Abstract
With drug resistance to available therapeutics continuing to develop against Plasmodium falciparum malaria, the development of an effective vaccine candidate remains a major research goal. Successful interruption of invasion of parasites into erythrocytes during the blood stage of infection will prevent the severe clinical symptoms and complications associated with malaria. Previously studied blood stage antigens have highlighted the hurdles that are inherent to this life-cycle stage, namely that highly immunogenic antigens are also globally diverse, resulting in protection only against the vaccine strain, or that naturally acquired immunity to blood stage antigens do not always correlate with actual protection. The blood stage antigen reticulocyte binding homolog RH5 is essential for parasite viability, has globally limited diversity, and is associated with protection from disease. Here we summarize available information on this invasion ligand and recent findings that highlight its candidacy for inclusion in a blood-stage malaria vaccine.
Introduction
Malaria, caused by protozoan parasites of the order Apicomplexa, remains one of the most important vector-borne human diseases to this day. Plasmodium falciparum infections alone account for over a million deaths annually,Citation1 and P. falciparum has had a profound impact on infants and children in sub-Saharan Africa, where the emergence of drug-resistant strains of the parasite have spread across the continent, rendering affordable chemotherapy such as chloroquine and sulfadoxine-pyrimethamine ineffective, and is threatening the effectiveness of artemisinin-based anti-malarials.Citation2 Malaria also wreaks havoc in many other epidemiological groups and population settings. It is a major international public health problem, dramatically undermining worker productivity and draining national budgets. In recent years there has been a shift from managing this disease and reducing severe symptomatic cases back toward eradication and elimination. A major goal in this approach is the development of new prophylactic agents such as drugs or vaccines. The spread of drug-resistant P. falciparum has made vaccine research all the more urgent as vaccines hold the greatest potential for reducing malaria-associated morbidity and mortality in areas with the most intense transmission, as well as preventing malaria among travelers to those regions.
The symptoms of malaria disease occur during the erythrocytic stage of the parasite, dominated by anaemia and associated complications, and are caused by the cyclical invasion, multiplication and release of merozoites from red blood cells (RBCs). Producing a successful blood stage vaccine that interrupts this cycle will reduce clinical disease, and many blood stage antigens have been identified as potential vaccine candidates, the most studied being MSP1 and AMA1. Both MSP1 and AMA1 are genetically diverse antigens, with multiple non-synonymous mutations, but are immunogenic and antibodies to these antigens in individuals from malaria endemic regions have been associated with naturally acquired immunity,Citation3-8 suggesting they could be potent vaccine candidates. However, early clinical trials have shown that although vaccine-induced anti-AMA1or anti-MSP1 antibodies are produced after immunization, they are not associated with protection against disease, or protection has been only toward the homologous (vaccine) strain and they do not elicit strain-transcending immunity.Citation9,10 Thus, the main barrier to blood stage malaria vaccine development is the identification of an antigen able to provoke a strong immune response which is also able to neutralize a wide range of parasite variants. An ideal blood stage vaccine antigen would be highly conserved across a broad spectrum of strains to increase the ability for successful heterologous challenge, and would be essential to parasite viability or reproduction so resistance could not be easily acquired by mutation, or by simply switching off expression of that antigen in favor of an alternative. RH5, a member of the reticulocyte binding homolog family is the latest blood stage antigen to be considered as a vaccine candidate, and is fast becoming a front runner as it appears to be essential to parasite invasion and limited diversity has been observed by sequencing naturally circulating, globally diverse parasite populations, with only 12 non-synonymous mutations currently identified.Citation11,12
Parasite Invasion
Invasion of free merozoites into new RBCs is a critical pinch-point in the parasite life cycle as the parasite is exposed to the peripheral blood stream, including immune cells and antibody, while they interact with and invade new erythrocytes, yet invasion is accomplished within about a minute.13 However, invasion is a complicated process that is not fully understood or delineated, and requires a series of steps at the molecular level, starting with the initial contact and recognition between merozoites and erythrocyte. The merozoite then reorientates itself so that the apical end of the parasite, where the micronemes, rhoptries and dense granules are situated, is closest to the erythrocyte surface. Some of these released proteins bind to RBC surface receptors and directly contribute to the formation of a dynamic tight junction, which moves across the merozoites surface from fore to aft. Invasion finally concludes in the resealing of the erythrocyte membrane and simultaneous formation of a parasitophorous vacuole encasing the parasite inside the host cell.Citation13,14 It is known that accumulation of intracellular calcium (Ca2+) is involved in the signaling pathways of invasion of multiple intracellular parasites.Citation16 One study suggests that the trigger for the Ca2+ accumulation and subsequent release of invasion proteins from the micronemes is associated with exposure to low potassium (K+) concentrations in the external micro-environment,Citation15 but actual commitment to invasion by proteins released after those in the micronemes may rely on triggers originating from actual contact with the RBC surface.Citation16 However, it has been shown that P. falciparum can thrive in vitro even when exposed to a broad range of K+ (as well as Na+ Cl-, and H+) concentrations in the culture medium, suggesting K+ concentration may not be the absolute trigger for Ca2+ accumulation in merozoites.Citation17 Further, the trigger for Ca2+ accumulation in Toxoplamsa gondii has been shown to be controlled by abscisic acid.Citation18 Erythrocyte attachment and entry pathways vary by parasite species, and even strain, with the most diversity observed so far in P. falciparum, which utilize multiple parasite ligands and red cell receptors.Citation19 The multiple ligand-receptor parings and subsequent redundancy observed within P. falciparum are likely an adaptive response to the heterogeneous nature of human RBC populations.Citation20-22 Given this redundancy within the invasion ligands, it is also possible that there is a redundancy in the triggers for egress leading to merozoite maturation and the release of invasion ligands.
The two main protein families have been identified as having crucial involvement during the invasion process are the Erythrocyte Binding-Like (EBL) proteins, homologous to the P. vivax Duffy Binding Protein (PvDBP), and the Reticulocyte binding homologues (RHs), homologous to the P. vivax reticulocyte binding proteins (PvRBP1 and PvRBP2). The EBL family consists of EBA-175,Citation23 EBA-140,Citation24,25 EBA-181,Citation26,27 and EBL-1,Citation28 which are located in the micronemes prior to invasion. Most members of the RH family were first recognized for their homology to the reticulocyte binding proteins of P. vivaxCitation29 and P. yoelliCitation30. In P. falciparum, the RH family comprises RH1,Citation31,32 RH2a, RH2b, RH4,Citation33 and RH5.Citation11,34,Citation35 RH2a and 2b are highly identical and are encoded by 2 genes that appear to have arisen from a duplication event as both genes are located adjacently on chromosome 13 in a head to head orientation.Citation20,36-38 EBA-165 and RH3 are pseudogenes within each family that are transcribed but not translated.Citation39,40
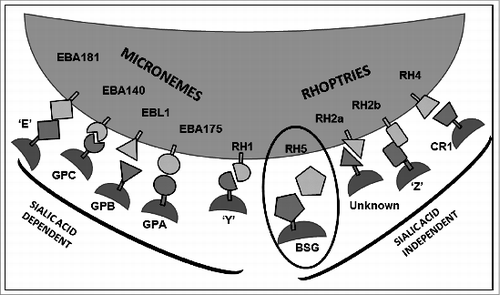
Not all parasite receptors on the RBC surface have been identified, but 2 major invasion pathways have been characterized: ligands from the EBL family largely bind to receptors which contain sialic acid residues and thus govern the sialic acid (SA) dependent pathway of invasion, and the RH family, except for RH1, mediate SA independent invasion as they bind to RBC receptors which do not contain sialic acid residues (Fig. 1). The glycophorins (GP) comprising GPA,Citation41 GPB,Citation42,43 and GPCCitation44 are the major sialylated proteins on the RBC surface and have been identified as the receptors to which EBA-175, EBL-1 and EBA-140 bind, respectively. Neuraminidase, chymotrypsin and trypsin are enzymes that respectively cleave sialic acid residues, specific peptide bonds, and general proteins, and are a useful tool in defining the characteristics of RBC receptors. The receptor to which EBA-181 binds has yet to be identified, but has been found to be neuraminidase and chymotrypsin sensitive, and trypsin resistant (“E”),Citation26,45 and it does also bind to band 4.1, a protein located inside the erythrocyte.Citation46 RH2a, RH2b, RH4 and RH5 all utilize the sialic acid independent pathway. RH1 is an exception as it binds to an as-yet unidentified receptor which is neuraminidase-sensitive, chymotrypsin- and trypsin-resistant receptor (“Y”),Citation31,32 thus utilizing the sialic acid dependent pathway (Fig. 1). The receptor(s) to which RH2a and RH2b bind have not yet been identified, but RH2b recognizes a neuraminidase and trypsin resistant but chymotrypsin sensitive (“Z”).Citation20,47, Citation48 RH4 binds to complement receptor 1,Citation49-51 and RH5 binds to basigin.Citation52 The release of ligands for invasion follows a specific sequence with the proteins of the micronemes (EBL and AMA proteins) being released first followed by release of the rhoptry proteins, including the RHs,Citation15,16 although the exact timing and function of RH5 within the invasion process has yet to be fully elucidated. RH5 lacks a transmembrane domain or membrane anchor but is indirectly linked to the membrane via a complex with P. falciparum RH5 interacting protein (PfRipr), a protein released from the micronemes,Citation53 and with Cysteine-rich protective antigen (CyRPA), a GPI-linked protein also localized to the micronemes.Citation54 The expression of the RH proteins has been shown to correlate with the invasion phenotypes of different parasite lines. The laboratory strains Dd2, MCamp and FCR3 express higher levels of RH1 and low levels of RH2a/2b and as such are sialic-acid dependent strains,Citation55-57 while 3D7, HB3, 7G8 are sialic acid independentCitation55-57 as they express higher levels of RH2a/2b and lower levels of RH1.Citation20,32,Citation58 The ability to switch between invasion pathways due to selective pressure is most clearly observed in the laboratory isolate Dd2, which switches to the non-sialic acid pathway in the presence of neuraminidase-treated RBCs,Citation59 and is associated with an up-regulation of RH4.Citation60,61
Figure 1. Schematic of invasion ligands strictly from the Erythrocyte Binding Like (EBL) and Reticulocyte binding Homologue (RH) families, and the erythrocyte receptors to which they bind, as far as they are known, visually organized to show whether they invade RBCs by the sialic acid dependent or independent pathways. Invasion ligands from the EBL and RH families are located at the apical end of merozoites prior to invasion within the micronemes and rhoptries, respectively. The only ligand essential for invasion identified so far is RH5, which is not anchored directly itself to the merozoite surface and binds to basigin (circled). GP – glycpophorin; ‘E’, ‘Y’, and ‘Z’ are unidentified receptors but their enzymatic profiles have been established; CR1 – complement receptor 1; BSG - basigin.
RH5
RH5 is located in a subtelomeric region of chromosome 4, upstream from RH4Citation61 that contains species-specific genes involved in host-parasite interactions.Citation62 It lacks a C-terminal transmembrane domain and the full-length translated protein is much smaller than the other RH proteins at ∼63 kDa.Citation11,34,Citation35 A processed fragment of 45 kDa product have been observed in native parasite lysates,Citation11,34,Citation35 thought to represent the C-terminus portion, with the N-terminus fragment being lost upon cleavage prior to binding to basigin,Citation35 but the exact sites of cleavage and the timing at which the protein is processed is not clear, and products of 28 and 31 kDaCitation11,63 were observed in the absence of the 45 kDa fragment, suggesting further cleavage events occur in RH5 at some point during the invasion process. Attempts at generating a RH5 knock-out have not been successful,Citation11,35 suggesting this protein pays an essential function in the overall invasion process. Although RH5 is located in the rhoptries at the apical end of merozoites,Citation11,34,Citation35 it does not co-localize with other rhoptry proteins, like the PfRhopH2 and PfRAP1, which are known to reside in the rhoptry bulb,Citation11,34,Citation35,64 or with those proteins in the rhoptry neckCitation63,65 but the processed 45 kDa fragment does form a complex with PfRipr in late-stage schizonts and in merozoites, and this RH5/PfRipr complex interacts with CyRPA at the apical end of merozoites during invasion.Citation53,54,Citation63 If co-localization with PfRipr then CyRPA, occurs at the formation of the moving junction, it would only be accessible to antibodies for a short time of about 30 seconds as the merozoites makes contact and re-orientates to form the junction.Citation66 If RH5 is exposed between reorientation and junction formation only, the time for antibody to access the ligand is even shorter, closer to 11 seconds.Citation66
RH5 mediates invasion through the sialic acid independent pathway and binds to basigin on the erythrocyte surface.Citation52 Basigin, also known as CD147, EMMPRIN and M6, is a member of the Ok blood group and has many biological functions including embryo implantation, spermatogenesis,Citation67 and retinal development.Citation68 It has been shown that all P. falciparum strains have the same dependency on basigin for invasion as anti-basigin inhibited parasite invasion in both lab and freshly isolated strains.Citation52 Although the basigin sequence is highly conserved among humans and other higher apes, such as chimpanzee (Pan troglodytes) and gorilla (Gorilla gorilla), it has been shown that the human ortholog bound RH5 with a fold10- greater affinity than the non-human basigins, which bound poorly or did not bind at all,Citation69 suggesting only a few residues are responsible for binding between RH5 and basigin. Further, all non-human primate basigins encode a glutamine at residue 191; in humans, this is a lysine residue. Mutation of the chimpanzee basigin to contain a lysine at this position allowed RH5 to bind, highlighting the importance of this residue within the RH5 binding specificity to human RBCs. Such a mutation in basigin that prevents RH5 binding but does not prevent basigin function could be a target for anti-malarial therapeutics. The crystalline structure of RH5 aloneCitation70 and of RH5 with basiginCitation71 have been determined. RH5 forms a flat ‘kite-shaped’ or ‘elliptical’ structure, with basigin (which exists on the surface of erythrocytes in its short isoforms containing 2 domainsCitation52) binding at one tip of RH5 with predominantly one domain only, creating an asymmetrical complex.Citation71 However, as it appears there is a limited involvement of the basigin side chains for binding, the potential for mutations within basigin to prevent RH5 binding and subsequent invasion is also limited.Citation71 The residues from each protein which are directly involved in binding have also been identified, including K191 of basigin as predicted, though none of the polymorphisms of RH5 observed from infections or cultures in human erythrocytes appear to be directly involved in binding with human basigin. Earlier work from Hayton et al.Citation11,72 showed that RH5 polymorphisms at residues I204K and I407V enable P. falciparum parasites to switch their ligand-receptor interactions and adapt to different RBC receptors, and the observed ability of strains from natural human infections in Africa to invade Aotus (New World) monkeys is likely from the diversity of ligand-receptor interactions exploited by P. falciparum in its various invasion pathways. The polymorphisms seen in the I204K and I407V codons may influence the binding to Aotus-specific RBC receptors, but they do not appear to be directly involved in binding to human basigin.Citation71
Anti-RH5 Antibodies from Natural Infection
The prevalence of anti-RH5 antibodies in individuals naturally exposed to malaria is not very high, suggesting this antigen is not a primary target of naturally acquired immunityCitation65,73 but native RH5 is vulnerable to naturally acquired antibody.Citation74 Antibody responses to RH proteins in individuals from Peru with malaria were significantly higher in asymptomatic vs. symptomatic individuals, suggesting an association with the development of clinical immunity, but only 11% of parasitaemic individuals had anti-RH5 antibodies, despite being naturally exposed malaria.Citation73 Others have shown a similarly low prevalence of RH5 antibodies in individuals from malaria endemic regions. In Kenya, only 16% of individuals with malaria had anti-RH5 antibodies present.Citation65 Anti-RH5 responses from low and high endemicity areas in Senegal showed there was a significant age-association with RH5 positivity in high but not low endemicity areas, suggesting repeated exposure does lead to production of anti-RH5 antibodies,Citation75 and anti-RH5 responses have been shown to be associated with protection from high density parasitaemia during re-infection after treatment in Papua New Guinea,Citation76 and has been associated with an extended time to re-infection in Malian populations.Citation74 This poor immunogenicity in natural infections may well be explained by the supposition that RH5 is exposed only transiently during cell attachment and entry.Citation53 Although these data show that naturally acquired antibodies are associated with protection from various indicators of disease, they are not sufficient to show that anti-RH5 antibodies specifically are protective, and despite these associations, it is possible that anti-RH5 antibodies are not significant causal contributors to protection. However, it is notable that RH5 is analogous to the cryptic neutralizing epitopes of some important viral agents, such as HPV L2,Citation77 HIV env,Citation78 Flu HA,Citation79 the epitopes of which are being intensively studied as the potential bases of vaccines against those agents. Like them, RH5 is intimately involved with the processes of cellular entry and even if it is only transiently exposed to the immune system during this time, it is nevertheless susceptible to neutralization by antibody. As with the broadly neutralizing antibodies of HIV, only a small percentage of patients produce a significant anti-RH5 response, and then only after prolonged chronic exposure, yet this does still correlate with relative resistance to disease progression.Citation73,75 A clear link between anti-RH5 titers and protection needs to be determined to establish the vaccine candidacy of RH5 further, but available data shows promise.
RH5 Recombinant Antigens
Large scale production of pure, correctly conformed antigens are required for successful in vitro and in vivo characterization of vaccine candidates, yet producing recombinant malarial antigens is not straight forward due to the AT-rich malarial genome and overall difficulty of expressing plasmodial proteins in heterologous expression systems.Citation80-83 Partial recombinant RH5 proteins, covering residues Asn-31 to Val-174Citation34 or Asn-191 to His-359Citation35 both produced by E. coli expression systems, did bind to erythrocytes, but anti-RH5 sera against these fragments did not inhibit parasite invasion in vivo, suggesting although these regions of RH5 do each contain epitopes required for binding to basigin, they do not contain the neutralizing epitopes targeted by anti-RH5 antibodies, or that truncated RH5 antigens are not able to fold correctly thus unable to produce specific antibodies. Full-length recombinant RH5 antigens have successfully been produced in the mammalian cell expression systems,Citation52,84 viral-vectored vaccine constructs,Citation65 wheat-germ cell freeCitation64,85 and E. coli expression systems,Citation86 and Drosophila S2 cells.Citation71 These recombinants are all based on the 3D7 sequence and are all cable of producing neutralizing antibodies, suggesting they are each fully conformed and functional antigens, and clearly showing that RH5 is both immunogenic and anti-RH5 sera does mediate potent inhibition of invasion: in vitro assays with purified rabbit IgG from, RH5 synthesized via the viral-vectored vaccine construct E. coli, mammalian, and Drosophila expression systems all showed ∼75% −90% (inferred) inhibition at 10 mg/mL.Citation65,71,Citation84,86 The wheat germ cell free system showed a neutralization effect of ∼70% inhibition of invasion in vitro at 0.5 mg/mL with purified IgG from mouse sera.Citation85 Further, production of monoclonal antibodies from mice immunized with the wheat germ recombinant RH5 that completely abrogate RBC invasion have been developed,Citation64 showing that there are critical epitopes within RH5 that are able to elicit specific antibodies with a high neutralizing activity.
Neutralizing epitopes on RH5 and potential mechanisms of action of inhibitory antibodies
Although it has been shown that antibody against RH5 can effectively neutralize parasites, the details of the exact mechanisms by which anti-RH5 antibody prevents parasite invasion have yet to be characterized fully, though the use of anti-RH5 mAbs, produced from mice immunized with a viral-vectored full-length recombinant RH5, have been employed to elucidate this process.Citation63,65 Monoclonal antibodies that were shown to be capable of blocking the RH5-basigin interaction completely in the avidity-based extracellular interaction screen (AVEXIS),Citation52,87 were not very potent inhibitors of invasion (∼40–50% inhibition). Conversely, potent inhibition of invasion was observed by anti-basigin mAbs that did not block the RH5-basigin interaction in the AVEXIS assay,Citation63 showing that absence of the ability to interrupt the RH5-basigin interaction was not a predictor of neutralizing ability in growth inhibition assays and does not fully reflect the in vivo dynamics of the invasion process. Thus it is possible that anti-RH5 mAbs that potently inhibit invasion may function by mechanisms other than direct inhibition of the RH5-basigin interaction. Mapping the targets of these mAbs, QA5 and 9AD4, revealed 2 linear epitopes within the RH5 antigen sequence: YGK(C/Y)IAVDAFIKKI (residues 200–213), and TNGIR(Y/F)HYD (residues 353–361), where C/Y and Y/F represent the location of 2 of the known non-synonymous changes in the RH5 antigen, suggesting immune pressure may be acting at these sites, though the presence of these changes did not affect binding of these mAbs to heterologous epitopes.Citation63 Although these epitopes are separated on the linear RH5 antigen, competitive binding assays suggest they are spatially related in the context of the fully confirmed RH5 protein, and likely close to the basigin binding site. Crystal structures of 9AD4 and QA1 in complex with RH5 provided further information the inhibitory epitopes targeted by these antibodies.Citation71 QA1, a mAb shown to block the RH5-basigin interaction and parasite growth, binds to residues on the RH5 tip, overlapping with sites where the N-terminus domain of basigin binds. However, the 9AD4 mAb, which does not block the RH5-basigin interaction but is a high inhibitor of parasite growth, actually binds to different epitopes, according to the crystal structure, compared to those identified in peptide binding assays. The epitopes are close to but not overlapping with the basigin binding epitopes, suggesting the high neutralizing activity of this mAb is likely due to steric hindrance preventing the RH5-basigin interaction.Citation71
Studies from our laboratory identified 3 anti-RH5 mAbs produced from mice immunized with the wheat germ recombinant RH5 antigen, 2E11, 5A03, and 5A08, that were found to completely block parasite invasion.Citation64 To identify the epitope to which the mAb 5A08 targets, a virus-like particle strategy was employed.Citation88–91 VLPs utilize the intrinsic ability of viral structural proteins to self-assemble into non-infective particles while retaining their antigenic similarity to the authentic virion, thus making VLPs an excellent vaccine candidate against the virus from which they were originally derived. However, the coat protein of the bacteriophage MS2 has the added advantage that it has surface-exposed loop in its coat protein that tolerates insertions without disruption of protein folding or VLP assembly, and it encapsids the nucleic acid encoding the coat protein and any guest peptide it displays within that loop.Citation89,90 When the guest peptides expressed in the surface of the MS2-VLP are random sequences of varying lengths, and when expressed in bacteria, each VLP that is assembled displays a different guest peptide on its surface, creating a large VLP-library to select against.
Although there is no definite limitation on the peptide size that can be accommodated within the AB-loop, the percentage of random peptide sequences that can support proper coat protein folding and assembly drops as the peptide gets longer,Citation90 and the sizes of insert tested so far are in the 6- to 10-mer range. The anti-RH5 mAb itself was then used against a VLP-library containing 6-, 7-, 8- and 10-mer inserts to affinity-select for its own target epitope,Citation89 identifying the 8-mer insert SAIKKPVT, with the RH5-spevcific epitope identified as being the central 4 amino acids, AIKK, correlating to residues 28–31, a linear tetrapeptide present at the N-terminus of the protein.Citation64 Sera from immunization with this epitope, again expressed in the context of a virus-like particle (VLP), was able to inhibit invasion to the same degree as the parent mAb, demonstrating that even a single epitope of RH5 could serve as an effective vaccine.Citation64 Thus, a systematic and comprehensive analysis of the entire RH5 protein must be carried out to identify other neutralization sensitive epitopes.
The main advantage of the VLP system is the high immunogenicity of peptides displayed multivalently on the MS2 surface permits the use of the affinity-selected VLP itself as a vaccine immunogen, thus integrating both epitope discovery and immunization functions into a single platform. An epitope-specific VLP-based vaccine may have advantages over a more traditional subunit vaccine as clinical trials conducted with VLP-based biologics have demonstrated that these products have good safety profiles,Citation92-95 and even at low doses, VLPs elicit high-titer and remarkably durable antibody responses, potentially obviating the need for adjuvants,Citation96 although the VLP immunogenicity does depend on high valency epitope display.Citation88-90,Citation96 Further, as the VLPs can be produced at high levels in bacteria, they remain easy and cheap to manufacture, and the particle itself is relatively stable. These features are particularly important in the developing world, where the burden of malaria is the highest.
RH5 as a Vaccine Candidate
RH5 shows limited sequence diversity with only 12 non-synonymous amino acids changes (of 526 residues) identified across multiple laboratory strainsCitation11 and naturally circulating isolates.Citation12 The prevalence of these non-synonymous amino acid changes show only 5 (Y147H, H148D, S197Y, C203Y, and I410M) are considered as the most common variants in global parasite populations, being present at more than 10% frequency in any given population.Citation84 Interestingly, only 2 of the 12 identified polymorphisms, Y148H and H148D, are from field isolates and are not observed in any laboratory strain sequenced so far, which suggests any as-yet unidentified polymorphisms would be at very low frequency. Although the level of sequence polymorphism is not the only factor that predicts whether a candidate antigen will prove to be a successful vaccine component, it is a significant contributor. As well having limited diversity, the support of RH5 as a promising vaccine candidate is strengthened with the fact that no inverse relationship between RH5 genotype and invasion inhibition from anti-RH5 antibodies has been observed, and anti-RH5 antibodies against 3D7 elicit potent strain-transcending parasite-neutralizing antibodies in vitro.Citation71,84,Citation86 Although these data are extremely promising, there is currently no absolute in vitro assay that can accurately predict human in vivo protection and the contrasting data available for other blood stage antigens raises uncertainties about the use of these inhibition assays to predict vaccine outcomes in vivo. AMA1 was originally identified as it was shown to be the target of parasite neutralizing antibodies in invasion assays,Citation97 and even though purified IgG from malaria-naïve adults immunized with various AMA1 vaccine formulations in a Phase 1/2a trial was inhibitory to homologous (but not heterologous) parasites, the inhibition observed was not correlated with in vivo protection after parasite challenge.Citation98 However, it has also been shown that antibody titer (determined by ELISA) and inhibition of parasites in vitro with purified IgG from animals immunized with the carboxyl terminal of MSP-1 did correlate with in vivo protection in the Aotus animal model against homologous and heterologous challenge.Citation99
It is not clear exactly how much antibody is required to protect individuals from disease and the invasion inhibition assay is currently the most commonly used tool used to directly determine the effect of antibody on parasites, especially in the selection process of vaccine candidates.Citation99,100 Some reassurance of using invasion assays as an endpoint to assess RH5 is provided by data suggesting that the EC50 of anti-RH5 antibody in invasion assays is lower than that of anti-AMA1 or anti-MSP1, both for vaccine-induced antibodyCitation101 and for naturally acquired antibody,Citation74 and that in combination with other antigens, such as EBA-175Citation85 or RH2 and RH4,Citation101 a synergistic effect is observed, where the ability to inhibit parasite invasion is observed at lower antibody concentrations. As RH5 interacts with PfRiprCitation53 and CyRRACitation54, neither of which can be knocked out, and antibodies against both are able to inhibit invasion, it is likely these proteins are under strict functional constraints preventing maintenance of multiple mutations, thus it may be difficult for the parasite to develop resistance to an effective RH5 vaccine by genetic drift driven by host immune responses.
Members of the P. falciparum EBL and RH families were first identified based on homologs on other malaria species, and one of the earliest examples of a protective blood-stage vaccine was actually purified parasite-derived Py235,Citation102 and anti-Py235 mAbs are protective against P. yoelii.Citation103 It is possible that all Plasmodium spp. have at least one member of these protein families in their invasion armory, even if as-yet unidentified or characterized. However, there are currently no known homologues to RH5 or alternative “RH5-like” antigens in rodent malarias that can be utilized to further characterize this antigen as a vaccine candidate in rodent animal models, leaving the non-human primate (NHP) model as the only opportunity to study in vivo RH5 efficacy before conducting human clinical trials. Previous NHP studies assessing blood stage antigens have shown limited protection was only achieved when adjuvants unsuitable for human administration were used, and protection was toward homologous strains only. However recent data on in vivo efficacy of RH5 in the NHP model not only supports a causal link between invasion inhibition and actual protection for this antigen but significantly show heterologous protection was achieved when immunized using a viral-vectored platform known to be compatible with humans, and suggest that a human RH5 vaccine does have the capacity to deliver protection that is strain-transcending.Citation104 A Phase 1a trial of RH5 using the viral-vectored delivery platform used in the NHP studies, boosted with modified vaccinia Ankara is underway (https://clinicaltrials.gov/ct2/show/NCT02181088), which will show whether the promising in vitro and NHP data will translate into the human system.
Future Challenges or Closing Remarks
Despite the desperate need for alternative therapeutics due to the emergence and spread of drug resistance, only one malaria vaccine, RTS,S AS01, from a portfolio of about 40Citation105 has achieved the necessary level of efficacy to reach Phase III clinical trials, and highlights the need to assess all vaccine candidates as thoroughly as possible. RTS,S, which targets the pre-erythrocytic stages, resulted in only 50% protection from clinical malaria in children aged 5–17 months, and only about 30% protection in children aged 6–12 weeks,Citation106,107 and 3 y after vaccination, no vaccine efficacy could be detected.Citation108 However, pre-erythrocytic stage vaccines such as RTS,S aim to prevent clinical disease by eliminating parasites before they can enter the cyclical erythrocytic stage. If they are not 100% effective, parasites that have ‘escaped’ will enter the peripheral blood stream, able to cause the full range of clinical complications associated with this stage of the parasite life-cycle. An efficacious blood stage vaccine would prevent these clinical symptoms, and reduction in parasitemia would be beneficial to individuals and the general public health. The main challenges to a blood stage vaccine development include, but are not limited to, selecting an antigen that is both essential and elicits a strong, protective immune response, yet is not so diverse that immunity to only the vaccine strain would develop, and presenting the antigen to the host immune system with a sufficiently immunogenic but safe vaccine adjuvant, the availability and suitability of animal models, as well as gaps in our knowledge of the nature of antimalarial immunity and how it is acquired.Citation109,110 The malaria antigen RH5 described in this review may well live up to this challenge.
Disclosure if Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are indebted to Drs. Gabriel Gutierrez, Amy Noe, David Peabody and Annie Mo for the many valuable discussions during the course of this project.
Funding
Work done in the authors' lab cited in this review was funded by NIAID/DMID contract AI-N01–045210 to Leidos.
References
- Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 2012; 379:413-31; PMID:22305225; http://dx.doi.org/10.1016/S0140-6736(12)60034-8
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 2009; 361:455-67; PMID:19641202; http://dx.doi.org/10.1056/NEJMoa0808859
- Cortes A, Mellombo M, Masciantonio R, Murphy VJ, Reeder JC, Anders RF. Allele specificity of naturally acquired antibody responses against Plasmodium falciparum apical membrane antigen 1. Infect Immun 2005; 73:422-30; PMID:15618180; http://dx.doi.org/10.1128/IAI.73.1.422-430.2005
- Polley SD, Mwangi T, Kocken CH, Thomas AW, Dutta S, Lanar DE, Remarque E, Ross A, Williams TN, Mwambingu G, et al. Human antibodies to recombinant protein constructs of Plasmodium falciparum Apical Membrane Antigen 1 (AMA1) and their associations with protection from malaria. Vaccine 2004; 23:718-28; PMID:15542195; http://dx.doi.org/10.1016/j.vaccine.2004.05.031
- Osier FH, Fegan G, Polley SD, Murungi L, Verra F, Tetteh KK, Lowe B, Mwangi T, Bull PC, Thomas AW, et al. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun 2008; 76:2240-8; PMID:18316390; http://dx.doi.org/10.1128/IAI.01585-07
- Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, Schoepflin S, Gilson PR, Murphy VJ, Anders RF, Mueller I, et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun 2009; 77:1165-74; PMID:19139189; http://dx.doi.org/10.1128/IAI.01129-08
- Moormann AM, Sumba PO, Chelimo K, Fang H, Tisch DJ, Dent AE, John CC, Long CA, Vulule J, Kazura JW. Humoral and cellular immunity to Plasmodium falciparum merozoite surface protein 1 and protection from infection with blood-stage parasites. J Infect Dis 2013; 208:149-58; PMID:23539744; http://dx.doi.org/10.1093/infdis/jit134
- Corran PH, O'Donnell RA, Todd J, Uthaipibull C, Holder AA, Crabb BS, Riley EM. The fine specificity, but not the invasion inhibitory activity, of 19-kgdalton merozoite surface protein 1-specific antibodies is associated with resistance to malarial parasitemia in a cross-sectional survey in The Gambia. Infect Immun 2004; 72:6185-9; PMID:15385530
- Thera MA, Doumbo OK, Coulibaly D, Laurens MB, Ouattara A, Kone AK, Guindo AB, Traore K, Traore I, Kouriba B, et al. A field trial to assess a blood-stage malaria vaccine. N Engl J Med 2011; 365:1004-13; PMID:21916638; http://dx.doi.org/10.1056/NEJMoa1008115
- Ogutu BR, Apollo OJ, McKinney D, Okoth W, Siangla J, Dubovsky F, Tucker K, Waitumbi JN, Diggs C, Wittes J, et al. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PloS one 2009; 4:e4708; PMID:19262754; http://dx.doi.org/10.1371/journal.pone.0004708
- Hayton K, Gaur D, Liu A, Takahashi J, Henschen B, Singh S, Lambert L, Furuya T, Bouttenot R, Doll M, et al. Erythrocyte binding protein PfRH5 polymorphisms determine species-specific pathways of Plasmodium falciparum invasion. Cell Host Microbe 2008; 4:40-51; PMID:18621009; http://dx.doi.org/10.1016/j.chom.2008.06.001
- Manske M, Miotto O, Campino S, Auburn S, Almagro-Garcia J, Maslen G, O'Brien J, Djimde A, Doumbo O, Zongo I, et al. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature 2012; 487:375-9; PMID:22722859; http://dx.doi.org/10.1038/nature11174
- Aikawa M, Miller LH, Johnson J, Rabbege J. Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J Cell Biol 1978; 77:72-82; PMID:96121; http://dx.doi.org/10.1083/jcb.77.1.72
- Dvorak JA, Miller LH, Whitehouse WC, Shiroishi T. Invasion of erythrocytes by malaria merozoites. Science 1975; 187:748-50; PMID:803712; http://dx.doi.org/10.1126/science.803712
- Singh S, Alam MM, Pal-Bhowmick I, Brzostowski JA, Chitnis CE. Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLoS Pathog 2010; 6:e1000746; PMID:20140184; http://dx.doi.org/10.1371/journal.ppat.1000746
- Riglar DT, Richard D, Wilson DW, Boyle MJ, Dekiwadia C, Turnbull L, Angrisano F, Marapana DS, Rogers KL, Whitchurch CB, et al. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe 2011; 9:9-20; PMID:21238943; http://dx.doi.org/10.1016/j.chom.2010.12.003
- Pillai AD, Addo R, Sharma P, Nguitragool W, Srinivasan P, Desai SA. Malaria parasites tolerate a broad range of ionic environments and do not require host cation remodelling. Mol Microbiol 2013; 88:20-34; PMID:23347042; http://dx.doi.org/10.1111/mmi.12159
- Nagamune K, Hicks LM, Fux B, Brossier F, Chini EN, Sibley LD. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature 2008; 451:207-10; PMID:18185591; http://dx.doi.org/10.1038/nature06478
- Iyer J, Gruner AC, Renia L, Snounou G, Preiser PR. Invasion of host cells by malaria parasites: a tale of two protein families. Mol Microbiol 2007; 65:231-49; PMID:17630968; http://dx.doi.org/10.1111/j.1365-2958.2007.05791.x
- Duraisingh MT, Triglia T, Ralph SA, Rayner JC, Barnwell JW, McFadden GI, Cowman AF. Phenotypic variation of Plasmodium falciparum merozoite proteins directs receptor targeting for invasion of human erythrocytes. Embo J 2003; 22:1047-57; PMID:12606570; http://dx.doi.org/10.1093/emboj/cdg096
- Mitchell GH, Hadley TJ, McGinniss MH, Klotz FW, Miller LH. Invasion of erythrocytes by Plasmodium falciparum malaria parasites: evidence for receptor heterogeneity and two receptors. Blood 1986; 67:1519-21; PMID:3516259;
- Perkins ME, Holt EH. Erythrocyte receptor recognition varies in Plasmodium falciparum isolates. Mol Biocheml Parasitol 1988; 27:23-34; http://dx.doi.org/10.1016/0166-6851(88)90021-7
- Camus D, Hadley TJ. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science 1985; 230:553-6; PMID:3901257; http://dx.doi.org/10.1126/science.3901257
- Maier AG, Duraisingh MT, Reeder JC, Patel SS, Kazura JW, Zimmerman PA, Cowman AF. Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nat Med 2003; 9:87-92; PMID:12469115; http://dx.doi.org/10.1038/nm807
- Adams JH, Blair PL, Kaneko O, Peterson DS. An expanding ebl family of Plasmodium falciparum. Trends Parasitol 2001; 17:297-9; PMID:11378038; http://dx.doi.org/10.1016/S1471-4922(01)01948-1
- Gilberger TW, Thompson JK, Triglia T, Good RT, Duraisingh MT, Cowman AF. A novel erythrocyte binding antigen-175 paralogue from Plasmodium falciparum defines a new trypsin-resistant receptor on human erythrocytes. J Biol Chem 2003; 278:14480-6; PMID:12556470; http://dx.doi.org/10.1074/jbc.M211446200
- Mayer DC, Mu JB, Kaneko O, Duan J, Su XZ, Miller LH. Polymorphism in the Plasmodium falciparum erythrocyte-binding ligand JESEBL/EBA-181 alters its receptor specificity. Proc Natl Acad Sci U S A 2004; 101:2518-23; PMID:14983041; http://dx.doi.org/10.1073/pnas.0307318101
- Peterson DS, Wellems TE. EBL-1, a putative erythrocyte binding protein of Plasmodium falciparum, maps within a favored linkage group in two genetic crosses. Mol Biochem Parasitol 2000; 105:105-13; PMID:10613703; http://dx.doi.org/10.1016/S0166-6851(99)00173-5
- Galinski MR, Medina CC, Ingravallo P, Barnwell JW. A reticulocyte-binding protein complex of Plasmodium vivax merozoites. Cell 1992; 69:1213-26; PMID:1617731; http://dx.doi.org/10.1016/0092-8674(92)90642-P
- Khan SM, Jarra W, Preiser PR. The 235 kDa rhoptry protein of Plasmodium (yoelii) yoelii: function at the junction. Mol Biochem Parasitol 2001; 117:1-10; PMID:11551627; http://dx.doi.org/10.1016/S0166-6851(01)00333-4
- Rayner JC, Vargas-Serrato E, Huber CS, Galinski MR, Barnwell JW. A Plasmodium falciparum homologue of Plasmodium vivax reticulocyte binding protein (PvRBP1) defines a trypsin-resistant erythrocyte invasion pathway. J Exp Med 2001; 194:1571-81; PMID:11733572; http://dx.doi.org/10.1084/jem.194.11.1571
- Triglia T, Duraisingh MT, Good RT, Cowman AF. Reticulocyte-binding protein homologue 1 is required for sialic acid-dependent invasion into human erythrocytes by Plasmodium falciparum. Mol Microbiol 2005; 55:162-74; PMID:15612925; http://dx.doi.org/10.1111/j.1365-2958.2004.04388.x
- Kaneko O, Mu J, Tsuboi T, Su X, Torii M. Gene structure and expression of a Plasmodium falciparum 220-kDa protein homologous to the Plasmodium vivax reticulocyte binding proteins. Mol Biochem Parasitol 2002; 121:275-8; PMID:12034462; http://dx.doi.org/10.1016/S0166-6851(02)00042-7
- Rodriguez M, Lustigman S, Montero E, Oksov Y, Lobo CA. PfRH5: a novel reticulocyte-binding family homolog of plasmodium falciparum that binds to the erythrocyte, and an investigation of its receptor. PloS one 2008; 3:e3300; PMID:18827878; http://dx.doi.org/10.1371/annotation/dde6c172-c9c3-43bb-8fc3-db54613d4424
- Baum J, Chen L, Healer J, Lopaticki S, Boyle M, Triglia T, Ehlgen F, Ralph SA, Beeson JG, Cowman AF. Reticulocyte-binding protein homologue 5 - an essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int J Parasitol 2009; 39:371-80; PMID:19000690; http://dx.doi.org/10.1016/j.ijpara.2008.10.006
- Rayner JC, Galinski MR, Ingravallo P, Barnwell JW. Two Plasmodium falciparum genes express merozoite proteins that are related to Plasmodium vivax and Plasmodium yoelii adhesive proteins involved in host cell selection and invasion. Proc Natl Acad Sci U S A 2000; 97:9648-53; PMID:10920203; http://dx.doi.org/10.1073/pnas.160469097
- Triglia T, Thompson J, Caruana SR, Delorenzi M, Speed T, Cowman AF. Identification of proteins from Plasmodium falciparum that are homologous to reticulocyte binding proteins in Plasmodium vivax. Infect Immun 2001; 69:1084-92; PMID:11160005; http://dx.doi.org/10.1128/IAI.69.2.1084-1092.2001
- Dvorin JD, Bei AK, Coleman BI, Duraisingh MT. Functional diversification between two related Plasmodium falciparum merozoite invasion ligands is determined by changes in the cytoplasmic domain. Mol Microbiol 2010; 75:990-1006; PMID:20487292; http://dx.doi.org/10.1111/j.1365-2958.2009.07040.x
- Triglia T, Thompson JK, Cowman AF. An EBA175 homologue which is transcribed but not translated in erythrocytic stages of Plasmodium falciparum. Mol Biochem Parasitol 2001; 116:55-63; PMID:11463466; http://dx.doi.org/doi:10.1016/S0166-6851(01)00303-6
- Taylor HM, Triglia T, Thompson J, Sajid M, Fowler R, Wickham ME, Cowman AF, Holder AA. Plasmodium falciparum homologue of the genes for Plasmodium vivax and Plasmodium yoelii adhesive proteins, which is transcribed but not translated. Infect Immun 2001; 69:3635-45; PMID:11349024; http://dx.doi.org/10.1128/IAI.69.6.3635-3645.2001
- Sim BK, Chitnis CE, Wasniowska K, Hadley TJ, Miller LH. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science 1994; 264:1941-4; PMID:8009226; http://dx.doi.org/10.1126/science.8009226
- Dolan SA, Proctor JL, Alling DW, Okubo Y, Wellems TE, Miller LH. Glycophorin B as an EBA-175 independent Plasmodium falciparum receptor of human erythrocytes. Mol Biochem Parasitol 1994; 64:55-63; PMID:8078523; http://dx.doi.org/10.1016/0166-6851(94)90134-1
- Mayer DC, Cofie J, Jiang L, Hartl DL, Tracy E, Kabat J, Mendoza LH, Miller LH. Glycophorin B is the erythrocyte receptor of Plasmodium falciparum erythrocyte-binding ligand, EBL-1. Proc Natl Acad Sci U S A 2009; 106:5348-52; PMID:19279206; http://dx.doi.org/10.1073/pnas.0900878106
- Lobo CA, Rodriguez M, Reid M, Lustigman S. Glycophorin C is the receptor for the Plasmodium falciparum erythrocyte binding ligand PfEBP-2 (baebl). Blood 2003; 101:4628-31; PMID:12576308; http://dx.doi.org/10.1182/blood-2002-10-3076
- Maier AG, Baum J, Smith B, Conway DJ, Cowman AF. Polymorphisms in erythrocyte binding antigens 140 and 181 affect function and binding but not receptor specificity in Plasmodium falciparum. Infect Immun 2009; 77:1689-99; PMID:19204093; http://dx.doi.org/10.1128/IAI.01331-08
- Lanzillotti R, Coetzer TL. The 10 kDa domain of human erythrocyte protein 4.1 binds the Plasmodium falciparum EBA-181 protein. Malar J 2006; 5:100; PMID:17087826; http://dx.doi.org/10.1186/1475-2875-5-100
- DeSimone TM, Jennings CV, Bei AK, Comeaux C, Coleman BI, Refour P, Triglia T, Stubbs J, Cowman AF, Duraisingh MT. Cooperativity between Plasmodium falciparum adhesive proteins for invasion into erythrocytes. Mol Microbiol 2009; 72:578-89; PMID:19400777; http://dx.doi.org/10.1111/j.1365-2958.2009.06667.x
- Sahar T, Reddy KS, Bharadwaj M, Pandey AK, Singh S, Chitnis CE, Gaur D. Plasmodium falciparum reticulocyte binding-like homologue protein 2 (PfRH2) is a key adhesive molecule involved in erythrocyte invasion. PloS one 2011; 6:e17102; PMID:21386888; http://dx.doi.org/10.1371/journal.pone.0017102
- Spadafora C, Awandare GA, Kopydlowski KM, Czege J, Moch JK, Finberg RW, Tsokos GC, Stoute JA. Complement receptor 1 is a sialic acid-independent erythrocyte receptor of Plasmodium falciparum. PLoS Pathog 2011; 6:e1000968; PMID:20585558; http://dx.doi.org/10.1371/journal.ppat.1000968
- Tham WH, Schmidt CQ, Hauhart RE, Guariento M, Tetteh-Quarcoo PB, Lopaticki S, Atkinson JP, Barlow PN, Cowman AF. Plasmodium falciparum uses a key functional site in complement receptor type-1 for invasion of human erythrocytes. Blood 2011; 118:1923-33; PMID:21685372; http://dx.doi.org/10.1182/blood-2011-03-341305
- Tham WH, Wilson DW, Lopaticki S, Schmidt CQ, Tetteh-Quarcoo PB, Barlow PN, Richard D, Corbin JE, Beeson JG, Cowman AF. Complement receptor 1 is the host erythrocyte receptor for Plasmodium falciparum PfRh4 invasion ligand. Proc Natl Acad Sci U S A 2010; 107:17327-32; PMID:20855594; http://dx.doi.org/10.1073/pnas.1008151107
- Crosnier C, Bustamante LY, Bartholdson SJ, Bei AK, Theron M, Uchikawa M, Mboup S, Ndir O, Kwiatkowski DP, Duraisingh MT, et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 2011; 480:534-7; PMID:22080952; http://dx.doi.org/10.1038/nature10606
- Chen L, Lopaticki S, Riglar DT, Dekiwadia C, Uboldi AD, Tham WH, O'Neill MT, Richard D, Baum J, Ralph SA, et al. An EGF-like protein forms a complex with PfRh5 and is required for invasion of human erythrocytes by Plasmodium falciparum. PLoS Pathog 2011; 7:e1002199; PMID:21909261; http://dx.doi.org/10.1371/journal.ppat.1002199
- Reddy KS, Amlabu E, Pandey AK, Mitra P, Chauhan VS, Gaur D. Multiprotein complex between the GPI-anchored CyRPA with PfRH5 and PfRipr is crucial for Plasmodium falciparum erythrocyte invasion. Proc Natl Acad Sci U S A 2015; 112(4):1179-84; PMID:25583518; http://dx.doi.org/10.1073/pnas.1415466112
- Gaur D, Mayer DC, Miller LH. Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Int J Parasitol 2004; 34:1413-29; PMID:15582519; http://dx.doi.org/10.1016/j.ijpara.2004.10.010
- Gaur D, Storry JR, Reid ME, Barnwell JW, Miller LH. Plasmodium falciparum is able to invade erythrocytes through a trypsin-resistant pathway independent of glycophorin B. Infect Immun 2003; 71:6742-6; PMID:14638759; http://dx.doi.org/10.1128/IAI.71.12.6742-6746.2003
- Jiang L, Gaur D, Mu J, Zhou H, Long CA, Miller LH. Evidence for erythrocyte-binding antigen 175 as a component of a ligand-blocking blood-stage malaria vaccine. Proc Natl Acad Sci U S A 2011; 108:7553-8; PMID:21502513; http://dx.doi.org/10.1073/pnas.1104050108
- Taylor HM, Grainger M, Holder AA. Variation in the expression of a Plasmodium falciparum protein family implicated in erythrocyte invasion. Infect Immun 2002; 70:5779-89; PMID:12228308; http://dx.doi.org/10.1128/IAI.70.10.5779-5789.2002
- Dolan SA, Miller LH, Wellems TE. Evidence for a switching mechanism in the invasion of erythrocytes by Plasmodium falciparum. J Clin Invest 1990; 86:618-24; PMID:2200806; http://dx.doi.org/10.1172/JCI114753
- Gaur D, Furuya T, Mu J, Jiang LB, Su XZ, Miller LH. Upregulation of expression of the reticulocyte homology gene 4 in the Plasmodium falciparum clone Dd2 is associated with a switch in the erythrocyte invasion pathway. Mol Biochem Parasitol 2006; 145:205-15; PMID:16289357; http://dx.doi.org/10.1016/j.molbiopara.2005.10.004
- Stubbs J, Simpson KM, Triglia T, Plouffe D, Tonkin CJ, Duraisingh MT, Maier AG, Winzeler EA, Cowman AF. Molecular mechanism for switching of P. falciparum invasion pathways into human erythrocytes. Science 2005; 309:1384-7; PMID:16123303; http://dx.doi.org/10.1126/science.1115257
- Kooij TW, Carlton JM, Bidwell SL, Hall N, Ramesar J, Janse CJ, Waters AP. A Plasmodium whole-genome synteny map: indels and synteny breakpoints as foci for species-specific genes. PLoS Pathog 2005; 1:e44; PMID:16389297; http://dx.doi.org/10.1371/journal.ppat.0010044
- Douglas AD, Williams AR, Knuepfer E, Illingworth JJ, Furze JM, Crosnier C, Choudhary P, Bustamante LY, Zakutansky SE, Awuah DK, et al. Neutralization of Plasmodium falciparum merozoites by antibodies against PfRH5. J Immunol 2014; 192:245-58; PMID:24293631; http://dx.doi.org/10.4049/jimmunol.1302045
- Ord RL, Caldeira JC, Rodriguez M, Noe A, Chackerian B, Peabody DS, Gutierrez G, Lobo CA. A malaria vaccine candidate based on an epitope of the Plasmodium falciparum RH5 protein. Malar J 2014; 13:326; PMID:25135070; http://dx.doi.org/10.1186/1475-2875-13-326
- Douglas AD, Williams AR, Illingworth JJ, Kamuyu G, Biswas S, Goodman AL, Wyllie DH, Crosnier C, Miura K, Wright GJ, et al. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat Commun 2011; 2:601; PMID:22186897; http://dx.doi.org/10.1038/ncomms1615
- Gilson PR, Crabb BS. Morphology and kinetics of the three distinct phases of red blood cell invasion by Plasmodium falciparum merozoites. Int J Parasitol 2009; 39:91-6; PMID:18952091; http://dx.doi.org/10.1016/j.ijpara.2008.09.007
- Igakura T, Kadomatsu K, Kaname T, Muramatsu H, Fan QW, Miyauchi T, Toyama Y, Kuno N, Yuasa S, Takahashi M, et al. A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis. Dev Biol 1998; 194:152-65; PMID:9501026; http://dx.doi.org/doi:10.1006/dbio.1997.8819
- Fadool JM, Linser PJ. 5A11 antigen is a cell recognition molecule which is involved in neuronal-glial interactions in avian neural retina. Dev Dyn 1993; 196:252-62; PMID:8219348; http://dx.doi.org/10.1002/aja.1001960406
- Wanaguru M, Liu W, Hahn BH, Rayner JC, Wright GJ. RH5-Basigin interaction plays a major role in the host tropism of Plasmodium falciparum. Proc Natl Acad Sci U S A 2013; 110:20735-40; PMID:24297912; http://dx.doi.org/10.1073/pnas.1320771110
- Chen L, Xu Y, Healer J, Thompson JK, Smith BJ, Lawrence MC, Cowman AF. Crystal structure of PfRh5, an essential ligand for invasion of human erythrocytes. eLife 2014; 3:e04187; PMID:25296023; http://dx.doi.org/10.7554/eLife.04187
- Wright KE, Hjerrild KA, Bartlett J, Douglas AD, Jin J, Brown RE, Illingworth JJ, Ashfield R, Clemmensen SB, de Jongh WA, et al. Structure of malaria invasion protein RH5 with erythrocyte basigin and blocking antibodies. Nature 2014; 515:427-30; PMID:25132548; http://dx.doi.org/10.1038/nature13715
- Hayton K, Dumoulin P, Henschen B, Liu A, Papakrivos J, Wellems TE. Various PfRH5 polymorphisms can support Plasmodium falciparum invasion into the erythrocytes of owl monkeys and rats. Mol Biochem Parasitol 2013; 187:103-10; PMID:23305874; http://dx.doi.org/10.1016/j.molbiopara.2012.12.005
- Villasis E, Lopez-Perez M, Torres K, Gamboa D, Neyra V, Bendezu J, Tricoche N, Lobo C, Vinetz JM, Lustigman S. Anti-Plasmodium falciparum invasion ligand antibodies in a low malaria transmission region, Loreto, Peru. Malar J 2012; 11:361; PMID:23110555; http://dx.doi.org/10.1186/1475-2875-11-361
- Tran TM, Ongoiba A, Coursen J, Crosnier C, Diouf A, Huang CY, Li S, Doumbo S, Doumtabe D, Kone Y, et al. Naturally acquired antibodies specific for Plasmodium falciparum reticulocyte-binding protein homologue 5 inhibit parasite growth and predict protection from malaria. J Infect Dis 2014; 209:789-98; PMID:24133188; http://dx.doi.org/10.1093/infdis/jit553
- Patel SD, Ahouidi AD, Bei AK, Dieye TN, Mboup S, Harrison SC, Duraisingh MT. Plasmodium falciparum merozoite surface antigen, PfRH5, elicits detectable levels of invasion-inhibiting antibodies in humans. J Infect Dis 2013; 208:1679-87; PMID:23904294; http://dx.doi.org/10.1093/infdis/jit385
- Chiu CY, Healer J, Thompson JK, Chen L, Kaul A, Savergave L, Raghuwanshi A, Li Wai Suen CS, Siba PM, Schofield L, et al. Association of antibodies to Plasmodium falciparum reticulocyte binding protein homolog 5 with protection from clinical malaria. Front Microbiol 2014; 5:314; PMID:25071730; http://dx.doi.org/10.3389/fmicb.2014.00314
- Ghim SJ, Young R, Jenson AB. Antigenicity of bovine papillomavirus type 1 (BPV-1) L1 virus-like particles compared with that of intact BPV-1 virions. J Gen Virol 1996; 77 ( Pt 2):183-8; PMID:8627221; http://dx.doi.org/10.1099/0022-1317-77-2-183
- Martin L, Stricher F, Misse D, Sironi F, Pugniere M, Barthe P, Prado-Gotor R, Freulon I, Magne X, Roumestand C, et al. Rational design of a CD4 mimic that inhibits HIV-1 entry and exposes cryptic neutralization epitopes. Nat Biotechnol 2003; 21:71-6; PMID:12483221; http://dx.doi.org/10.1038/nbt768
- Han T, Sui J, Bennett AS, Liddington RC, Donis RO, Zhu Q, Marasco WA. Fine epitope mapping of monoclonal antibodies against hemagglutinin of a highly pathogenic H5N1 influenza virus using yeast surface display. Biochem Biophys Res Commun 2011; 409:253-9; PMID:21569761; http://dx.doi.org/10.1016/j.bbrc.2011.04.139
- Mehlin C, Boni E, Buckner FS, Engel L, Feist T, Gelb MH, Haji L, Kim D, Liu C, Mueller N, et al. Heterologous expression of proteins from Plasmodium falciparum: results from 1000 genes. Mol Biochem Parasitol 2006; 148:144-60; PMID:16644028; http://dx.doi.org/10.1016/j.molbiopara.2006.03.011
- Aguiar JC, LaBaer J, Blair PL, Shamailova VY, Koundinya M, Russell JA, Huang F, Mar W, Anthony RM, Witney A, et al. High-throughput generation of P. falciparum functional molecules by recombinational cloning. Genome Res 2004; 14:2076-82; PMID:15489329; http://dx.doi.org/10.1101/gr.2416604
- Vedadi M, Lew J, Artz J, Amani M, Zhao Y, Dong A, Wasney GA, Gao M, Hills T, Brokx S, et al. Genome-scale protein expression and structural biology of Plasmodium falciparum and related Apicomplexan organisms. Mol Biochem Parasitol 2007; 151:100-10; PMID:17125854; http://dx.doi.org/10.1016/j.molbiopara.2006.10.011
- Birkholtz LM, Blatch G, Coetzer TL, Hoppe HC, Human E, Morris EJ, Ngcete Z, Oldfield L, Roth R, Shonhai A, et al. Heterologous expression of plasmodial proteins for structural studies and functional annotation. Malar J 2008; 7:197; PMID:18828893; http://dx.doi.org/10.1186/1475-2875-7-197
- Bustamante LY, Bartholdson SJ, Crosnier C, Campos MG, Wanaguru M, Nguon C, Kwiatkowski DP, Wright GJ, Rayner JC. A full-length recombinant Plasmodium falciparum PfRH5 protein induces inhibitory antibodies that are effective across common PfRH5 genetic variants. Vaccine 2013; 31:373-9; PMID:23146673; http://dx.doi.org/10.1016/j.vaccine.2012.10.106
- Ord RL, Rodriguez M, Yamasaki T, Takeo S, Tsuboi T, Lobo CA. Targeting sialic acid dependent and independent pathways of invasion in Plasmodium falciparum. PloS one 2012; 7:e30251; PMID:22253925; http://dx.doi.org/10.1371/journal.pone.0030251
- Reddy KS, Pandey AK, Singh H, Sahar T, Emmanuel A, Chitnis CE, Chauhan VS, Gaur D. Bacterially expressed full-length recombinant Plasmodium falciparum RH5 protein binds erythrocytes and elicits potent strain-transcending parasite-neutralizing antibodies. Infect Immun 2014; 82:152-64; PMID:24126527; http://dx.doi.org/10.1128/IAI.00970-13
- Bartholdson SJ, Crosnier C, Bustamante LY, Rayner JC, Wright GJ. Identifying novel Plasmodium falciparum erythrocyte invasion receptors using systematic extracellular protein interaction screens. Cell Microbiol 2013; 15:1304-12; PMID:23617720; http://dx.doi.org/10.1111/cmi.12151
- Caldeira Jdo C, Medford A, Kines RC, Lino CA, Schiller JT, Chackerian B, Peabody DS. Immunogenic display of diverse peptides, including a broadly cross-type neutralizing human papillomavirus L2 epitope, on virus-like particles of the RNA bacteriophage PP7. Vaccine 2010; 28:4384-93; PMID:20434554; http://dx.doi.org/10.1016/j.vaccine.2010.04.049
- Chackerian B, Caldeira Jdo C, Peabody J, Peabody DS. Peptide epitope identification by affinity selection on bacteriophage MS2 virus-like particles. J Mol Biol 2011; 409:225-37; PMID:21501621; http://dx.doi.org/10.1016/j.jmb.2011.03.072
- Peabody DS, Manifold-Wheeler B, Medford A, Jordan SK, do Carmo Caldeira J, Chackerian B. Immunogenic display of diverse peptides on virus-like particles of RNA phage MS2. J Mol Biol 2008; 380:252-63; PMID:18508079; http://dx.doi.org/10.1016/j.jmb.2008.04.049
- Peabody, DS. Subunit fusion confers tolerance to peptide insertions in a virus coat protein. Arch Biochem Biophys 1997; 347:85-92; PMID:9344468; http://dx.doi.org/10.1006/abbi.1997.0312
- Hillman RJ, Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Jr., Vardas E, Aranda C, Jessen H, Ferris DG, Coutlee F, et al. Immunogenicity of the quadrivalent human papillomavirus (type 6/11/16/18) vaccine in males 16 to 26 years old. Clin Vaccine Immunol 2012; 19:261-7; PMID:22155768; http://dx.doi.org/10.1128/CVI.05208-11
- Lopez-Macias C. Virus-like particle (VLP)-based vaccines for pandemic influenza: performance of a VLP vaccine during the 2009 influenza pandemic. Hum Vaccin Immunother 2012; 8:411-4; PMID:22330956; http://dx.doi.org/10.4161/hv.18757
- Wang JW, Roden RB. Virus-like particles for the prevention of human papillomavirus-associated malignancies. Expert Rev Vaccines 2013; 12:129-41; PMID:23414405; http://dx.doi.org/10.1586/erv.12.151
- Wu T, Li SW, Zhang J, Ng MH, Xia NS, Zhao Q. Hepatitis E vaccine development: a 14 year odyssey. Hum Vaccin Immunother 2012; 8:823-7; PMID:22699438; http://dx.doi.org/10.4161/hv.20042
- Tumban E, Peabody J, Peabody DS, Chackerian B. A universal virus-like particle-based vaccine for human papillomavirus: longevity of protection and role of endogenous and exogenous adjuvants. Vaccine 2013; 31:4647-54; PMID:23933337; http://dx.doi.org/10.1016/j.vaccine.2013.07.052
- Deans JA, Alderson T, Thomas AW, Mitchell GH, Lennox ES, Cohen S. Rat monoclonal antibodies which inhibit the in vitro multiplication of Plasmodium knowlesi. Clin Exp Immunol 1982; 49:297-309; PMID:6751636;
- Spring MD, Cummings JF, Ockenhouse CF, Dutta S, Reidler R, Angov E, Bergmann-Leitner E, Stewart VA, Bittner S, Juompan L, et al. Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A. PloS one 2009; 4:e5254; PMID:19390585; http://dx.doi.org/10.1371/journal.pone.0005254
- Singh S, Miura K, Zhou H, Muratova O, Keegan B, Miles A, Martin LB, Saul AJ, Miller LH, Long CA. Immunity to recombinant plasmodium falciparum merozoite surface protein 1 (MSP1): protection in Aotus nancymai monkeys strongly correlates with anti-MSP1 antibody titer and in vitro parasite-inhibitory activity. Infect Immun 2006; 74:4573-80; PMID:16861644; http://dx.doi.org/10.1128/IAI.01679-05
- Duncan CJ, Hill AV, Ellis RD. Can growth inhibition assays (GIA) predict blood-stage malaria vaccine efficacy? Hum Vaccin Immunother 2012; 8:706-14; PMID:22508415; http://dx.doi.org/10.4161/hv.19712
- Williams AR, Douglas AD, Miura K, Illingworth JJ, Choudhary P, Murungi LM, Furze JM, Diouf A, Miotto O, Crosnier C, et al. Enhancing blockade of Plasmodium falciparum erythrocyte invasion: assessing combinations of antibodies against PfRH5 and other merozoite antigens. PLoS Pathog 2012; 8:e1002991; PMID:23144611; http://dx.doi.org/10.1371/journal.ppat.1002991
- Holder AA, Freeman RR. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature 1981; 294:361-4; PMID:7312033;
- Freeman RR, Trejdosiewicz AJ, Cross GA. Protective monoclonal antibodies recognising stage-specific merozoite antigens of a rodent malaria parasite. Nature 1980; 284:366-8; PMID:7360274;
- Douglas AD, Baldeviano GC, Lucas CM, Lugo-Roman LA, Crosnier C, Bartholdson SJ, Diouf A, Miura K, Lambert LE, Ventocilla JA, et al. A PfRH5-Based Vaccine Is Efficacious against Heterologous Strain Blood-Stage Plasmodium falciparum Infection in Aotus Monkeys. Cell Host Microbe 2015; 17:130-9; PMID:25590760; http://dx.doi.org/10.1016/j.chom.2014.11.017
- Schwartz L, Brown GV, Genton B, Moorthy VS. A review of malaria vaccine clinical projects based on the WHO rainbow table. Malar J 2012; 11:11; PMID:22230255; http://dx.doi.org/10.1186/1475-2875-11-11
- Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmuller B, Issifou S, Kremsner PG, et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 2012; 367:2284-95; PMID:23136909; http://dx.doi.org/10.1056/NEJMoa1208394
- Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BG, Doucka Y, Flamen A, Mordmuller B, et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 2011; 365:1863-75; PMID:22007715; http://dx.doi.org/10.1056/NEJMoa1102287
- Bejon P, White MT, Olotu A, Bojang K, Lusingu JP, Salim N, Otsyula NN, Agnandji ST, Asante KP, Owusu-Agyei S, et al. Efficacy of RTS,S malaria vaccines: individual-participant pooled analysis of phase 2 data. Lancet Infect Dis 2013; 13:319-27; PMID:23454164; http://dx.doi.org/10.1016/S1473-3099(13)70005-7
- de Souza JB. Protective immunity against malaria after vaccination. Parasite Immunol 2014; 36:131-9; PMID:24188045; http://dx.doi.org/10.1111/pim.12086
- Sheehy SH, Douglas AD, Draper SJ. Challenges of assessing the clinical efficacy of asexual blood-stage Plasmodium falciparum malaria vaccines. Hum Vaccin Immunother 2013; 9:1831-40; PMID:23778312; http://dx.doi.org/10.4161/hv.25383
