Abstract
Currently marketed Streptococcus pneumoniae vaccines are based on polysaccharide capsular antigens from the most common strains. Pneumococcal histidine triad protein D (PhtD) is a conserved surface protein that is being evaluated as a candidate for a vaccine with improved serotype coverage. Here, we measured the functional activity of human anti-PhtD antibodies in a passive protection model wherein mice were challenged with a lethal dose of S. pneumoniae by intravenous injection. This functional activity was compared with anti-PhtD antibody concentrations measured by enzyme-linked immunosorbent assay (ELISA) to estimate the 50% protective dose (ED50). Anti-PhtD antibodies affinity purified from pooled normal human sera passively protected mice with an ED50 of 1679 ELISA units/ml (95% confidence interval, 1420–1946). Sera from subjects injected with aluminum-adjuvanted PhtD in a phase I trial had similar activity per unit of antibody (ED50 = 1331 ELISA units/ml [95% confidence interval, 762–2038]). Vaccine-induced activity in the passive protection model was blocked by pre-incubation with recombinant PhtD but not by a control S. pneumoniae antigen (LytB). These results show that human anti-PhtD antibodies, whether naturally acquired or induced by the PhtD candidate vaccine, are functional. This supports the development of the PhtD candidate as part of a broadly protective pneumococcal vaccine.
Each year, Streptococcus pneumoniae causes more than 800,000 deaths worldwide in children under 5 years of age.Citation1 Currently marketed S. pneumoniae vaccines, which are based on polysaccharide capsular antigens from the most common strains, have substantially reduced pneumococcal disease rates.Citation2 However, because serotypes can vary between countries or regions, coverage may be incomplete in some cases.Citation3 Moreover, serotype replacement might eventually render these vaccines less effective.Citation4,5 To provide broader, more diverse, and possibly infection stage-specific protection, vaccines based on conserved proteins are being investigated.Citation2,6,Citation7
Pneumococcal histidine triad protein D (PhtD) is a conserved surface protein that mediates attachment to respiratory epithelial cellsCitation6,7 and can elicit a protective immune response.Citation8-11 In mice, intranasal immunization with PhtD generates robust serum antibody and CD4 Th1-biased immune memory responses and confers protection against pneumococcal colonization.Citation12 A second study in mice showed that vaccination with PhtD protects against nasopharyngeal and lung colonization.Citation13 In a primate study, vaccination with PhtD and chemically detoxified pneumolysin induced high levels of antibodies and protected against a challenge with S. pneumoniae serotype 19F.Citation14 A phase I trial in adults 18–50 years of age showed that a aluminum phosphate-adjuvanted PhtD vaccine candidate was well tolerated, immunogenic, and could be boosted by a second vaccine dose.Citation15 During development of an enzyme-linked immunosorbent assay (ELISA) to measure antibody responses in the phase I trial, we found that individual and pooled serum from unimmunized healthy adults contained substantial PhtD-binding antibody (data not shown). To further investigate the immune response elicited by a PhtD-based pneumococcal vaccine, we developed a murine passive protection sepsis model for assessing the functional activity of human anti-PhtD antibodies.
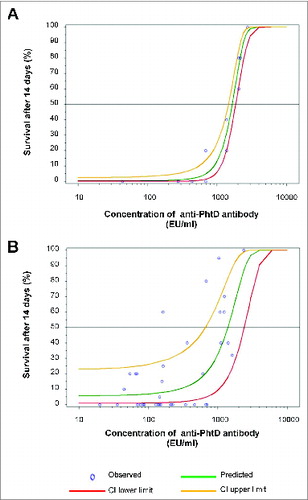
Naturally occurring human PhtD-binding antibody was purified from a commercial pooled serum (obtained from approximately 200 healthy individuals; Sigma, St. Louis, MO). The concentration of anti-PhtD antibody was determined by ELISA, and its purity and specificity were confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blotting, and competition with recombinant PhtD (data not shown). The purified PhtD-binding antibodies were passively transferred by intraperitoneal injection (200 µl) to 6- to 8-week-old female CBA/CaHN-Btk xid /J (CBA/N) mice (Jackson Laboratories, Bar Harbor, ME). After 1 h, the mice were challenged by intravenous injection with a lethal dose (50 colony forming units [cfu] in 200 µl) of S. pneumoniae strain A66.1 (serotype 3) (obtained from D. Briles, University of Alabama-Birmingham). The proportion of mice surviving at 14 days post-challenge increased with the concentration of anti-PhtD antibody (). The dose providing 50% survival (ED50) was estimated to be 1679 ELISA units (EU)/ml (95% confidence interval [CI], 1420–1946) by logistic regression with probit link.
Figure 1. Dose-response of antibody activity in the passive protection model. 6- to 8-week-old female naïve CBA/N mice (n = 5/group) received an intraperitoneal injection of 200 µl test sample or PBS. Control mice received PBS. After 1 h, mice were challenged intravenously with a lethal dose of S. pneumoniae strain A66.1 (serotype 3), and survival was monitored for 14 days. Data were analyzed using logistic regression with probit link under PROC GLIMMIX in SAS version 8.2 to determine the ED50. In each plot, circles indicate survival data for individual samples, and the best fit regression is shown as a green line, with the upper and lower limits of the 95% confidence interval shown as yellow and red lines, respectively. The horizontal line indicates a median response of 50% survival. In (A), mice were injected with 2.8–27.5 EU (1–10 µg) of purified anti-PhtD antibody or PBS. Survival data were from 4 passive protection experiments, which included 13 survival points. In five of the 13 cases (206, 275, 687, 1375, 2063, and 2750 EU/ml), the passive protection experiment was performed twice, and for 2 of these (275 and 2750 EU/ml), the points overlap and appear as a single data point. Protection experiments for 3 cases (43, 435, and 2178 EU/ml) were not repeated and were included to provide a more appropriate range of antibody concentrations. In (B), mice were injected with 1:20 to 1:60 post-immune sera. To account for the baseline levels of protection seen in the pre-immune sera, the analysis was corrected for over-dispersion using PROC GENMOD with the SCALE = option in SAS version 9.13 and based on survival data from mice that received pre- and post-immune sera.
We next examined the activity of vaccine-induced antibodies in the passive protection model. In the phase I clinical trial, adults were vaccinated on weeks 0 and 4 with 6, 25, or 100 μg of the candidate aluminum phosphate-adjuvanted PhtD vaccine.Citation15 As described above, we found substantial pre-existing anti-PhtD antibodies in pooled sera from vaccine-naïve healthy adults. We therefore selected subjects who had the lowest pre-immune passive protection activity and the highest post-immune PhtD antibody titers for testing in the passive protection assay. For testing in subsequent experiments, we selected a dilution for each subject where the pre-immune serum was not protective in the passive protection assay. All samples were initially tested at a dilution of 1:20, and survival was compared to control mice injected with PBS. Human pre-immune sera that were protective at a 1:20 dilution and for which corresponding post-immune sera (week 8) had an increase in titer of at least 1000 EU/ml were further tested at a 1:40 dilution. Pre-immune sera shown to be protective at a 1:40 dilution and for which corresponding post-immune sera had an increase in titer of at least 2000 EU/ml over baseline were further tested at a 1:60 dilution.
Of the 54 serum pairs tested, we identified appropriate dilutions for 18 (). Vaccination increased the anti-PhtD antibody titer in all subjects at all vaccine doses (6, 25, and 100 µg). Post-immune sera from 10 of these subjects (nos. 21, 36, 37, 41, 43, 45, 51, 57, 58, and 61) significantly delayed death in the murine challenge model compared to the same dilution of the corresponding pre-immune serum. In addition, 2 of these post-immune sera (from subject nos. 37 and 43) significantly improved survival compared to the corresponding pre-immune serum. As with the naturally acquired antibody, survival increased with the concentration of anti-PhtD antibody as determined by ELISA (). The ED50 in this case was estimated to be 1331 EU/ml (95% CI, 762–2038).
Table 1. Anti-PhtD titers and survival mediated by the 18 selected paired pre-/post-immune sera
Finally, we performed ligand competition experiments to investigate the specificity of the vaccine-induced activity. For these experiments, we selected 5 serum pairs (subject nos. 4, 21, 37, 43, and 61) that spanned the different vaccine doses and functional activities. Sera from subject nos. 4 and 61 were selected because the post-immune sera significantly increased survival at day 14 compared to the pre-immune sera, and sera from subject nos. 37 and 43 were selected because the post-immune sera increased survival at day 14 and delayed death compared to the pre-immune serum. We also selected sera from subject no. 21 because the post-immune serum had weak or no functional activity, as indicated by a non-significant delay to death (P = 0.06) and small increase (79.9 EU/ml) in anti-PhtD titer compared to the pre-immune serum. Prior to testing in the passive protection model, the selected serum pairs were incubated for 1 h with recombinant PhtD to block PhtD-binding antibodies. In parallel reactions, sera were incubated with LytB, another S. pneumoniae surface protein, as a control. Compared to the pre-immune sera, post-immune sera from all subjects except no. 21 significantly increased mouse survival when incubated with LytB, whereas survival was not significantly increased by any of the post-immune sera incubated with PhtD (). Therefore, most or all of the protective activity in the post-immune sera was due to anti-PhtD antibodies.
In summary, our results show that the candidate PhtD vaccine induces anti-PhtD antibodies that can protect against S. pneumoniae infection. Another PhtD candidate vaccine, adjuvanted with AS02V, was recently reported to be immunogenic and well tolerated in adults and to induce antibodies that passively protect mice against a lethal dose of S. pneumoniae.Citation16,17 Vaccination with the AS02V-adjuvanted candidate vaccine increased the protective activity in sera from older but not younger adults. The authors concluded that the difference between the 2 age groups was due to higher baseline activity in the younger adults. In the current study, we tried to avoid interference from such baseline functional activity by testing serum dilutions at which the corresponding pre-immune sera was not protective. In addition, we performed competition experiments to confirm that the functional activity in the sera was specifically due to anti-PhtD antibodies and not to antibodies to other pneumococcal antigens, which could have arisen from a S. pneumoniae infection during the trial.
Table 2. Specificity of vaccine-induced antibody
According to logistic analysis, naturally acquired and vaccine-induced antibodies had overlapping activities in the passive protection model (ED50 = 1679 EU/ml [95% CI, 1420–1946] for naturally acquired antibodies vs. 1331 EU/ml [95% CI, 762–2038] for vaccine-induced antibodies). Another study also found that naturally acquired anti-PhtD antibody from human serum can protect mice against a lethal S. pneumoniae intranasal challenge; however, the study did not examine the relationship between functional activity and vaccination, antibody quantity, or antibody quality.Citation13 Accordingly, our current results indicate that the candidate PhtD vaccine increases functional activity, at least in part, by increasing the antibody concentration. Whether vaccination also improves antibody quality is less clear. Our preliminary epitope mapping studies and experiments measuring serum binding to bacterial surfaces suggest that vaccination can also expand the epitope repertoire of anti-PhtD antibodies (data not shown).
Collectively, these results showed that human anti-PhtD antibodies, whether naturally acquired or induced by the PhtD candidate vaccine, are functional. This supports the development of the PhtD candidate as part of a broadly protective pneumococcal vaccine.
Disclosure of Potential Conflicts of Interest
All authors are employees of Sanofi Pasteur.
Acknowledgments
Medical writing was provided by Dr. Phillip Leventhal (4Clinics, Paris, France). The authors thank Elena Newman for purification of antibody from human serum.
Funding
Medical writing was funded by Sanofi Pasteur.
References
- O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009; 374:893-902; PMID:19748398; http://dx.doi.org/10.1016/S0140-6736(09)61204-6
- Moffitt KL, Malley R. Next generation pneumococcal vaccines. Curr Opin Immunol 2011; 23:407-13; PMID:21514128; http://dx.doi.org/10.1016/j.coi.2011.04.002
- Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, Muenz LR, O'Brien KL. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010: 7(10):e1000348
- Mehr S, Wood N. Streptococcus pneumoniae–a review of carriage, infection, serotype replacement and vaccination. Paediat Respir reviews 2012; 13:258-64; PMID:23069126; http://dx.doi.org/10.1016/j.prrv.2011.12.001
- Pittet LF, Posfay-Barbe KM. Pneumococcal vaccines for children: a global public health priority. Clin Microbiol Infect: Off Pub Eur Soc Clin Microbiol Infect Dis 2012; 18 Suppl 5:25-36; PMID:22862432; http://dx.doi.org/10.1111/j.1469-0691.2012.03938.x
- Kallio A, Sepponen K, Hermand P, Denoel P, Godfroid F, Melin M. Role of Pht proteins in attachment of Streptococcus pneumoniae to respiratory epithelial cells. Infect Immun 2014; 82:1683-91; PMID:24491577; http://dx.doi.org/10.1128/IAI.00699-13
- Khan MN, Pichichero ME. Vaccine candidates PhtD and PhtE of Streptococcus pneumoniae are adhesins that elicit functional antibodies in humans. Vaccine 2012; 30:2900-7; PMID:22349524; http://dx.doi.org/10.1016/j.vaccine.2012.02.023
- Adamou JE, Heinrichs JH, Erwin AL, Walsh W, Gayle T, Dormitzer M, Dagan R, Brewah YA, Barren P, Lathigra R, et al. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect Immun 2001; 69:949-58; PMID:11159990; http://dx.doi.org/10.1128/IAI.69.2.949-958.2001
- Turner P, Turner C, Green N, Ashton L, Lwe E, Jankhot A, Day NP, White NJ, Nosten F, Goldblatt D. Serum antibody responses to pneumococcal colonization in the first 2 years of life: results from an SE Asian longitudinal cohort study. Clin Microbiol Infect: Off Pub Eur Soc Clin Microbiol Infect Dis 2013; 19:E551-8; PMID:24255996; http://dx.doi.org/10.1111/1469-0691.12286
- Simell B, Ahokas P, Lahdenkari M, Poolman J, Henckaerts I, Kilpi TM, Käyhty H. Pneumococcal carriage and acute otitis media induce serum antibodies to pneumococcal surface proteins CbpA and PhtD in children. Vaccine 2009; 27:4615-21; PMID:19524618; http://dx.doi.org/10.1016/j.vaccine.2009.05.071
- Beghetto E, Gargano N, Ricci S, Garufi G, Peppoloni S, Montagnani F, Oggioni M, Pozzi G, Felici F. Discovery of novel Streptococcus pneumoniae antigens by screening a whole-genome lambda-display library. FEMS Microbiol Lett 2006; 262:14-21; PMID:16907734; http://dx.doi.org/10.1111/j.1574-6968.2006.00360.x
- Khan MN, Pichichero ME. CD4 T cell memory and antibody responses directed against the pneumococcal histidine triad proteins PhtD and PhtE following nasopharyngeal colonization and immunization and their role in protection against pneumococcal colonization in mice. Infect Immun 2013; 81:3781-92; PMID:23897609; http://dx.doi.org/10.1128/IAI.00313-13
- Godfroid F, Hermand P, Verlant V, Denoel P, Poolman JT. Preclinical evaluation of the Pht proteins as potential cross-protective pneumococcal vaccine antigens. Infect Immun 2011; 79:238-45; PMID:20956575; http://dx.doi.org/10.1128/IAI.00378-10
- Denoel P, Philipp MT, Doyle L, Martin D, Carletti G, Poolman JT. A protein-based pneumococcal vaccine protects rhesus macaques from pneumonia after experimental infection with Streptococcus pneumoniae. Vaccine 2011; 29:5495-501; PMID:21624422; http://dx.doi.org/10.1016/j.vaccine.2011.05.051
- Seiberling M, Bologa M, Brookes R, Ochs M, Go K, Neveu D, Kamtchoua T, Lashley P, Yuan T, Gurunathan S. Safety and immunogenicity of a pneumococcal histidine triad protein D vaccine candidate in adults. Vaccine 2012; 30:7455-60; PMID:23131206; http://dx.doi.org/10.1016/j.vaccine.2012.10.080
- Leroux-Roels G, Maes C, De Boever F, Traskine M, Ruggeberg JU, Borys D. Safety, reactogenicity and immunogenicity of a novel pneumococcal protein-based vaccine in adults: a phase I/II randomized clinical study. Vaccine 2014; 32:6838-46; PMID:24607003; http://dx.doi.org/10.1016/j.vaccine.2014.02.052
- Leroux-Roels I, Devaster JM, Leroux-Roels G, Verlant V, Henckaerts I, Moris P, Hermand P, Van Belle P, Poolman JT, Vandepapelière P, et al. Adjuvant system AS02V enhances humoral and cellular immune responses to pneumococcal protein PhtD vaccine in healthy young and older adults: randomised, controlled trials. Vaccine 2015; 33:577-84; PMID:24176494; http://dx.doi.org/10.1016/j.vaccine.2013.10.052
