Abstract
To characterize different tissue MSCs as sources of cell immunomodulatory therapy. Examined the effects of IFN-γ on WJ-MSC and their immunomodulatory function characteristics. We compared human fetal bone marrow (F-BM), adipose tissue (AT), and Warton's Jelly-derived MSCs (WJ-MSCs) for surface antigen expression, differentiation ability, proliferation capacity, clonality, tolerance for aging, gene expression, and whether IFN-γ affected WJ-MSC gene expression, as determined by real time quantitative PCR. Fifteen geneswere examined. We further assess WJ-MSCs-mediated immunomodulatory on peripheral blood mononuclear, stimulated by PHA, IL-2 and CD3Ab after 5 days of co-cultured in a 5:1 ratio (PBMC:MSCs). Examined the effect of WJ-MSCs on the Th1, Th2, Th17 cytokines production and Treg augument. MSCs from different tissues have similar levels of cell surface antigen expression and differentiation ability, while F-BM-MSCs and WJ-MSC had higher rates of cell proliferation and clonality than AD-MSCs. All 15 genes were expressed at similar levels in WJ-MSCs and AD-MSCs (P > 0.05). 9 genes were upregulated in WJ-MSCFor F-MSC, including IL-6, CXCL9, CXCL10, CXCL11, ICAM-1, IDO1, HLA-G5, SDF1A, and NOTCH were down expression, but VCAM-1 was lower expressionin WJ-MSCS. After IFN-γ treatment, 7 genes were upregulated in WJ-MSC, including chemokine ligands CXCL9, CXCL10 and CXCL11, and the adhesion protein VCAM1and ICAM1. Additionally, immunosuppressive factors, such as HLA-G and IDO were both increased. When cocultured with peripheral blood mononuclear, WJ-MSCs showed an immunosuppressive function by inhibit the proliferative response of Th1 and Th17 but augment Th2 and Treg. Primed WJ-MSCs by IFNγ caused a greater reduction in IFNγ and TNFα than untreated WJ-MSCs, also the effect on augument in Treg and inhibit Th17 (P < 0.01). Our results demonstrate that primitive F-BM-MSCs and WJ-MSCs have biological advantages as compared to adult cells, WJ-MSCs have a gene expression pattern similar to AT-MSCs but not F-BM MSCs, and that inflammatory stimuli regulate gene expression in WJ-MSCs. WJ-MSC showed the immunosuppressive function in co-cultured system with PBMC, and IFNγ can promoted the immunosuppressive function.
Introduction
Human MSCs are a population of multilineage progenitor cells, which can differentiate into chondrocytes, osteoblasts, or adipocytes.Citation1,2 Friedenstein et al.Citation3 proposed that human bone marrow (BM) cells contain a precursor for multiple mesenchymal cell lineages first. MSCs have recently been applied for for repairing or regenerating damaged or mutated tissues in clinical.Citation4 In addition, MSCs can affect immunomodulatory functions, such as reduce inflammation, suppress lymphocyte alloreactivity in vitro in mixed lymphocyte reaction (MLR) assays.Citation5,6 MSCs also be able to repair tissue damage caused by the immune system in autoimmune diseases. MSC can be used as a candidate for treatment of autoimmune diseases such as ulcerative colitis, Crohn's disease and prevent rejection of organ transplants and graft vs. host disease after allogeneic HSC transplantation.Citation7 Krampera et al.Citation8 also demonstrated in the presence of inflammatory factor IFN-γ, human MSCs can interact with HLA-unrelated immune cells, and modulate their proliferative activity, thus suggesting that MSCs play an important role in activating immunomodulatory effects.
However, the use of BM-derived cells is not always acceptable, due to their high probability of viruls exposure and significant decreases in cell number and proliferation/differentiation capacity with age.Citation9 In addition, obtaining a BM sample is a painful, invasive procedure.Citation10 Thus, investigators have sought various substitutes of BM. Previous reports indicate there are several human tissues that are readily accessible for MSCs,Citation11,12 including adult tissues such as adipose tissues, and fetal stage tissues such as cord blood, amniotic fluid, amniotic membrane placentas, umbilical cord and fetal BM.Citation13-17 Increasing evidence demonstrates different characteristics of MSCs, depending on tissue location, donor health, and their source.
Previous studies have demonstrated significant differences in proliferation and gene expression among different tissue sources of MSCs. Even within the same source of MSCs, certain factors such as the local environment and cytokines affect their immunomodulatory function. Therefore, the source and local environment should be taken into account when using MSC in treating immune disease.Citation16-18
MSCs isolated from umbilical cord Wharton's jelly (WJ-MSCs) are more primitive and acceptable than those isolated from other tissue sources. Furthermore, obtaining WJ-MSC does not employ any invasive procedure which may be potential harmful to the donor. Therefore, WJ-MSCs represent a promising alternative cell source for the future MSC-based therapies. Deuse T et al.Citation18 has found that WJ-MSCs are superior potential cells for immunomodulation, because of their higher proliferative capacity, lower immunogenicity, stronger production of soluble tolerogenic factors. Since they are rarely exposed to infectious agents, WJ MSCs also present as a safely donor.Citation15,19,20
In order to determine whether WJ–MSC will candidate as a potential source for immunotherapy, we compare the WJ–MSC and other sources of adult and fetal tissue MSC gene expression, further assess whether WJ–MSC exert immune regulating function ex vivo, we compare the WJ–MSC and other sources of adult and fetal tissue MSC gene expression differences, and assess whether WJ–MSC has immune regulating function. We cultured and identified MSCs from adipose tissue (n = 5), WJ (n = 5), and F-BM (n = 4), ex vivo, and, investigated 15 gene expression differences from these multiple MSC sources (AT, WJ and F-BM). We also examined the effects of IFN-γ on WJ-MSC and their immunomodulatory function characteristics, as compared to other sources. We further attempted to assess WJ-MSCs-mediated immunomodulatory on peripheric blood mononuclear, which were stimulated by PHA, IL-2 and, CD3Ab. Both WJ-MSC and WJ-MSC stimulated with the proinflammatory cytokines IFNγrepresented a controlled and reproducible method of immune function after 5 days of co-cultured in a 5:1 ratio (MNC:MSCs). We attempted to examine the effect of WJ-MSCs on the Th1, Th2, Th17 cytokines production and Treg augument.
Results
Isolation and characterization MSCs from 3 sources
MSCs were isolated from 4 F-BM samples, 5 AD samples, and 5 WJ samples in the same culture medium. All isolated MSCs populations displayed a spindle-shaped morphology ().
Figure 1. (A) Morphologic comparison of MSCs isolated under different conditions. Mesenchymal stem cells were isolated from the umbilical cord (WJ-MSCs), adipose tissue (AT-MSCs) and fetal bone marrow (F-BM) under same conditions. All cells were plastically adherent with a spindle-shaped morphology. In all 3 MSCs populations adipogenic and osteogenic differentiation could be induced as examined by Oil Red-Ostaining and von Kossa staining. (B) Immunophenotyping of MSCs. AT-MSCs, fetal BM-MSCs and WJ-MSCs, were labeled with antibodies against the indicated antigens, and analyzed by flow cytometry. The markers including CD90 and CD105 showed positive and the markers CD34, CD14, HLA-DR and CD45 showed negative. The staining pattern of MSCs preparations was highly consistent to the markers of MSCs
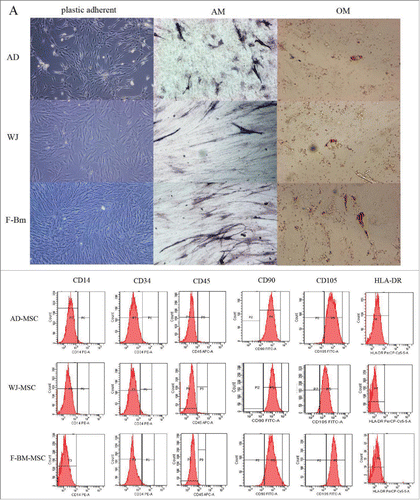
To evaluate MSC properties, their immunophenotypes and differentiation capacities were considered. A panel of surface markers was tested by flow cytometry, with MSCs defined as having a cell surface protein expression immunonegative for CD34, CD45, CD14 and HLA-DR, and immunopositive for CD90 and CD105. In vitro differentiation analysis confirmed that all isolated MSCs from different sources exhibited a comparable capacity to differentiate into osteoblasts and adipocytes, thus confirming their multipotentency ().
Growth Profiling and Cellular Senescence
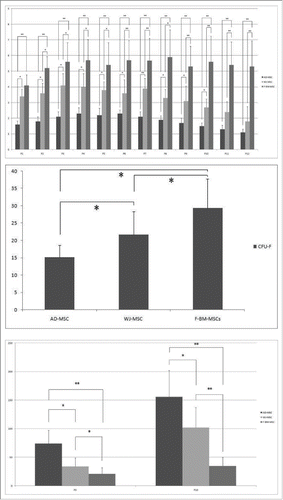
For therapeutic purposes, large-scale expansion and slow senescence are important. Here, we determined cell proliferation rates and cellular senescence in all isolated MSCs, with cells continually cultured until growth ceased. F-BM-MSCs and WJ-MSC could be cultured for significantly longer periods and exhibited the greatest expansion capacity, whereas AT-MSCs had the shortest culture time and lowest growth rate (). In most F- BM-MSCs, cell growth arrested by passages 22∼24, WJ-MSCs growth arrested by passages 17∼18, and AD-MSC proliferation stopped by passage 11∼12. A comparison of the clonogenic potential of the different tissue MSCs, by colony forming unit-fibroblast (CFU-F) assay, showed that by passage 3, more colonies formed from F-BM-MSC (33.9 ± 7.8) and WJ-MSCs (25.7 ± 8.9) than from AT-MSCs AT-MSCs (18.4 ± 4.6) (). Growth profiling of all MSCs was summarized as the final PD number through long-term cultivation, with PD measured for every passage. The final PD of the AT-MSCs was found to be significantly less than that of F- BM and WJ MSCs. Therefore, F-BM-MSCs were the most proliferative, while the results showed the long PD time, calculated at passages 3 and 10, of AD-MSCs ().
Figure 2. Growth kinetics ofF- BM-, AT-, and WJ-MSCs. (A) F-BM-MSCs and WJ-MSC showed more population-doubling (PD) than AD-MSC in all passages; (B) Clonogenetic capacity was measured by colony forming unit-fibroblast (CFU-F) assay. F-BM-MSCs formed more colonies than WJ- or AT-MSCs in Passage 3; (C) Each population doubling time (PDT) was analyzed in Passage 3 to 4 and in Passage 10 to 11, respectively. In both passages, the PDT of F-BM-MSCs was significantly lower. Error bars represent the means ± SD, n = 5; ** P < 0.01; P, passage.
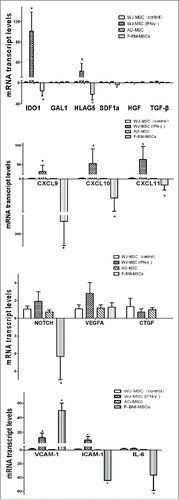
Differences in the gene expression between WJ-MSCs, AD-MSC, F-MSCs and interferon-γ treatment WJ-MSCs
It is well recognized that multiple factors are involved in the immunosuppressive function of MSCs. To investigate the effect of inflammatory conditions on MSCs gene expression, real-time RT–PCR analysis of 15 genes was performed on the 3 types of MSCs cultured with or without IFN-γ. The expression of these 15 genes, as determined by real-time PCR, did not change significantly in WJ-MSCs, compared to AT-MSCs (P > 0.05). However, 9 genes were upregulated in WJ-MSCs, as F-MSCs compared to WJ-MSC, including IL-6 (0.03 ± 0.01 fold), CXCL9 (0.005 ± 0.0001 fold), CXCL10 (0.03 ± 0.004 fold), CXCL11 (0.08 ± 0.02 fold), ICAM-1 (0.02 ± 0.001 fold), IDO1 (0.07 ± 0.01 fold), HLA-G5 (0.05 ± 0.01 fold), SDF1A (0.25 ± 0.09 fold), and NOTCH (0.23 ± 0.05 fold) were down expression. Only VCAM-1 was downregulated (49.84 ± 10.27-fold) in WJ-MSCS.
After IFN-γtreatment, 7 genes were upregulated in WJ-MSCs, including CXCL9, CXCL10, CXCL11, ICAM-1, VCAM-1, IDO1 and HLA-G5, without changing the expression of GAL1, SDF1a, IL-6, TGF-b, NOTCH, VEGFA and HGF. Quantifiably, in WJ-MSCs, IFN-γ upregulated the expression of HLA-G (22.07 ± 15.41-fold) and IDO 1(101.72 ± 37.35-fold), the T lymphocyte attractant genes CXCL9 (51.27 ± 26.82-fold), CXCL10 (85.04 ± 59.95-fold) and CXCL11 (101.72 ± 37.35 fold), and 2 adhesion protein-encoding genes, VCAM-1 (12.49 ± 6.50-fold) and ICAM-1 (9.27 ± 4.85-fold) ().
Immunomodulatory capacity of WJ-MSCs after IFN-γ expression on T cells proliferation, cytokines secretion
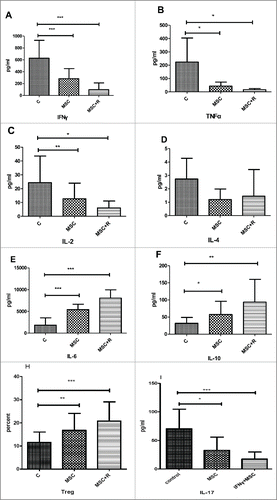
To examine the effect of IFN-γon the immunomodulatory capacity of WJ-MSCs, PBMCs cells were stimulated with PHA, CD3Ab, and IL-2 in the absence or presence of pretreated WJ-MSCs (PBMCs:MSC = 5:1) for 5 days. Th1, Th2, Th17 cytokines secretion profiles and Treg of activated lymphocytes was assessed after 5 days of co-culture. WJ-MSCs inhibit the proliferative response of Th1 and Th17 but augment Th2 and Treg. WJ-MSCs produced a reduction in secreted levels of IFNγ, TNFα, and IL-2 in activated PMBC cultures, compared to the control. Primed WJ-MSCs caused a greater reduction in IFNγ and TNFα than untreated WJ-MSCs (P < 0.01). Co-cultures with WJ-MSCs resulted in an increase in IL-10 and IL-6 levels, compared to the control samples. Furthermore, a significant increase in IL-10 and IL-6 levels were observed in co-cultures with IFNγ treated WJ-MSCs. WJ-MSCs did not cause a significant change in the kinetics and threshold levels of IL-4, compared to the control samples, except for the primed WJ-MSC co-cultures().
Figure 4. A-F: The levels of Th1 and Th2 Cytokine secretion from activated PMBC cultured in the absence or presence of MSCs were measured at 5 days by CBA cytokine detection. The levels of IFNγ, TNF-a, and IL-2 secreted from activated Th1 cells cultured in the absence of MSCs increased over time. In contrast, levels were significantly reduced at 5 days when co-cultured with MSCs. Mean values of 3 repetitive measures are shown. Statistically significant differences are specified by asterisks, compared to the fraction co-cultured with MSCs (*P < 0.05, **P < 0.01, **P < 0.001). H: MSCs pretreated by IFN-γ increased the frequency of CD4 + CD25 + CD127dim/− T cells and decreased the frequency of Th17. I: Flow cytometry diagrams in PB-MNC; also in the CBA assay, IL-17 showed a decrease after co-culture with MSC and IFNγ pretreated MSCs.
Discussion
Aging adult BM-MSCs have been reported to lack proliferation capacity,Citation10 and in this study, we sought to find an easily accessible, multipotent alternative tissue source of MSCs. The differentiation capacity of MSCs isolated and cultured from WJ was evaluated and compared to those from adult AD and F-BM. The results showed all cells to exhibit the original MSCs' features, as defined by the ISCT minimum criteria: spindle shape, multi-lineage differentiation, and surface marker expression.Citation1,2 Both F-BM- and WJ-MSCs had similar capacities for differentiation into osteoblasts and adipocytes, as compared to AT-MSCs. The growth kinetics and clonality of F-BM-MSC and WJ-MSCs could be sustained for longer periods in culture than in AD-MSCs (). The greater proliferation of MSCs allow for shorter culture time or large expansion to generate a sufficient population of MSCs for clinical usage. In addition, F-BM-MSC and WJ-MSCs are able to expand over 20 passages with normal karyotypes. Fetal tissues are thought to possess relatively more primitive MSCs, compared to adult AT, because of their higher growth rate. Moreover, neonatal tissues exhibit certain biological properties that differ from MSCs originating from adult sources.
Previous studies have indicated that different sources of MSCs seem to differ in proliferation, differentiation potential, gene expression, and protein translation.Citation23-26 Nakanishi et al.Citation26 previously showed that genes associated with mitosis, inflammation, and stress response are upregulated in AD-MSCs, while genes associated with regulation of organ development, morphogenesis and cell migration are upregulated in BM-MSCs. Other reports found that different sourced MSCs were all able to differentiate into adipocytes and osteocytes, despite differential gene expression of adhesion molecules, chemokines, and proinflammatory factors.Citation23,24,28–30 In our study, the expressional levels of 15 genes in WJ-MSCs were similar in AD-MSC but different in F-BM-MSC, and gene expression was also changed after IFNγ stimulation.
We further found that the expressional levels of immunomodulatory factors such as IDO1, HLA-G5, SDF1α and IL-6 were different in WJ-MSCs compared to F-BM MSCs. Furthermore, IFN-γ upregulated expression of IDO1 and HLA-G5 significantly, confirming the results of Yoo et al.Citation25 IDO1 catalyzes the conversion of tryptophan to kynurenine, and inhibits T-cell proliferation by tryptophan depletion after induction by IFNγ.Citation24 In our study, the IDO1 expression level in F-BM-MSCs was lower (0.07 ± 0.01-fold) than that in WJ-MSCs, implying that the immunomodulatory functions of F-BM-MSCs are different from WJ-MSCs and adult MSCs.
HLA-G is a nonclassical major histocompatiblity complex (MHC) class I protein which is expressed in both membrane-bound and soluble isoforms that can display tolerogenic properties via interaction with inhibitory receptors on dendritic cells, natural killer cells, and T-cells. Soluble HLA-G exerts an immunosuppressive effect by inducing apoptosis in CD8+ T-cells, while also down-modulating CD4+ T-cell proliferation.Citation16 While NakanishiCitation 30 and CoropCitation28 both detected HLA-G in adult tissues, but Gotherstrom et al. did not detect the same results.Citation16 Interestingly, the expressional level of HLA-G is higher in adult tissue compared to fetal tissue MSC.Citation28,31 In this study, we found that the level of HLA-G mRNA expression in F-BM-MSCs was 0.05 ± 0.01-fold compared to WJ-MSCs. In additional, IFN-γ upregulated WJ-MSC HLA-G5 expression by 22.07 ± 15.41-fold, further suggesting that IFN-γ can promote a WJ-MSC through HLA-G5-mediated immunosuppressive effection. Additionally, Kadri T et al.Citation32-34,37 demonstrated that galectin-1 (GAL1) was constitutively secreted by MSCs, and the secretion was markedly increased during direct coculture with activated T-cells. While we did not evaluate GAL1 protein levels, the mRNA levels remained the same in the 3 types of MSCs, even after IFN- γ treatment.
The mRNA levels of IL-6 and SDF1α were both higher in WJ-MSCs, compared to F-BM-MSCs. IL-6 and SDF1α are the soluble factors in MSCs that are related to T-cell survival and differentiation.IL-6 secretion is dependent on cell-cell contact and can promote Th-cell differentiation.Citation24,25 The lower expression of IL-6 and SDF1α in F-BM-MSCs might also contribute to different immunomodulatory function of between F-BM-MSC and WJ-MSC.
Although the mRNA levels of CXCL9, CXCL10 and CXCL11 in WJ-MSCs were higher than those in F-BM MSCs, they were the same as in AD-MSCs, and IFN-γupregulated all 3 chemokine ligands. The cell membrane interactions between MSC and immune cells via adhesion molecules and chemokines also play a crucial role in the immunomodulatory capacity of MSCs. As chemokine ligands, T-lymphocyte attractants are CXCL9, CXCL10 and CXCL11. Both WJ-MSCs and AD-MSCs may further benefit the immunomudulatory effects by the higher level of chemokine ligands than F-BM MSCs, which might result in the upregulation of chemokines for attraction of T lymphocytes. Moreover, closely contacting of activated immune cells and MSCs may increase the efficacy of the immunomodulatory function of WJ-MSCs.Citation35
Adhesion molecules play important roles in the specific and effective immune response to foreign pathogens. MSCs expressed a large number of cell surface molecules, including those of the integrin families and adhesion molecules responsible for cellular interactions via binding to receptors on T-cells. The cell adhesion proteins ICAM-1 and VCAM-1 both are commonly regarded as markers of MSCs,Citation26,36,37 and are reported to be important for MSC homing in tissue repair. Real-time PCR analysis revealed that MSCs constitutively expressed these molecules, at different levels in different tissues. Furthermore, Ren et al.Citation26 found that ICAM-1 and VCAM-1 in MSCs were upregulated by inflammatory cytokines, rendering MSCs more adhesive to T-cells. We found that mRNA levels of ICAM-1 and VCAM-1 were different in MSCs. In WJ-MSCs, IFN-γ increased the expression of VCAM-1 (12.49 ± 6.50 fold) and ICAM-1 (9.27 ± 4.85 fold), while VCAM-1 mRNA levels in F-BM-MSCs were higher than those in WJ-MSCs. We are the first to report different expression levels of VCAM-1 in WJ-MSCs and F-BM-MSCs. VCAM-1 is a prototype marker for endothelial cell activation, and ICAM-1, a CD18 receptor, mediates tight leukocyte adhesion to endothelium, transendothelial migration, and attachment to parenchymal cells. Differential expression of VCAM-1 between adult and fetal tissues indicate different functions of the different sources of MSCs, while WJ-MSCs have characteristics similar to adult MSCs.
Besides of their immunomodulatory properties, MSCs have a potential capacity to support tissue regeneration. While this is mediated partially via their differentiation into other cell types, there is now increasing evidence that the regenerative effect of MSCs is also the result of their production of trophic factors, which stimulate resident progenitor cells.Citation6,38–40 In this study, we evaluated the mRNA levels of NOTCH, VEGFA, HGF, CTGF, TGF-β in WJ-MSCs, as compared to the other 2 types of MSCs and stage of inflammation. The results showed that there were no significant differences in the mRNA levels of all the above genes except CTGF, which was not detected in F-BM MSCs. Moreover, inflammatory conditions did not affect the expression of all the growth factors mentioned above. Thus, while WJ-MSCs gain immunosuppressive capacity under inflammatory conditions, their regenerative capacity is preserved.
MSCs isolated from umbilical cord Wharton's jelly (WJ-MSCs) are more primitive and acceptable than those isolated from other tissue sources. Furthermore, obtaining WJ-MSC employ no invasive procedure which may be potential harmful to the donor, WJ can be acted as the rich source of MSC for clinical using compared to AD-MSC and fetal BM MSC. For further evaluate the immune regulating function of WJ-MSC, we applied in vitro coculture with WJ-MSC and PBMC and assay IFNγon WJ–MSC immunomodulatory effects. In the present study, we found that MSCs were able to inhibit the proliferative response of Th1 and Th17 lymphocytes. WJ-MSCs exerted their suppressive activity by inducing Treg cells augmentation within the target population. In the previous study on immunomodulatory effects of MSC, different MSC ratios produced opposite results. The higher dosage of MSCs inhibited T cell proliferation but the lower ratio of MSCs promoted T cell proliferation.Citation18,19 Furthermore, cell-cell contact was not a necessary condition for the MSC immunomodulatory function.Citation5,18,19,22 In our study, we used a higher ratio of PMBC:WJ-MSC (5:1), and we found the 5:1 ratio of PMBC and WJ-MSC can effectively inhibit the proliferation of Th1 and Th17.
In addition, Krampera et al.Citation5 demonstrated that human MSCs could interact with HLA-unrelated immune cells, thus, modulating their proliferative response. They also reported that IFN-γplayed an important role in activating the immunomodulatory effects of MSCs. Recent reports have noted that the different sources MSC have different immunomodulatory capacities. Therefore, when using MSC for the treatment of immune diseases, the source and local environment should be taken into account. In the pathogenesis of MS, the cytokines of Th1 are the key factors. Th1 immune responses were thought to mediate inflammatory demyelination in MS and EAE; both cell-to-cell contact and soluble factors have been demonstrated to be implicated in MSC-mediated immunomodulatory properties.Citation41-43 In our study, WJ-MSCs or WJ-MSCs pretreated by 1,000 u/ml IFN γcan significantly reduce the levels of Th1 cytokines, and WJ-MSCs pretreated by IFNγachieved a stronger capacity. The cytokines, which are produced by Th1, including IFN-γ, TNF-α, and IL-2, decreased significantly, compared to the decrease of Th1-related cytokines. The cytokines of Th2 increased after co-culture with WJ-MSCs. The WJ-MSCs pretreated with IFNγ showed this effect. In addition, IL-10 and IL-6 significantly modified the results; however, we did not find a significant change of IL-4 under the same stimulating conditions.
Evidence continues to accumulate that implicates the newly-characterized IL-17A and IL17F-producing Th cell population (Th17) in the pathogenesis of these and other autoimmune diseases. Guo et al.Citation20 reported that F-BM-MSC suppressed the proliferation of CD4+ T cells, but stimulated the secretion of IL-17 by CD4+ T cells. Eljaafari et al.44 found that BM-MSC could promote IL-17A secretion and inhibit IL-10 secretion when co-cultured with BM-MSC and T cells of rheumatoid arthritis, this finding was in contrast to the effect of MSC on other diseases. Our study showed that WJ-MSC inhibited the proliferation of the activated cells as well as the cytokine production specific for Th1 and Th17. The higher immunomodulatory action of WJ-MSC on the proliferation and the cytokine production profile was also observed on WJ-MSCs pretreated by IFN-γ. Using CD4+, CD25+, CD127dim/− as the Treg populations, we found that after co-culture with WJ-MSCs, Treg was increased, and the same results was found when the MSCs which were pretreated with the IFNγ, even the WJ-MSC pretreatment by IFN-γ had more highly modulated function. We demonstrated that WJ-MSCs have the impact on the Treg and Th17 balances.Citation5,22,29,41-43
The present study demonstrates that gene expression in WJ-MSCs is similar to that in adult adipose MSCs but different from fetal-BM MSCs. The different expression between WJ-MSC and F-MSC in chemokines, and adhesion factor, which show WJ-MSC have the strong ability in inducing cells and promoting and have stronger immune function. Immunosuppressive activity gene expression can be enhanced by culturing WJ-MSCs with proinflammatory cytokines. The potential regenerative capacity of WJ-MSCs is also similar to that of adult MSCs and fetal BM MSCs, and is not affected by inflammatory conditions. WJ-MSC showed immunosuppressive activity when co-culture with PBMC by ratio 1:5, and their immunosuppressive activity can be enhanced further by culturing WJ-MSCs with proinflammatory cytokines.
Conclusion, the immune activation of WJ-MSCs could be of benefit for potential clinical immunotherapy with WJ-MSCs. Therefore, immune activation of WJ-MSCs could be of benefit for potential clinical immunotherapy. The immunoregulatory effects of WJ–MSC in different autoimmune diseases need further research.
Materials and Methods
Isolation of human MSCs from F-BM, AT and WJ
The use of human tissues for the purpose of this research was approved by the Medical Ethics Review Board of the Chinese Academy of Medical Sciences and China Medical University. Donors were screened by questionnaire to be healthy and without blood infection and immune system disease. All donors provided written informed consent.
For AT-MSCs culture, adult adipose tissues were obtained by liposuction, and MSCs then isolated and cultured according to a previous protocol.Citation1 Briefly, lipoaspirates were washed extensively with equal volumes of Dulbecco's phosphate-buffered saline (DPBS) and the extracellular matrix then digested with 0.1% collagenase IV (Roche Applied Science, Germany) at 37°C for 30 min. LG-DMEM containing 10% FBS with 100 U/ml penicillin/streptomycin, neutralized Enzyme digestion, the samples were then centrifuged at 1200 × g for 10 min. Deposited cells were washed with DPBS and incubated in a humidified atmosphere at 37°C at 5% CO2 in MesenCult MSC Basal Medium (Stem Cell, Canada).When the adherent cells were covered the bottle over 80%, cells detached using 0.05% trypsin–EDTA (Hyclone, USA) and re-plated at a 1:3 ratio under the same culture conditions.
WJ-MSCs were isolated using an explantation culture methodCitation19 in which umbilical cords are washed in DPBS to remove blood components and vessels removed to avoid endothelial cell contamination. The wharton's jelly were then cut into 0.5–1 cm3 pieces and placed directly into culture wells for expansion in MesenCult MSC Basal Medium and 100 U/ml penicillin/streptomycin. When colonies of cells reached 70% confluency, the cells were detached using 0.05% trypsin–EDTA and re-plated at a 1:3 ratio under the same culture conditions, as described previously.Citation20 For fetal BMC culture, BM was collected from fetal long bones and the mononuclear cell fractions isolated using Ficoll-Paque gradient centrifugation. Human F-BM-MSCs were separated based on their adherence to plastic flasks, in MesenCult MSC Basal Medium, 100 U/ml penicillin/streptomycin, while the non-adherent cells were discarded after 3 days. At about 80–85% confluency, the adherent cells were detached by treatment with 0.125% trypsin and 0.1% EDTA and re-plated at a 1:3 dilution under the same culture conditions.Citation20 Time-dependant cell expansion was determined by the trypan blue exclusion method. In each passage, MSCs were cultured for 7 days, followed by collection using trysin-EDTA (Gibco), counting and reseeding at the initial cell density (2000 cells/cm2). Culture medium was replaced twice weekly. The number of PDs (population doublings) were calculated based on the total cell number at each passage by dividing the logarithm of the fold-increase value obtained at the end of the passage by the logarithm of 2. This procedure was repeated until the cells stopped proliferating, at which time the cells were counted to calculate the final PD.Citation21
Population doubling time was examined using the formula: (t − t0)·log2/log(N − N0), where t − t0 is the total culture time (h), N is the number of harvested cells, and N0 is the initial number of cells.
CFU-F (colony forming unit-fibroblast) assays were performed by seeding cells in a culture dish (BD Biosciences, San Jose, CA, USA) and incubating in humidified 5% CO2 at 37°C; culture medium was exchanged every 3 days. After 2 weeks, the dishes were washed twice with phosphate-buffered saline (PBS, Invitrogen, La Jolla, CA, USA), fixed with 100% methanol, and stained with 3% Crystal violet (Sigma) for counting the number of colonies.
Osteogenic differentiation
Cells were plated at 10,000 cells/cm2 in 6-well plates and cultured under osteogenic conditions for the first 8 days. Differentiation medium consisted of growth medium supplemented with 10−8 M dexamethasone and 50 μg/mL glycerophosphate. Half the medium was changed in 5 days, and in 8 days, 3.5 mM mascorbate-2-phosphate was added to the medium for 5 weeks. To assess their osteogenic differentiation, cells were fixed in 70% ethanol for 1 h and stained with Von Kossa.Citation22
Adipogenic differentiation
Cells were plated at 10,000 cells/cm2 in 6-well plates and cultured for 14 days with adipogenic medium, consisting of MesenCult MSC basal Medium supplemented with 1 μM dexamethasone, 500 mM isobutylmethylxanthine, 1.0 mg/ml insulin, and 100 mM indomethacin. To examine their adipogenic differentiation, the cells were fixed with 10% formalin (Sigma) for 15 min and stained with 0.3% oil red O for 30 min at 37°C.Citation22
Immunophenotyping characterization of MSCs
A total of 6 surface markers, including CD14, CD34, CD45, CD90, CD105 and the HLA-DR, were analyzed by flow cytometry (BD Biosciences, USA). Cells were suspended in PBS at 106 cells/mL, and 50 μl aliquots of cells were transferred to flow cytometry tubes and incubated for 15 min at 4°C with mouse anti-human CD14-FITC, CD34-FITC, CD45-PE, CD90-FITC, CD105-FITC, and HLA-FITC monoclonal antibodies). The negative control stain was FITC-conjugated mouse IgG1-isotype (all from BD Biosciences, USA). Subsequently, the cells were washed with PBS, diluted in 500 μl PBS, and the samples analyzed after 5 min of incubation at room temperature. 5000 gated events were acquired on a biexponential fluorescence scale. Positive staining for the CD markers was defined as the emission of a fluorescence signal that exceeded levels obtained by >95% of control population cells stained with matched isotype (non-fluorescent) antibodies. Dot-plots were generated using the software CELLQUEST (BD Biosciences, USA).
Gene expression by real-time quantitative PCR analysis of WJ-MSC, AD-MSC, F-BM-MSC and WJ-MSC pretreated with IFN-γ
The three types of MSCs were extracted from cultured cells at indicated time points. For IFN-γ- pretreated MSCs, 1000 U/mL human recombinant IFN-γ was added to WJ-MSCs for 24 h. Cells were then washed twice with PBS to remove IFN-γ and collected and stored at −80°C. Total cellular RNA was extracted using TRIzol (Takala, Japan), according to the manufacturer's instructions, and quantified spectrophotometrically. Cell to cDNA II kit (Ambion, USA) was used to generate cDNA, according to the manufacturer's instructions, and the cDNA generated was then analyzed by real-time PCR. An aliquot of cDNA, equivalent to 200 ng starting RNA, was subjected to polymerase chain reaction (PCR) amplification of a total of 15 genes, including genes encoding inflammation factors such as IL-6, chemokine ligands such as CXCL9, CXCL10, and CXCL11, the adhesion molecule ICAM-1, and immune response molecules such as IDO1, HLA-G5, SDF1α and NOTCH. The primer sequences and expected product sizes were designed using Primer Express 2.0 software (Applied Biosystems, Foster City, CA, USA). Primer information, accession numbers for mRNA sequences and amplicon sizes are shown in . All of the reactions were performed in a total volume of 10-μl, with 2-μl of cDNA as template and 300 nM forward and 300 nM reverse primers. Amplification of the cDNA was achieved following the manufacturer's conditions: an initial activation and denaturation step of 20 s at 95°C followed by 45 cycles consisting of 3 s at 95°C and 30 s at 60°C. A dissociation curve protocol was run after every reaction to determine the purity of the PCR products. Gene expression levels were determined by the comparative δδCt method, and a normalization factor (NF), calculated as the geometric mean of the quantity of housekeeping genes (GAPDH), was used to normalize the expression of each gene. All RT-PCR assays were performed at each time point 3 times, with MSCs obtained from 4 or 5 independent donors.
Table 1. Primer information, accession numbers for mRNA sequences and amplicon sizes
PBMC isolation and MSCs and PBMC co-culture
Stimulated PBMC were isolated from the buffy coat of healthy donors by the Ficoll-Paque method. Isolated cells were cultured at a density of 1 × 106 cells/ml in RPMI-1640 medium (Hyclone, Thermo) supplemented with 10% fetal bovine serum (Hyclone, Thermo), 50 IU/ml penicillin, and 50 IU/ml streptomycin (Invitrogen, USA).
For mitogenic stimulation, the cells were stimulated with 5 μg/mL phytohemagglutinin (PHA, Biological Industries, Israel) and 20 U/mL IL-2 and 1 ug/ml anti-CD3 mAb (all from ProTech, USA). After the third passage of MSCs, the MSCs were plated at 4 × 103 cells/cm2, which corresponded to 1 × 104 MSCs/ml, in a flat-bottomed 24-well plate. After a short period of adherence, 1,000 u/ml IFNγ(ProTech, USA) was added to some of the wells for 24 hours. Then, the PBMC were incubated with the plated MSCs with or without IFN-γfor the next 5 days of co-culture in RPMI-1640 medium supplemented with 10% FBS. The ratio of MSCs: PBMC at 1:5 was used to investigate the MSC-mediated effects. Only the control wells were planted with PBMC in RPMI-1640 medium supplemented with 10% FBS.
Cytokine quantification assay
Supernatants were collected from PBMC co-cultured with MSCs or without MSCs and frozen at −70°C. Multiplex human cytokine detection (BD Biosciences) was utilized to measure the production of interleukin (IL-2, IL-4, IL-6, IL-10, IL-17), IFN-γ,and TNF-α, according to the manufacturer's instructions. The cytokines secreted by Th1, Th2, and Th17 assay were detected simultaneously using the human Th1/Th2/Th17 cytokine kit (Cytometric Bead Array (CBA); BD PharMingen, USA). Briefly, 50 μl of each sample was mixed with 50 μl of a mixture of capture beads and 50 μl of the human Th1/Th2/ Th17 PE detection reagent consisting of PE-conjugated anti-human IL-2, IL-4, IL-6, IL-10, IL-17, IFN-γ, and TNF-α. The samples were incubated at room temperature for 3 hours in the dark. After incubation with the PE-labeled detection reagent, the samples were washed once and resuspended in 300 μl of wash buffer before acquisition in the FACS CantoII flow cytometer (BD Bio). Data were analyzed using CBA software. Standard curves were generated for each cytokine using the mixed cytokine standard provided by the kit. The concentration for each cytokine in cell supernatants was determined by interpolation from the corresponding standard curve. The range of detection was 20–5,000 pg/ml for each cytokine measured by CBA. For the cytokines with > 5000 pg/ml, the average MGN was calculated for comparison.
Detection of CD4+, CD25+, and CD127dim/−regulatory T cells
FACS Lysing Solution, Monoclonal antibodies (mAbs) CD25-PC5, CD127-PE, CD4-FITC, mouse IgG1-PC5, and mouse IgG1-PE were provided by Beckman Coulter in the United States. 20 μl CD4-FITC, mouse IgG1-PC5, and mouse IgG1-PE were added to the control tube, 20 μl CD4-FITC, CD25-PC5, and CD127-PE were added to the test tube, and then a 100 μl cell suspension was added. Samples were incubated for 20 minutes at room temperature (20–25°C) and then 2 ml of PBS was added twice to wash the cells. At this point, the PBS resuspended cells were ready for the FACS Canto II flow cytometry (BD Bio). From each tube, we obtained 5 × 104 cells for the following analysis: lymphocytes were gated in the FSC-SSC scatter plot, and then CD4+ T cells were gated in the CD4-SSC scatter plot to analyze the CD4+, CD25+, and CD127 dim/− cell mass proportion. The detection result was indicated by the percentage of CD4+, CD25+, and CD127dim/− cells in CD4+ T cells.
Statistical analysis
Parametric data was expressed as means ± standard deviation (SD), while non-parametric data was expressed as medians (interquartile range). Differences and statistical significance were verified by one-way ANOVA followed by the Fisher's least significant difference (LSD) post hoc test. Cytokine quantification assay data were analyzed using the paired t-test, depending on the distribution of the data as tested with the t test for normality. Parametric data were expressed as mean ± standard deviation (SD), while non-parametric data were expressed as median (interquartile range). Statistical significance was defined as P < 0.05 (2-tailed).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
WQS conceived of the study and participated in its design and coordination and performed the statistical analysis. WZ carried out cell differentiation. YQN carried out cell cultured and participated in drafting the manuscript. THX carried out FCM assay. MLY carried out the immunoassays. ZY carried out the molecular genetic studies. YZW carried out the design of the study. All authors read and approved the final manuscript.
Funding
This work was supported by the Science Council of Liaonong Province (grants:2011225020 and 2012225014) and Science Council of Shenyang (grants, F11-262-9-53).
References
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001; 7(2):211-28; PMID:11304456; http://dx.doi.org/10.1089/107632701300062859
- Sottile V, Halleux C, Bassilana F, Keller H, Seuwen K. Stem cell characteristics of human trabecular bone-derived cells. Bone 2002; 30(5):699-704; PMID:11996907; http://dx.doi.org/10.1016/S8756-3282(02)00674-9
- Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968; 6(2):230-47; PMID:5654088; http://dx.doi.org/10.1097/00007890-196803000-00009
- Bassi EJ, Aita CA, Câmara NO. Immune regulatory properties of multipotent mesenchymal stromal cells: where do we stand? World J Stem Cells 2011; 3(1):1-8; PMID:21607131; http://dx.doi.org/10.4252/wjsc.v3.i1.1
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memoryantigen-specific T cells to their cognate peptide. Blood 2003; 101(9):3722-9; PMID:12506037; http://dx.doi.org/10.1182/blood-2002-07-2104
- Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implicationsin transplantation. Transplantation 2003; 75(3):389-97; PMID:12589164; http://dx.doi.org/10.1097/01.TP.0000045055.63901.A9
- Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, Genrich K, Mehrotra S, Setty S, Smith B, et al. IFN-γ activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol 2008; 38:1745-55; PMID:18493986; http://dx.doi.org/10.1002/eji.200738129
- Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, et al. Role for interferon-γ in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 2006; 24(2):386-98; PMID:16123384; http://dx.doi.org/10.1634/stemcells.2005-0008
- Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 2003; 33(6):919-26; PMID:14678851; http://dx.doi.org/10.1016/j.bone.2003.07.005
- Rubinstein P, Rosenfield RE, Adamson JW, Stevens CE. Stored placental blood for unrelated bone marrow reconstitution. Blood 1993; 81(7):1679-90; PMID:8096404
- Da Silva Meirelles L, Chagastelles P C, Nardi N B. Mesenchymal stem cells reside invirtually all post-natal organs and tissues. J Cell Sci 2006; 119(Pt11):2204-13; PMID:16684817; http://dx.doi.org/10.1242/jcs.02932
- Hoogduijn MJ, Crop MJ, Peeters AM, Van Osch GJ, Balk AH, Ijzermans JN, Weimar W, Baan CC. Human heart, spleen, and perirenal fat-derived mesenchymal stem cells have immunomodulatory capacities. Stem Cells 2007; 16(4):597-604; PMID:17784833; http://dx.doi.org/10.1089/scd.2006.0110
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001; 7(2):211-28; PMID:11304456; http://dx.doi.org/10.1089/107632701300062859
- Yen BL, Huang HI, Chien CC, Jui HY, Ko BS, Yao M, Shun CT, Yen ML, Lee MC, Chen YC. Isolation of multipotent cells from human term placenta. Stem Cells 2005; 23(1):3-9; PMID:15625118; http://dx.doi.org/10.1634/stemcells.2004-0098
- Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells 2004; 22(7):1330-7; PMID:15579650; http://dx.doi.org/10.1634/stemcells.2004-0013
- Gotherstrom C, Ringden O, Tammik C, Zetterberg E, Westgren M, Le Blanc K. Immunologic properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol 2004; 190(1):239-45; PMID:14749666; http://dx.doi.org/10.1016/j.ajog.2003.07.022
- Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 2001; 98(8):2396-402; PMID:11588036; http://dx.doi.org/10.1182/blood.V98.8.2396
- Deuse T, Stubbendorff M, Tang-Quan K, Phillips N, Kay MA, Eiermann T, Phan TT, Volk HD, Reichenspurner H, Robbins RC, et al. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Trans 2011; 20(5):655-67; PMID:21054940; http://dx.doi.org/10.3727/096368910X536473
- Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, et al. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells 2003; 21(1):50-60; PMID:12529551; http://dx.doi.org/10.1634/stemcells.21-1-50
- Guo Z, Zheng C, Chen Z, Gu D, Du W, Ge J, Han Z, Yang R. Fetal BM-derived mesenchymal stem cells promote the expansion of human Th17 cells, but inhibit the production of Th1 cells. Eur J Immunol 2009; 39(10):2840-9; PMID:19637224; http://dx.doi.org/10.1002/eji.200839070
- Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, Eom Y, Lee JE, Kim YJ, Yang SK, Jung HL, et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009; 259(2):150-6; PMID:19608159; http://dx.doi.org/10.1016/j.cellimm.2009.06.010
- Nakanishi C, Nagaya N, Ohnishi S, Yamahara K, Takabatake S, Konno T, Hayashi K, Kawashiri MA, Tsubokawa T, Yamagishi M. Gene and protein expression analysis of mesenchymal stem cells derived from rat adipose tissue and bone marrow. Circ J 2011; 75(9):2260-8; PMID:21747191; http://dx.doi.org/10.1253/circj.CJ-11-0246
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998; 281(5380):1191-3; PMID:9712583; http://dx.doi.org/10.1126/science.281.5380.1191
- Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2, 3-dioxygenase-mediated tryptophan degradation. Blood 2004; 103(12):4619-21; http://dx.doi.org/10.1182/blood-2003-11-3909
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008; 2(2):141-50; PMID:18371435; http://dx.doi.org/10.1016/j.stem.2007.11.014
- Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Pescatori M, Stubbs AP, van Ijcken WF, Dahlke MH, Eggenhofer E, Weimar W, et al. Inflammatory conditions affect gene expression and function of human adipose tissue-derived mesenchymal stem cells. Clin Exp Immunol 2010; 162(3):474-86; PMID:20846162; http://dx.doi.org/10.1111/j.1365-2249.2010.04256.x
- Najar M, Rouas R, Raicevic G, Boufker HI, Lewalle P, Meuleman N, Bron D, Toungouz M, Martiat P, Lagneaux L. Mesenchymal stromal cells promote or suppress the proliferation of T lymphocytes from cord blood and peripheral blood: the importance of low cell ratio and role of interleukin-6. Cytotherapy 2009; 11(5):570-83; PMID:19565371; http://dx.doi.org/10.1080/14653240903079377
- Hemeda H, Jakob M, Ludwig A, Giebe B, Lang S, Brandau S. Interferon-gamma and tumor necrosis factor-alpha differentially affect cytokine expression and migration properties of mesenchymal stem cells. Stem Cells Dev 2010; 19(5):693-706; PMID:20067407; http://dx.doi.org/10.1089/scd.2009.0365
- Nasef A, Mathieu N, Chapel A, Frick J, François S, Mazurier C, Boutarfa A, Bouchet S, Gorin NC, Thierry D, et al. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation 2007; 84(2):231-7; PMID:17667815; http://dx.doi.org/10.1097/01.tp.0000267918.07906.08
- Kadri T, Lataillade JJ, Doucet C, Marie A, Ernou I, Bourin P, Joubert-Caron R, Caron M, Lutomski D. Proteomic study of Galectin-1 expression in human mesenchymal stem cells. Stem Cells Dev 2005; 14(2):204-12; PMID:15910247; http://dx.doi.org/10.1089/scd.2005.14.204
- Sioud M, Mobergslien A, Boudabous A, Floisand Y. Evidence for the involvement of galectin-3 in mesenchymal stem cell suppression of allogeneic T-cell proliferation. Scan J Immun 2010; 71(2):267-74; http://dx.doi.org/10.1111/j.1365-3083.2010.02378.x
- Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA 1996; 93(13):6737-42; PMID:8692888; http://dx.doi.org/10.1073/pnas.93.13.6737
- Rice CM. Scolding NJ. Adult human mesenchymal cells proliferate and migrate in response to chemokines expressed in demyelination. Cell Adh Migr 2010; 4(2):235-40; PMID:20234187; http://dx.doi.org/10.4161/cam.4.2.11404
- Ren G, Zhao X, Zhang L, Zhang J, L'Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol 2010; 184(5):2321-8; PMID:20130212; http://dx.doi.org/10.4049/jimmunol.0902023
- Najar M, Raicevic G, Id Boufker H, Stamatopoulos B, De Bruyn C, Meuleman N, Bron D, Toungouz M, Lagneaux L. Modulated expression of adhesion molecules and galectin-1: role during mesenchymal stromal cell immunoregulatory functions. Exp Hematol 2010; 38(10):922-32; PMID:20570633; http://dx.doi.org/10.1016/j.exphem.2010.05.007
- Isobe M, Suzuki J, Yamazaki S, Yazaki Y, Horie S, Okubo Y, Maemura K, Yazaki Y, Sekiguchi M. Regulation by differential development of Th1 and Th2 cells in peripheral tolerance to cardiac allograft induced by blocking ICAM-1/LFA-1 adhesion. Circulation 1997; 96(7):2247-53; PMID:9337197; http://dx.doi.org/10.1161/01.CIR.96.7.2247
- Shimada Y, Hasegawa M, Kaburagi Y, Hamaguchi Y, Komura K, Saito E, Takehara K, Steeber DA, Tedder TF, Sato S. L-selectin or ICAM- 1 deficiency reduces an immediate-type hypersensitivity response by preventing mast cell recruitment in repeated elicitation of contact hypersensitivity. J Immunol 2003; 170(8):4325-34; PMID:12682269; http://dx.doi.org/10.4049/jimmunol.170.8.4325
- Caplan AI, Dennis JE. Mesenchymal stem cells astrophic mediators. J Cell Biochem 2006; 98(5):1076-84; http://dx.doi.org/10.1002/jcb.20886
- Cheng H, Qiu L, Ma J, Zhang H, Cheng M, Li W, Zhao X, Liu K. Replicative senescence of human bone marrow and umbilical cord derived mesenchymal stem cells and their differentiation to adipocytes and osteoblasts. Mol Biol Rep 2011; 38:5161-8; PMID:21188535; http://dx.doi.org/10.1007/s11033-010-0665-2
- Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB. Pro-inflammatory cytokines, IFNγ and TNFα, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One 2010; 5(2):e9016; http://dx.doi.org/10.1371/journal.pone.0009016
- Kassis I, Grigoriadis N, Gowda-Kurkalli B, et al. Neuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitis[J]. ARCH NEUROL 2008; 65(6):753-761
- Oreja-Guevara C, Sindern E, Raulf-Heimsoth M, Malin JP. Analysis of lymphocyte subpopulations in cerebrospinal fluid and peripheral blood inpatients with multiple sclerosis and inflammatory diseases of the nervous system[J]. Acta Neurol Scand, 1998; 98(5):310-313
- Rafei M, Campeau PM, Aguilar-Mahecha A. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol 2009; 182(10):5994-6002
- Eljaafari A, Tartelin ML, Aissaoui H, Chevrel G, Osta B, Lavocat F, Miossec P. Bone marrow-derived and synovium-derived mesenchymal cells promote Th17 cell expansion and activation through caspase 1 activation: Contribution to the chronicity of rheumatoid arthritis. Arthritis Rheum 2012; 64(7):2147-2157