Abstract
The common pathological hallmark of Alzheimer's disease (AD) is β-amyloid plaques deposition. Immunotherapy is a revolutionary pharmacological treatment for AD, aiming at improving plaque clearance while concomitantly decreasing inflammation. Our previous study prepared antigen 4Aβ1-15 and found that it could alleviate pathologic alterations in APP/PS1 transgenic mice. The objective of our study was to research the changing processes induced by immunotherapy, including the inflammatory factor levels and microglial activation that is closely associated with Aβ burdens clearance. APP/PS1 mice were injected with 4Aβ1-15 6 times. Each time, the inflammatory factors in sera were detected, and a specific fluctuation that first increased and then decreased was found, in which there was a turning point after the third injection. It prompted us to further detect the indicators in the brains after the third injection and the sixth injection. The results showed that the therapeutic effects for Aβ burdens and behaviors were continuously improved during the whole immune processes, whereas the inflammatory factor levels and microglial activation experienced similar specific fluctuations. The novel discovery may provide convenient methods for further detection and evaluation of immunotherapy in disease courses.
Abbreviations
| 4Aβ1-15 | = | 4 alternative tandem repeats of GPGPG-linked Aβ1-15 sequences |
| Aβ | = | β-amyloid |
| APP | = | amyloid precursor protein |
| IFN-γ | = | interferon-γ |
| IgG | = | immunoglobulin G |
| IL-1β | = | interleukin-1β |
| IL-4 | = | interleukin-4 |
| PS1 | = | presenilin-1 |
| Th | = | T-helper type |
| TNF-α | = | tumor necrosis factor-α |
Introduction
Alzheimer's disease (AD), the most common form of neurodegenerative disorder disease, leads to a progressive loss of memory and cognitive functions in the elderly.Citation1 The neuropathological hallmark of AD is ascribed to the deposition of extracellular β-amyloid plaques in the brain with an excessively activated inflammatory immune response. Therapies aim to improve the burden clearance from the brain while concomitantly decreasing inflammation.Citation2 A revolutionary pharmacological treatment for AD is immunotherapy. Theoretically, antigen immunity plays a long-term role and can be used as prophylactic agent.Citation3
In our previous study, we prepared antigen 4Aβ1-15, which recognizes an epitope generating the majority of anti-Aβ antibodies.Citation4 Six treatments of 4Aβ1-15 in APP/PS1 transgenic mice induced microglial cell activation and decreased inflammatory response, and the Aβ burdens were markedly cleared.
Inflammatory factors are excessively activated by microglial in APP/PS1 mice, which is closely associated with the depositions Aβ plaque elimination through phagocytosis.Citation5 Clinical Alzheimer's diagnosis can be classified and predicted based on some biological indicators, such as plasma signaling proteins.Citation6,7 The convenience and safety of inflammatory factors detection prompted us to question whether the inflammatory levels in the periphery would also reflect the pathologic alterations in the CNS. It is unknown how the Aβ burdens are cleared in the condition of decreased inflammatory response and what are the changing processes of the microglial activation and the inflammatory factor levels.
With these questions, we conducted this study to detect the levels of inflammatory factors, including IL-1β, IL-4, TNF-α and IFN-γ, after each injection in 4Aβ1-15-immunized mice. The Aβ antibodies, burdens and microglial cell activation, as well as the behaviors, were detected to reveal the specific changing processes of pathologic alterations in 4Aβ1-15-immunized APP/PS1 mice and to further help us to formulate a safer and more effective immune procedure for detecting and evaluating AD.
Results
4Aβ1-15 increases anti-Aβ antibody concentrations in sera and brain tissue
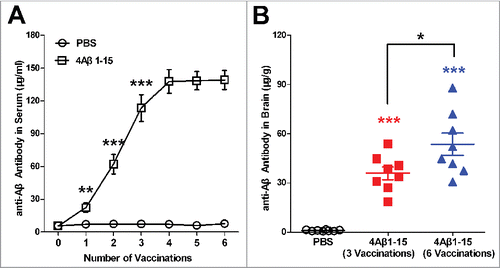
After injections, the Aβ antibody levels of the 4Aβ1-15 group continued increasing until the fourth treatment and then entered a platform stage. The anti-Aβ antibodies of the controls showed a slight change (). The anti-Aβ antibody level in the brain tissue was significantly higher in the 4Aβ1-15 group than in the controls after the 3rd and 6th injection, whereas the latter was higher than that in the former (). This indicated that the 4Aβ1-15 antigen vaccinated successfully, and some of the antibodies generated in the periphery enter the CNS through the blood-brain barrier.
Figure 1. Levels of anti-Aβ antibodies in sera and brain. (A) Anti-Aβ antibodies in blood samples one week after each vaccination markedly increased and then entered a platform stage after the third vaccination. (B) Anti-Aβ antibodies in brains were higher after the sixth vaccination compared with the third vaccination. The data are presented as the means ± SD (n = 8, *P < 0.05, **P <0.01, *** P < 0.001).
4Aβ1-15 regulated the changing process of inflammatory factors levels that first increase with Th1-polarization then decrease later with Th2-polarization
To further study the changing processes of inflammatory factors, the levels of the proinflammatory factors IL-1β, TNF-α and IFN-γ and anti-inflammatory IL-4 in sera were detected one week after each injection and standardized to the control mice.Citation8
The results showed that compared with the controls, the levels of the inflammatory factors in 4Aβ1-15 mice all gradually increased significantly until the 3rd treatment and then decreased to approach the normal standard (). These changes reveal the inflammatory response to antigen immunity of 4Aβ1-15 is a dynamic process, in which the vital turning point occurs after the third injection.
Figure 2. Inflammatory factors in sera and brain. (A) Levels of inflammatory factors in sera present specific fluctuations that first increase and then decrease. (B) Levels of inflammatory factors in the brain present a similar variation trend as in the sera. (C) The standardized ratio of IFN-γ to IL-4 in the brain, representing the balance of Th1/Th2, showed Th1-polarized immune response first and then Th2-polarized immune response compared with the controls. The data are presented as the means ± SD (n = 8, *P < 0.05, **P <0.01, *** P < 0.001).
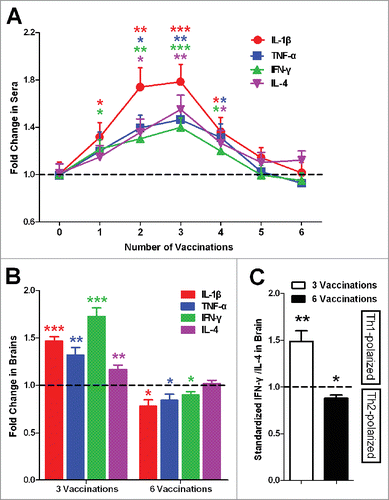
We detected the levels of the inflammatory factors in brains one week after the 3rd and after 6 treatments. Similar to the sera, the levels of the inflammatory factors in the brains were all significantly increased until the third injection and then decreased (). After the sixth treatment, the levels of proinflammatory factors IL-1β, TNF-α and IFN-γ were lower, whereas anti-inflammatory IL-4 was no difference compared with the control mice.
The ratio of IFN-γ to IL-4 in the brain, representing the balance of Th1/Th2,Citation8-10 was calculated and standardized to controls. The bias presented a similar fluctuation that showed Th1-polarized and then Th2-polarized immune response ().
4Aβ1-15 induced cerebral Aβ plaque clearance during continuous vaccinations
Active immunity stimulates the release of inflammatory factors involving the clearance of Aβ burdens. According to the above detection of the inflammatory factors, immunohistochemical and quantitative image analyses were employed to determine the amyloid burden.
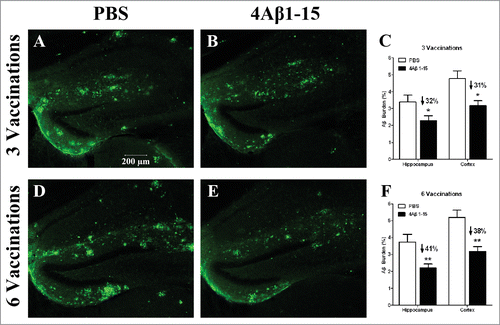
The effect of treatment on plaque burden is demonstrated in the confocal micrographs of brain sections obtained from the mouse with the median level of Aβ plaque burden within each group. The confocal micrographs showed that the majority of the larger compacted plaques were diffused into deposits in the 4Aβ1-15 group compared to the controls (). Quantitative image analysis showed more Aβ burdens were eliminated after 6 treatments than 3 treatments () compared with their respective controls. These results demonstrated that although the inflammatory factors were no longer increased after the 3rd treatment, the clearance of Aβ plaques lasted through the whole course of 6 continuous treatments.
Figure 3. Confocal micrographs of brain sections with immunohistochemical staining represent the cerebral Aβ pathology in APP/PS1 mice after the third injection (A–C) and the sixth injection (D–F). Micrographs of brain sections obtained from mice with the median level of Aβ plaque burden in their respective groups. Scale bars are indicated in the figures. Histograms show the percentages of Aβ burden calculated by quantitative image analysis (n = 8). The reduction percentage relative to the controls is indicated in the figure. More Aβ plaque burdens were cleared after the sixth injection in 4Aβ1-15-treated mice (*P <0.05).
4Aβ1-15 continuous improved learning and memory ability for 6 treatments in APP/PS1 mice
The Morris water maze tests were administered 1 week after the 3rd and 6th vaccination to assess the spatial learning and memory task abilities related to hippocampal function.
After the 3rd injection, no difference was observed between the 2 groups in finding the visible platform, indicating that the treatments did not affect mouse vision. There was still no significant difference between the groups in the hidden platform-swimming test the next 2 days, but on the 4th day, the 4Aβ1-15-treated mice spent significantly less time finding the hidden platform (). In the spatial probe trial (5th day), the platform was removed from the water maze, the mean values of time and distance proportions of 4Aβ1-15-treated mice spent in the target quadrant that formerly contained the platform were a little higher compared with the controls (). In the reversal phase (6th and 7th days), the platform was placed in a position opposite to its former location, and no significant difference was observed ().
Figure 4. 4Aβ1-15 improves the spatial learning and memory ability in APP/PS1 mice as shown by the Morris water-maze (MWM). After three injections, (A) 4Aβ1-15-treated mice show less escape latency in the mean value, except for the fourth day in the place navigation. Meanwhile, (B) on the 5th day of the spatial probe trial, mice in the 4Aβ1-15 group spent a little larger proportion of time and distance in the target quadrant. Compared with controls, mice injected 6 times present markedly improvement in both place navigation (C) and the spatial probe trial (D) (n = 8, *P < 0.05).
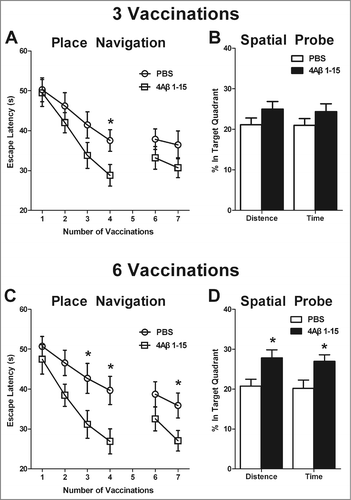
Meanwhile, after the 6th injection, there was no difference between the 2 groups on the 1st and 2nd days. However, mice in 4Aβ1-15-treated group showed less escape latency to find the hidden platform on the 3rd and 4th days compared with the controls (). The proportions of time and distance in the target quadrant in the spatial probe trial were also larger (). In the reversal phase, no significant difference was observed on the 6th day, whereas mice in the 4Aβ1-15 group displayed significantly less escape latency on the 7th day (). The swimming speed was no difference between them.
4Aβ1-15 induced microglial cells activation first increases and then decreases
Previous studies have demonstrated that the precursors of inflammatory factors could be activated by microglial cells, which could communicate with Aβ burdens by phagocytosis.Citation5 The above results show that 4Aβ1-15 induced the inflammatory factors to increase first and then decrease, whereas the Aβ burdens and behaviors were improved through the whole course of injections. These results made us question how the microglial cell activation changes during the continuous vaccinations.Citation11
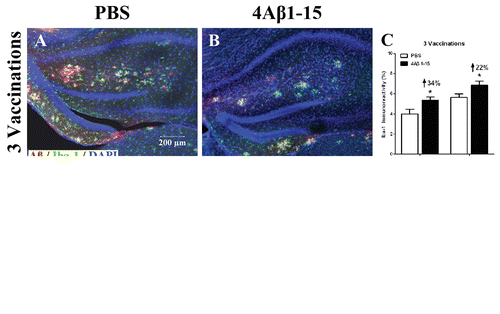
Aβ plaques and activated microglia were double-labeled by immunohistochemical method. The confocal micrographs showed microglia cells in the APP/PS1 transgenic mice were activated and tended to surround Aβ plaques. Representative immunoreactivity of Iba-1 positive cells (green) demonstrated microglial activation was a little increased in the hippocampal and cortical brain regions of 3 times 4Aβ1-15-treated mice (). After the 6th injection, the immunoreactivity of microglial activation was significantly decreased compared with the controls (). The specific fluctuation was similar to the inflammatory response, which verified the microglial cells were closely associated with the inflammatory factors.
Figure 5. Confocal micrographs show Aβ plaques (red) and activated microglial cells (green) representing the immunoreactivity of Iba-1 positive cells and doubly labeled immunohistochemically. Cell nuclei are labeled with DAPI (blue) (A, B, D, E). Scale bars are indicated in the figures. The microglial are activated into amebocyte morphology and tend to surround Aβ plaques. The histogram shows a larger degree of microglial activation after the third injection (C), whereas it is significantly reduced after (F) compared with the controls. The reduction percentage is indicated in the figure (n = 8, *P < 0.05).
Discussion
Aβ burdens are the vital factor influencing the development of Alzheimer's disease. Large numbers of trials in mouse models and in the clinic have indicated that immunotherapy is a promising strategy for improving AD, which aims at eliminating Aβ plaques with low inflammation levels.Citation4,12
We previously found that the antigen 4Aβ1-15 markedly inhibited inflammatory factors activated excessively in APP/PS1 transgenic mice, which is associated with the phagocytosis of microglial to clear the Aβ plaques.Citation9 Meanwhile, clinical Alzheimer's diagnosis can be classified and predicted based on some biological indicators,Citation6,13 and the anti-inflammation antigen can be used to treat AD. Considering the convenience of inflammatory factors detection in sera, we conducted this study to research the relationship between the changing processes of the inflammatory response and the pathologic alterations.
In this study, 4Aβ1-15 was injected to APP/PS1 mice 6 times. After the third vaccination, Aβ antibodies reached a platform stage in sera but continued increasing in the brain, demonstrating that peripherally generated antibodies against Aβ entered the CNS through the blood-brain barrier over a long term.Citation5,14 Furthermore, the change processes of pathologies in 6 continuous treatments of 4Aβ1-15 in APP/PS1 mice showed that the inflammation response and microglial activation experienced specific fluctuations that rose in the first stage and then decreased to approach the normal levels. Meanwhile, the Th-bias in the brain showed a fluctuation of Th1-polarization first and then Th2-polarization, which presented a shift from proinflammatory response to anti-inflammatory response. The above results indicate that the third injection may play a vital role as the turning point. Even so, the detection of 6 injections induced more Aβ clearance compared with 3 injections. Along with the Aβ plaques decreasing during the whole process of injection, the behavioral test was consistent with the results. The Morris water maze showed 4Aβ1-15 improved spatial learning and memory in APP/PS1 transgenic mice after 6 treatments compared to the third injection, which demonstrated that treating AD is a process of continuous immunotherapy and repeated treatments may be more effective.
These results illustrated that as Aβ antibodies were generated, microglial cells were activated to eliminate Aβ burdens through phagocytosis.Citation15 Under normal conditions, microglia scavenge the CNS for plaques, damaged neurons and infectious agents constantly, but when they are activated too much, large numbers of inflammatory factors associated with serious pathologies are released, aggravating nerve injury and causing harm to normal organisms.Citation11,16 In other words, microglial cells activate the precursors of inflammation factors. With Aβ plaques continuously cleared, the cognitive abilities were improved, and the inflammatory responses and microglia declined.
When A β antigen was administered to the organism, the generated anti-A β antibodies induced microglia activation to release the proinflammatory factors IL-1β, TNF-α and IFN-γ, and eliminate A β burdens simultaneously, but why the anti-inflammatory factor IL-4 was also increased is unclear. We suspect that the organism is in a condition of dynamic balance, and there may be some competition between the positive and negative sides as proinflammatory and anti-inflammatory. As 4A β1-15 was continuously injected into the mice, the A β burdens were cleared, the stimulation for microglial was weaker and the microglial cells were activated within a controlled degree to decline. The levels of inflammatory factors were also inhibited in the brain and periphery. After the sixth treatment, the levels of proinflammatory factors were lower, whereas the anti-inflammatory factors were a little higher compared with the controls.
It is all known that intrinsic inflammatory factors are existent naturally in organisms with physiological functions and biological activities such as provide protection,Citation17 and Th2 cells support intrinsic anti-inflammatory properties of the brain.Citation18 Meanwhile, it has been demonstrated that the levels of proinflammatory factors in APP/PS1 mice are higher than the age-matched wild-type mice.Citation19 Considered the possibility of activated intrinsic inflammatory factors impacting the result in APP/PS1 mice, we detected inflammatory factors of 4A β1-15-group by calculating the fold changes which were standardized to the PBS-group.
Several proteins associated with inflammation have been found in the pathologies of A β burdens in Alzheimer's disease. Inflammatory response is the reaction to resist pathologic alterations, which demonstrated that inflammation plays a vital role in influencing AD.Citation20 Studies have verified that the effects of inflammatory response are double-sided. Microglial are activated, releasing harmful inflammatory factors, whereas inflammatory factors induce phagocytosis of microglial, stimulating A β clearance. Therefore, the detection of inflammatory response is very important in evaluating AD course.
In summary, continuous vaccinations of 4A β1-15 generate effective anti-A β antibodies in the periphery and enter the CNS through the blood-brain barrier, stimulate microglial activation to clear A β burdens by phagocytosis and induce inflammatory factor releasing with Th1-polarized immune response. On the other hand, as A β burdens are cleared, microglial activation is controlled and then decreases, thus reducing the levels of the inflammatory factors with Th2-polarized immune response.Citation21 The series of reactions further induce the continuous improvements of cognitive ability.
In conclusion, our study found the specific fluctuations of inflammatory factors that first increase and then decrease and may play a pivotal role in evaluating A β burdens clearance and behavior improvement in continuous vaccinations of active immunity 4A β1-15. These results indicate that in the early course of AD pathology, increasing levels of inflammatory response may benefit treatment, whereas for immunotherapy, decreasing levels of inflammatory response may represent good prognosis in the later courses. The novel discovery presents an original finding and warrants further investigations into the convenience of detecting and evaluating immunotherapy in the course of Alzheimer's disease.
Materials and Methods
Samples and Immunization protocol
The APP/PS1 transgenic mouse models of AD obtained from the Animal Experimental Center of Guangdong Academy of Medical Science were divided into 2 groups (n = 8 for each group, males). One group was randomly chosen to be vaccinated with 4A β1-15 antigen supported by Livzon pharmaceutical company (the purity of the peptides >95%), and the remaining mice were injected with germfree PBS (pH 7.4) as control.
Since A β plaques can be detected from the 6th month in the brains of the transgenic mice, a total of 6 injections were performed every 2 weeks from the months 6. The 4A β1-15 was emulsified 1:1 with MF59 adjuvant to 1 mg/ml.Citation4,9 Each mouse was treated with 0.1 ml subcutaneously. Indexes in sera were detected after each vaccination during a total 6 immunizations. Since the detection in brain would sacrifice the mice, we designed to detect these indexes in the turning point at about the third vaccination, and compare with the mice immunized 6 times. Mice of 3-dose group and 6-dose group are in different batches with the same immunization schedules of biweekly injection from the months 6.
All animals were housed in a light cycle-controlled and temperature-controlled facility. The experiments were administered in accordance with the guidelines of the Animal Ethical and Welfare Committee of Sun Yat-sen University.
Enzyme-linked immunosorbent assay analysis for levels of anti-A β antibodies and inflammatory factors in sera and brain
One week after each injection, blood samples were collected from the animals' caudal veins. The samples were stored at 4°C overnight and centrifuged at 8000 r/min for 10 min to extract the sera. For biochemical analysis and immunohistochemical analysis,Citation16 mice were sacrificed under deep anesthesia and then perfused transcardially with normal saline. The brains were removed and bisected in the midsagittal plane. The right hemisphere was snap frozen for biochemical analysis.
For indirect ELISA, primary antibodies were diluted, added to each well of the plate and then incubated at 37°C for 1 h; the plate was then washed 3 times. An HRP-conjugated secondary antibody was added to incubate at 37°C for 30 min, and then the plate was washed 5 times. TMB reagent was added to each well, followed by the stop buffer. After 15-30 min for color development, the absorbance of each well was measured.
The inflammatory factors in the serum and brain were determined, respectively, with the ELISA kit. The calibration curve for the determination of the anti-A β antibody concentration was based on the monoclonal antibody 6E10 diluted in different known concentrations and used as a positive control.Citation9,10 Absorbance was measured at 450 nm with a spectrophotometer. Anti-A β antibody titers were calculated using the statistics software Curve Expert 1.3. OD450 values that were 2 times greater than the background were considered positive.Citation9
Immunohistochemical Staining
For the immunohistochemical analysis, the left hemisphere was postfixed in 4% paraformaldehyde overnight and then equilibrated in phosphate-buffered 30% sucrose.Citation22 Serial coronal sections of 40 μm thick, 240 μm apart were collected on a freezing microtome (Leica SM2000R).Citation23 The sections were stored at 4°C before immunohistochemistry was performed. Actual section thickness was measured, and appropriate guard zones of the section were defined.
For the staining, floating sections were blocked in 1% BSA containing 0.25% Triton X-100 (Sigma) and then incubated with the primary antibody overnight at 4°C, followed by incubation with the applicable secondary antibody at 37°C for 2 h. Then, the sections were washed in PBS 3 times and mounted with fluorescence mounting media.
Antibodies and Reagents for Immunohistochemistry. The following primary antibodies were used: anti-A β42-A β deposits (1:2000; Invitrogen), rabbit anti-Iba1 (1:1000; Wako Chemicals USA). The secondary antibodies were: Alexa Fluor 488 or 555 goat anti-mouse (1:400; Invitrogen) and Alexa Fluor 488 goat anti-rabbit (1:400; Invitrogen).
Quantitative Image Analysis
For the microscopic analysis, a Zeiss LSM 710 confocal laser scanning microscope was used.
For the quantitative image analysis of A β plaque and Iba-1 immunoreactivity, 6 40 μm coronal sections at 240 μm intervals were immunostained from each mouse. The dorsal hippocampus and the cerebral cortex were chosen for quantitative analysis.Citation9 Sections of each anatomic region of interest (hippocampus and cortex) were quantified using Image-Pro Plus 6.0 image analysis software, which was capable of color segmentation and automation via programmable macros.Citation19 The A β immunoreaction stained areas were expressed as a percentage of the total brain region tissue area, as was the quantitative image analysis of the microglial cell immunoreactivity.
Open-Field Behavioral Test and Morris Water-Maze Behavioral Test
The Morris water-maze behavioral test (MWM) was employed to assess the APP/PS1 transgenic mice one week after the 3rd and 6th injection. It is a test of hippocampus-dependent spatial learning and memory for rodents that relies on distal cues for navigation.Citation24 The mice were trained to find a submerged escape platform in a 0.8 m diameter, white, non-toxic pool filled with colored water (temperature: 23°C). Water maze tests were administered on 7 consecutive days. The place navigation included the acquisition phase from the 1st to the 4th days and a reversal phase on the 6th and 7th days, whereas the spatial probe occurred on the 5th day.Citation11 On the first day, the mice were required to locate a visible platform located 1 cm above the water surface to test whether immunity influenced motility or vision in APP/PS1 mice. On the next 3 days, the mice were required to find a hidden platform located 1 cm below the water surface in each trial. The escape latency is the average amount of time required in 4 quadrants to locate the platform and climb onto it, recorded up to 60 s. If the mouse did not find the platform within 60 s, it was manually placed on the platform and returned to its home cage after 30 s. On the fifth day, the platform was removed from the pool and each mouse was tested by a probe trial for 60 s. On the last 2 days, the platform was placed in the quadrant opposite of the location on days 1−4, and the mice were then retrained in 4 sessions per day. The data were recorded with an Etho Vision automated tracking system.
Statistical Analysis
The data were collected by investigators blinded to the treatment. The data were analyzed by one-way ANOVA or 2-way ANOVA with repeated measures.Citation23,25 All data are expressed as the means ± SEM, and P < 0.05 was considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
We thank Prof. Huaiyu Gu from Sun Yat-sen University for sharing his expertise, and technician Qunfang Yuan for her helpful suggestions.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81000545, No. 81071033); Technology Project of Guangdong Province (No. 0911220600567); Science and Technology Planning Project of Guangdong Province (No. 2009B080701089).
References
- Robert R, Wark KL. Engineered antibody approaches for Alzheimer's disease immunotherapy. Arch Biochem Biophys 2012; 526:132-8; PMID:22475448; http://dx.doi.org/10.1016/j.abb.2012.02.022
- Lemere CA. Immunotherapy for Alzheimer's disease: hoops and hurdles. Mol Neurodegener 2013; 8:36; PMID:24148220; http://dx.doi.org/10.1186/1750-1326-8-36
- Mohajeri MH, Saini K, Schultz JG, Wollmer MA, Hock C, Nitsch RM. Passive immunization against beta-amyloid peptide protects central nervous system (CNS) neurons from increased vulnerability associated with an Alzheimer's disease-causing mutation. J Biol Chem 2002; 277:33012-7; PMID:12068009; http://dx.doi.org/10.1074/jbc.M203193200
- Guan X, Zou J, Gu H, Yao Z. Short amyloid-beta immunogens with spacer-enhanced immunogenicity without junctional epitopes for Alzheimer's disease immunotherapy. Neuroreport 2012; 23:879-84; PMID:22922658; http://dx.doi.org/10.1097/WNR.0b013e328358a044
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 2000; 6:916-9; PMID:10932230; http://dx.doi.org/10.1038/78682
- Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, Friedman LF, Galasko DR, Jutel M, Karydas A, et al. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nat Med 2007; 13:1359-62; PMID:17934472; http://dx.doi.org/10.1038/nm1653
- Folkesson R, Malkiewicz K, Kloskowska E, Nilsson T, Popova E, Bogdanovic N, Ganten U, Ganten D, Bader M, Winblad B, et al. A transgenic rat expressing human APP with the Swedish Alzheimer's disease mutation. Biochem Biophys Res Commun 2007; 358:777-82; PMID:17506994; http://dx.doi.org/10.1016/j.bbrc.2007.04.195
- Browne TC, McQuillan K, McManus RM, O'Reilly JA, Mills KH, Lynch MA. IFN-gamma Production by amyloid beta-specific Th1 cells promotes microglial activation and increases plaque burden in a mouse model of Alzheimer's disease. J Immunol 2013; 190:2241-51; PMID:23365075; http://dx.doi.org/10.4049/jimmunol.1200947
- Guan X, Yang J, Gu H, Zou J, Yao Z. Immunotherapeutic efficiency of a tetravalent Abeta1-15 vaccine in APP/PS1 transgenic mice as mouse model for Alzheimer's disease. Hum Vaccin Immunother 2013; 9:1643-53; PMID:23732905; http://dx.doi.org/10.4161/hv.24830
- Xing X, Sha S, Li Y, Zong L, Jiang T, Cao Y. Immunization with a new DNA vaccine for Alzheimer's disease elicited Th2 immune response in BALB/c mice by in vivo electroporation. J Neurol Sci 2012; 313:17-21; PMID:22029939; http://dx.doi.org/10.1016/j.jns.2011.09.040
- Butovsky O, Koronyo-Hamaoui M, Kunis G, Ophir E, Landa G, Cohen H, Schwartz M. Glatiramer acetate fights against Alzheimer's disease by inducing dendritic-like microglia expressing insulin-like growth factor 1. Proc Natl Acad Sci U S A 2006; 103:11784-9; PMID:16864778; http://dx.doi.org/10.1073/pnas.0604681103
- Du J, Sun B, Chen K, Fan L, Wang Z. Antagonist of peroxisome proliferator-activated receptor gamma induces cerebellar amyloid-beta levels and motor dysfunction in APP/PS1 transgenic mice. Biochem Biophys Res Commun 2009; 384:357-61; PMID:19422805; http://dx.doi.org/10.1016/j.bbrc.2009.04.148
- Gruden MA, Davidova TB, Malisauskas M, Sewell RD, Voskresenskaya NI, Wilhelm K, Elistratova EI, Sherstnev VV, Morozova-Roche LA. Differential neuroimmune markers to the onset of Alzheimer's disease neurodegeneration and dementia: autoantibodies to Abeta((25-35)) oligomers, S100b and neurotransmitters. J Neuroimmunol 2007; 186:181-92; PMID:17477976; http://dx.doi.org/10.1016/j.jneuroim.2007.03.023
- Park SM, Shin JH, Moon GJ, Cho SI, Lee YB, Gwag BJ. Effects of collagen-induced rheumatoid arthritis on amyloidosis and microvascular pathology in APP/PS1 mice. BMC Neurosci 2011; 12:106; PMID:22029666; http://dx.doi.org/10.1186/1471-2202-12-106
- Ahmed AA, Subaiea MG, Eid A, Li L, Seeram PN, Zawia HN. Pomegranate Extract Modulates Processing of Amyloid-beta Precursor Proteinin an Aged Alzheimer's Disease Animal Model. Curr Alzheimer Res 2014; 11(9):834-43; PMID:25274111
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci 2006; 9:268-75; PMID:16415867; http://dx.doi.org/10.1038/nn1629
- Galea E, Heneka MT, Dello Russo C, Feinstein DL. Intrinsic regulation of brain inflammatory responses. Cell Mol Neurobiol 2003; 23:625-35; PMID:14514020; http://dx.doi.org/10.1023/A:1025084415833
- Gimsa U, Wolf SA, Haas D, Bechmann I, Nitsch R. Th2 cells support intrinsic anti-inflammatory properties of the brain. J Neuroimmunol 2001; 119:73-80; PMID:11525802; http://dx.doi.org/10.1016/S0165-5728(01)00343-5
- Lok K, Zhao H, Shen H, Wang Z, Gao X, Zhao W, Yin M. Characterization of the APP/PS1 mouse model of Alzheimer's disease in senescence accelerated background. Neurosci Lett 2013; 557 Pt B:84-9; PMID:24176881; http://dx.doi.org/10.1016/j.neulet.2013.10.051
- Wilcock DM, Jantzen PT, Li Q, Morgan D, Gordon MN. Amyloid-beta vaccination, but not nitro-nonsteroidal anti-inflammatory drug treatment, increases vascular amyloid and microhemorrhage while both reduce parenchymal amyloid. Neuroscience 2007; 144:950-60; PMID:17137722; http://dx.doi.org/10.1016/j.neuroscience.2006.10.020
- McGeer PL, McGeer EG. Inflammation of the brain in Alzheimer's disease: implications for therapy. J Leukoc Biol 1999; 65:409-15; PMID:10204568
- He F, Zou JT, Zhou QF, Niu DL, Jia WH. Glatiramer acetate reverses cognitive deficits from cranial-irradiated rat by inducing hippocampal neurogenesis. J Neuroimmunol 2014; 271:1-7; PMID:24713401; http://dx.doi.org/10.1016/j.jneuroim.2014.03.015
- Xia Y, Qi F, Zou J, Yang J, Yao Z. Influenza vaccination during early pregnancy contributes to neurogenesis and behavioral function in offspring. Brain Behav Immun 2014; PMID:25014010
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 2006; 1:848-58; PMID:17406317; http://dx.doi.org/10.1038/nprot.2006.116
- Wiessner C, Wiederhold KH, Tissot AC, Frey P, Danner S, Jacobson LH, Jennings GT, Lüönd R, Ortmann R, Reichwald J, et al. The second-generation active Abeta immunotherapy CAD106 reduces amyloid accumulation in APP transgenic mice while minimizing potential side effects. J Neurosci 2011; 31:9323-31; PMID:21697382; http://dx.doi.org/10.1523/JNEUROSCI.0293-11.2011
