Abstract
A novel combined Haemophilus influenzae type b-Neisseria meningitidis serogroups A and C-tetanus-toxoid conjugate vaccine (Hib-MenAC vaccine) has been developed to protect children against diseases caused by Hib, MenA, and MenC. This study investigated the safety and immunogenicity of the Hib-MenAC vaccine administered in 2-dose series to children aged 6–23 months and in a single dose to children aged 2–5 y. A randomized, positive-controlled, non-inferiority clinical trial was conducted for 1200 healthy participants in each age group. Within each age group, participants were randomly allocated to the Hib-MenAC group or the control group at a ratio of 1:1. Adverse reactions were recorded within 28 d after each dose. Blood samples were obtained to assess immunogenicity on day 0 and at 28 d after a complete vaccination course. For the investigational vaccine, the incidence of total adverse reactions in vaccinees aged 6–23 months was 46.8% and that in vaccinees aged 2–5 y was 29.8%. Most adverse reactions were mild or moderate. One non-fatal serious adverse event occurred in the Hib-MenAC group, but was unrelated to vaccination. The seroconversion rate to the 3 components reached 94.0%, and the proportion of vaccinees with rSBA titers ≥ 1:8 and PRP ≥ 0.15 g/mL reached 97.0% in both age groups. The safety and immunogenicity of the Hib-MenAC vaccine were non-inferior when compared to the licensed vaccines. It was concluded that the novel vaccine would be expected to protect children against all of the targeted diseases.
Abbreviations:
- Hib, Haemophilus influenzae
- type b
- MenA, Neisseria meningitidis serogroup A
- MenC, Neisseria meningitidis serogroup C
- Hib-MenAC vaccine, combined Haemophilus influenzae
- type b–Neisseria meningitidis serogroups A and C-tetanus-toxoid conjugate vaccine
- PRP, polyribosylribitol phosphate
- rSBA, a serum bactericidal assay using baby rabbit complement
- SAEs, serious adverse events
- GMTs, geometric mean titers
- GMCs, geometric mean concentrations
- EPI, Expanded Program on Immunization
- ATP, according to protocol
- CI, confidence interval
- RD, rate difference
Introduction
Haemophilus influenzae type b (Hib) and Neisseria meningitidis remain serious global health threats associated with high mortality and morbidity in young children. Globally, Hib is estimated to account for 8 million cases of serious illnesses and 371,000 deaths per year,Citation1 and N. meningitidis is reported to infect between 500,000 to 1.2 million people, killing between 50,000 and 135,000 per year.Citation2 Young children with less mature immune systems are among those who suffer the greatest disease burden.Citation3
In China, meningococcal disease outbreaks have previously occurred in a cyclical pattern at intervals of 8–10 y.Citation4 Serogroup A has been responsible for 4 pandemics, occurring in 1959, 1967, 1977, and 1984.Citation5 By contrast, serogroup C emerged in China in 2003 and caused disease outbreaks with more severe symptoms than did serogroup A.Citation6,7 Although serogroups B, W, and Y have been isolated from both patients and healthy carriers, they are not among the predominant serogroups in China.Citation8 The overall impact of Hib-related infections and the extent of coverage of Hib conjugate vaccines are unclear because of limited epidemiologic study in most developing countries, including China. One study in a northern China city reported that the incidence of Hib infection in children with acute upper respiratory tract infections was 19.5%.Citation9
Generally, vaccination has been considered the most effective way to prevent infection against Hib and N. meningitidis. However, according to China's Expanded Program on Immunization (EPI), up to 12 vaccines (amounting to 25 doses) are recommended in a child's first 18–24 months of life.Citation10 As a result of this strict immunization schedule, many problems related to the compliance, acceptance, and coverage of vaccines have arisen. Therefore, the development of combined vaccines would be advantageous for increasing coverage. To provide children direct protection against Hib and N. meningitidis serogroups A (MenA) and C (MenC), without adding additional injections to the immunization schedule, a new combined Hib-MenA and MenC-tetanus toxoid conjugated (Hib-MenAC) vaccine was recently developed by Royal (Wuxi) Biological Co., Ltd. It is the first freeze-dried, trivalent, combined vaccine against Hib, MenA, and MenC to have gone through phase 3 clinical trials. This article reports the available safety and immunogenicity data of the novel Hib-MenAC vaccine, which is administered to children aged 6–23 months through a 2-dose immunization package, and to those aged 2–5 y through a single dose immunization package. (Clinical Trial number: NCT01428908).
Results
Participants and demography
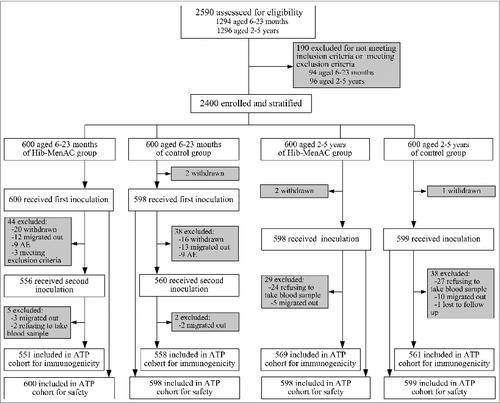
In June 2011, 1294 children aged 6–23 months and 1296 children aged 2–5 y were recruited and assessed for eligibility as potential study participants. According to the inclusion and exclusion criteria, 94 children aged 6–23 months and 96 children aged 2–5 y were excluded. Of the 1200 participants aged 6–23 months who were enrolled and randomized into the Hib-MenAC group or the control group, 1198 (99.8%) received a first dose, 1116 (93.0%) received a second dose, and 1109 (92.4%) participants were included in the follow-up analysis of immunogenicity. Of the 1200 participants aged 2–5 years, 1197 (99.8%) participants received the vaccination, and 1130 (94.2%) were included in the follow-up analysis of immunogenicity (). The rates of compliance to the protocol between the treatment groups for both age groups were comparable.
Demographic characteristics at the time of enrollment for the groups receiving the investigational vaccine (6–23-month-old group: 57.5% male participants, mean age 10.8 months; 2–5-year-old group: 56.0% male participants, mean age 36.4 months) and the control vaccine (6–23-month-old group: 58.4% male participants, mean age 11.1 months; 2–5-year-old group: 53.4% male participants, mean age 35.5 months) were similar for age and sex.
Safety
For the 6–23-month-old vaccinees, the incidence of overall adverse reactions after dose 2 was 46.8% (281/600) for the Hib-MenAC group and 49.8% (298/598) for the control group, as presented in . Most of the adverse reactions were grade 1 or grade 2. The incidence of grade 3 adverse reactions was 2.3% in the Hib-MenAC group and 2.2% in the control group. No grade 4 adverse reactions were observed during the clinical trial. In the Hib-MenAC group and the control group, fever was the most frequently reported systemic adverse reaction (36.5% and 40.1%, respectively), with redness (1.2% and 1.5%, respectively) and swelling (1.2% for both groups) being the most frequently reported local adverse reactions. The incidence of other systemic reactions was higher in the control group (1.2%) than in the Hib-MenAC group (0%), and the difference was statistically significant. No statistically significant differences were observed for other systemic and local adverse reactions.
Table 1. Summary of serious adverse event and adverse reactions
For the 2–5-year-old vaccinees, the incidence of overall adverse reactions after dose 1 for the Hib-MenAC and control groups was 29.8% (178/598) and 30.9% (185/599), respectively, as presented in . Most of the adverse reactions were grade 1 or grade 2. The incidence of grade 3 adverse reactions was 0.5% in the Hib-MenAC group and 1.0% in the control group. Fever was the most frequently reported systemic adverse reaction in the 2 treatment groups (21.2% in Hib-MenAC and 23.5% in control), with redness (1.7% and 1.0%, respectively) and swelling (1.7% and 2.0%, respectively) being the most frequently reported local adverse reactions. No statistically significant differences were observed for any systemic or local adverse reactions between treatment groups.
In total, 4 non-fatal serious adverse events (SAEs) occurred during the study period: one in the Hib-MenAC group and 3 in the control group. Among them, one participant, aged 30 months, from the control group had a body temperature of over 39°C 13 h after the first dose. Investigators concluded that this fever was likely related to vaccination. However, the fever resolved within 72 h after hospital treatment, and there were no sequelae. The other 3 SAEs were considered to be unrelated to the vaccinations.
Immunogenicity
Response to polyribosylribitol phosphate
The seroconversion rate of polyribosylribitol phosphate (PRP) in the 6–23-month-old group was greater than 95.0%, and in the 2–5-year-old group, it was greater than 94.0%. There was no significant difference between the treatment groups according to the pre-specified non-inferiority margin ().
Table 2. The seroconversion rate of PRP, MenA , MenC after vaccination
The proportion of children retaining pre-vaccination anti-PRP concentrations ≥ 0.15 and 1.0 μg/mL and the overall anti-PRP geometric mean concentrations (GMCs) were comparable between the treatment groups for both age groups. More than 98.0% of the vaccinees achieved post-vaccination anti-PRP concentrations ≥ 0.15 and 1.0 μg/mL, with no statistical difference between the treatment groups of both age groups. However, the post-vaccination anti-PRP GMCs of the Hib-MenAC group were significantly lower than those of the control group in both age groups ().
Table 3. Porportion of vaccinees with responses to PRP, MenA , MenC during the pre- and post-vaccination phases
Response to MenA
The seroconversion rate of rSBA (a serum bactericidal assay using baby rabbit complement)-MenA was greater than 95.0% in the 6–23-month-old group and over 93.0% in the 2–5-year-old group (). No significant differences between treatment groups were noticed.
Less than 7.0% of the vaccinees had pre-vaccination rSBA-MenA titers ≥ 1:8 and less than 2.0% had pre-vaccination rSBA-MenA titers ≥ 1:128. At 28 d post-vaccination, these values rose such that over 97.0% of vaccinees had titers ≥ 1:8 and over 80.0% of vaccinees had titers ≥ 1:128 for both age groups. Non-inferiority of the proportion of participants with rSBA-MenA titers ≥ 1:8 and ≥ 1:128 was observed in the Hib-MenAC group and the control group between both age groups, before and after vaccination. In addition, the pre- and post-vaccination rSBA-MenA geometric mean titers (GMTs) were comparable between the 2 different vaccination groups for each age group ().
Response to MenC
The seroconversion rate of rSBA-MenC was greater than 96.0% in the 6–23-month-old group and over 94.0% in the 2–5-year-old group (). No significant differences between treatment groups were noticed.
Within the 6–23-month-old group, the proportion of vaccinees with pre-vaccination rSBA-MenC titers ≥1:8 in the Hib-MenAC group was 1.1%, which was significantly lower than that in the control group (2.5%). By contrast, in the 2–5-year-old group, the proportion of vaccinees with pre-vaccination rSBA-MenC titers ≥ 1:8 in the Hib-MenAC group were significantly higher (6.5%) than that in the control group (3.9%). However, the proportion of vaccinees with post-vaccination rSBA-MenC titers ≥ 1:8 and ≥ 1:128 was greater than 97.0% and 66.0%, respectively, with no statistically significant differences between treatment groups for both age groups. Although slightly higher rSBA-MenC GMTs were measured in the Hib-MenAC group of the 2–5-year-old group before vaccination, the post-vaccination rSBA-MenC GMTs between the treatment groups were comparable().
Discussion
The first meningococcal disease vaccine and Hib vaccine were available in the late 1960s and in the early 1980s, respectively. However, these polysaccharide-based vaccines are not sufficiently immunogenic in infants to warrant universal application.Citation11,12 Effective polysaccharide-protein conjugate vaccines for these targeted diseases were later developed and resulted in a marked decrease in the incidence.Citation13,14 To provide protection to infants against both meningococcal diseases and Hib disease while minimizing the overall number of required injections, the combination Hib-MenC and Hib-MenCY vaccines were successively developed.Citation15,16 There are currently no licensed combination vaccines for the prevention of MenA, MenC, and Hib. If licensed, the new Hib-MenAC conjugate vaccine developed by Royal (Wuxi) Biological Co., Ltd, can provide protection to children against 2 of the major serogroups contributing to meningococcal diseases and Hib in China and in other countries where there is a risk for the endemic outbreaks of these diseases. This study investigated the safety and immunogenicity of a novel trivalent combination vaccine against Hib, MenA, and MenC diseases and was administered in a 2-dose series to children aged 6–23 months and in a single dose to children aged 2–5 y.
The safety profile of the novel vaccine was comparable to the profile of 2 licensed vaccines. The overall incidence of SAEs was low. Moreover, local and systemic reactions were mild and resolved rapidly. The incidence of adverse reactions was noticeably higher in the 6–23-month-old group than in the 2–5-year-old group. This may be expected, as the protocol for younger children involved a 2-dose series, and the higher number of doses of vaccinees received correlated with a higher number of adverse reactions. Another factor may be that older toddlers are more resistant to potential adverse effects caused by vaccination than are infants.Citation9,17
Considering the seroconversion and seroprotection rates, the immunogenicity of each of the 3 components of the investigational Hib-MenAC vaccine were non-inferior to the licensed MenAC conjugate vaccine and Hib conjugate vaccine currently available in China. In the novel vaccine, the seroconversion rates for the 3-component vaccine surpassed a rate of 94.0% for children aged 6 months-5 y. The proportion of vaccinees with rSBA titers ≥ 1:8 and PRP ≥ 0.15 g/mL was greater than 97.0%, and that of vaccinees with rSBA titers ≥ 1:128 and PRP ≥ 1.0 g/mL was over 80.0%, 65.0%, and 95.0% for MenA, MenC, and Hib, respectively.
Interpretation of the results presented here requires the consideration of 2 important issues. First, the overall post-vaccination anti-PRP GMCs for both age groups were lower in the Hib-MenAC group than in the control group. As this may have a clinical impact on the persistence of protection, we recommend a follow-up study to evaluate Hib antibody persistence. Second, in comparison to published data for other vaccines,Citation18-21 the rSBA GMTs in this study appear to be lower both before and after vaccination. The reason for this difference may be related to a number of factors, including the facts that the populations selected in each of the studies differed with regard to race and potential previous contact with meningococci; that the vaccines were produced by different manufactures; and that there may have been differences in the laboratories performing the assays.
One major limitation of this study is that children previously exposed to MenA polysaccharide vaccines were not excluded. The routine immunization schedule for MenA polysaccharide vaccine in China includes 4 doses; 2 doses of the MenA monovalent polysaccharide vaccine are given to children aged 6–18 months, and 2 booster doses of the MenA and MenC bivalent polysaccharide vaccine are administered at the age of 3 y and at the age of 6 years, respectively. Induction of MenC hypo-responsiveness by a previous dose of polysaccharide vaccine has been observed across all ages,Citation22-24 and there is conflicting data about hypo-responsiveness induced by the MenA polysaccharide vaccine.Citation25,26 To evaluate specific immunogenicity of the novel vaccine, a randomized, parallel-controlled clinical trial would effectively eliminate the disturbance and influence of the immunologic hypo-responsiveness. In the current study, the proportion of participants aged 2–5 y with post-vaccination rSBA-MenA titers ≥1:8 was greater than 97.0%. The reasons for this phenomenon remain uncertain and are worthy of further study.
The second limitation is a lack of consistency in the pre-vaccination rSBA-MenC titers between different treatment groups, which cannot be explained. The optimum vaccinees should be seronegative participants, who are both naive to vaccination and have no history of contact with target pathogens. Because of China's EPI schedule and the limited study period, it is not possible to screen participants by detecting antibody levels prior to vaccination in order to enroll only seronegative participants. Therefore, interpretations of these results must consider these aspects.
In conclusion, the data show that the novel vaccine requiring only a single injection will be an appropriate replacement for the currently licensed MenAC and Hib vaccines in China to enhance the coverage of Hib vaccine without complicating the current Chinese immunization schedule.
Participants and Methods
Study design
We conducted a single-center, randomized, observer-blinded, non-inferiority clinical trial in Funing County, in the Jiangsu Province of China. In the control group, a licensed bivalent MenAC conjugate vaccine was administered concomitantly with a licensed Hib conjugate vaccine. In the Hib-MenAC group, a novel Hib–MenAC conjugate vaccine was co-administered with a placebo injection in order to maintain participant and investigator blindness. The co-administered vaccines were injected intramuscularly in the deltoids with one shot in each arm.
The protocol was approved by the Jiangsu Provincial Center of Disease Control and Prevention's institutional review board and was conducted according to the principles put forth by the International Conference on Harmonization Guideline for Good Clinical Practice 1996 and the Declaration of Helsinki. Written informed consent was obtained from the guardians of all participants prior to enrollment.
Randomization and masking
Eligible participants were stratified by age (6–23 months and 2–5 years) and randomly allocated to the Hib-MenAC group or the control group in a ratio of 1:1. Each dose of vaccine and placebo was assigned a code from a random number table by block randomization (block size = 10). The random number table was generated by an external statistician by using SAS (version 9.1). All packages of the investigational and control vaccines were identical in appearance, with the code being the only identifier. Although the inner packaging and injection syringes in the 2 treatment groups were different, nurses who prepared and administered the vaccination signed nondisclosure agreements to not discuss the allocated assignment.
Every participant was assigned a sequential number according to the sequence of enrollment and received the investigational or control vaccine labeled with the same numbers. Investigators involved in randomization and masking did not participate in any other part of the trial. Allocation was masked from all participants, their guardians, and investigators.
Participants
Eligible participants were healthy children aged from 6 months to 5 y. The exclusion criteria included the following: a history of Hib or bivalent MenA and MenC vaccination; Hib or meningococcal diseases; acute febrile disease on the day of enrolment; allergies to any of the vaccine components; acute infections within the past 1 week; receipt of blood products, including immunoglobulin, within the past 2 weeks; hematological, neurological, or autoimmune disease; immunodeficiency; malnutrition; congenital defects; and inability to comply with the study's schedule. The participants were screened through a medical history inquiry and a physical examination.
Vaccine
The investigational Hib-MenAC vaccine, the licensed bivalent MenAC vaccine, and the placebo (0.5 mL of phosphate buffered saline) were developed and manufactured by Royal (Wuxi) Biological Co., Ltd. The licensed Hib vaccine was manufactured by Sanofi Pasteur Biological Products Co., Ltd. All of the conjugate vaccines were conjugated to tetanus-toxoid without any adjuvant. Each dose of the Hib-MenAC vaccine contained 10 μg of plain polysaccharide PRP, 10 μg MenA, and 10 μg MenC polysaccharides, supplied as freeze-dried powder to be reconstituted with the supplied diluent. The two control vaccines contained the same amounts of MenA and MenC polysaccharides, and PRP.
Procedure
Two different immunization schedules were followed, based on China's EPI schedule and the results of animal experimentation with the novel vaccine before the clinical trial. For participants aged 2–5 years, one dose was administered on day 0. For participants aged 6–23 months, 2 doses were administered, one each on days 0 and 28.
Following each dose, participants were observed for 30 min for any adverse reactions. The site staff members instructed parents or guardians of the participants on how to measure the local reactions at the injection sites with a gauge and how to measure the axilla temperature with a thermometer. Parents or guardians were also asked to record any adverse reactions that may have occurred on days 0–28 after each dose on diary cards. Home visits were conducted by the study's staff at 6 h, 24 h, 48 h, and 72 h following each dose, to ensure that any adverse reactions were recorded properly on the diary card. Any SAEs that occurred during the study period were reported and followed. Adverse reactions were rated according to the Adverse Reaction Rating Scale Guideline for Preventative Vaccine Clinical Trials,Citation27 which was derived from the pediatric toxicity table of US. National Institutes of Health Microbiology and Infectious Disease Division of Microbiology and Infectious Diseases.Citation28
Blood samples were collected immediately before and 28 d after the complete vaccination course. The National Institute for Food and Drug Control conducted the antibody analysis of the blood samples.
Serology
Functional anti-MenA and MenC activities were measured by rSBA.Citation29 The cut-off used for rSBA was a 1:8 dilution, an antibody titer that is considered indicative of seroprotection for rSBA-MenCCitation30 and has also been previously applied to other serogroups.Citation31 An rSBA threshold of 1:128 was also evaluated.Citation32 The rSBA assay employed the 29019 strain and the 29026 strain for serogroups A and C, respectively. The lower limit of the rSBA detection was 1:4. Both positive and negative control samples were run on each rSBA assay plate to assure quality control of the assay.
For Hib, ELISA was used to measure the antibodies binding to PRP. The seroprotection thresholds against Hib were defined as follows: anti-PRP ≥ 0.15 g/mL (short-term protection threshold) and ≥ 1.0 g/mL (long-term protection threshold).Citation33,34
Seronegativity was defined as an anti-PRP concentration < 0.15 μg/mL or rSBA titer < 1:8. Seropositivity was defined as anti-PRP concentrations ≥ 0.15 μg/mL or an rSBA titer ≥ 1:8. Seroconversion was defined as a > 4-fold rise in antibody concentration (titer) in the population that was seropositive prior to vaccination, or when seropositivity was observed in the population that was seronegative prior to vaccination.
Statistical analysis
The sample size calculation was conducted on the basis of one-sided non-inferiority tests with an α value of 0.025, a power of 90%, and a non-inferiority margin of −10%, using PASS software (version 8.0, NCSS, USA). Assuming a seroconversion rate of 95% could be achieved for each vaccine antigen in the control vaccine, a sample size of 435 in each treatment group was needed. The investigational vaccine could be considered as immunogenic as the licensed vaccines, if the 95% CI difference of the 2 treatment groups’ seroconversion rates was within the non-inferiority limit of −10%. Other statistical analyses were performed on the basis of a 2-sided test with α value of 0.05. Either the Chi-square test or the Fisher Exact test was used for analyzing categorical data. The Student's t-test was used for analyzing continuous data between groups.
The primary safety endpoint was the incidence of adverse reactions and SAEs within 28 d after each dose. The primary endpoint for immunogenicity was the seroconversion rate, while the seroprotection rate was considered the secondary endpoint for immunogenicity.
The safety analysis was performed on the basis of intention-to-treat cohort, including all participants who had received at least one injection, with their safety data available. The according-to-protocol cohort for immunogenicity analysis included all eligible participants who completed the immunization schedule and had available assay results for antibodies of pre-and post-immunization.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all the infants and their families as well as the staff members and nurses, who were involved in this study. We would also like to thank Christina L. Meyer of Johns Hopkins University Zanvyl Krieger School of Arts and Sciences for her help with editing.
Funding
This work was supported by the China 12-5 National Major Infectious Disease Programs (2012ZX10002-001) and Royal (Wuxi) Biological Co., Ltd.
References
- Watt JP, Wolfson LJ, O'Brien KL, Henkle E, Deloria-Knoll M, McCall N, Lee E, Levine OS, Hajjeh R, Mulholland K, et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet 2009; 374:903-11; PMID:19748399; http://dx.doi.org/10.1016/S0140-6736(09)61203-4
- Rouphael NG, Stephens DS. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods Mol Biol 2012; 799:1-20; PMID:21993636; http://dx.doi.org/10.1007/978-1-61779-346-2_1
- Nadel S. Prospects for eradication of meningococcal disease. Arch Dis Childhood 2012; 97:993-8; PMID:22984187; http://dx.doi.org/10.1136/archdischild-2012-302036
- Su LY, Huang RN, Wang C, Zhang XC. Epideiology of Meningococcal Disease in Chengdu,1950-2008 (in Chinese). J Prev Med Inf 2010; 26:38-42
- Hu XJ, Li XW, Ji YD, Xi W, Gao LH. Study on periodically prevaclent features for epidemic cerebrospinal meningitis in China (In Chinese). J Chin Epidemiol 1991; 12:136-9.
- Shao Z, Li W, Ren J, Liang X, Xu L, Diao B, Li M, Lu M, Ren H, Cui Z, et al. Identification of a new Neisseria meningitidis serogroup C clone from Anhui province, China. Lancet 2006; 367:419-23; PMID:16458767; http://dx.doi.org/10.1016/S0140-6736(06)68141-5
- Xu XH, Ye Y, Hu LF, Jin YH, Jiang QQ, Li JB. Emergence of serogroup C meningococcal disease associated with a high mortality rate in Hefei, China. BMC Infect Dis 2012; 12:205; PMID:22943188; http://dx.doi.org/10.1186/1471-2334-12-205
- Li J, Li Y, Shao Z, Li L, Yin Z, Ning G, Xu L, Luo H. Prevalence of meningococcal meningitis in China from 2005 to 2010. Vaccine 2014.
- Li G, Zhang H, Zhou W, Ye Q, Li F, Wang H, Hou Q, Xu Y, Ma X, Tan Y, et al. Safety and immunogenicity of a diphtheria, tetanus, acellular pertussis and Haemophilus influenzae Type b combination vaccine compared with separate administration of licensed equivalent vaccines in Chinese infants and toddlers for primary and booster immunization. Vaccine 2010; 28:4215-23; PMID:20399240; http://dx.doi.org/10.1016/j.vaccine.2010.03.061
- Ministry of Health. Expanded programmer on immunization in China. Available from: http://www.gov.cn/gzdt/2008-02/19/content_893572.htm. Accessed February 19, 2008.
- Chandran A, Watt JP, Santosham M. Prevention of Haemophilus influenzae type b disease: past success and future challenges. Exp Rev Vaccines 2005; 4:819-27; PMID:16372878; http://dx.doi.org/10.1586/14760584.4.6.819
- Pollard AJ. Global epidemiology of meningococcal disease and vaccine efficacy. Pediatric Infect Dis J 2004; 23:S274-9; PMID:15597069
- Campbell H, Borrow R, Salisbury D, Miller E. Meningococcal C conjugate vaccine: the experience in England and Wales. Vaccine 2009; 27 Suppl 2:B20-9; PMID:19477053; http://dx.doi.org/10.1016/j.vaccine.2009.04.067
- Trotter CL, Ramsay ME. Vaccination against meningococcal disease in Europe: review and recommendations for the use of conjugate vaccines. FEMS Microbiol Rev 2007; 31:101-7; PMID:17168998; http://dx.doi.org/10.1111/j.1574-6976.2006.00053.x
- Schmitt HJ, Maechler G, Habermehl P, Knuf M, Saenger R, Begg N, Boutriau D. Immunogenicity, reactogenicity, and immune memory after primary vaccination with a novel Haemophilus influenzae-Neisseria meningitidis serogroup C conjugate vaccine. Clin Vaccine Immunol 2007; 14:426-34; PMID:17287313; http://dx.doi.org/10.1128/CVI.00377-06
- Bryant KA, Marshall GS. Haemophilus influenzae type b-Neisseria meningitidis serogroups C and Y tetanus toxoid conjugate vaccine for infants and toddlers. Exp Rev Vaccines 2011; 10:941-50; PMID:21806393; http://dx.doi.org/10.1586/erv.11.90
- Zhu FC, Liang ZL, Li XL, Ge HM, Meng FY, Mao QY, Zhang YT, Hu YM, Zhang ZY, Li JX, et al. Immunogenicity and safety of an enterovirus 71 vaccine in healthy Chinese children and infants: a randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet 2013; 381:1037-45; PMID:23352749; http://dx.doi.org/10.1016/S0140-6736(12)61764-4
- Knuf M, Pantazi-Chatzikonstantinou A, Pfletschinger U, Tichmann-Schumann I, Maurer H, Maurer L, Fischbach T, Zinke H, Pankow-Culot H, Papaevangelou V, et al. An investigational tetravalent meningococcal serogroups A, C, W-135 and Y-tetanus toxoid conjugate vaccine co-administered with Infanrix hexa is immunogenic, with an acceptable safety profile in 12-23-month-old children. Vaccine 2011; 29:4264-73; PMID:21420417; http://dx.doi.org/10.1016/j.vaccine.2011.03.009
- Ostergaard L, Lebacq E, Poolman J, Maechler G, Boutriau D. Immunogenicity, reactogenicity and persistence of meningococcal A, C, W-135 and Y-tetanus toxoid candidate conjugate (MenACWY-TT) vaccine formulations in adolescents aged 15-25 years. Vaccine 2009; 27:161-8; PMID:18834910; http://dx.doi.org/10.1016/j.vaccine.2008.08.075
- Pace D, Snape M, Westcar S, Oluwalana C, Yu LM, Begg N, Wysocki J, Czajka H, Maechler G, Boutriau D, et al. A novel combined Hib-MenC-TT glycoconjugate vaccine as a booster dose for toddlers: a phase 3 open randomised controlled trial. Arch Dis Childhood 2008; 93:963-70; PMID:18463125; http://dx.doi.org/10.1136/adc.2007.136036
- Gatchalian S, Palestroque E, De Vleeschauwer I, Han HH, Poolman J, Schuerman L, Dobbelaere K, Boutriau D. The development of a new heptavalent diphtheria-tetanus-whole cell pertussis-hepatitis B-Haemophilus influenzae type b-Neisseria meningitidis serogroups A and C vaccine: a randomized dose-ranging trial of the conjugate vaccine components. Inter J Infect Dis 2008; 12:278-88; PMID:17981067; http://dx.doi.org/10.1016/j.ijid.2007.08.007
- Gold R, Lepow ML, Goldschneider I, Draper TL, Gotschlich EC. Clinical evaluation of group A and group C meningococcal polysaccharide vaccines in infants. J Clinical Investigation 1975; 56:1536-47; PMID:1202084; http://dx.doi.org/10.1172/JCI108235
- Richmond P, Kaczmarski E, Borrow R, Findlow J, Clark S, McCann R, Hill J, Barker M, Miller E. Meningococcal C polysaccharide vaccine induces immunologic hyporesponsiveness in adults that is overcome by meningococcal C conjugate vaccine. J Infect Dis 2000; 181:761-4; PMID:10669372; http://dx.doi.org/10.1086/315284
- MacDonald NE, Halperin SA, Law BJ, Forrest B, Danzig LE, Granoff DM. Induction of immunologic memory by conjugated vs plain meningococcal C polysaccharide vaccine in toddlers: a randomized controlled trial. Jama 1998; 280:1685-9; PMID:9832000; http://dx.doi.org/10.1001/jama.280.19.1685
- Jokhdar H, Borrow R, Sultan A, Adi M, Riley C, Fuller E, Baxter D. Immunologic hyporesponsiveness to serogroup C but not serogroup A following repeated meningococcal A/C polysaccharide vaccination in Saudi Arabia. Clin Diagn Lab Immunol 2004; 11:83-8; PMID:14715549
- Broker M, Veitch K. Quadrivalent meningococcal vaccines: hyporesponsiveness as an important consideration when choosing between the use of conjugate vaccine or polysaccharide vaccine. Travel Med Infect Dis 2010; 8:47-50; PMID:20188306; http://dx.doi.org/10.1016/j.tmaid.2009.12.001
- SFDA. The guideline for rating scales of adverse reaction of clinical trials of preventive vaccines.Available at: http://www.sda.gov.cn/WS01/CL0055/9350_5.html. Accessed October 14, 2005.
- NIH. Division of microbiology and infectious diseases pediatric toxicity table-DRAFT. Available at: http://www.niaid.nih.gov/LabsAndResources/resources/DMIDClinRsrch/Pages/toxtables.aspx. Accessed November 11, 2007.
- Maslanka SE, Gheesling LL, Libutti DE, Donaldson KB, Harakeh HS, Dykes JK, Arhin FF, Devi SJ, Frasch CE, Huang JC, et al. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin Diagn Lab Immunol 1997; 4:156-67; PMID:9067649
- Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection–serum bactericidal antibody activity. Vaccine 2005; 23:2222-7; PMID:15755600; http://dx.doi.org/10.1016/j.vaccine.2005.01.051
- Inadvertent misadministration of meningococcal conjugate vaccine–United States, June-August 2005. MMWR Morb Mortal Wly Rep 2006; 55:1016-7; PMID:16988640
- Borrow R, Andrews N, Goldblatt D, Miller E. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect Immu 2001; 69:1568-73; PMID:11179328; http://dx.doi.org/10.1128/IAI.69.3.1568-1573.2001
- Kayhty H, Peltola H, Karanko V, Makela PH. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis 1983; 147:1100; PMID:6602191; http://dx.doi.org/10.1093/infdis/147.6.1100
- Anderson P. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis 1984; 149:1034-5; PMID:6610714; http://dx.doi.org/10.1093/infdis/149.6.1034