100% of subjects achieving this titer, compared to a significantly lower proportion in those who received a single dose of Menjugate®, 84.72%. These findings may relate to differences in the ability of these vaccines to generate memory B-cells following primary immunisations. The memory B cells measured by ELISpot in the peripheral blood at 5 months of age following a single dose of NeisVac-C® at 3 months of age were compared to 2 doses of Menjugate® at 3 and 4 months of age.Citation19 Responses following a booster dose, showed significantly higher SBA GMTs following boosting in those primed with a single dose of NeisVac-C® at 3 months of age compared to 2 doses of Menjugate® at 3 and 4 months of age. Children who had been primed with NeisVac-C® produced the greatest memory B cell response to the Menitorix® booster. One-dose priming with Menjugate® appears to be at least as good as 2-dose priming with respect to the memory B cell response to the Menitorix® booster; with evidence for a more rapid booster response.
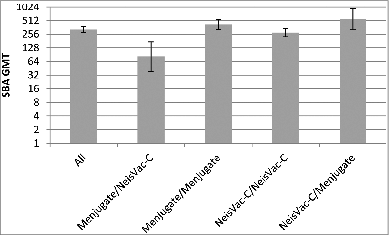
The benefit of a single dose priming schedule would prevent a use of mixed manufacturer MCC schedules. Between September 2006-June 2013, the UK infant immunisation program included a 5-in-1 vaccine against diphtheria, tetanus, polio, Haemophilus influenzae type b and pertussis (Pediacel®) at 2, 3 and 4 months, pneumococcal conjugate vaccine at 2 and 4 months, and MCC vaccine at 3 and 4 months. Infants in the UK, could receive any one of 3 MCC vaccines interchangeably, with the potential for immune interference or enhancements when co-administered with combination vaccines. A trial conducted in the UK evaluated the MenC response following a 2 dose infant priming schedule at 3 and 4 months of age by the MCC vaccine administered, 2 doses of NeisVac-C® or Menjugate® or NeisVac-C® followed by Menjugate® or vice versa, Menjugate® followed by NeisVac-C®.Citation20 The results of this trial showed that MenC responses differed significantly according to MCC schedule. Those vaccinated with Menjugate® followed by NeisVac-C® had the lowest SBA titres, while those receiving NeisVac-C®/Menjugate® had the highest; suggesting the order in which the infants received the MCC affected their responses (). Despite the impact of mixed priming vaccines on the magnitude of the SBA GMTs, the percentage of infants achieving a protective titre of ≥8 was similar (≥99%) between the 4 study groups.
Figure 5. SBA GMTs 1 month post 2 doses of MCC at 3 and 4 months of age (Data from ref. 20Citation).
Adolescent Booster
Both the UK and Netherlands introduced MCC vaccines with a catch-up campaign, and both programmes had high vaccine coverage >94 % resulting in control of MenC disease in both countries. The successful control of MenC disease within both of these countries is attributed to the large scale catch-up campaigns that included adolescents. Teenagers and young adults have the highest nasopharyngeal meningococcal carriage levels and are considered the main source of spread. MCC vaccines were shown to not only protect against disease but reduction in the acquisition of nasopharyngeal carriage has also been demonstratedCitation4 and large scale vaccination of adolescents was very effective in preventing circulation of MenC in the population.
The UK seroprevalence data,Citation12 along with the antibody persistence dataCitation10 shows that those immunised in infancy with single or multiple doses followed by a booster dose in the second year of life, do not have antibody levels which will be sufficient enough to maintain protection or herd effects until adolescence. This waning of SBA titres may adversely affect the direct protection of adolescents and also threatens the herd protection achieved by reduced nasopharyngeal carriage acquisition rates of MenC following immunisation with glycoconjugate vaccines. Additional MCC vaccination at an older age may be required in order to maintain control of MenC. In 2013, the UK introduced an adolescent booster (at age 13–14 years) of a MCC vaccine, in order to keep the young adults protected when subjects who were vaccinated at an early age reach adulthood.Citation21,22 At the same time, a booster dose of MCC vaccine was recommended to first time university entrants under the age of 25 years, for a limited time to offer protection to those who would not have received an adolescent booster. It is expected that if this adolescent booster was not introduced, protection in adolescents will wane dramatically resulting in a new peak in MenC disease not only in this age group but in infants and young children if MenC circulation increases. Continued meningococcal disease surveillance and seroprevalence studies will indicate whether modifications to this approach will be required.
Summary
Cases of meningococcal disease within the UK have been controlled by an effective vaccination program, which included an initial catch-up campaign and routine immunisation. The routine immunisation campaign has had several modifications over the last decade, with the most recent change being the reduction to a one dose infant priming schedule. Trials have shown that a single dose schedule of MCC (either NeisVac-C® or Menjugate®) is sufficient for priming in infancy. NeisVac-C® has been shown to be more immunogenic than Menjugate®, resulting in higher SBA GMTs, however, no significant difference in proportion of subjects achieving SBA titres of ≥8. As those vaccinated in infancy move into adolescence, the antibody levels in which will have waned, maintaining the effects of direct and indirect protection is of value. A booster dose of MCC in early adolescence should maintain high levels of bactericidal antibodies throughout adolescence and early adulthood.Citation22
The meningococcal serogroup B vaccine, Bexsero® received licensure in Europe in 2013Citation23 and has been recommended by the UK JCVI for inclusion into the infant immunisation program.Citation24 Bexsero® has the potential to provide some protection to other serogroups of meningococci, (due to fact that the vaccine antigens are subcapsular proteins) including serogroup C and that infant MCC offered at 3 months of age could potentially be removed from the schedule. Furthermore, given the currently very good herd protection and the low level of MenC carriage in the population, the need for the 3 month dose is uncertain, and the JCVI noted that a number of countries have maintained control of MenC disease without any doses before 12 months of age. However, the JCVI agreed that removal of the infant MCC vaccine should only be undertaken once the program of MCC vaccination in adolescents was established, so that herd protection would be sustained by achieving high levels of antibody in adolescents in the future.
Disclosure of Potential Conflicts of Interest
Both authors have performed contract research as employees of Public Heath England for GlaxoSmithKline, Novartis Vaccines and Diagnostics, Merck, Alexion, and Sanofi Pasteur.
References
- Chiappini E, Venturini E, Bonsignori F, Galli L, de Martino M. Serogroup C Neisseria meningitidis invasive infection: analysis of the possible vaccination strategies for a mass campaign. Acta Paediatr 2010:1609-1614; PMID:20545931; http://dx.doi.org/10.1111/j.1651-2227.2010.01908.x
- Miller E, Salisbury D, Ramsay M. Planning, registration and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine 2001; 20:S58-67; PMID:11587814; http://dx.doi.org/10.1016/S0264-410X(01)00299-7
- Ramsay ME, Andrews NJ, Trotter CL, Kaczmarski EB, Miller E. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. BMJ 2003; 326:365-366; PMID:12586669; http://dx.doi.org/10.1136/bmj.326.7385.365
- Maiden MC, Ibarz-Pavón AB, Urwin R, Gray SJ, Andrews NJ, Clarke SC, Walker AM, Evans MR, Kroll JS, Neal KR, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis 2008; 197:737-743; PMID:18271745; http://dx.doi.org/10.1086/527401
- Richmond P, Borrow R, Miller E, Clark S, Sadler F, Fox A, Begg N, Morris R, Cartwright K. Meningococcal serogroup C conjugate vaccine is immunogenic in infancy and primes for memory. J Infect Dis 1999; 179:1569-1572; PMID:10228085; http://dx.doi.org/10.1086/314753
- Campbell H, Andrews N, Borrow R, Trotter C, Miller E. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol 2010; 17:840-847; PMID:20219881; http://dx.doi.org/10.1128/CVI.00529-09
- Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin Diagn Lab Immunol 2003; 10:780-786; PMID:12965904
- Borrow R1, Goldblatt D, Andrews N, Southern J, Ashton L, Deane S, Morris R, Cartwright K, Miller E. Antibody persistence and immunological memory at age 4 years after meningococcal group C conjugate vaccination in children in the United kingdom. J Infect Dis 2002; 186:1353-1357; PMID:12402208; http://dx.doi.org/10.1086/344324
- Auckland C, Gray S, Borrow R, Andrews N, Goldblatt D, Ramsay M, Miller E. Clinical and immunologic risk factors for meningococcal C conjugate vaccine failure in the United Kingdom. J Infect Dis 2006; 194:1745-1752; PMID:17109348; http://dx.doi.org/10.1086/509619
- Borrow R, Andrews N, Findlow H, Waight P, Southern J, Crowley-Luke A, Stapley L, England A, Findlow J, Miller E. Kinetics of antibody persistence following administration of a combination meningococcal serogroup C and Haemophilus influenzae type b conjugate vaccine in healthy infants in the United Kingdom primed with a monovalent meningococcal serogroup C vaccine. Clin Vaccin Immunol 2010; 17:154-159; PMID:19906895; http://dx.doi.org/10.1128/CVI.00384-09
- Trotter CL1, Borrow R, Findlow J, Holland A, Frankland S, Andrews NJ, Miller E. Seroprevalence of antibodies against serogroup C meningococci in England in the postvaccination era. See comment in PubMed Commons below. Clin Vaccine Immunol 2008; 15:1694-8; PMID:18827191; http://dx.doi.org/10.1128/CVI.00279-08
- Ishola DA Jr, Borrow R, Findlow H, Findlow J, Trotter C, Ramsay ME. Prevalence of serum bactericidal antibody to serogroup C Neisseria meningitidis in England a decade after vaccine introduction. Clin Vaccine Immunol 2012; 19:1126-1130; PMID:22647271; http://dx.doi.org/10.1128/CVI.05655-11
- Southern J, Borrow R, Andrews N, Morris R, Waight P, Hudson M, Balmer P, Findlow H, Findlow J, Miller E. Immunogenicity of a reduced schedule of meningococcal group C conjugate vaccine given concomitantly with the Prevenar and Pediacel vaccines in healthy infants in the United Kingdom. Clin Vaccine Immunol 2009; 16:194-199; PMID:19091990; http://dx.doi.org/10.1128/CVI.00420-08
- Findlow H, Borrow R, Andrews N, Waight P, Sheasby E, Matheson M, England A, Goldblatt D, Ashton L, Findlow J, et al. Immunogenicity of a single dose of meningococcal group C conjugate vaccine given at 3 months of age to healthy infants in the United Kingdom. Pediatr Infect Dis J 2012; 31:616-622; PMID:22333698; http://dx.doi.org/10.1097/INF.0b013e31824f34e6
- Poellabauer EM, Pavlova BG, Fritsch S, Singer J, Neubauer C, Doralt J, Valenta-Singer B, Ehrlich HJ. Single priming dose of meningococcal group C conjugate vaccine (NeisVac-C®) in infants. Vaccine 2013; 31:3611-3616; PMID:23672977; http://dx.doi.org/10.1016/j.vaccine.2013.04.070
- Borrow R, Goldblatt D, Finn A, Southern J, Ashton L, Andrews N, Lal G, Riley C, Rahim R, Cartwright K, et al. Immunogenicity of, and immunologic memory to, a reduced primary schedule of meningococcal C-tetanus toxoid conjugate vaccine in infants in the United Kingdom. Infect Immun 2003; 71:5549-5555; PMID:14500473; http://dx.doi.org/10.1128/IAI.71.10.5549-5555.2003
- Díez-Domingo J1, Cantarino MV, Torrentí JM, Sansano MI, Rosich AJ, Merino AH, de Miguel AG, González JB, Marcos MD; MenC Study Group. A randomized, multicenter, open-label clinical trial to assess the immunogenicity of a meningococcal C vaccine booster dose administered to children aged 14 to 18 months. Pediatr Infect Dis J 2010; 29:148-152; PMID:19927040; http://dx.doi.org/10.1097/INF.0b013e3181b9a831
- Pace D, Khatami A, McKenna J, Campbell D, Attard-Montalto S, Tajar A, Birks J, White C, Finn A, Macloed E, et al. Immunogenicity of a reduced conjugate Meningococcal Serogorup C vaccination schedule in infancy. European Society of Pediatric Infectious Diseases, Milan, Italy; 2013.
- Khatami A, Clutterbuck EA, Thompson AJ, McKenna JA, Pace D, Birks J, Snape MD, Pollard AJ. Evaluation of the induction of immune memory following infant immunisation with aerogroup C Neisseria meningitidis conjugate vaccines - exploratory analyses within a randomised controlled trial. PLoS One 2014; 9:e101672; EPUB; PMID:25020050; http://dx.doi.org/10.1371/journal.pone.0101672
- Ladhani S, Andrews N, Waight P, Hallis B, Matheson M, England A, Findlow H, Bai X, Borrow R, Goldblatt D, et al. Interchangeability of meningococcal group C conjugate vaccines with different carrier proteins in the United Kingdom infant immunisation schedule. Vaccine 2015; 33:648-55.
- Department of Health/Public Health England. Changes to the schedule for meningococcal serogroup C conjugate vaccine 7 May 2013.
- de Whalley PC1, Snape MD, Plested E, Thompson B, Nuthall E, Omar O, Borrow R, Pollard AJ. Long-term seroprotection after an adolescent booster meningococcal serogroup C vaccination. Arch Dis Child 2013; 98:686-691; PMID:23853000; http://dx.doi.org/10.1136/archdischild-2013-303893
- EMEA:http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002333/human_med_001614.jsp& Accessed 18/07/2014
- JCVI. JCVI position statement on use of Bexsero® meningococcal B vaccine in the UK. 21st March 2014
