Abstract
CIGB-247 is a cancer vaccine that is a formulation of a recombinant protein antigen representative of the human vascular endothelial growth factor (VEGF) with a bacterially-derived adjuvant (VSSP). The vaccine has shown an excellent safety profile in mice, rats, rabbits, not-human primates and in recent clinical trials in cancer patients. Response to the vaccine is characterized by specific antibody titers that neutralize VEGF/VEGFR2 binding and a cytotoxic tumor-specific response. To expand our present anti-VEGF active immunotherapy strategies, we have now studied in mice and non-human primates the effects of vaccination with a formulation of our recombinant VEGF antigen and aluminum phosphate adjuvant (hereafter denominated CIGB-247-A). Administered bi-weekly, CIGB-247-A produces high titers of anti-VEGF IgG blocking antibodies in 2 mice strains. Particularly in BALB/c, the treatment impaired subcutaneous F3II mammary tumor growth and reduced the number of spontaneous lung macro metastases, increasing animals' survival. Spleen cells from specifically immunized mice directly killed F3II tumor cells in vitro. CIGB-247-A also showed to be immunogenic in non-human primates, which developed anti-VEGF blocking antibodies and the ability for specific direct cell cytotoxic responses, all without impairing the healing of deep skin wounds or other side effect. Our results support consideration of aluminum phosphate as a suitable adjuvant for the development of new vaccine formulations using VEGF as antigen.
Abbreviations
| VEGF | = | vascular endothelial growth factor |
| VSSP | = | very small sized proteoliposomes |
| VEGFR2 | = | vascular endothelial growth factor receptor 2 |
| HPLC | = | High-performance liquid chromatography |
| ANOVA | = | Analysis of Variance |
| GST | = | Glutathione S-transferase |
| KDR | = | kinase domain receptor |
| CTL | = | Cytotoxic T lymphocyte |
| ELISA | = | Enzyme-linked immune-sorbent assay |
| PBMC | = | Peripheral blood mononuclear cells |
| Ni-NTA | = | nickel-nitrilotriacetic acid |
| FACS | = | Fluorescence-activated cell sorting |
| CFSE | = | Carboxyfluorescein succinimidyl ester |
Introduction
Therapeutic cancer vaccine preparations based on cells, complex antigen preparations, or on defined DNA, peptides or proteins, are the subject of intense research with the goal of controlling tumor growth, progression, and or dissemination of malignant cells, via the stimulus of the patient's humoral and/or T-cell response against its tumor.Citation1 Because of the self-nature of most of the used antigens, the presence of a strong negative regulatory effect of the tumor on the immune system, and the immune suppression produced by other previous or concomitant anti-neoplastic treatments, these procedures very seldom induce immediate reductions in tumor burden, and effects are apparent only after many months, even beyond initial cancer progression. Within this complex scenario, research on positive immune modulation strategies is essential for the future success or failure of active immunotherapy as a generalized cancer treatment modality.Citation2
CIGB-247 is a cancer vaccineCitation3-7 that is a formulation of a recombinant protein antigen representative of the human vascular endothelial growth factor (VEGF) with VSSP, a powerful bacterially-derived immune modulatory preparation.Citation8-10 CIGB-247 elicits anti-VEGF IgG antibodies that block VEGF interaction with VEGF receptor 2, and stimulates in mice direct cell cytotoxicity and/or CD8 T lymphocyte responses. In C57Bl/6 and BALB/c mice challenged with experimental tumors, CIGB-247 produces anti-tumor and anti-metastatic effects.Citation3-6 CIGB-247 is also immunogenic in rats, rabbits, and non-human primates.Citation5-6 The VSSP-adjuvanted CIGB-247 vaccine was recently evaluated in a Phase I clinical trial with patients bearing advanced solid tumors, showing to be safe and immunogenic.Citation7 Since these clinical data revealed an unexploited immune reaction potential against VEGF in cancer patients, further exploration was needed in order to increase such response. This can be accomplished by using other adjuvants with recognized abilities to induce higher antibody responses, as in the case of aluminum salts.
Aluminum adjuvants have been employed for more than 80 y in human vaccine, and it's efficacy is mainly attributed to a depot effect,Citation11 which allowed prolonged and effective stimulation of the immune system. Recently new evidence demonstrated that insoluble aluminum salts can also activate innate immune cells in a manner that ultimately results in both a T helper 1 and a T helper 2 (Th1 and Th2) immune response to protein antigens.Citation12
In an effort to increase our knowledge on the immune response mechanisms that follow immunization with VEGF, and to allow us to expand the possibilities of VEGF-targeted active immunotherapy, we have now formulated our recombinant VEGF antigen with aluminum phosphate adjuvant, and studied its immunogenicity in mice and non-human primates, as well as the anti-tumor and anti-metastatic properties of immunization in a BALB/c mouse tumor metastasizing model.
Results
P64K-hVEGFKDR− antigen adsorption to aluminum phosphate
Quality control of antigen incorporation to Aluminum salts was assessed using HPLC. An adsorption rate of 0.422 ± 0.032 mg of P64K-hVEGFKDR− per mg of 3+Al was obtained, with no differences between antigen batches (P > 0.05, one-way ANOVA, n = 3). For 100 μg and 200 μg of P64K-hVEGFKDR− the full amount of antigen was adsorbed to the aluminum phosphate employed (not more than 0.70 mg of 3+Al per dose). For the 400 μg dose, 75% of the antigen used was found to be incorporated.
Anti-VEGF antibody response in C57Bl/6 mice immunized with CIGB-247-A
For the weekly and biweekly schemes anti-VEGF antibodies were evaluated in serum samples obtained after the eighth and fourth immunizations, respectively. shows that healthy CIGB-247-A vaccinated C57Bl/6 mice developed anti-GST-hVEGF IgG antibodies with mean titer values of 1:15,000, 1:29,000 and 1:32,000, for the 100, 200, or 400 μg antigen groups, respectively. The average titer in the 100 μg antigen group was significantly lower than those of other 2 cohorts (P < 0.05, Bonferroni post-test), that were not statistically different. In agreement, a significant trend to higher titers was detected as dose increase (p = 0 .0008, Post-test for linear trend). Mice immunized weekly with CIGB-247-A (400 μg of antigen) showed no antibody titer advantage.
Figure 1. C57BL/6 mice immunized with CIGB-247-A using different antigen doses or placebo, and 2 vaccination regimes. (A) Serum anti-GST-hVEGF IgG antibody titers. (B) GST-hVEGF/KDR-Fc interaction inhibition activity by serum. The symbols represent individual mice; data are presented as mean ± standard deviation of the mean (SEM); horizontal truncated lines depict the vaccination regimes.
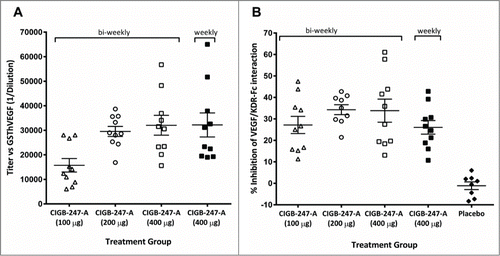
Serum samples from all antigen vaccinated animals blocked GST-hVEGF/KDR-Fc interaction (), with inhibitions significantly higher than those found for samples of the placebo group (p < 0.05, Bonferroni post-test). Mice vaccinated either bi-weekly with 100 μg of antigen, or weekly with 400 μg, showed similar average inhibition percentages (26.67% and 26.05%, respectively). A slight but non-significant increase in average inhibition percent was found for the biweekly 200 μg or 400 μg of antigen groups (33.8% and 34.3%, respectively), as compared to the lower dose cohorts.
Anti-VEGF antibody response in BALB/c mice immunized with CIGB-247-A
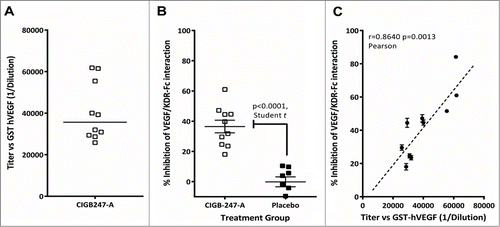
An average anti-IgG antibody titer of 1:39,000 against GST-hVEGF was estimated in serum of healthy BALB/c mice immunized biweekly with 400 μg of antigen (). Sera from these mice blocked GST-hVEGF/KDR-Fc interaction () with inhibition values statistically different from those obtained for the placebo. IgG average titer value and inhibition ability of individual sera correlated, as shown in (Pearson r = 0 .8640, p = 0 .0013).
Figure 2. BALB/c mice immunizations with CIGB-247-A using 400 μg of antigen or placebo. (A) Serum anti-GST-hVEGF IgG antibody titers. (B) GST-hVEGF/KDR-Fc interaction inhibition activity in serum. (C) Correlation between individual titer values and inhibition activity. The symbols represent individual mice; data are presented as mean ± standard deviation of the mean (SEM).
Antibodies produced in both, C57Bl/6 and BALB/c immunized mice were mainly of the IgG1 subclass, cross-reacted with GST-mVEGF, and inhibited the interaction between GST-mVEGF and KDR-Fc. All animals serum were screened for antibodies to GST proteins, and specific antibody titers after CIGB-247-A immunizations were undetectable (below 1:50) (results not shown in detail).
Direct cell cytotoxicity in BALB/c mice vaccinated with CIGB-247-A
Spleen cells from animals vaccinated with CIGB-247-A (400 μg of antigen) reduced the viability of F3II target tumor cells, with respect to their counterparts from placebo-treated mice (). Activation of mouse splenocytes using recombinant hVEGFKDR− and IL-2 further increased cytotoxicity ability of antigen-immunized mouse cells on F3II.
Figure 3. Administration of the CIGB-247-A vaccine significantly reduce tumor growth and spontaneous metastases in BALB/c mice challenged with F3II mammary tumor cells. (A) Tumor mean volume kinetics ± standard deviation of the mean (SEM) (n = 20, Student t test, P < 0.005). (B) Number of metastases per lung, per animal group. The symbols represent individual mice, n = 10. (C) Mean survival time of the animals relative to control, in 10 mice from each group. Fisher and Logrank test analysis are included in each graph. (D) Evaluation of F3II specific cytotoxicity of spleen cells from BALB/c mice immunized with CIGB-247-A (400 μg of antigen) or placebo, activated or not “in vitro.” Data are presented as percentage of dead cells as compared to un-treated target cells, mean ± standard deviation of the mean (SEM) is depicted; p-values were calculated according to Student's t-test (n = 5).
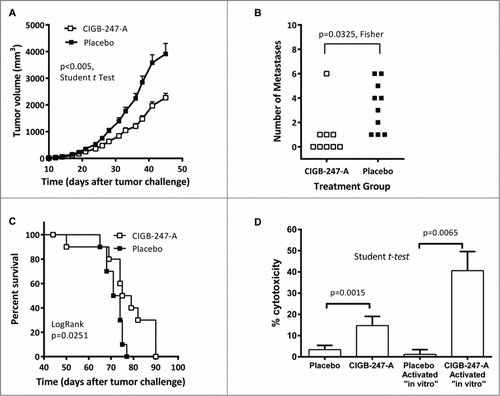
Anti-tumor and anti-metastatic effects of the CIGB-247-A vaccine in mice
The antitumor effects of the alum based vaccine were evaluated in a syngeneic model of metastatic breast sarcomatoid carcinoma: F3II.Citation13 All tumor challenged BALB/c mice developed subcutaneous tumors. illustrate that vaccination with CIGB-247-A (400 μg of antigen) significantly decreased tumor volume (P < 0.005, Student t test) and the amount of lung metastases (p = 0 .0325, Fisher test), with respect to placebo. Lung metastases were also found to be smaller in size in the CIGB-247-A cohort. Finally, survival was higher (p = 0 .0251, LogRank) in the CIGB-247-A vaccinated cohort, with respect to placebo ().
The average anti-GST-hVEGF IgG titer of serum samples taken after 7 CIGB-247-A vaccinations was 1:97,000. Inhibition of GST-hVEGF/KDR-Fc interaction by serum was statistically higher for CIGB-247-A immunized mice, with respect to placebo (P < 0.05, unpaired t test), with 59.65% and 1.41% average values, respectively.
Vaccine immunogenicity and safety profile in monkeys
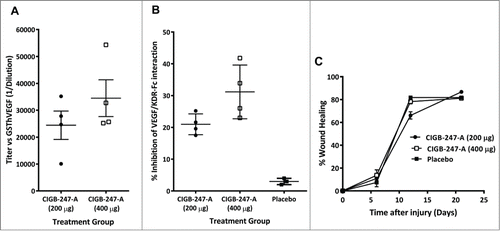
All monkeys in the 2 antigen-immunized cohorts developed IgG antibodies specific for human VEGF (). At 1:200 dilutions, serum from CIGB-247-A vaccinated animals inhibited GST-hVEGF/KDR-Fc interaction (). Because of individual data dispersion, there were no statistical differences (P > 0.05, unpaired t test) in antibody titers or inhibition values between the 2 antigen-dose groups, despite the fact that animals immunized with 400 μg showed a trend toward higher values.
Figure 4. African Green monkeys immunized with CIGB-247-A (200 or 400 μg of antigen) or placebo. (A) Serum anti-GST-hVEGF IgG antibody titers. (B) GST-hVEGF/KDR-Fc interaction inhibition activity by serum. The symbols in (A) and (B) represent individual monkeys; data are presented as mean ± standard deviation of the mean (SEM). (C) Skin deep wound closing dynamics, evaluated at days 0, 6, 12, and 21. Percent of wound healing was calculated as the percent of wound area reduction as compared to the initial values. Data are presented as mean ± standard deviation of the mean (SEM).
Cell cytotoxicity studies were performed using PBMC both as effector and target (incubated with hVEGFKDR) cells, isolated from the animals reported above one week after the last immunization. show that 3 of the 4 monkeys immunized with 400 μg of antigen in CIGB-247-A have higher cytotoxicity levels than the only placebo-treated monkey that exhibited cytolysis effect. In the 200 μg of antigen group, monkey one showed higher cytotoxicity than the placebo-positive animal, the blood sample from monkey 4 was not useful due to a manipulation problem in the extraction.
Table 1. Direct cytolysis of autologous VEGF-charged monkey PBMC
Finally, the study of inflicted deep skin wound closure dynamics () showed no differences (P > 0.05, Bonferroni post-test) when comparing CIGB-247-A vaccinated animals and placebo.
During the whole experiment observational time period, no differences were seen in the immunized monkeys with respect to the initial body weight, rectal temperature, and respiratory and cardiac rates. No lesions appeared at the inoculation site, and no changes in hematologic or blood biochemical parameters were observed.
Discussion
As mentioned in the introductory part of this article, it is well recognized that the complexity of stimulating the immune system to fight an established tumor is enormous, and exploring not only the selection of the best possible target tumor antigens, but also developing accompanying tools to specifically boost the immune response in patients with cancer, are fundamental for the future of cancer therapeutic vaccines.Citation14-15
In the development process of our CIGB-247 vaccine, and because of its reported immune modulatory properties,Citation8-9 VSSP was initially selected among other possible adjuvants to be formulated with the P64K-hVEGFKDR− antigen.Citation3 Different from most cancer therapeutic vaccines approaches, including anti-angiogenic active immunotherapy,-discussed in,Citation7 that rely almost exclusively in developing anti-tumor CTL responses, the production of specific anti-VEGF antibodies able to block the effects of this pro-angiogenic soluble growth factor produced by the tumor and tumor stroma cells plays a key role in our vaccination approach. Anti-VEGF antibodies are important potential mediators of the vaccine's possible anti-tumor effect, as mouse tumor studies with CIGB-247 have highlighted.Citation3,6 Polyclonal immunoglobulins against VEGF can reduce free growth factor bioavailability, and block its interaction with receptors in activated endothelial cells. The importance of antibody in VEGF therapy is absolutely demonstrated by Bevacizumab.Citation16 Therefore, because of the particular adjuvantation properties of aluminum-based formulations, this could be of interest when a preferential humoral response against VEGF, is needed.
Additionally, complementation of the existing VSSP-based anti-VEGF vaccine with a different adjuvant formulation could be important in several scenarios. While we have not found it to be a case (unpublished), extended (chronic) vaccination could lead to a decrease in patients' antibody titers or quality.Citation17 A new form of presentation of the antigen (i.e., a different adjuvant) would be an interesting possibility toward recovering vaccine immunogenicity.
Aluminum salts are an attractive option for adjuvant suitable for human use.Citation18 There are effective because they allow antigen to remain in the body for a longer time slowly releasing it from the insoluble salt particles (depot effect).Citation11 This phenomenon prolongs the stimulation of the immune system, and improves the kinetics of antigen uptake and presentation by antigen presenting cells, allowing the induction of a good antibody (Th2) response.Citation12,14,18,19 Nevertheless, the recent described capacity to stimulate cellular (Th1) immune of aluminum-based formulations could be also of interest.Citation18 In particular, this property let a safe and non-toxic option for stimulate cellular response which is important for cancer treatment.Citation20
As reported in a previous paper of our group,Citation3 vaccination with P64K-hVEGFKDR− formulated with aluminum hydroxide elicited specific antibodies and an anti-tumor effect against C57Bl/6 mouse melanoma B16 F10. Due to des-adsorption of P64K-hVEGFKDR− from the aluminum hydroxide preparation (unpublished results), this adjuvant was discarded for further studies and an aluminum phosphate vaccine formulation was developed. Foreseeing a possible future human testing of the new vaccine, the amount of totalCitation3+Al was adjusted to limits per dose recommended by the FDA for conventional preventive immunizations (0.7 mg 3+Al per dose).Citation18
In this work we show that anti VEGF titers in primates are higher for CIGB-247-A than those previously reported for CIGB-247,Citation5 The use of allo-antigens is in fact one of the causes of the low titers generally detected when vaccines based on autologous proteins are used, but this was not our case. Titers in both mice strains and non-human primates where high and their quality in terms of neutralization of VEGF/VEGFR2 binding parallel that of other immunization schemes previously conducted with VSSP as adjuvant. Given the high homology between human and monkey VEGF (99 %) we can conclude that B cell tolerance can be also broken with a Phosphate Aluminum adjuvant and CIGB-247combination.
Un-published results from our lab indicates that immunizing mice with mouse VEGF produced in the same recombinant format, result in similar anti-VEGF titers with the same specific activity in terms of VEGF/VEGFR2 neutralization
In C57Bl/6 mice and non-human primates, increasing the frequency of vaccination from a bi-weekly to a weekly schedule did not produce a detectable increase in titer or antibody blocking ability. In general, bi-weekly schemes using aluminum as an adjuvant have been shown as the most favorable for antibody induction, the bi-weekly aluminum-based formulation behaves similar to VSSP-adjuvanted preparation described by us in previous papers.Citation5-6 Hence, it seems possible to reduce the final total antigen dose to half the amount employed with the VSSP formulations.
While a direct experimental comparison with the VSSP-adjuvanted vaccine was not our main objective, it is interesting to comment that CIGB-247-A antibody titers, estimated with the same ELISA test and methodology, seem to be higher than those induced by CIGB-247.Citation3 This increase was not necessarily translated into an increase in GST-hVEGF/KDR-Fc interaction blocking values, a phenomenon for which we have no clear explanation at present.
Based on our previous experience with the VSSP-adjuvanted vaccine,Citation6 we selected the F3II mammary tumor to evaluate the anti-tumor potential of CIGB-247-A. These studies in BALB/c mice were done with the maximum antigen dose tested (400 μg), and the bi-weekly scheme, and demonstrated that the new vaccine formulation reduced tumor growth, as well as the number of lung macro metastases, compared to placebo treated animals. As an expression of the reduced metastatic load, CIGB-247-A administration also increased animal survival as compared to the placebo cohort. The presence of tumor in the vaccinated animals did not impair the development of anti-VEGF antibodies able to block the interaction between the growth factor and soluble KDR-Fc. The higher antibody titers and inhibition values reported in this experiment, compared with the study done in healthy BALB/c mice, could be due to the 3 additional CIGB-247-A immunizations given to these animals. Overall, the results of the anti-tumor experiments done now resemble those reported with CIGB-247.Citation6
Side effects of anti-angiogenic drugs have raised concerns because of the important role that the VEGF/VEGFR2 system plays in the maintenance of the functionality of the fenestrated endothelium lining several organs.Citation21-22 In spite of the high anti-VEGF IgG blocking antibodies developed in monkeys no impairment of deep skin wounds healing was observed. Any of the commonly reported toxicities or adverse events for commercial anti-VEGF and anti-angiogenic drugs was found.Citation22 CIGB-247-A administration led to no clinical, histological, or blood biochemistry alterations. As discussed previously,Citation4-7 the high safety profile of our vaccine preparations is probably due to the low amount and polyclonal nature of the anti-VEGF antibodies elicited by immunization, when compared to the very high blood concentration of infused IgG produced, for example, after iv infusion of Bevacizumab.Citation23 Within this scenario, the constant pharmacological availability of “metronomic” amounts of VEGF-blocking antibodies produced by vaccination is nevertheless enough for the vaccine's possible therapeutic effect.
Finally, we found that vaccination with CIGB-247-A not only elicited anti-VEGF antibodies with anti-angiogenic potential, but also produced specific cell cytotoxic responses. In the BALB/c mouse experiments, spleen cells from vaccine immunized (but not from placebo) animals were directly cytotoxic against F3II tumor cells. Together with our finding that monkey PBMC were cytotoxic for autologous cells loaded with a VEGF variant antigen, these evidences suggest that while aluminum salts rarely induce cellular immune responses, and their utility for therapeutic cancer vaccines has been questioned,Citation15 there may be instances where this adjuvant could be used for this purpose.
Overall, testing aluminum salts with our recombinant VEGF antigen is an effort to increase our knowledge on the immune response mechanisms that follow immunization with this growth factor, and opens new possibilities of VEGF-targeted active immunotherapy. Our experimental results in mice and monkeys support the consideration of aluminum phosphate as a suitable adjuvant for the development of new vaccine combinations, to be tested in diseases associated with pathological angiogenesis.
Materials and Methods
Vaccine antigen and adjuvant
P64K-hVEGFKDR− is a mutated form of human VEGF isoform 121.Citation3-4 This recombinant vaccine antigen is supplied by the Technological Development Department of the Center for Genetic Engineering and Biotechnology (TD-CIGB, Havana) in lyophilized form at 0.4 mg/vial. The desired antigen amount is mixed at the moment of vaccination with aluminum phosphate (Brenntag Biosector, Denmark; not more than 0.70 mg of 3+Al per dose). Aluminum phosphate was formulated in Tris/HCL 10mM, pH 7.4 and provided in sterile vials at 6.33 or 12.66 mg/mL by the TD-CIGB. The formulation of P64K-hVEGFKDR− with aluminum adjuvant will be hereafter denominated CIGB-247-A.
Antigen adsorption studies
P64K-hVEGFKDR− was reconstituted using 0.5 mL of water for injection and 0.5 mL of aluminum phosphate at 0.70 mg of 3+Al per dose. After 5 minutes, the mixtures were centrifuged for 10 minutes at 12,000 rpm. Adsorption was calculated from the soluble P64K-hVEGFKDR− concentration.Citation24
Recombinant proteins used in ELISA and cell cytotoxicity techniques
GST-hVEGF and GST-mVEGF
GST-hVEGF is an E. coli recombinant fusion of GST and human VEGF isoform 121.Citation25 GST-mVEGF is a fusion of GST and mouse VEGF isoform 120. GST-mVEGF interacts with human and mouse VEGFR2 (unpublished).
hVEGFKDR−
The hVEGFKDR− recombinant protein is a version of the P64K-hVEGFKDR− antigen, devoid of the Nm P64K domain. hVEGFKDR− is expressed in E. coli and purified by Ni-NTA chromatography (Qiagen), Triton-X114 based washings and an overnight incubation on Cellufine ET clean S matrix (Cellufine, Japan), according to the manufacturer instructions. Resulting protein exhibit endotoxin levels below 0.1 UE/mL in 7.5 μg/mL of protein.Citation26
Animals
Animals from the National Center for Animal Breeding (Havana, Cuba) were adapted to laboratory conditions and maintained in accordance with the Cuban guidelines for the care and use of laboratory animals. Female C57Bl/6 and BALB/c mice (18–20 g; 6–8 weeks), were maintained at 5 animals per cage. African Green monkeys (Chlorocebus, formerly Cercopithecus–aethiops sabaeus) of either sex weighting from 3 to 7 kg, and naïve for antibodies against P64K and GST-hVEGF were caged individually.Citation5-6 Monkeys were anesthetized by intramuscular injection of ketamine hydrochloride (10 mg/kg) before immunization and/or blood extraction.
Vaccination doses and regimes
Healthy Mice: Mice were assigned to groups of 10 animals each and received: (a) 4 sc injections of CIGB-247-A with 100, 200 or 400 μg of antigen (C57Bl/6), or only 400 μg (BALB/c), (b) a mixture of antigen excipient and aluminum phosphate (both strains; hereafter denominated placebo), in a bi-weekly immunization schedule, or (c) 8 injections of CIGB-247-A with 400 μg of antigen, or placebo, in a weekly immunization scheme (only for C57Bl/6). All immunizations were done in a total volume of 500 μL (2 sites).
Tumor challenged mice: 40 BALB/c mice were divided in 2 groups, to receive 7 or 8 biweekly sc doses of CIGB-247-A (400 μg of antigen), or placebo. Animals were injected sc with of 2 × 105 F3II mammary carcinoma cells,Citation13 one week after the fourth immunization. Tumor volumes at different times were calculated as described before.Citation3-4 Forty 5 d after tumor cell injection, half of the animals in each group were sacrificed, and lung macrometastasis counting done after fixation in Bouin's solution. Remaining animals received an eighth injection, and their survival recorded.
Monkeys: Animals ranked by weight and age were assigned to 2 vaccination groups of 4 animals each, that received 4 sc biweekly injections of CIGB-247-A with 200 or 400 μg of antigen (1000 μL of volume per injection), and one group of 3 for placebo.
Blood samples were collected from all the animals before starting treatment and one week after each immunization, or at the end of the experiment. Serum was separated and stored at −20˚C until used.
ELISA for specific anti-human and murine VEGF antibody titers in animal's sera
Specific IgG antibodies were detected by ELISA as previously described,Citation4-6 using GST-hVEGF, GST or GST-mVEGF as coating antigens. Linear regression curves were adjusted for the OD values obtained from dilutions of individual sera and the value corresponding to 5 times the standard deviation of the average of pre-immune sera was interpolated as the titer.
Specific anti-VEGF antibody class and subclass
Antibody subclasses were determined using a mouse antibody isotyping kit (ISO2–1KT, Sigma). ELISA plates were coated with 20 μg/mL of GST-hVEGF in PBS. Mouse sera were added to wells at a 1:4000 dilution.
Competition ELISA test to measure inhibition of VEGF/KDR-Fc interaction
Plates were coated with 10 μg/mL of GST-hVEGF or GST-mVEGF in PBS, as previously described.Citation4-6 Serial dilutions of sera were incubated for 1 h at 22˚C, and recombinant human VEGF receptor 2/Fc chimera (KDR-Fc; Sigma) added. After washings, plates were incubated either with rabbit anti-human Fc HRPO conjugated antibodies (Jackson ImmunoResearch) for mouse experiments, or with goat biotinylated anti-human KDR antibody (R&D) for monkeys, the latter followed by avidin peroxidase (Peprotech). Percent inhibition of GST-VEGF/KDR-Fc interaction was: 100 (A492nm immune serum/A492nm pre-immune serum).
In vitro cytotoxicity assays
BALB/c mice
As previously described,Citation4 BALB/c spleen effector cells were aseptically obtained and used immediately after isolation, or after in vitro activation for 6 d with 7.5 μg/mL of hVEGFKDR− and 10 units/mL of human IL-2 (CIGB, Havana). F3II tumor target cells were labeled with 10 nM CFSE and combined with effector cells at a 100:1 effector/target ratio. Results were expressed as the mean percent of the cytotoxicity found for 5 animals per group, for each condition.
Monkeys
Peripheral blood mononuclear cells (PBMC) were isolated 7 d after the last immunization using Ficoll (Amersham). PBMC to be used as targets were incubated or not with hVEGFKDR− for 2 h, and later labeled with CFSE. Subsequently, these cells were mixed with autologous effectors PBMC at a 2:1 ratio and direct cytolysis was evaluated by FACS, expressed as the percent reduction of the CFSE labeled population.Citation5-6
Follow up of clinical, behavioral, and other parameters in monkeys
As done before,Citation4-6 body weight, rectal temperature, respiratory and cardiac rates were measured daily, and general clinical examinations, including the vaccination site, were done before and after each vaccine administration. Blood biochemistry analyses were also performed to determine: hemoglobin, hematocrit, platelets, white blood cell count, neutrophils, monocytes, eosinophils, reticulocytes, alkaline phosphatase, aspartate aminotransferase, alanine animotransferase, total bilirubin, albumin, total proteins, glucose and creatinine.Citation4
Skin wound repair assessment in monkeys
Immunized or placebo animals had 4 symmetric ulcers inflicted in the dorsum using disposable 8 mm diameter punch biotomes (Biopunch®, Fray).Citation5-6 Wound closure dynamics was studied at days 0, 6, 12 and 21 using the standard cutaneous round ulcer model. Healing measurements and histopathological characterization in monkey wounds were performed by technical personnel unaware of the animal's treatment.
Statistical analysis
Statistics used methods are described in the legend footnotes and Results text.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The study was supported by Heber Biotec (http://www.heber-biotec.com), and Chemo (http://www.chemogroup.com).
References
- Schlom J. Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst 2012; 104:599-13; PMID:22395641; http://dx.doi.org/10.1093/jnci/djs033
- Berzofsky JA, Terabe M, Wood LV. Strategies to use immune modulators in therapeutic vaccines against cancer. Semin Oncol 2012; 39:348-57; PMID:22595057; http://dx.doi.org/10.1053/j.seminoncol.2012.02.002
- Morera Y, Bequet-Romero M, Ayala M, Lamdan H, Agger EM, Andersen P, Gavilondo JV. Anti-tumoral effect of active immunotherapy in C57BL/6 mice using a recombinant human VEGF protein as antigen and three chemically unrelated adjuvants. Angiogenesis 2008; 11:381-93; PMID:19034678; http://dx.doi.org/10.1007/s10456-008-9121-5
- Morera Y, Bequet-Romero M, Ayala M, Castro J, Puente P, Suárez J, et al. Immunogenicity and some safety features of a VEGF-based cancer therapeutic vaccine in rats, rabbits and non-human primates. Vaccine 2010; 28:3453-61; PMID:20197134; http://dx.doi.org/10.1016/j.vaccine.2010.02.069
- Morera Y, Bequet-Romero M, Ayala M, Pérez PP, Castro J, Sánchez J, Alba JS, Ancízar J, Cosme K, Gavilondo JV. Antigen dose escalation study of a VEGF-based therapeutic cancer vaccine in non-human primates. Vaccine 2012; 30:368-77; PMID:22075086; http://dx.doi.org/10.1016/j.vaccine.2011.10.082
- Bequet-Romero M, Morera Y, Ayala-Ávila M, Ancizar J, Soria Y, Blanco A, Suárez-Alba J, Gavilondo JV. CIGB-247: A VEGF-based therapeutic vaccine that reduces experimental and spontaneous lung metastasis of C57Bl/6 and BALB/c mouse tumors. Vaccine 2012; 30:1790-99; PMID:22240345; http://dx.doi.org/10.1016/j.vaccine.2012.01.006
- Gavilondo JV, Hernández-Bernal F, Ayala-Ávila M, de la Torre AV, de la Torre J, Morera-Díaz Y, Bequet-Romero M, Sánchez J, Valenzuela CM, Martín Y, et al. Specific active immunotherapy with a VEGF vaccine in patients with advanced solid tumors. Results of the CENTAURO antigen dose escalation phase I clinical trial. Vaccine 2014; 32:2241-50; PMID:24530151; http://dx.doi.org/10.1016/j.vaccine.2013.11.102
- Mesa C, de LJ, Rigley K, Fernandez LE. Very small size proteoliposomes derived from Neisseria meningitidis: an effective adjuvant for Th1 induction and dendritic cell activation. Vaccine 2004;22:3045-52; PMID:15297054; http://dx.doi.org/10.1016/j.vaccine.2004.02.010
- Mesa C, de LJ, Fernandez LE. Very small size proteoliposomes derived from Neisseria meningitidis: an effective adjuvant for generation of CTL responses to peptide and protein antigens. Vaccine 2006; 24:2692-99; PMID:16316710; http://dx.doi.org/10.1016/j.vaccine.2005.08.111
- Fernandez A, Mesa C, Marigo I, Dolcetti L, Clavell M, Oliver L, Fernández LE, Bronte V. Inhibition of tumor-induced myeloid derived suppressor cell function by a nanoparticulated adjuvant. J Immunol 2011; 186:264-74; PMID:21135171; http://dx.doi.org/10.4049/jimmunol.1001465
- Marrack Ph, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol 2009; 9:287-93; PMID:19247370; http://dx.doi.org/10.1038/nri2510
- Grun JL, Maurer PH. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: the role of endogenous interleukin 1 in proliferative responses. Cell Immunol 1989; 121:134-45; PMID:2524278; http://dx.doi.org/10.1016/0008-8749(89)90011-7
- Alonso DF, Farias EF, Urtreger A, Ladeda V, Vidal MC, Bal De Kier Joffe E. Characterization of F3II, a sarcomatoid mammary carcinoma cell line originated from a clonal subpopulation of a mouse adenocarcinoma. J Surg Oncol 1996; 62: 288-97; PMID:8691844; http://dx.doi.org/10.1002/(SICI)1096-9098(199608)62:4%3c288::AID-JSO14%3e3.0.CO;2-1
- Blankenstein T, Coulie PG, Gilboa E, Jaffee EM. The determinants of tumour immunogenicity. Nat Rev Cancer 2012; 12:307-13; PMID:22378190; http://dx.doi.org/10.1038/nrc3246
- Nitcheu Tefit, J, Vincent Serra, V. Outlining novel cellular adjuvant products for therapeutic vaccines against cancer. Expert Rev Vaccines 2011; 10:1207-20; PMID:21854313; http://dx.doi.org/10.1586/erv.11.84
- Yaning W, David F, Martin V, An Song. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis 2004; 7: 335-45; PMID:15886877; http://dx.doi.org/10.1007/s10456-004-8272-2
- Zinkernagel RM. Localization dose and time of antigens determine immune reactivity. Seminars in Immunology 2000; 12:163-71; PMID:10910735; http://dx.doi.org/10.1006/smim.2000.0253
- Baylor NW, Egan W, Richman P. Aluminum salts in vaccines-US perspective. Vaccine 2002; 20: S18-S23; PMID:12184360; http://dx.doi.org/10.1016/S0264-410X(02)00166-4
- Petrovsky N, Aguilar JC. Vaccine adjuvants: Current state and future trends. Immunol Cell Biol 2004; 82:488-96; PMID:15479434; http://dx.doi.org/10.1111/j.0818-9641.2004.01272.x
- Mannhalter JW, Neychev HO, Zlabinger GJ, Ahmad R, Eibl MM. Modulation of the human immune response by the non-toxic and non-pyrogenic adjuvant aluminium hydroxide: effect on antigen uptake and antigen presentation. Clin Exp Immunol 1985; 61:143-51; PMID:3876178
- Eskens FA, Verweij J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors: a review. Eur J Cancer 2006; 42:3127-39; PMID:17098419; http://dx.doi.org/10.1016/j.ejca.2006.09.015
- Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol 2009; 6:465-77; PMID:19581909; http://dx.doi.org/10.1038/nrclinonc.2009.94
- Lin YS, Nguyen C, Mendoza JL, Escandon E, Fei D, Meng YG, Modi NB. Preclinical pharmacokinetics, interspecies scaling, and tissue distribution of a humanized monoclonal antibody against vascular endothelial growth factor. J Pharmacol Exp Ther 1999; 288:371-8; PMID:9862791
- Zor T, Selinger Z. “Linearization of the Bradford Protein Assay Increases Its Sensitivity: Theoretical and Experimental Studies.” Anal Biochem 1996; 236:302-8; PMID:8660509; http://dx.doi.org/10.1006/abio.1996.0171
- Morera Y, Lamdan H, Bequet M, Ayala M, Rojas G, Muñoz Y, Gavilondo JV. Biologically active vascular endothelial growth factor as a bacterial recombinant glutathione S-transferase fusion protein. Biotechnol Appl Biochem 2006; 44:45-53; PMID:16401185; http://dx.doi.org/10.1042/BA20050169
- Zimmerman T, Petit Frère C, Satzger M, Raba M, Weisbach M, Döhn K, Popp A, Donzeau M. Simultaneous metal chelate affinity purification and endotoxin clearance of recombinant antibody fragments. J Immunol Methods 2006; 314:67-3; http://dx.doi.org/10.1016/j.jim.2006.05.012
