Abstract
Invasive pneumococcal disease (IPD) and pneumonia are the major causes of morbidity and deaths in children in the world. The management of IPD and pneumonia is an important economic burden on healthcare systems and families. The aim of this study was to assess the economic burden of IPD and pneumonia among younger children in Taiwan. We used a cost-illness approach to identify the cost categories for analysis in this study according to various perspectives. We obtained data of admission, outpatient, and emergency department visit data from the National Health Insurance Research (NHIR) database for children <5 y of age between January 2008 and December 2008. A prospective survey was administered to the families of patients to obtain detailed personal costs. All costs are presented in US dollars and were estimated by extrapolating 2008 cost data to 2013 price levels. We estimated the number of pneumococcal disease cases that were averted if the PCV-13 vaccine had been available in 2008. The total annual social and hospital costs for IPD were US $4.3 million and US $926,000, respectively. The total annual social and hospital costs for pneumonia were US $150 million and US $17 million, respectively. On average, families spent US $653 or US $218 when their child was diagnosed with IPD or pneumonia, respectively. This cost is approximately 27%–81% of the monthly salary of an unskilled worker. In conclusion, a safe and effective pediatric pneumococcal vaccine is needed to reduce the economic burden caused by pneumococcal infection.
Definitions
Invasive pneumococcal disease (IPD) was defined as an acute illness associated with the isolation of pneumococcus from a normally sterile body site (e.g., blood, cerebrospinal fluid, synovial fluid, pericardial fluid, pleural fluid, lung tissue, or peritoneal fluid).Citation1
Pneumonia was defined as an acute illness with the presence of new or progressive infiltrates on chest radiograph, plus at least 2 of the following symptoms: fever, cough, dyspnea, or pleuritic chest pain. Blood culture-negative pneumonias were divided into 2 subgroups according to the chest radiograph results, the white blood cell (WBC) counts, and the amount of C-reactive protein (CRP) for the purpose of determining the relative likelihood that S. pneumoniae was the causative organism. The pneumococcal infection group presented lobar/focal (unilateral) consolidation on chest radiographs and ≥15 × 109 WBC/liter (with <60% neutrophils) or ≥8 mg/l CRP. The possible pneumococcal infection group had either lobar/focal or unilateral consolidation on chest radiograph and <15 × 109 WBC/l (or ≥15 × 109 WBC/liter with <60% neutrophils) and ≥8 mg/l CRP or patchy or widespread, bilateral consolidation on chest radiograph and ≥15 × 109 WBC/l (with ≥60% neutrophils) or ≥8 mg/l CRP.Citation2
Introduction
Streptococcus pneumoniae is an important cause of bacterial pneumonia, meningitis, and sepsis in children in developed and developing countries.Citation3-7 In 2000, approximately 14.5 million cases of serious pneumococcal disease occurred. Cases with pneumococcal infection were responsible for approximately 826,000 deaths in children 1–59 months of age, of which 91,000 deaths occurred in HIV-positive children and 735,000 deaths (with estimates ranging from 519,000–825,000) occurred in HIV-negative children. Of the deaths in HIV-negative children, over 61% occurred in 10 African and Asian countries.Citation2 Pneumococcal disease is associated with substantial morbidity and mortality. Furthermore, it results in numerous hospitalizations, outpatients, and antibiotic prescriptions and leads to productivity losses for parents and other informal caregivers.Citation8 The 7-valent pneumococcal conjugate vaccine (PCV-7) was first approved in the United States (US) in 2000 to prevent diseases due to Streptococcus pneumoniae in infants and young children. In 2007, the World Health Organization (WHO) recommended incorporating the vaccine into their own national childhood immunization programs.Citation9 In 2009, 10-valent (PCV-10) and 13-valent (PCV-13) pneumococcal conjugate vaccines with extended serotype coverage were also introduced. Since their introduction, the PCV-7 vaccine has been replaced by these newer vaccines gradually.Citation10 The PCV-10 is a mixture of conjugates of 10 capsular polysaccharides (CP) to the non-typable Haemophilus influenza carrier protein, whereas the PCV-13 is a mixture of conjugates of each of 13 CPs to the same carrier protein (CRM197) as that of PCV-7. To encourage introduction of the PCV-10 or PCV-13 vaccine, cost-effectiveness analysis has been conducted in many different countries. In general, these studies have given the results that vaccination with the PCV-10 or PCV-13 vaccine is cost-effective or cost-saving compared with the PCV-7 vaccine in the prevention of diseases with S. pneumonia infection.Citation11–14 In Taiwan, the PCV-7 vaccine was adopted in October 2005. The coverage rate of PCV-7 vaccination for Taiwanese infants is considered to be markedly low (approximately 10%) because vaccination costs must be paid out of pocket.Citation15
As more effective PCV vaccines become available, policy makers will need to concern regarding the relative cost-benefit relationship of vaccination while also considering the clinical effectiveness of this prevention measure. Policy makers should take account of the economic burden of disease, the impact of vaccination on health and economic outcomes due to vaccination and the net benefit of vaccination. The costs of vaccination must be evaluated with respect to its health impacts.Citation16 However, very rare data on pneumococcal disease and its economic burden is available to provide policy maker for making appropriate decisions regarding the implementation of a vaccination program in Taiwan. Therefore, we analyzed economic data on pneumococcal infections in Taiwan to investigate the direct cost of healthcare systems for pneumococcal infections and the indirect costs borne by individual patients in Taiwan.
Results
During January 2008 and December 2008, a total of 29,991 children <5 y of age with IPD or pneumonia were included in the study. Of these children, 15,389 (51%) were male and 14,602 (49%) were female. A total of 172 (0.6%) children were diagnosed with IPD, of which 5 (29%) died. A total of 29,819 children were diagnosed with pneumonia, of which 15,335 (51%) were hospitalized, 14,299 (48%) received outpatient treatment, and 185 died. We estimated the number of pneumococcal disease cases that were averted if the PCV-13 vaccine had been available in 2008. The incidence rates for IPD, hospitalized pneumonia, and outpatient pneumonia were 19.5 cases/100,000 individuals, 17.4 cases/1,000 individuals, and 16.2 cases/1,000 individuals, respectively. The fatality rates for IPD and pneumonia were 2.9 cases/100 individuals and 0.6 cases/100 individuals, respectively. The estimated cumulative incidence of pneumococcal infection was 3%. In other words, approximately 3 in 100 children were hospitalized or attended the OPD for pneumococcal infections by their fifth year of life.
We calculated the costs according to the various types of disease, which included a group of only IPD cases (n = 172) and a group of only pneumonia cases (n = 29,819). All costs are presented in US dollars and were estimated by extrapolating 2008 cost data to 2013 price levels. shows the overall cost of the 29,991 children with hospitalization. The total cost of medical care from January 2008 to December 2008 was estimated as US $925,535 for children with only IPD and $16,958,301 for children with only pneumonia. Furthermore, the indirect costs were US $3,416,658 for children with only IPD and $132,797,362 for children with only pneumonia.
Table 1. Hospital and clinic healthcare costs per visit used to calculate the social and private costs of pneumococcal infection in Taiwan
Table 2. Total social and private costs of IPD and pneumococcal pneumonia in Taiwan
A breakdown of the costs at the single patient level is showed in . The costs of medical care per child from January 2008 to December 2008 were US $5,381 (1,156) for children with only IPD and $568 (304) for children with only pneumonia. Furthermore, the indirect costs were US $611,487 (128,716) for children with only IPD and $600,397 (78,616) for children with only pneumonia. For patients with IPD, the mean (SD) total social cost was US $616,868 (123,276), which was derived from a mean (SD) total direct cost of US $5,381 (1,156) and a mean (SD) total indirect cost of US $611,487 (128,716). The mean (SD) total medical cost for patients with IPD was US $5,381 (1,156), and the mean total cost paid by family was US $653 (400). Therefore, up to 12% (US $653) of the costs for medical care was the family expenditure. This value is approximately 81% of the monthly salary (US $806) of an unskilled worker. For patients with pneumonia, the mean (SD) total social cost was US $600,965 (77,812), which was derived from a mean (SD) total direct cost of US $568 (304) and a mean (SD) total indirect cost of US $600,397 (78,616). The mean (SD) total medical cost for patients with pneumonia was US $568 (304), and the mean total cost paid by family was US $218 (134). Therefore, up to 38% (US $218) of the total costs for medical care was the family expenditure. This value is approximately 27% of the monthly salary of an unskilled worker.
Table 3. Mean social and private costs of IPD and pneumococcal pneumonia in Taiwan
We estimated the number of pneumococcal disease cases that were averted because of the PCV-13 vaccine. We estimated the number of sp-pneumococcal disease cases in Taiwan with serotypes that were covered by the PCV-13 vaccine. First, we assumed the serotype contained in the vaccine was 80% (95% CI 59–90%).Citation17,18 The next step, we multiplied the cases with PCV-13 serotype coverage by the efficacy rate of the vaccine for pneumococcal disease, which was assumed to be 80% for IPD, 33% for pneumonia with hospitalization, and 9% for pneumonia using the 4-dose schedule (3 dose primary before 6 months of age plus a booster at 12 to 15 months of age).Citation19,20 The estimated number of pneumococcal disease cases that were prevented by vaccination was equal to the serotype coverage rate multiplied by the efficacy rate multiplied by the estimated number of cases attributable to sp. Therefore, the estimated number of IPD cases that were prevented by vaccination was 110 (0.8 × 0.8 × 172 = 110). Vaccination prevented approximately 4,048 (0.8 × 0.33 × 15,335 = 4,048) pneumonia cases that would have required hospitalization. In addition, vaccination prevented approximately 1,029 (0.8 × 0.09 × 14,299 = 1,029) pneumonia cases that would have required outpatient treatment. There were 52 ([0.8 × 0.8 × 5] + [0.8 × 0.33 × 185] = 52) deaths from IPD and pneumonia prevented by vaccination.
Discussion
Healthcare systems primarily reduce morbidity and premature death due to pneumococcal diseases. Studies on the cost of these illness can provide insight into the characteristics and item spending for specific diseases, leading to important strategies to reduce the costs of therapy. In this study, we have extended our knowledge of the pneumococcal disease burden in Taiwan by evaluating the cost of pneumococcal infections and by promoting the application of a pneumococcal vaccine program. In Taiwan, the burden of pneumococcal disease burden is considerable: approximately 30,000 cases (15,502 hospitalizations and 14,299 OPD visits and 190 fatal cases) per year among children <5 y of age.Citation21,22 In parallel, the healthcare costs of pneumococcal diseases are substantial. The total annual direct medical costs for admissions with IPD and pneumonia, including direct medical costs and direct non-medical costs (e.g., travel cost), were approximately US $4.3 million (NT $133.3 million) and US $149.8 million (NT $4.6 billion), which equates to approximately US $25,245/child and $5,021/child, respectively. The healthcare costs of IPD and pneumonia represent 79.1% and 15.7%, respectively, of the gross national income in 2008 ($31,900 per capita). In this study, we estimated the total social costs, the total indirect costs, and the total costs paid by family, which have not been estimated for cases of pneumococcal disease previously. These cost estimates demonstrate that most costs related to pneumococcal disease are indirect medical costs, which mainly consist of the cost of productivity loss (time cost of the caregivers and PMC).
In Taiwan, routine immunizations in children are funded by the government through a system of public health centers. Very few children receive their routine immunizations privately. The rationale for this study from the H/C perspective was that a substantial cost to the H/C, and therefore to the Taiwan government, would persuade the government to adopt a pneumococcal vaccine into the routine immunization schedule for children. In addition, we documented the extra expenditures incurred by the families of children with pneumococcal infections and who used the highly subsidized NHI system (i.e., the total cost paid by family). Our study demonstrate that the families incurred total costs of US $653 or US $218 when their child was diagnosed with IPD or pneumonia, respectively. The monthly salary for unskilled workers in Taiwan is US $806 (NT $25,000); therefore, IPD or pneumonia with hospitalization resulted in costs equivalent to approximately 80% and 30% of their monthly salary, respectively. However, this study only assessed the costs of IPD and pneumonia cases that required hospital admission or physician visits, and the costs associated with cases in children who were cared for in the home or who remained undiagnosed were not included.
Socioeconomic studies have several limitations, and caution must be exercised in interpreting these results. The cost per unit of service and the value of resources used in the treatment of pneumococcal disease may be difficult to accurately quantify. Information regarding all major direct expenditures and indirect costs are required for calculating the cost of an illness. Only costs for hospitalized patients, outpatient care and mortality were estimated in this study. Costs from the sequelae of meningitis and encephalitis were not included in the study. In addition, although otitis media is one of the important pneumococcal disease, it was not included in the study due to their diagnosis was unspecific to pneumococal infection. The absence of such data was a weakness of the current study. These data may represent a conservative estimate of the overall costs. To the best of our knowledge, this study is the first to evaluate the economic burden of childhood pneumococcal disease in Taiwan.
The findings of this study extend the results of previous research on the economic burden of pneumococcal infection. Several cost-of-illness studies have been conducted in other countries; Citation13,23-25 however, direct comparisons between these studies and the present study are difficult. The estimated burden of IPD (19.5 cases/100,000 individuals) and hospitalized pneumonia (17.4 cases/1,000 individuals) in this study is similar to reported rates in Hong Kong (23.7 cases/100,000 individuals and 931.6 cases/100,000 individuals, respectively)Citation26 and Japan (11.7 cases/100,000 individuals and 17.6 cases/1,000 individuals, respectively).Citation27 A comparison between the present study in Taiwan and studies in Japan may be feasible because of them have similar socioeconomic environments and approaches to healthcare delivery in these countries.Citation23,24 In this study, the estimated costs, including direct and indirect costs, were derived from data on the disease burden of pneumococcal infection with hospitalization according to detailed cost data. The annual direct costs associated with pneumococcal infection were estimated to be US $42.1 million for hospitals based on a birth cohort of 480,000 new born babies and assumed rates of 19.6 cases and 1,854.2 cases per 100,000 general pediatric hospitalizations related to IPD and pneumonia, respectively.Citation24 For a cohort with population of 220,000 new born babies, the equivalent admission costs were approximately US $15.1 million. Our hospital costs (US $9.9 million) were much lower than the estimates of admission costs in Japan. The main reason is due to the differences in the estimates of direct cost. In children with pneumococcal infection-associated illnesses, hospitalizations accounted for the most costs to the healthcare system; therefore, it will be important to evaluate the effect of a pneumococcal vaccine on the admission rate when more data become available.
The implementation of a national pneumococcal vaccination program for children in Taiwan would prevent approximately 64% (110/172) of IPD, 17% (5,077/29,819) of pneumonia, and 27% (52/190) of deaths due to pneumococcal infection. Pneumococcal infection imposes a significant economic burden in Taiwan. The adoption of a safe and effective PCV vaccine may not prevent all cases with pneumococcal infection; however, a vaccine would be expected to result in significant cost savings for both the society and families.
Methods
Data on the disease burden
The Taiwanese government implemented a mandatory national health insurance program in 1995. By 1999, approximately 96% of the population in Taiwan was covered by the program.Citation28 The program provides comprehensive coverage. It covers admission care, ambulatory care, laboratory tests, prescription drugs and certain nonprescription drugs, dental services, traditional Chinese medicine, and certain preventative services. A co-payment is required for ambulatory care, inpatient care, and pharmaceuticals. No co-payment is required for low-income households and veterans or for services associated with catastrophic diseases, childbirth, or preventative healthcare. In addition, medical services in specific mountain areas or on offshore islands are exempt from co-payments. To obtain a national estimate of the mean number of invasive pneumococcal disease (IPD) and pneumonia cases that required care, we collected the data for children less than 5 y of age from the Taiwan National Health Insurance Research (NHIR) database between January 2008 and December 2008.
The eligible study population was selected from NHIR database in 2008 according to the International Classification of Disease, Ninth Revision, and Clinical Modification (ICD-9-CM) diagnosis (ICD-9 CM easy coder 2002). Classifications for major pneumococcal disease include pneumococcal meningitis (ICD-9-CM code, 320.1), streptococcal meningitis (ICD-9-CM code, 320.2), streptococcal septicemia (ICD-9-CM code, 038.0), pneumococcal septicemia (ICD-9-CM code, 038.2), unspecified septicemia (038.9), bacteremia (ICD-9-CM code, 790.7), and pneumonia (ICD-9-CM codes, 481–486). All pneumococcal disease events were considered discrete cases, even when patients were treated for a pneumococcal infection on more than one occasion. The sum of the cases of pneumococcal septicemia (code 038.2) and pneumococcal meningitis (code 320.1) was used to estimate the rates of IPD. Although otitis media is one of the important pneumococcal disease, it was not included in the study due to their diagnosis was unspecific to pneumococal infection.
Population figures from the 2008 census by the Statistics Department, Interior Ministry, Taiwan were used in all of the calculations. The number of children younger than 5 y of age was 880,000. Age was defined as the number of complete years since birth.
Because discharge coding is known to be imprecise,Citation29 data from the Notifiable Diseases Surveillance System (NNDSS) were used to assess the sensitivity of the coding data as a surrogate for IPD and to obtain the proportion of radiological pneumonia diagnosed under code 486, which indicates unspecific pneumonia. Since 1990, the National Notifiable Diseases Surveillance System (NNDSS) has been developed in Taiwan described in the literature previously.Citation30 In 2007, the reporting system for invasive pneumococcal disease (IPD) was established. Physicians are required by law to report all cases of IPD to the Taiwan CDC within one week of case identification using Taiwan CDC-developed software.Citation31–33 After these reports are received by the CDC, a public health workers is assigned to verify the diagnosis, collect patient information, and traced the contacts. We cross-checked these data on IPD with those in the NHIR database to confirm the reporting accuracy. All cases with IPD had been reported to both Taiwan CDC and NHI program. The reporting accuracy was 100%.
Institutional Review Board approval for this study was obtained from of National Cheng Kung University Hospital.
Data on cost
Cost and wages published before 2008 were updated according to the consumer price index.Citation34 Future disease costs and IPD and pneumonia cases were modeled to decrease at an annual discount rate of 3%. The medical costs of IPD and pneumonia cases over a 5-year period were calculated according to the age-specific incidence estimates for each outcome. Pneumonia mortality and the productivity costs of IPD were estimated using the average life expectancy of a child younger than 5 y of age. All of the costs were estimated by extrapolating the 2008 US dollars to 2013 price levels. The monetary value in New Taiwan dollars (NT$) was converted into United States dollars (US$) based on the average exchange rate in 2013 (1 US$ = 31.0 NT$).
We obtained hospitalization, outpatient, and emergency department visit data from the National Health Insurance Research (NHIR) database from 2008. The NHIR database contains every medical claim record (inpatient and outpatient care) for patients, including the patient ID number, gender, birth date, date of visit, and length of hospital stay (LOS). The direct medical costs for patients were collected from the NHRI database. The NHIR database contains records of every medical claim (inpatient and outpatient care).
Indirect costs were collected from all cases of IPD and a representative sample of cases with pneumonia. Taiwan was divided into 4 regions including Northern, Central, Southern and Eastern region. In 2008, 100 patients in NHIR data bank were randomly selected in each region as the patient sample. The inclusion criteria were the patient who aged <5 y of age and diagnosed with pneumonia in 2008. Informed consent was obtained from participating families. Information of demographic characteristics, clinical picture, and costs related to pneumococcal infection was collected by a case report form designed by authors. Information on the costs associated with each IPD or pneumonia episode in each child was completed by the families, and the families were to fill the form and return within 7 d of study recruitment. The public health worker demonstrated how to complete the case report form, which included all of the costs relevant to the illness episode, extra travel costs; the other additional costs (e.g., food supplements or nonprescription drugs); time off work due to the illness; and the salary lost was estimated by hour, day, week, or month and the length of stay (LOS) in hospital; visits to other hospitals or clinics. The indirect costs were estimated by extrapolating the sample cost data to study population price levels.
Data analysis
The cost analysis incorporated both monetary and time costs when applicable. Pneumococcal infection-related costs include direct expenditures for medical care and medical costs.Citation35 In direct expenditures for medical care included costs and charges (as proxy for cost) for hospitalization, outpatient services and medication. Information on drugs that were not directly related to pneumococcal infection (e.g., treatment for fungus infection) was difficult to obtain and was therefore not considered. Indirect costs included the cost of time lost from work by caretakers due to the illness of pneumococcal infections.
The premature mortality cost (PMC) was calculated as follows: the human capital approach equates productivity lost to the wage rate of an individual and is based on the assumption that an individual produces a stream of output over a working lifetime, which is cut short by premature death. Years of potential productive life lost (YPLL) were calculated by multiplying the number of pneumococcal infection-specific deaths for a given age group (<1, 1–2, 3–4 y) by the expected productive life years that remained at the mid-point for each age group. Expected productive life years were derived from a standard modeled life table with a retirement age of 65.Citation36,37 On average, the monthly salary for unskilled workers in Taiwan was US $806 (NT $25,000) in 2008.
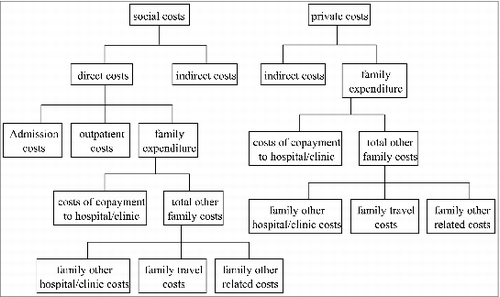
presents the flow chart of estimated costs. The costs were estimated social costs (total direct costs + total indirect costs) and private costs (total indirect costs + total costs paid by family). The individual cost was calculated as followings:
| 1. | Total direct costs included total hospital/clinic (H/C) costs and total costs paid by family:
| ||||||||||||||||||||||||||||||||||
| 2. | The total costs paid by family included all co-payments made by the family to the hospitals or clinics (family H/C costs) and the total other family costs. This parameter was estimated as followings:
| ||||||||||||||||||||||||||||||||||
| 3. | Total indirect costs were estimated as the parent's time cost + other caretaker's time cost, where parent's time cost = (hours/days off work) (parent's estimated hour/day salary), and other caretaker's time cost = (hours/days off work) (other's estimated hour/day salary). | ||||||||||||||||||||||||||||||||||
Statistical analysis
The cost data are presented as the total and mean (SD). The costs were classified as the following types of disease: only IPD cases, only pneumonia cases, and total cases. All costs are presented in US dollars and were estimated by extrapolating 2008 cost data to 2013 price levels. We estimated the number of pneumococcal disease cases that were averted if the PCV-13 vaccine had been available in 2008.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by a grant (MOST 103-2314-B-217-001) from the National Science Council of Taiwan and in part by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002), China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092), NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039 -006), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo AoshimaMemorial Funds, Japan. The funder played no role in the study design, data collection, data analysis, decision to publish, or preparation of the manuscript.
References
- Kellner JD, Vanderkooi OG, MacDonald J, Church DL, Tyrrell GJ, Scheifele DW. Changing epidemiology of invasive pneumococcal disease in Canada, 1998-2007: update from the Calgary-area Streptococcus pneumoniae research (CASPER) study. Clin Infect Dis 2009; 49(2):205-12; PMID:19508165; http://dx.doi.org/10.1086/599827
- O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009; 374:893-902; PMID:19748398; http://dx.doi.org/10.1016/S0140-6736(09)61204-6
- Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995-2003. Pediatr Infect Dis J 2004; 23(9):824-8; PMID:15361720; http://dx.doi.org/10.1097/01.inf.0000136871.51792.19
- Block SL, Hedrick J, Harrison CJ, Tyler R, Smith A, Findlay R, Keegan E. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr Infect Dis J 2004; 23(9):829-33; PMID: 15361721
- Murphy TF. Vaccine development for non-typeable Haemophilus influenza and Moraxella catarrhalis: progress and challenges. Expert Rev Vaccines 2005; 4(6):843-53[Review]; PMID:16372880; http://dx.doi.org/10.1586/14760584.4.6.843
- O’Neill JM, St Geme JW 3rd, Cutter D, Adderson EE, Anyanwu J, Jacobs RF, Schutze GE. Invasive disease due to nontypeable Haemophilus influenzae among children in Arkansas. J Clin Microbiol 2003; 41(7):3064-9; PMID:12843045; http://dx.doi.org/10.1128/JCM.41.7.3064-3069.2003
- Ladhani S, Slack MP, Heath PT, von Gottberg A, Chandra M, Ramsay ME; European Union Invasive Bacterial Infection Surveillance participants. Invasive Haemophilus influenzae Disease, Europe, 1996-2006. Emerg Infect Dis 2010. 16(3):455-63; PMID:20202421
- Morrow A, De Wals P, Petit G, Guay M, Erickson LJ. The burden of pneumococcal disease in the Canadian population before routine use of the seven-valent pneumococcal conjugate vaccine. Can J Infect Dis Med Microbiol 2007; 18(2):121-7; PMID:18923713
- World Health Organization. Pneumococcal conjugate vaccine for childhood immunization - WHO position paper. Wkly Epidemiol Rec 2007; 82(12):93-104; PMID:17380597
- Pneumococcal vaccines WHO position paper -2012. Wkly Epidemiol Rec 2012; 87(14):129-44; PMID:24340399
- Blank PR, Szucs TD. Cost-effectiveness of 13-valent pneumococcal conjugate vaccine in Switzerland. Vaccine 2012; 30(28):4267-75; PMID:22521287; http://dx.doi.org/10.1016/j.vaccine.2012.04.028
- Strutton DR, Farkouh RA, Earnshaw SR, Hwang S, Theidel U, Kontodimas S, Klok R, Papanicolaou S. Cost-effectiveness of 13-valent pneumococcal conjugate vaccine: Germany, Greece, and The Netherlands. J Infect 2012; 64(1):54-67; PMID:22085813; http://dx.doi.org/10.1016/j.jinf.2011.10.015
- Rubin JL, McGarry LJ, Strutton DR, Klugman KP, Pelton SI, Gilmore KE, Weinstein MC. Public health and economic impact of the 13-valent pneumococcal conjugate vaccine (PCV13) in the United States. Vaccine 2010; 28(48):7634-43. PMID:20883739; http://dx.doi.org/10.1016/j.vaccine.2010.09.049
- Tyo KR, Rosen MM, Zeng W, Yap M, Pwee KH, Ang LW, Shepard DS. Cost- effectiveness of conjugate pneumococcal vaccination in Singapore: comparing estimates for 7-valent, 10-valent, and 13-valent vaccines. Vaccine 2011; 29(38):6686-94; PMID:21745516; http://dx.doi.org/10.1016/j.vaccine.2011.06.091
- Chen YY. Streptococcus Pneumoniae infection in Taiwan, 2008-2010. Taiwan Epidemiol Bull 2011;27(22):297-304.
- Trueman P, Drummond M, Hutton J. Developing guidance for budget impact analysis. Pharmacoeconomics 2001; 19:609-21; PMID:11456210; http://dx.doi.org/10.2165/00019053-200119060-00001
- Chen YY, Yao SM, Chou CY, Chang YC, Shen PW, Huang CT, Su HP, Li SY. Surveillance of invasive Streptococcus pneumonia in Taiwan, 2002-2003. J Med Microbiol 2006; 55(Pt 8):1109-14; PMID:16849732; http://dx.doi.org/10.1099/jmm.0.46530-0
- Lucero MG, Dulalia VE, Nillos LT, Williams G, Parreño RA, Nohynek H, Riley ID, Makela H. Pneumococcal conjugate vaccines for preventing vaccine-type invasive pneumococcal disease and X-ray defined pneumonia in children less than two years of age. Cochrane Database Syst Rev 2009; (4):CD004977; PMID:19821336; http://dx.doi.org/10.1002/14651858.CD004977.pub2
- Jansen AG, Hak E, Veenhoven RH, Damoiseaux RA, Schilder AG, Sanders EA. Pneumococcal conjugate vaccines for preventing otitis media. Cochrane Database Syst Rev 2009; (2):CD001480; PMID:19370566; http://dx.doi.org/10.1002/14651858.CD001480.pub3
- Nuorti JP, Whitney CG, Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - use of 13-valent vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2010; 59(RR-11):1-18
- Chen KT, Chen PY, Tang RB, Huang YF, Lee PI, Yang JY, Chen HY, Bresee J, Hummelman E, Glass R. Sentinel hospital surveillance for rotavirus diarrhea in Taiwan, 2001-2003. J Infect Dis 2005; 92:s44-8; PMID:16088804; http://dx.doi.org/10.1086/431495
- Bureau of National Health Insurance, R.O.C. (Taiwan). The national health insurance annual statistical report, 2013. Accessed on 15 August 2014 by http://www.nhi.gov.tw/00english/e_index.htm
- Sohn HS, Suh DC, Jang E, Kwon JW. Economic evaluation of childhood 7-valent pneumococcal conjugate vaccination in Korea. J Manag Care Pharm 2010; 16:32-45; PMID:20044845
- Hoshi SL, Kondo M, Okubo I. Economic evaluation of vaccination programme of 13-valent pneumococcal conjugate vaccine to birth cohort in Japan. Vaccine 2013; 31:2762-71; PMID:23588088; http://dx.doi.org/10.1016/j.vaccine.2013.03.052
- Kulpeng W, Leelahavarong P, Rattanavipapong W, Sornsrivichai V, Baggett HC, Meeyai A, Punpanich W, Teerawattananon Y. Cost-utility analysis of 10- and 13-valent pneumococcal conjugate vaccines: Protection at what price in the Thai context? Vaccine 2013; 31:2839-47; PMID:23588084; http://dx.doi.org/10.1016/j.vaccine.2013.03.047
- Ho PL, Chiu SS, Chow FK, Mak GC, Lau YL. Pediatric hospitalization for pneumococcal diseases preventable by 7-valent pneumococcal conjugate vaccine in Hong Kong. Vaccine 2007; 25:6837-41; PMID:17714837; http://dx.doi.org/10.1016/j.vaccine.2007.07.039
- Tanaka J, Ishiwada N, Wada A, Chang B, Hishiki H, Kurosaki T, Kohno Y. Incidence of childhood pneumonia and serotype and sequence-type distribution in Streptococcus pneumoniae isolates in Japan. Epidemiol Infect 2012; 140:1111-21; PMID:21875450; http://dx.doi.org/10.1017/S0950268811001592
- Bureau of National Health Insurance, R.O.C. (Taiwan). The national health insurance annual statistical report, 2008. Accessed on 15 August 2014 by http://www.nhi.gov.tw/00english/eindex.htm
- Guevara RE, Butler JC, Marston BJ, Plouffe JF, File TM Jr, Breiman RF. Accuracy of ICD-9-CM codes in detecting community-acquired pneumococcal pneumonia for incidence and vaccine efficacy studies. Am J Epidemiol 1999; 149:282-9; PMID:9927225; http://dx.doi.org/10.1093/oxfordjournals.aje.a009804
- Chen SC, Hsiao-Ling Chang HL, Chen KT. The epidemiology of imported malaria in Taiwan between 2002-2013: the importance of sensitive surveillance and implications for pre-travel medical advice. Int J Environ Res Public Health 2014; 11(6):5651-64; PMID:24871257; http://dx.doi.org/10.3390/ijerph110605651
- Centers for Disease Control, R.O.C. (Taiwan). Notifiable infectious disease statistical system. Available at http://nidss.cdc.gov.tw ( accessed on 14 May 2014).
- Chang YK, Chen JY, Chang HL, Yu MC, Hsiao HF, Hou CC, Liu SY, Chen KT. Absence of endemic measles transmission in a highly vaccinated population from 1999 to 2008: implications of sustained measles elimination in Taiwan. Vaccine 2010; 28:5332-7; PMID:20665978; http://dx.doi.org/10.1016/j.vaccine.2010.05.047
- Chen KT, Chang HL, Wang ST, Cheng YT, Yang JY. Epidemiologic features of hand-foot-mouth disease and herpangina caused by enterovirus 71 in Taiwan, 1998-2005. Pediatrics 2007; 120:e244-52; PMID:17671037; http://dx.doi.org/10.1542/peds.2006-3331
- International Monetary Fund, Taiwan province of China: inflation, average consumer prices. World Economic Outlook Database. Available online at: www.imf.org. Accessed January 2014.
- Rice DP, Hodgson TA, Kopstein AN. The economic costs of illness: a replication and update. Health Care Finance Rev 1985; 7:61-80; PMID:10311399
- Murray CJ. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ 1994; 72:429-45; PMID:8062401
- Chiu SS, Ho PL, Khong PL, Ooi C, So LY, Wong WH, Chan EL. Population-based incidence of community-acquired pneumonia hospitalization in Hong Kong children younger than 5 years before universal conjugate pneumococcal immunization. J Microbiol Immunol Infect 2014 Jul 25. pii: S1684-1182(14)00104-2. http://dx.doi.org/10.1016/j.jmii.2014.05.007