Abstract
The immune system becomes less effective with age, and older age is associated with an increased susceptibility to diseases and reduced responses to vaccination. Furthermore, some adult populations, such as those with diabetes mellitus, are at increased risk of acute hepatitis B virus (HBV) infection. Decreasing responses to vaccination with advanced age have been described, but it is not known at what age immunogenicity starts to reduce, or until what age immunogenicity remains acceptable (for example ≥80 % seroprotection post-vaccination). We characterized the relationship between age and seroprotection rate induced by recombinant HBV vaccination by conducting a pooled analysis of clinical trial data. Healthy adults aged ≥20 y who had been vaccinated with 20μg HBV vaccine (Engerix™ B, GSK Vaccines, Belgium) in a 0, 1, 6 months schedule in 11 studies since 1996 were included. The observed seroprotection rate, defined as an anti-HBV surface antigen antibody concentration ≥10 mIU/ml was 94.5% in the whole population (N = 2,620, Total vaccinated cohort), ranging from 98.6% in adults vaccinated at age 20–24 years, to 64.8% in those vaccinated at age ≥65 y A model on seroprotection rates showed a statistically significant decrease with age, and predicted that the anti-HBs seroprotection rate remains ≥90% up to 49 y of age and ≥80% up to 60 y of age. Individuals at risk of HBV infection should be vaccinated as early in life as possible to improve the likelihood of achieving seroprotection. Additional studies are needed to identify whether unvaccinated individuals older than 60 y would benefit from regimens that include additional or higher vaccine doses.
Introduction
The global population is aging, with the proportion of those aged 60 y and older expected to double between 2000 and 2050.Citation1 The immune system becomes less effective with age, and old age is associated with reduced responses to vaccination, increased susceptibility to infectious diseases, and an increased risk of developing severe or complicated illnesses as a result.Citation2 Age-related immune defects may also be exacerbated by co-existing morbidities and chronic diseases that can lead to immune dysfunction, such as chronic renal disease requiring hemodialysis,Citation3 underlying liver diseaseCitation4 and diabetes: adults with diabetes mellitus are at increased risk of acute hepatitis B infection and suffer 2–3-fold higher rates of complications including progression to cirrhosis and hepatocellular cancer, compared to non-diabetics.Citation5-9
Immune senescence is characterized by altered composition of bone marrow with reduced capacity to produce and nurture stem cells, and atrophy of the thymus gland with reduced output of T-cells.Citation10 The response to vaccination is impaired in the elderly due to functional defects at multiple levels in innate and adaptive immune responses. These include reduced capacity of antigen presenting cells to take up and present antigen, lower numbers of T-cells capable of responding to new antigen, reduced magnitude and quantity of the B-cell antibody response, and poor effector T-cell activation and signaling due to reduced numbers and loss of receptor diversity.Citation11-13
Decreasing responses to vaccination with increasing age have been described for numerous vaccines including those targeting seasonal influenza,Citation14 diphtheria,Citation15 tetanus,Citation16 pertussis,Citation17 pneumococcal disease,Citation18 and others.Citation19 Several studies describe decreased responses to hepatitis B vaccination in older adults, although the studied age ranges vary between 40 and 80 y.Citation20-26 Indeed, it is not known at what age the magnitude of the immune response to hepatitis B vaccination starts to reduce, or until what age the immune response remains acceptable (for example ≥80 % seroprotection post-vaccination). In order to answer these questions for currently recommended doses and schedules, we undertook a pooled analysis of clinical trial data (E-track 201931). All GSK-sponsored interventional vaccine studies using hepatitis B vaccine (Engerix™ B, GSK Vaccines, Belgium) reported after 1996 were included. All studies were to be completed (published or unpublished), with a database and clinical study report available. Eligible study groups were those in which healthy adults aged ≥20 y received 20 μg of a licensed formulation of Engerix™ B according to a 0, 1 and 6 month immunization schedule. Data from subjects who received other hepatitis B vaccines, combination vaccines such as combined hepatitis A and hepatitis B vaccines, or who belonged to special populations (for example hemodialysis patients), or who were vaccinated according to an alternative schedule, were excluded from the analysis.
All adults vaccinated with Engerix™ B and whose immune results were available one month after the third vaccine dose were included in the analysis (Total vaccinated cohort). Seroprotection results with exact 95% confidence intervals (CI) were summarized by age in 5-y intervals. All studies measured antibodies to hepatitis B surface antigen (anti-HBs) one month post-dose 3 using either an enzyme-linked immunosorbent assay or chemiluminescence immunoassay. An anti-HBs threshold of 10 mIU/ml defined seroprotection. The 10 mIU/mL threshold is accepted as a serological correlate of protection for HBV infection and was used in our study as indicative of immunogenicity.Citation27,28
A logistic model on seroprotection rate was used to assess whether age influenced the seroprotection rate. A 2-sided p-value <0.05 for the null hypothesis, that there is no age effect, was used to indicate a statistical significant effect of age. With ≥2, 300 subjects aged between 20 and 83 years, and an expected seroprotection rate at ages 35 and 45 y of 90% and 87.8%, respectively (corresponding to a 0.8 odds ratio for a decennial age increase), the analysis had 89% power to identify a statistically significant age effect on the hepatitis B seroprotection rate (PASS 2005, logistic regression with a normally distributed covariable, 2-sided alpha = 5 %). If a significant age effect was observed, a piecewise linear model was used to determine whether an age cut-off exists for the seroprotection rate decrease. The age cut-off was estimated from the best fitted piecewise model based on the Akaike Information Criterion (a measure of the relative quality of a statistical model for a given set of data).
The analysis included 2,620 eligible adults (Total vaccinated cohort) who participated in 11 studies conducted in 10 countries (). The mean age was 34.3 y (standard deviation 12.3 years), 57.7% of subjects were female and 91.8% were Caucasian. The seroprotection rate in the whole population was 94.5% (95% CI 93.5; 95.3), ranging from ≥98.6% in adults 20–24 y of age to 64.8% in those aged ≥65 y ().
Figure 1. Observed (A) and predicted (B) seroprotection rates with 95% CI (Total vaccinated cohort). Predicted seroprotection rate (%) = Predicted percentage of subjects with anti-HBs concentration ≥10 mIU/ml evaluated from the best fit logistic regression model where Ln (P/1-P) = 4.23−0.07*age and P represents seroprotection rate. Vertical lines indicate 95% confidence intervals.
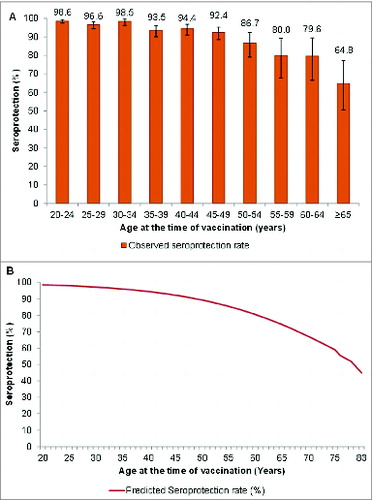
Table 1. Summary of subject characteristics by age group (total vaccinated cohort)
The best model fit was achieved using a log-linear curve with an age effect beginning at 20 y of age where Ln (P/1-P) = 4.23 − 0.07*age (). The model predicted that the anti-HBs seroprotection rate remains ≥90% up to 49 y of age and ≥80% up to 60 y of age ().
This pooled analysis of clinical trial data allows robust assessment of the effect of age upon the immune response to hepatitis B vaccination in a large population. The clinical trials were conducted in healthy adults without underlying disease or known immune deficiencies, allowing assessment of the effect of age alone on the immune response. The decline in anti-HBs seroprotection rate with age followed a log linear curve, indicating that the decrease in hepatitis B seroprotection progresses throughout all age groups. Our study suggests that immune senescence starts in early adulthood, but accelerates after 50–60 y of age.
The thymus gland has a pivotal role in immune functioning and produces diverse populations of naïve T-cells necessary for humoral and cellular immune responses.Citation10,29 Thymic involution is one of the most important events that contributes to immune dysfunction in the elderly.Citation10,29 Evidence of early and continual decreases in vaccine responses with age is broadly consistent with the process of thymic involution, which begins in early adulthood. By 50 y of age around 80% of functional thymus tissue has been lost.Citation10
Our model predicts that the hepatitis B seroprotection rate remains ≥90% up to 49 y of age, and above 80% until 60 y of age in adults who are otherwise healthy. While we did not investigate interactions between gender and immune senescence, our results are comparable to results of a modeling study of anti-HBs concentrations in more than 11,000 healthy adults in the Netherlands.Citation26 In the Netherlands study the response rate (anti-HBs ≥10 mIU/ml) was also predicted to be 90% until approximately 40 y of age and 80% at around 60 y of age.Citation26 This information has implications for vaccination policy. Individuals at risk of hepatitis B infection should be vaccinated as early in life as possible to improve the likelihood of achieving seroprotection, and because of the ease of identifying an at-risk individual before exposure occurs. This is particularly relevant for at-risk older populations, including patients with co-morbidities such as chronic renal or liver disease, and patients with diabetes, who have higher rates of acute hepatitis B and are at greater risk of developing complications of hepatitis B than healthy individuals.Citation3-8 Further investigations are needed to identify if unvaccinated individuals over 60 y of age, in whom hepatitis B vaccination is recommended, would benefit from regimens that include additional or higher doses, as adopted for patients on hemodialysis,Citation30,31 in order to improve seroprotection rates.Citation32
Engerix is a trademark of the GlaxoSmithKline group of companies.
Disclosure of Potential Conflicts of Interest
All authors are employees of the GlaxoSmithKline group of companies. MDR, BC and OVDM declare to be in receipt of GSK stocks.
Acknowledgments
The authors thank Rashmi Jain (GSK India) for performing the statistical analysis. Writing support was provided by Joanne Wolter (Independent medical writer on behalf of GlaxoSmithKline group of companies) and editorial support and publication management was provided by Julia Donnelly (freelance on behalf of the GlaxoSmithKline group of companies).
Funding
This study was sponsored and funded by GlaxoSmithKline Biologicals S.A. GlaxoSmithKline Biologicals S.A. was involved in all stages of the study conduct and analysis and also took charge of all costs associated with the development and the publishing of the manuscript.
References
- United Nations Population Division. World Population Ageing: 1950-2050 [Internet]. New York: 2001 [cited 2014 Nov 3]. Available from: http://www.un.org/esa/population/publications/worldageing19502050/
- Gavazzi G, Krause K-H. Ageing and infection. Lancet Infect Dis 2002; 2:659-66; PMID:12409046; http://dx.doi.org/10.1016/S1473-3099(02)00437-1
- Urbánek P. Viral hepatitis infections in chronic kidney disease patients and renal transplant recipients. Kidney Blood Press Res 2012; 35:454-67; http://dx.doi.org/10.1159/000338309
- Keeffe EB. Acute hepatitis A and B in patients with chronic liver disease: prevention through vaccination. Am J Med 2005; 118 (Suppl 10A):21S-27S; PMID:16271537; http://dx.doi.org/10.1016/j.amjmed.2005.07.013
- Huo TI, Wu JC, Lee PC, Tsay SH, Chang FY, Lee SD. Diabetes mellitus as a risk factor of liver cirrhosis in patients with chronic hepatitis B virus infection. J Clin Gastroenterol 2000; 30:250-4; PMID:10777182; http://dx.doi.org/10.1097/00004836-200004000-00009
- El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004; 126:460-8; PMID:14762783; http://dx.doi.org/10.1053/j.gastro.2003.10.065
- Huang Y-W, Wang T-C, Lin S-C, Chang H-Y, Chen D-S, Hu J-T, Yang S-S, Kao J-H. Increased risk of cirrhosis and its decompensation in chronic hepatitis B patients with newly diagnosed diabetes: a nationwide cohort study. Clin Infect Dis 2013; 57:1695-702; PMID:24051864; http://dx.doi.org/10.1093/cid/cit603
- Reilly ML, Schillie SF, Smith E, Poissant T, Vonderwahl CW, Gerard K, Baumgartner J, Mercedes L, Sweet K, Muleta D, et al. Increased risk of acute hepatitis B among adults with diagnosed diabetes mellitus. J Diabetes Sci Technol 2012; 6:858-66; PMID:22920812; http://dx.doi.org/10.1177/193229681200600417
- Centers for Disease Control and Prevention (CDC). Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011; 60:1709-11; PMID:22189894.
- Rezzani R, Nardo L, Favero G, Peroni M, Rodella LF. Thymus and aging: morphological, radiological, and functional overview. Age (Dordr) 2014; 36:313-51; PMID:23877171; http://dx.doi.org/10.1007/s11357-013-9564-5
- Goldstein DR. Role of aging on innate responses to viral infections. J Gerontol A Biol Sci Med Sci 2012; 67:242-6; PMID:22042722; http://dx.doi.org/10.1093/gerona/glr194
- Kogut I, Scholz JL, Cancro MP, Cambier JC. B cell maintenance and function in aging. Semin Immunol 2012; 24:342-9; PMID:22560930; http://dx.doi.org/10.1016/j.smim.2012.04.004
- Lord JM. The effect of ageing of the immune system on vaccination responses. Hum Vaccin Immunother 2013; 9:1364-7; PMID:23584248; http://dx.doi.org/10.4161/hv.24696
- Haq K, McElhaney JE. Immunosenescence: Influenza vaccination and the elderly. Curr Opin Immunol 2014; 29:38-42; PMID:24769424; http://dx.doi.org/10.1016/j.coi.2014.03.008
- Theeten H, Rümke H, Hoppener FJP, Vilatimó R, Narejos S, Van Damme P, Hoet B. Primary vaccination of adults with reduced antigen-content diphtheria-tetanus-acellular pertussis or dTpa-inactivated poliovirus vaccines compared to diphtheria-tetanus-toxoid vaccines. Curr Med Res Opin 2007; 23:2729-39; PMID:17897485; http://dx.doi.org/10.1185/03007X233034
- Hoel T, Wolter JM, Schuerman LM. Combined diphtheria-tetanus-pertussis vaccine for tetanus-prone wound management in adults. Eur J Emerg Med 2006; 13:67-71; PMID:16525231; http://dx.doi.org/10.1097/01.mej.0000184993.51799.ad
- Van Damme P, McIntyre P, Grimprel E, Kuriyakose S, Jacquet J-M, Hardt K, Messier M, Van Der Meeren O. Immunogenicity of the reduced-antigen-content dTpa vaccine (Boostrix®) in adults 55 years of age and over: a sub-analysis of four trials. Vaccine 2011; 29:5932-9; PMID:21718738; http://dx.doi.org/10.1016/j.vaccine.2011.06.049
- Jackson LA, Gurtman A, van Cleeff M, Jansen KU, Jayawardene D, Devlin C, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine 2013; 31:3577-84; PMID:23688526; http://dx.doi.org/10.1016/j.vaccine.2013.04.085
- Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis 2008; 46:1078-84; PMID:18444828; http://dx.doi.org/10.1086/529197
- De Rave S, Heijtink RA, Bakker-Bendik M, Boot J, Schalm SW. Immunogenicity of standard and low dose vaccination using yeast-derived recombinant hepatitis B surface antigen in elderly volunteers. Vaccine 1994; 12:532-4; PMID:8036828; http://dx.doi.org/10.1016/0264-410X(94)90313-1
- Williams RE, Sena AC, Moorman AC, Moore ZS, Sharapov UM, Drobenuic J, Hu DJ, Wood HW, Xing J, Spradling PR. Hepatitis B vaccination of susceptible elderly residents of long term care facilities during a hepatitis B outbreak. Vaccine 2012; 30:3147-50; PMID:22421557; http://dx.doi.org/10.1016/j.vaccine.2012.02.078
- Denis F, Mounier M, Hessel L, Michel JP, Gualde N, Dubois F, Barin F, Goudeau A. Hepatitis-B vaccination in the elderly. J Infect Dis 1984; 149:1019; PMID:6234369; http://dx.doi.org/10.1093/infdis/149.6.1019
- Wolters B, Junge U, Dziuba S, Roggendorf M. Immunogenicity of combined hepatitis A and B vaccine in elderly persons. Vaccine 2003; 21:3623-8; PMID:12922091; http://dx.doi.org/10.1016/S0264-410X(03)00399-2
- Stoffel M, Lievens M, Dieussaert I, Martin I, André F. Immunogenicity of Twinrix in older adults: a critical analysis. Expert Rev Vaccines 2003; 2:9-14; PMID:12901592; http://dx.doi.org/10.1586/14760584.2.1.9
- Van der Wielen M, Van Damme P, Chlibek R, Smetana J, von Sonnenburg F. Hepatitis A/B vaccination of adults over 40 years old: comparison of three vaccine regimens and effect of influencing factors. Vaccine 2006; 24:5509-15; PMID:16725234; http://dx.doi.org/10.1016/j.vaccine.2006.04.016
- Vermeiren APA, Hoebe CJPA, Dukers-Muijrers NHTM. High non-responsiveness of males and the elderly to standard hepatitis B vaccination among a large cohort of healthy employees. J Clin Virol 2013; 58:262-4; PMID:23895931; http://dx.doi.org/10.1016/j.jcv.2013.07.003
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol 2010; 17:1055-65; PMID:20463105; http://dx.doi.org/10.1128/CVI.00131-10
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis 1999; 179:489-92; PMID:9878036; http://dx.doi.org/10.1086/314578
- Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol 2007; 211:144-56; PMID:17200946; http://dx.doi.org/10.1002/path.2104
- Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep 2001; 50:1-43
- Hepatitis B vaccines: WHO position paper–recommendations. Vaccine 2010; 28:589-90; PMID:19896455; http://dx.doi.org/10.1016/j.vaccine.2009.10.110
- Van der Meeren O, Dionne M, Peterson J, Ebeling P, Beasley R, Rhealt P, Nissen M, De Ridder M, Crasta PD, Miller J, et al. A prospective clinical trial of hepatitis B vaccine in adults with type II diabetes mellitus. Presented at National Foundation for Infectious Disease - 18th Annual Congress on Vaccine Research, Bethesda MD. 13-15 April 2015.
- Tsai IJ, Chang MH, Chen HL, Ni YH, Lee PI, Chiu TY, Safary A. Immunogenicity and reactogenicity of the combined hepatitis A and B vaccine in young adults. Vaccine 2000; 19:437-41; PMID:11027806; http://dx.doi.org/10.1016/S0264-410X(00)00205-X
- Joines RW, Blatter M, Abraham B, Xie F, De Clercq N, Baine Y, Reisinger KS, Kuhnen A, Parenti DL. A prospective, randomized, comparative US trial of a combination hepatitis A and B vaccine (Twinrix) with corresponding monovalent vaccines (Havrix and Engerix-B) in adults. Vaccine 2001; 19:4710-9; PMID:11535321; http://dx.doi.org/10.1016/S0264-410X(01)00240-7
- Tatochenko VK, Il'ina NI, Romanenko VV, Alikova OA, Fassakhov RS, Miasnikova TN, Patlusova VV, Zima II, Reshetnikova ID, Frolova GS, et al. [Results of Russian multicenter trial of immunogenicity, reactogenicity and safety of new combination vaccine against hepatitis A and B (Twinrix)]. Zh Mikrobiol Epidemiol Immunobiol 2006; 6:30-5; PMID:17163135.
- Gloriani NG, Srinivasa K, Bock HL, Hoet B. Immunogenicity of HBV vaccine during stated shelf-life. Southeast Asian J Trop Med Public Health 2010; 41:876-82; PMID:21073062
