Abstract
This phase 2 study assessed the immunogenicity, safety, and reactogenicity of investigational formulations of meningococcal ABCWY vaccines, consisting of recombinant proteins (rMenB) and outer membrane vesicle (OMV) components of a licensed serogroup B vaccine, combined with components of a licensed quadrivalent meningococcal glycoconjugate vaccine (MenACWY-CRM). A total of 495 healthy adolescents were randomized to 6 groups to receive 2 doses (Months 0, 2) of one of 4 formulations of rMenB antigens, with or without OMV, combined with MenACWY-CRM, or 2 doses of rMenB alone or one dose of MenACWY-CRM then a placebo. Immunogenicity was assessed by serum bactericidal assay with human complement (hSBA) against serogroups ACWY and serogroup B test strains; solicited reactions and any adverse events (AEs) were assessed. Two MenABCWY vaccinations elicited robust ACWY immune responses, with higher seroresponse rates than one dose of MenACWY-CRM. Bactericidal antibody responses against the rMenB antigens and OMV components were highest in subjects who received 2 doses of OMV-containing MenABCWY formulations, with ≥68% of subjects achieving hSBA titers ≥5 against each of the serogroup B test strains. After the first dose, solicited local reaction rates were higher in the MenABCWY or rMenB groups than the MenACWY-CRM group, but similar across groups after the second dose, consisting mainly of transient injection site pain. Fever (≥38.0°C) was rare and there were no vaccine-related serious AEs. In conclusion, investigational MenABCWY formulations containing OMV components elicited highly immunogenic responses against meningococcal serogroups ACWY, as well as serogroup B test strains, with an acceptable safety profile. [NCT01210885]
Abbreviations::
- CI, confidence interval
- GMT, geometric mean titer
- hSBA, serum bactericidal assay with human complement
- IMD, invasive meningococcal disease
- fHbp, factor H-binding protein
- NadA, Neisseria adhesin A
- NHBA, Neisserial Heparin Binding Antigen
- OMV, outer membrane vesicle
- PP, per-protocol set
- AE, adverse event
- SAE, serious adverse event
Introduction
Infection with Neisseria meningitidis can lead to invasive meningococcal disease (IMD), which can cause permanent disability or death in an otherwise healthy person in a matter of hours.Citation1,2 The highest incidence of this disease occurs in infants in their first year of life, but a second peak occurs in adolescents,Citation3,4 and higher case fatality rates are generally reported in this age group. The vast majority of IMD can be attributed to infection by one of 5 immunologically-distinct serogroups: A, B, C, W and Y.Citation5 The geographic distribution of serogroups and disease incidence varies widely, and can change over time, making meningococcal epidemiology rather unpredictable and disease control strategies more complicated.Citation6
The development of effective vaccines targeted against the most common meningococcal serogroups is the most rational strategy to prevent IMD. To this end, meningococcal capsular polysaccharide-protein carrier conjugate vaccines specific to 4 of the 5 most common serogroups (ACWY) have been developed and are approved in various countries.Citation7-9 Meningococcal glycoconjugate vaccines have been demonstrated to elicit protection and immunologic memory,Citation10 as well as to reduce the acquisition of asymptomatic nasopharyngeal carriage, interrupting transmission and thereby inducing herd protection.Citation11–13
The results of clinical evaluations of MenACWY-CRM (Menveo®, Novartis Vaccines, Siena, Italy), a quadrivalent meningococcal oligosaccharide-CRM197 conjugate vaccine,Citation9 demonstrate that this vaccine is highly immunogenic and well tolerated in a wide range of age populations beginning from 2 months of age.Citation14-16 MenACWY-CRM is currently licensed for use in children, adolescents and adults in many countries, including the US and countries in the EU, Latin America and Asia.
Due to the poor immunogenic potential of the capsular polysaccharide of meningococcal serogroup B organisms, a glycoconjugate vaccine approach cannot be applied to serogroup B.Citation17,18 Therefore, a novel strategy was employed to develop 4CMenB (Bexsero®, Novartis Vaccines, Siena, Italy), a multicomponent vaccine targeted against meningococcal serogroup B.Citation18,19 4CMenB consists of 4 components, including 3 recombinant proteins that were identified by genome mining as surface antigens common to many serogroup B strains, factor H-binding protein (fHbp), Neisseria adhesin A (NadA) and Neisserial Heparin Binding Antigen (NHBA) (the latter 2 antigens are included as fusion proteins with other Neisseria proteins), combined with outer membrane vesicle (OMV) components from the New Zealand outbreak strain NZ98/254.Citation19-22 Results from Phase 2 and 3 clinical studies demonstrated that 4CMenB elicited strong bactericidal responses against the vaccine antigens and was generally well tolerated when used in infants and children, adolescents and adults.Citation21-26 4CMenB has been approved in the EU, Australia, Canada, Chile, and Uruguay for use in individuals beginning from 2 months of age and in the US for use in individuals 10 to 25 y of age.
In this report we present the results of the first Phase 2 clinical study to assess the immunogenicity, safety and reactogenicity of 4 investigational formulations of a meningococcal ABCWY vaccine, containing oligosaccharides from meningococcal serogroups ACWY conjugated to a CRM197 carrier protein, as well as recombinant serogroup B proteins (rMenB) and OMV components, in healthy adolescents.
Results
Enrollment, study flow and demographics
A total of 495 subjects were enrolled and randomized (n = 80−85 across study groups) into 6 groups to receive 2 doses, 2 months apart, of one of 4 investigational MenABCWY formulations (Groups I, II, III, and IV) or 2 doses of the rMenB vaccine alone (Group V) or one dose of MenACWY-CRM followed by a placebo (Group VI; ). Of these subjects, 494 were vaccinated and 485 subjects completed the study. Reasons for premature withdrawal were withdrawal of consent (n = 6), loss to follow up (n = 1), protocol deviation (n = 1), adverse event (AE; n = 1, vasovagal reaction pre-vaccination) and administrative reason (n = 1) (). The baseline demographic characteristics of the enrolled subjects were similar across the groups ().
Table 1. Study population demographics and baseline characteristics
Immunogenicity
Immune responses to N. meningitidis serogroups ACWY and serogroup B test strains that were chosen to measure immune responses to specific vaccine antigens (44/76-SL for fHbp; 5/99 for NadA; M07–0241084 for NHBA; and NZ98/254 for PorA P1.4, the major outer-membrane protein antigen) were assessed by a serum bactericidal assay with human complement (hSBA).
Immunogenicity against serogroups ACWY
One month following 2 doses of MenABCWY formulations, the percentage of subjects who achieved seroresponse (i.e. at least a 4-fold increase in hSBA titers from a prevaccination titer ≥4 or a rise to ≥8 in those with a prevaccination titer <4), against serogroups ACWY in Groups I-IV were high, with comparable results across the groups: A, 97–98%; C, 95–97%; W, 74–86%; and Y, 92–96% (). Comparatively, one month following one dose of MenACWY-CRM (Group VI), 86%, 68%, 60% and 78% of subjects achieved a seroresponse against serogroups A, C, W, and Y, respectively.
Figure 2. (A) Percentage of subjects (95% CI) with seroresponse and percentage of subjects (95% CI) with hSBA titers ≥8 against serogroups ACWY at one month after first dose (Day 31) and 1 month after the second dose (Day 91), by vaccination group. (B) hSBA geometric mean titers (95% CI) against serogroups ACWY at baseline (Day 1) and one month after the first and second doses, by vaccination group.
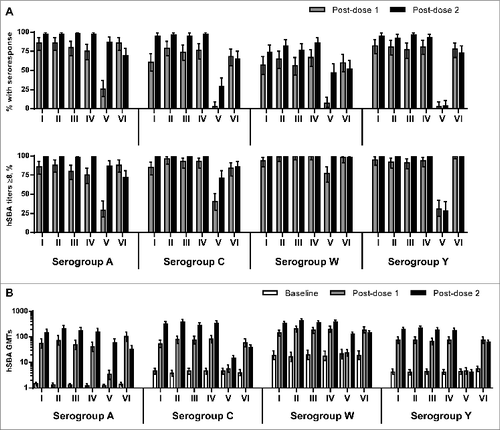
One month after the first dose of a MenABCWY formulation, there was no evidence of a difference in seroresponse rates in Groups I-IV against serogroups ACWY (A: 75–86%; C: 61–79%; W: 56–67%; Y: 77–82%), as compared to those in Group VI one month after one dose of MenACWY-CRM. Of note, following 2 doses of rMenB (Group V), 87%, 29% and 47% of subjects achieved seroresponse against serogroups A, C, and W, respectively.
Following two doses of investigational MenABCWY formulations, nearly all subjects had hSBA titers ≥8 against serogroups ACWY in Groups I-IV (98–100%), higher than that observed in Group VI after a single dose of MenACWY-CRM for serogroup A (88%) and serogroup C (84%) but comparable for serogroups W (98%) and Y (100%) ().
Following two vaccinations, large increases in the hSBA geometric mean titers (GMTs) against serogroups ACWY in Groups I-IV were demonstrated, ranging from ∼18–150-fold, with significantly higher GMTs against serogroups C, W, and Y than those observed in subjects one month following the single MenACWY-CRM vaccination; GMTs against serogroup A appeared to be higher in Groups I-IV, as compared to those of Group VI, although the difference was not significant (). In subjects who received 2 doses of rMenB (Group V), increases in GMTs relative to baseline were observed for serogroup A (49-fold), with smaller increases in GMTs for serogroups C and W (3–6-fold).
Immunogenicity against serogroup B test strains
One month following 2 doses of all investigational MenABCWY vaccines (Groups I-IV), or the rMenB vaccine alone (Group V), nearly all subjects had hSBA titers ≥5 against the fHbp test strain H44/76 (96–100%) and the NadA test strain 5/99 (99–100%). An overall higher percentage of subjects in Groups III and IV, which were administered vaccines containing NZ OMV components, achieved hSBA titers ≥5 against the NHBA test strain M07–0241084 (68–75%), as compared to those groups administered the MenABCWY formulations without the OMV components (Group I: 38%; Group II: 47%) or the rMenB vaccine alone (Group V: 54%). Only subjects in Groups III and IV exhibited hSBA titers ≥5 against the NZ OMV test strain NZ98/254 (71–78%) ().
Figure 3. (A) Percentage of subjects (95% CI) with hSBA titers ≥5 and (B) hSBA geometric mean titers (95% CI) for serogroup B test strains 44/76-SL (fHbp), 5/99 (NadA), M07–0241084 (NHBA) and NZ98/254 (PorA) at baseline (Day 1), one month after the first dose (Day 31), and one month after the second dose (Day 91), by vaccination group.
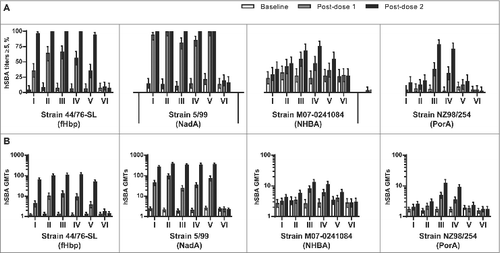
The hSBA GMTs against the serogroup B test strains at baseline were low and comparable between vaccine groups (). At one month after the second vaccine dose, robust immune responses against the fHbp and NadA antigens were observed in all groups administered MenABCWY formulations (Groups I-IV), or the rMenB vaccine alone (Group V), as evidenced by 51–88-fold and 111−174-fold increases in GMTs for the fHbp and NadA test strains, respectively. Increases in GMTs against the NHBA and NZ OMV test strains followed a similar trend to that observed for the percentages of subjects achieving hSBA titers ≥5 against these strains, with the most substantial increases in post-vaccination GMTs against these strains observed in Groups III and IV, as compared to Groups I, II, and V.
Safety and reactogenicity
Of 495 enrolled subjects, 494 (>99%) were exposed to at least one study vaccination and contributed to the safety analyses. Overall, 89−96% of subjects across vaccine groups reported at least one solicited reaction within 7 d after any study vaccination. The rates of solicited local reactions were higher in Groups I-IV (84−93%) and Group V (80%), as compared to Group VI (60%) after the first vaccination, but similar across vaccine groups after the second vaccination (65−84%) (). The highest frequencies of systemic reactions following the first vaccination were observed in the groups that received the investigational MenABCWY formulations including the OMV components (Groups III and IV: 76% versus Groups I, II, V, VI: 55–67%). Overall, solicited reactions in Groups I-V were mostly mild to moderate in severity and did not increase after the second vaccine dose; whereas, in Group VI a higher percentage of subjects reported reactogenicity following the aluminum hydroxide-containing placebo dose (80%), as compared to the MenACWY-CRM dose (68%). Across groups, the percentages of subjects who used any analgesic/antipyretic medications within 7 d of each study vaccination or who stayed home due to any reason were low (up to 14% and 2–6%, respectively).
Table 2. Overview of reactogenicity within 7 d of each vaccination, by group
The most commonly reported local reaction was injection site pain, reported by 50–92% (including 5−21% severe) and 63–83% (including 11−20% severe) of subjects across groups after the first and second vaccination, respectively (). Myalgia and headache were the most commonly-reported systemic reactions, with similar percentages of subjects reporting these reactions across groups (). Rash was reported by a small number of subjects (≤5 %), equally distributed across vaccine groups. Fever ≥38.0°C was rare, ranging from 2−7% across groups after any vaccination, with one report of severe fever ≥40°C after the second vaccination with rMenB (Group VI).
Table 3. Percentage of subjects in Groups I to VI reporting solicited local and systemic reactions within 7 d of each vaccination, by group
During the study period (91 days), unsolicited AEs were reported in 32−37% of subjects with comparable rates across vaccine groups, of which 7−17% were considered at least possibly related to study vaccination. Overall, the most frequently reported unsolicited AEs were nasopharyngitis (up to 13%), followed by myalgia, induration, swelling, headache, erythema and abdominal pain (all ≤5 %), judged mostly unrelated to study vaccination.
Three subjects experienced a serious AE (SAE; loss of consciousness and traumatic brain injury [Group V]; gastroenteritis [Group V], intentional drug misuse [Group VI]), none of which was considered related to study vaccination. Two cases of pregnancy were reported: one subject in Group V, who was identified as pregnant approximately one month following the second rMenB vaccination and subsequently delivered a healthy baby at 41 weeks without any congenital abnormalities, and one subject in Group IV, who tested positive for pregnancy 65 d after the first (and only) MenABCWY vaccination (¼ dose OMV formulation), and subsequently experienced a spontaneous abortion after study termination (72 d after the vaccination), that was considered as not related to study vaccination. There were no deaths reported in this study.
Discussion
The introduction of meningococcal glycoconjugate vaccines against serogroups ACWY has provided an opportunity to substantially reduce the overall incidence of meningococcal disease globally. The recent development of protein-based meningococcal vaccines targeting serogroup B represents an important step toward reducing the remaining prominent cause of childhood bacterial meningitis and septicemia.Citation27 The clinical data support the immunogenicity and tolerability of licensed ACWY conjugate vaccines and licensed multicomponent serogroup B vaccines; however, administration of both vaccines are needed to obtain protection against a majority of circulating strains from the 5 most common serogroups that cause IMD. A single combination meningococcal vaccine that includes the antigens of the licensed MenACWY-CRM and 4CMenB vaccines would simplify immunization schedules, potentially increase meningococcal immunization rates, and, therefore, provide public health benefits of increasing breadth of coverage, as compared to immunization with only one of either of these vaccines.
In the present study, we evaluated the immunogenicity and safety of investigational formulations of a meningococcal ABCWY vaccine in healthy adolescents. The results demonstrate that 2 doses of all of the investigational formulations, administered 2 months apart, elicited highly immunogenic responses against serogroups ACWY. Seroresponse rates following 2 doses of MenABCWY formulations against serogroups ACWY were high, overall higher than the observed response following one dose of MenACWY-CRM. Moreover, across Groups I-IV, nearly all (98–100%) of the subjects administered 2 doses of the investigational MenABCWY formulations achieved hSBA titers ≥8 against each of serogroups ACWY. Overall, immune responses against serogroups ACWY were comparable across the groups administered the MenABCWY investigative formulations.
The results of this study also demonstrate that 2 doses of MenABCWY investigational formulations elicited substantial immune responses against the serogroup B antigens. In particular, we observed that all 4 MenABCWY formulations induced high proportions of subjects with hSBA titers ≥5 against test strains for the fHbp and NadA antigens, similar to those responses induced by the rMenB vaccine alone. While there was no evidence for a difference in responses to the fHbp and NadA antigens among participants who received any of the MenABCWY investigational formulations, protective hSBA titers against the test strains for the NHBA and NZ OMV components were greatest in participants who received formulations including OMV. In comparing serogroup B immune responses following administration of MenABCWY formulations containing either a full dose or a one-quarter dose of the OMV components, GMTs against the NHBA and NZ OMV test strains appeared to be lower in participants who had received the formulation containing the reduced OMV content; however, further analyses will be needed to assess a possible difference between these formulations in immunogenicity against serogroup B test strains.
The recombinant antigens used in the 4CMenB vaccine are also present in other pathogenic serogroups of N. meningitidis, thus indicating that this vaccine might confer protection against non-serogroup B strains. For example, isolated epidemic strains from serogroups A, W, and X (a recently identified cause of IMD in Africa, for which no vaccine exists), express fHbp proteins that share significant sequence homology with the fHbp antigen used in the 4CMenB vaccine and, therefore, may be susceptible to the bactericidal activity of 4CMenB-induced antibodies.Citation28,29 Indeed, results of an analysis of the bactericidal activity of sera from 4CMenB-vaccinated individuals against a panel of serogroup X epidemic strains indicated that 4CMenB may indeed confer protection against strains of this serogroup, and that this protection is likely attributable to fHbp-specific antibody responses.Citation30 While 4CMenB coverage of non-serogroup B strains has not been studied extensively, the susceptibility of any meningococcal strain to bactericidal antibodies elicited by 4CMenB is dependent upon both the degree of similarity between vaccine antigens and the respective target proteins, as well as the expression level of the target proteins, in each strain.Citation31 In the current study, following 2 doses of the rMenB vaccine alone, we observed bactericidal antibody responses against the serogroup A test strain, in particular, and to a lesser extent the serogroup C and W test strains. Given the robust immune responses against serogroups ACWY following vaccination with MenACWY-CRM, the potential additive cross-protective benefit against these serogroups, as well as against serogroup X, conferred by combining MenACWY-CRM with the rMenB and OMV components deserves further clinical evaluation.
Vaccines incorporating outer membrane vesicles are known to be more reactogenic than other types of vaccines.Citation32 In the present study, reactions to MenABCWY formulations including OMV were more frequent relative to the other groups. Lowering the dosage of the OMV components from a full dose to a one-quarter dose resulted in only small differences in the proportions of subjects with reactions. Nonetheless, the overall frequency and type of reactions to the MenABCWY formulations containing OMV were consistent with previously-reported reactogenicity profiles of 4CMenB in adolescents.Citation25 In general, across groups administered the investigational MenABCWY formulations, frequencies of solicited reactions were higher after the first vaccination than after the second vaccination and the majority of the reactions reported in these groups were mild to moderate in severity. The most frequently reported local reaction after any vaccine formulation was pain at the injection site; most common reported systemic reactions were myalgia and headache. Fever was rare and there was no vaccine-related SAE throughout the study period.
There are several limitations to the present study. First, the small sample size limited inter-group comparisons for immunogenicity, reactogenicity, and safety results. Second, the accepted correlate for protection against IMD, hSBA titers ≥4,Citation33 is based on clinical efficacy data for monovalent serogroup A or C and bivalent serogroup AC meningococcal vaccines, and is inferred for serogroups B, W and Y, for which no efficacy data are available, as indicative of protection. Thus, while hSBA titers ≥4 is the standard correlate of protection, here we used a more stringent criterion of hSBA titers ≥8 or ≥5 for serogroups ACWY or serogroup B test strains, respectively, to assess the immunogenicity of the MenABCWY vaccine formulations. Third, as the antigens used in 4CMenB are variably expressed by serogroup B strains, the 4 test strains used in this study do not represent all circulating serogroup B strains, and, thus, the immunogenicity results cannot be generalized. Estimates of MenABCWY vaccine coverage against serogroup B strains necessitates further evaluation against larger panels that represent diverse circulating strains.
In summary, 2 doses of all investigational formulations of meningococcal ABCWY vaccines, administered 2 months apart, elicited highly immunogenic responses to meningococcal serogroups ACWY, comparable to or higher than those anticipated from the MenACWY-CRM vaccine. While ACWY immune responses were comparable for all MenABCWY formulations, we observed that those formulations containing the OMV components elicited overall higher bactericidal antibody responses to most of the serogroup B antigen test strains. All investigative MenABCWY formulations had an acceptable tolerability profile, with evidence of self-limited local and systemic reactions of mild to moderate severity. Collectively, these findings support the further development of an OMV-containing MenABCWY vaccine formulation as the first meningococcal vaccine that targets strains of the 5 most common serogroups that cause invasive meningococcal disease.
Patients and Methods
Study design and objectives
This Phase 2 randomized, observer-blinded, controlled study was conducted at 6 sites in Panama, 3 sites in Colombia and 2 sites in Chile between December 2010 and July 2011 (Clinicaltrials.gov identifier: NCT01210885). The study was undertaken according to Good Clinical Practice and the Declaration of Helsinki. Ethics review committees of participating centers approved the study protocol and study-specific documents, and written informed consent was obtained from every participant and/or parents or legal guardians, where appropriate, prior to enrollment.
The primary study objectives were to evaluate the immunogenicity, safety and reactogenicity of 2 doses of each of the 4 MenABCWY investigational vaccine formulations. A secondary immunogenicity objective was to assess the immune responses to each of the investigational vaccine formulations at one month after the first vaccine dose. The licensed quadrivalent meningococcal glycoconjugate vaccine MenACWY-CRM and the investigational rMenB vaccine, which included 3 recombinant proteins but no OMV components, were used as comparators.
Study participants
A total of 495 adolescents were randomized to one of the 6 study groups to receive 2 vaccinations, 2 months apart, of the indicated vaccine formulations (). Randomization was performed in a 1:1:1:1:1:1 ratio according to a centrally held randomization list from the sponsor.
Eligible study participants were healthy 11–18 year-old males and females. Subjects were excluded if they received any meningococcal vaccine or had a history of meningococcal disease or intimate exposure to an individual with meningococcal disease. Other exclusion criteria were: a significant acute infection or use of antibiotic within 7 d prior to enrolment; fever ≥38°C within 3 d of enrollment; known adverse reactions to vaccine components; any serious chronic or progressive disease; immune-mediated disease or impairment of the immune system from any cause, and previous receipt or intent to receive any investigational product. Pregnant or breast-feeding women were excluded from enrollment and women of childbearing potential had to be committed to using birth control measures for the duration of the study and for at least 2 months before study participation.
Vaccines
The investigational MenABCWY vaccines were prepared just prior to injection by reconstituting the lyophilized powder containing capsular oligosaccharide of serogroups ACWY conjugated to CRM197, a nontoxic mutant of diphtheria toxin, and either one or 2 doses of liquid suspension containing purified recombinant proteins of meningococcal serogroup B (rMenB: NadA (subvariant 3.1), fHbp-GNA2091 (subvariant 1.1) and NHBA-GNA1090 (subvariant 1.2) adsorbed on aluminum hydroxide), or one dose of rMenB with one or one-quarter dose of OMV from serogroup B strain NZ98/254. All MenABCWY formulations administered to Groups I-IV contained 10 μg of MenA-CRM197 and 5 μg each of MenC-CRM197, MenW-CRM197, and MenY-CRM197. Formulations administered to Groups I-V contained different amounts of rMenB proteins, OMV components, and aluminum hydroxide:
Group I [MenACWY-CRM + rMenB(1×)]: 50 μg each of NadA, fHbp-GNA2091 and NHBA-GNA1090 and 1.5 mg of aluminum hydroxide;
Group II [MenACWY-CRM + rMenB(2×)]: 100 μg each of NadA, fHbp-GNA2091 and NHBA-GNA1090, and 3.0 mg of aluminum hydroxide;
Group III [MenACWY-CRM + rMenB(1×) + OMV(1×)]: 50 μg each of NadA, fHbp-GNA2091 and NHBA-GNA1090, 1.5 mg of aluminum hydroxide and 25 μg (“full dose”) of OMV;
Group IV [MenACWY-CRM + rMenB(1×) + OMV(¼×)]: 50 μg each of NadA, fHbp-GNA2091 and NHBA-GNA1090, 1.5 mg of aluminum hydroxide and 6.25 μg (“¼ dose”) of OMV;
Group V [rMenB(1×)]: 50 μg each of NadA, fHbp-GNA2091 and NHBA-GNA1090, 1.5 mg of aluminum hydroxide.
All rMenB vaccines were supplied in pre-filled syringes.
Subjects in groups I to V were to receive 2 doses of investigational vaccines 2 months apart. Subjects in Group VI received one dose of the comparator MenACWY-CRM vaccine (Menveo®, Novartis Vaccines), which was prepared by mixing the lyophilized MenA-CRM197 component with the liquid MenCWY-CRM197 component just prior to injection, followed by a liquid suspension placebo that contained 1.5 mg of aluminum hydroxide and was supplied in a pre-filled syringe.
Vaccine formulations were prepared as a 0.5 mL (Groups I, III, IV, V, VI) or 1.0 mL dose (Group II) and were administered intramuscularly in the deltoid muscle of the nondominant arm. Designated unblinded study personnel, who otherwise did not participate in the evaluation of the subjects during the trial, prepared the vaccine formulations (“observer blind” design).
Immunogenicity analyses
Blood samples (15–20 mL) were obtained for immunogenicity analyses before and one month after each vaccination. Sera were analyzed at the Novartis Clinical Laboratory Sciences (Marburg, Germany). Immune responses to N. meningitidis serogroups ACW and serogroup B test strains were assessed using a high-throughput serum bactericidal assay with human complement (hSBA) as described previouslyCitation34 with some modifications. In brief, the high-throughput hSBA assay adheres to the same principles as the conventional hSBA assay but differs in that the bacterial outgrowth step is in a liquid suspension containing the cell-permeable redox substrate resazurin dye (the active ingredient of alamarBlue®). Resazurin is reduced to the fluorescent product resorufin through bacterial respiration, and, thus, the postreaction bacterial viability can be quantified over time by measuring the resorufin fluorescent signal. Each bactericidal reaction was performed in a 15 μl volume in a 384-well microtiter, black flat-bottom plate and contained serial dilutions of the serum sample and the exogenous complement source obtained from human plasma. The starting inoculum of relevant N. meningitidis strain was added subsequently. The starting bacterial inoculum was prepared by growing the relevant strain to an OD620 of 0.65–0.8, followed by dilution of the culture 1:500 in phosphate-buffered saline (PBS) supplemented with glucose and bovine serum albumin (BSA); a 5 μl volume of the diluted culture was added to each well of the microtiter plate. Following a 60 minute incubation period for the bactericidal reaction, 20 μl of PBS buffer supplemented with glucose, BSA and alamarBlue® (DAL1100; Invitrogen; prepared fresh the day of use) was added to each well. The plate was then sealed with a gas-permeable, optically-clear film and incubated at 37°C in 5% CO2 and 90% humidity. Fluorometric readings were then collected for 16 hours, conducted on an hourly basis, using a fully automated robotic platform (Tecan Evo 100). As controls for resorufin fluorescence-quenching activity of serum, a serum normalization series, which lacked bactericidal activity against the tested bacterial strain, was included in each experiment. Analysis of the fluorescence readouts and computation of high-throughput hSBA titers was conducted as described previously.Citation34 The reciprocal serum dilution that resulted in a 50% reduction of fluorescence (IC50) relative to the normalized maximum signal was reported as the high-throughput hSBA titer of the sample. Immune responses to N. meningitidis serogroup Y were assessed using the conventional hSBA assay.Citation14
The hSBA antibody responses against meningococcal serogroups ACWY were expressed as the percentage of subjects with seroresponse, percentage of subjects with hSBA titers ≥8, and hSBA geometric mean titers (GMTs) (see for additional information on the serogroup A, C, W, and Y test strains). Seroresponse was defined as the percentage of subjects per group achieving at least a 4-fold increase in titers from a prevaccination titer ≥4 or a rise to ≥8 in those with a prevaccination titer <4. The hSBA antibody responses to the serogroup B test strains (44/76-SL for fHbp; 5/99 for NadA; M07–0241084 for NHBA; and NZ98/254 for PorA P1.4, the major outer-membrane protein antigen) were expressed as percentages of subjects with hSBA titers ≥5 and hSBA GMTs.
Table 4. 4CMenB vaccine antigen genotypic and phenotypic characteristics of serogroup A, C, W, and Y test strains
Safety analyses
Subjects were observed for 30 minutes after each vaccination by blinded site personnel to monitor for any immediate adverse reactions. Subjects or guardians recorded solicited local reactions, systemic reactions, and any adverse event (AE) on diary cards for 7 d after each vaccination. Reports of any unsolicited AEs, medically-attended AEs, serious AEs (SAEs) and AEs leading to study withdrawal were collected throughout the study (91 days). The investigators assessed the AEs for severity and relation to study vaccines.
Statistical analyses
Statistical analyses were performed using Statistical Analyses System (SAS) software version 9.1 (SAS Institute, Cary, NC, US). A minimal sample of 80 subjects per vaccine group was to be enrolled, assuming a 10% dropout rate. No formal statistical hypothesis was tested. For the immunogenicity endpoints, seroresponse rates, percentage of subjects with hSBA titers ≥8 (serogroups ACWY), hSBA titers ≥5 (serogroup B test strains) and GMTs, as well as corresponding 95% Clopper-Pearson confidence intervals (CI), were calculated by vaccine group and time point. Titers below the limit of detection were set at half the limit of detection.
Immunogenicity analyses were run on the per-protocol (PP) set (n = 478; 97%), which consisted of subjects who received all of the relevant doses of vaccine correctly, provided at least one evaluable serum sample at the appropriate time points, and had no major protocol violations.
All subjects who received at least one study vaccination and provided safety data were included in the safety analyses. Safety data were evaluated descriptively, and expressed as the percentage and number of subjects with solicited reactions or AEs in each groups.
Disclosure of Potential Conflicts of Interest
EM, MGG, EH, IS, and PMD are permanent employees of Novartis companies. EM was a permanent employee of Novartis Pharma B.V. during study conduct and data analysis and interpretation. PMD was a permanent employee of Novartis Vaccines and Diagnostics, Inc. during study conduct and data analysis and interpretation. The institutions of XS-L, DCAV, and KA received grants from Novartis Vaccines and Diagnostics, Inc. for study conduct. XS-L has received honoraria from GSK and Sanofi Pasteur for participation in advisory boards.
Authors' Contributions
MGG, EH, and PMD designed the study. XS-L, DCAV, and KA conducted the study and participated in the acquisition of data. All authors participated in the analysis and interpretation of the data. EM and IS provided biostatistical expertise.
Acknowledgments
The authors are grateful to all volunteers who participated in the clinical trial. The authors would like to acknowledge investigators Javier Nieto, Adriana Chung, Albino Salas, Luis Marquez, Ivonne Abadía, Juan Carlos Velásquez, Gonzalo Franco, and Jose Manuel Novoa. The authors also wish to thank the following Novartis personnel: Vas Narasimhan, Francesca Carini, Gordon Brestrich, Victor Sales, Jose Jimeno, Sanne de Ridder, and Colin Malcolm. Patricia de Groot, PhD (CtrlP Scientific Writing; funded by Novartis Vaccines and Diagnostics, Inc.) and Jessica Tyler, PhD (Novartis Vaccines) are thanked for medical writing and editorial assistance in the preparation of this manuscript.
Funding
Novartis Vaccines and Diagnostics, Inc. funded the trial, was involved in all stages of the study design and conduct, the collection, analysis, and interpretation of data, and paid all costs associated with the development and submission of this manuscript.
References
- Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med 2001; 344:1378-88; PMID:11333996; http://dx.doi.org/10.1056/NEJM200105033441807
- Thompson MJ, Ninis N, Perera R, Mayon-White R, Phillips C, Bailey L, Harnden A, Mant D, Levin M. Clinical recognition of meningococcal disease in children and adolescents. Lancet 2006; 367:397-403; PMID:16458763; http://dx.doi.org/10.1016/S0140-6736(06)67932-4
- Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, Baker CJ, Messonnier NE; Centers for Disease Control and Prevention. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Motal Wkly Rep 2013; 62:1-28.
- Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, Pondo T, Arnold KE, Baumbach J, Bennett N, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease. Clin Infect Dis 2010; 50:184-91; PMID:20001736; http://dx.doi.org/10.1086/649209
- World Health Organization. Meningococcal vaccines: WHO position paper, November 2011. Weekly Epidemiol Rec. 2011; 86:521-39; PMID:22128384
- Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009; 27:B51-63; PMID:19477562; http://dx.doi.org/10.1016/j.vaccine.2009.04.063
- Keyserling H, Pollard AJ, DeTora LM, Gilmet GP. Experience with MCV-4, a meningococcal, diphtheria toxoid conjugate vaccine against serogroups A, C, Y and W-135. Expert Rev Vaccines 2006; 5:445-59; PMID:16989625; http://dx.doi.org/10.1586/14760584.5.4.445
- Croxtall JD, Dhillon S. Meningococcal quadrivalent (serogroups A, C, W135 and Y) tetanus toxoid conjugate vaccine (Nimenrix). Drugs 2012; 72:2407-30; PMID:23231026; http://dx.doi.org/10.2165/11209580-000000000-00000
- Broker M, Cooper B, Detora LM, Stoddard JJ. Critical appraisal of a quadrivalent CRM(197) conjugate vaccine against meningococcal serogroups A, C W-135 and Y (Menveo) in the context of treatment and prevention of invasive disease. Infect Drug Resist 2011; 4:137-47; PMID:21904459; http://dx.doi.org/10.2147/IDR.S12716
- Kelly DF, Snape MD, Clutterbuck EA, Green S, Snowden C, Diggle L, Yu LM, Borkowski A, Moxon ER, Pollard AJ. CRM197-conjugated serogroup C meningococcal capsular polysaccharide, but not the native polysaccharide, induces persistent antigen-specific memory B cells. Blood 2006; 108:2642-7; PMID:16675705; http://dx.doi.org/10.1182/blood-2006-01-009282
- Trotter CL, Maiden MC. Meningococcal vaccines and herd immunity: lessons learned from serogroup C conjugate vaccination programs. Expert Rev Vaccines 2009; 8:851-61; http://dx.doi.org/10.1586/erv.09.48
- Daugla D, Gami J, Gamougam K, Naibei N, Mbainadji L, Narbe M, Toralta J, Kodbesse B, Ngadoua C, Coldiron M, et al. Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: a community trial. Lancet 2014; 383:40-7; PMID:24035220; http://dx.doi.org/10.1016/S0140-6736(13)61612-8
- Read RC, Baxter D, Chadwick DR, Faust SN, Finn A, Gordon SB, Heath PT, Lewis DJM, Pollard AJ, Turner DPJ et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet 2014; 384:2123-31; PMID:25145775; http://dx.doi.org/10.1016/S0140-6736(14)60842-4
- Snape MD, Perrett KP, Ford KJ, John TM, Pace D, Yu LM, Langley JM, McNeil S, Dull PM, Ceddia F, et al. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. JAMA 2008; 299:173-84; PMID:18182599; http://dx.doi.org/10.1001/jama.2007.29-c
- Jackson LA, Baxter R, Reisinger K, Karsten A, Shah J, Bedell L, Dull PM; V59P13 Study Group. Phase III comparison of an investigational quadrivalent meningococcal conjugate vaccine with the licensed meningococcal ACWY conjugate vaccine in adolescents. Clin Infect Dis 2009; 49:e1-10; PMID:19476428; http://dx.doi.org/10.1086/599117
- Stamboulian D, Lopardo G, Lopez P, Cortes-Barbosa C, Valencia A, Bedell L, Karsten A, Dull PM. Safety and immunogenicity of an investigational quadrivalent meningococcal CRM197 conjugate vaccine, MenACWY-CRM, compared with licensed vaccines in adults in Latin America. Int J Infect Dis 2010; 14:e868-75; PMID:20655261; http://dx.doi.org/10.1016/j.ijid.2010.03.017
- Finne J, Bitter-Suermann D, Goridis C, Finne U. An IgG monoclonal antibody to group B meningococci cross-reacts with developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissues. J Immunol 1987; 138:4402-7; PMID:3108388
- Tan LK, Carlone GM, Borrow R. Advances in the development of vaccines against Neisseria meningitidis. N Engl J Med 2010; 362:1511-20; PMID:20410516; http://dx.doi.org/10.1056/NEJMra0906357
- Pizza M, Scarlato V, Masignani V, Giuliani MM, Arico B, Comanducci M, Jennings GT, Baldi L, Bartolini E, Capecchi B, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 2000; 287:1816-20; PMID:10710308; http://dx.doi.org/10.1126/science.287.5459.1816
- Giuliani MM, Adu-Bobie J, Comanducci M, Aricò B, Savino S, Santini L, Brunelli B, Bambini S, Biolchi A, Capecchi B, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Scie U S A 2006; 103: 10834-39; http://dx.doi.org/10.1073/pnas.0603940103
- Snape MD, Dawson T, Oster P, Evans A, John TM, Ohene-Kena B, Findlow J, Yu LM, Borrow R, Ypma E, et al. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: a randomized comparative trial. Pediatr Infect Disease J 2010; 29:e71-9; http://dx.doi.org/10.1097/INF.0b013e3181b0602e
- Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, Evans A, Telford KL, Ypma E, Toneatto D, et al. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis 2010; 51:1127-37; PMID:20954968; http://dx.doi.org/10.1086/656741
- Gossger N, Snape MD, Yu LM, Finn A, Bona G, Esposito S, Principi N, Diez-Domingo J, Sokal E, Becker B, et al. Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA 2012; 307:573-82; PMID:22318278; http://dx.doi.org/10.1001/jama.2012.85
- Kimura A, Toneatto D, Kleinschmidt A, Wang H, Dull P. Immunogenicity and safety of a multicomponent meningococcal serogroup B vaccine and a quadrivalent meningococcal CRM197 conjugate vaccine against serogroups A, C, W-135, and Y in adults who are at increased risk for occupational exposure to meningococcal isolates. Clin Vaccine Immunol 2011; 18:483-6; PMID:21177912; http://dx.doi.org/10.1128/CVI.00304-10
- Santolaya ME, O'Ryan ML, Valenzuela MT, Prado V, Vergara R, Munoz A, Toneatto D, Grana G, Wang H, Clemens R, et al. Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled study. Lancet 2012; 379:617-24; PMID:22260988; http://dx.doi.org/10.1016/S0140-6736(11)61713-3
- Vesikari T, Esposito S, Prymula R, Ypma E, Kohl I, Toneatto D, Dull P, Kimura A; EU Meningococcal B Infant Vaccine Study group. Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. Lancet 2013; 381:825-35; PMID:23324563; http://dx.doi.org/10.1016/S0140-6736(12)61961-8
- Racloz VN, Luiz SJ. The elusive meningococcal meningitis serogroup: a systematic review of serogroup B epidemiology. BMC Infect Dis 2010;10:175; PMID:20565757; http://dx.doi.org/10.1186/1471-2334-10-175
- Beernink PT, Caugant DA, Welsch JA, Koeberling O, Granoff DM. Meningococcal factor H-binding protein variants expressed by epidemic capsular group A, W-135, and X strains from Africa. J Infect Dis 2009; 199(9):1360-8; PMID:19302008; http://dx.doi.org/10.1086/597806
- Pajon R, Fergus AM, Koeberling O, Caugant DA, Granoff DM. Meningococcal factor H binding proteins in epidemic strains from Africa: implications for vaccine development. PLoS Negl Trop Dis 2011; 5:e1302; PMID:21909444; http://dx.doi.org/10.1371/journal.pntd.0001302
- Hong E, Giuliani MM, Deghmane AE, Comanducci M, Brunelli B, Dull P, Pizza M, Taha MK. Could the multicomponent meningococcal serogroup B vaccine (4CMenB) control Neisseria meningitidis capsular group X outbreaks in Africa? Vaccine 2013; 31:1113-6; PMID:23261039; http://dx.doi.org/10.1016/j.vaccine.2012.12.022
- Donnelly J, Medini D, Boccadifuoco G, Biolchi A, Ward J, Frasch C, Moxon ER, Stella M, Comanducci M, Bambini S, et al. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc Natl Acad Sci USA 2010; 107:19490-95; http://dx.doi.org/10.1073/pnas.1013758107
- Holst J, Martin D, Arnold R, Huergo CC, Oster P, O'Hallahan J, Rosenqvist E. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 2009; 27:B3-12; PMID:19481313; http://dx.doi.org/10.1016/j.vaccine.2009.04.071
- Frasch CE, Borrow R, Donnelly J. Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine 2009; 27S:B112-16; http://dx.doi.org/10.1016/j.vaccine.2009.04.065
- Mak PA, Santos GF, Masterman K-A, Janes J, Wacknov B, Vienken K, Giuliani M, Herman AE, Cooke M, Mbow ML, et al. Development of an automated, high-throughput bactericidal assay that measures cellular respiration as a survival readout for Neisseria meningitidis. Clin Vaccine Immunol 2011; 18:1252-60; PMID:21715580; http://dx.doi.org/10.1128/CVI.05028-11