Abstract
The aim of this study was to compare humoral and cellular immune responses to influenza vaccination in cancer survivors with and without severe symptoms of fatigue. Severely fatigued (n = 15) and non-fatigued (n = 12) disease-free cancer survivors were vaccinated against seasonal influenza. Humoral immunity was evaluated at baseline and post-vaccination by a hemagglutination inhibition assay. Cellular immunity was evaluated at baseline and post-vaccination by lymphocyte proliferation and activation assays. Regulatory T cells were measured at baseline by flow cytometry and heat-shock protein 90 alpha levels by ELISA. Comparable humoral immune responses were observed in fatigued and non-fatigued patients, both pre- and post-vaccination. At baseline, fatigued patients showed a significantly diminished cellular proliferation upon virus stimulation with strain H3N2 (1414 ± 1201 counts), and a trend in a similar direction with strain H1N1 (3025 ± 2339 counts), compared to non-fatigued patients (3099 ± 2401 and 5877 ± 4604 counts, respectively). The percentage of regulatory T lymphocytes was significantly increased (4.4 ± 2.1% versus 2.4 ± 0.8%) and significantly lower amounts of interleukin 2 were detected prior to vaccination in fatigued compared to non-fatigued patients (36.3 ± 44.3 pg/ml vs. 94.0 ± 45.4 pg/ml with strain H3N2 and 28.4 ± 44.0 pg/ml versus 74.5 ± 56.1 pg/ml with strain H1N1). Pre-vaccination heat-shock protein 90 alpha concentrations, post-vaccination cellular proliferation, and post-vaccination cytokine concentrations did not differ between both groups. In conclusion, influenza vaccination is favorable for severely fatigued cancer survivors and should be recommended when indicated. However, compared to non-fatigued cancer survivors, fatigued cancer survivors showed several significant differences in immunological reactivity at baseline, which warrants further investigation.
Abbreviations:
- CBT, cognitive behavior therapy
- CIS-fatigue, Checklist Individual Strength fatigue subscale
- HI, hemagglutination-inhibition
- HSP90α, human heat shock protein 90 alpha
- IFN- γ, interferon gamma
- IL-2, interleukin 2
- IL-4, interleukin 4
- IL-5, interleukin 5
- IL-10, interleukin 10
- PBMC, peripheral blood mononuclear cells
- PHA, phytohemagglutinin
- Radboudumc, Radboud University Medical Center
- Treg, regulatory T lymphocytes
Introduction
Severe fatigue is one of the long-term problems of cancer survivors. Postcancer fatigue is an invalidating problem, which occurs frequently and impairs the quality of life.Citation1,2 Severe fatigue is defined by a cut-off score of at least 35 points on the fatigue severity subscale of the Checklist Individual Strength (CIS-fatigue).Citation3,4 Already during cancer treatment 70–96% of the patients suffer from fatigue symptoms.Citation5,6 Postcancer fatigue has a prevalence ranging from 19 to 39% as observed in longitudinal studies.Citation7-11 The cancer diagnosis and treatment characteristics are not related to postcancer fatigue.Citation6,12-16 There is evidence, however, that patients who receive only surgery have a decreased risk for postcancer fatigueCitation11 and patients who receive both surgery and adjuvant treatment have an increased risk for postcancer fatigue.Citation7,17 Cognitive behavior therapy (CBT), particularly composed for postcancer fatigue, is proven to be efficacious in treating severely fatigued cancer survivors.Citation14,18 However, the nature of the underlying physiology of postcancer fatigue remains unclear.
Possible explanations for the pathophysiological mechanism of cancer-related fatigue are dysregulation of brain serotonin, dysregulation of the hypothalamic-pituitary-adrenal axis responsiveness, disruption of the circadian rhythm, alterations in muscle and ATP metabolism, activation of the vagal afferent nerve, and dysregulation of cytokines (for a review, see Ryan et al 2007Citation19, and for a meta-analysis, see Schubert et al 2007Citation20).
The presence of an immunological imbalance was also put forward as an explanation for postcancer fatigue. As a response to the presence of the tumor or its treatment, the immune system is activated, which results in the release of cytokines and other immune factors, including receptor antagonists, soluble receptors, and products of cellular activation.Citation21 Cytokines steer both the innate and adaptive immune response, but also mediate neural symptoms like fatigue.Citation22 Alterations in pro-inflammatory cytokines have been observed in fatigue-related disorders like chronic fatigue syndrome and depression.Citation23,24 Most of these changes in immune parameters resolve following completion of cancer treatment, but an imbalance in the immune system persists, which could explain the fatigue.
An immunological imbalance in postcancer fatigue patients may be reflected in an altered response to vaccination. Hence, the aim of this study was to compare humoral and cellular immune responses upon vaccination, using seasonal influenza vaccination as a representative vaccination, in severely fatigued and non-fatigued disease-free cancer survivors.
Results
Baseline characteristics of the participants
Patient characteristics at baseline are presented in . The group suffering from severe postcancer fatigue did not significantly deviate from the non-fatigued group in terms of sex, age, average time since cancer treatment, and history of influenza vaccination.
Table 1. Baseline characteristics
Humoral immune responses in postcancer fatigue upon influenza vaccination
Participants were vaccinated against seasonal influenza. Pre- and post-vaccination, humoral immune responses were assessed by the hemagglutination-inhibition (HI) antibody test. Comparable antibody titres were observed for the vaccination viral strains in fatigued and non-fatigued patients, both pre- and post-vaccination (). Influenza vaccination caused a near identical increment in antibody titres for strains H3N2 and H1N1 in patients suffering from postcancer fatigue as well as in non-fatigued patients (, respectively). Fatigued and non-fatigued patients showed comparable seroprotection rates (pre-vaccination and post-vaccination, ) and similar seroresponse rates against the 3 influenza strains (). The number of severely fatigued and non-fatigued patients without a humoral response to influenza vaccination was not significantly different ().
Figure 1. Hemagglutination inhibition antibody responses before (day 1) and after (day 22) influenza vaccination, and cellular proliferation before (day 1) and after (day 8) influenza vaccination. Both immune responses are presented for the 3 influenza strains of the vaccine (H3N2, H1N1, and B), in fatigued (A and C) and non-fatigued (B and D) cancer survivors. Antibody titres are presented as absolute numbers and mean on a linear scale, the dotted line indicates the protective titer cut-off value. Proliferation counts are presented as absolute numbers and mean (horizontal lines) on a logarithmic scale.
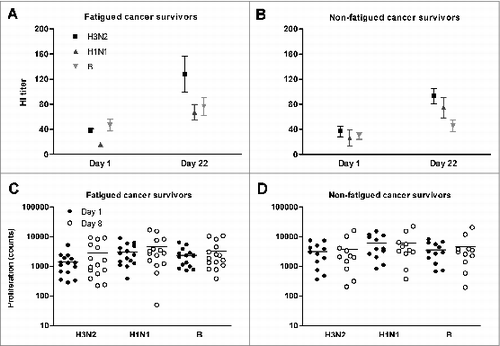
Table 2. Humoral and cellular immune responses
Cellular immune responses in postcancer fatigue upon influenza vaccination
Pre-vaccination
T cell proliferation upon stimulation with phytohemagglutinin (PHA) and influenza virus in both patient groups, indicate the presence of functional T cells (, ). At baseline, postcancer fatigue patients showed a diminished cellular proliferation upon stimulation with virus-strain H3N2 and with virus-strain H1N1, compared to non-fatigued controls ().
To investigate the cytokine secretion of peripheral blood mononuclear cells (PBMC) at baseline, interleukin 2 (IL-2) and interleukin 10 (IL-10) concentrations were measured. We detected significantly less IL-2 in patients suffering from postcancer fatigue compared to non-fatigued patients upon stimulation with virus-strains H3N2 and H1N1, whereas detected IL-10 concentrations were identical between the 2 groups ().
Regulatory T lymphocytes (Treg), which are known to negatively regulate T cell responses, were measured at baseline. Compared to non-fatigued controls, the percentage of Treg was significantly increased at baseline in severely fatigued patients ().
To test our hypothesis that impaired human heat shock protein 90 alpha (HSP90α) functioning resulted in Treg accumulation, HSP90α concentrations were measured in serum at baseline. Human HPS90α concentrations were not significantly different between fatigued and non-fatigued patients ().
In postcancer fatigue patients, the high percentage of Treg indeed correlated significantly with lower cellular proliferation upon stimulation with virus-strain H3N2 (p = 0.015), H1N1 (p = 0.031), and B (p = 0.005). Also, the percentage of Treg correlated significantly with IL-2 concentrations upon stimulation with virus-strain H3N2 (p = 0.017) and H1N1 (p = 0.001) in fatigued patients, but not upon stimulation with virus-strain B (p > 0.05). Non-fatigued patients did not demonstrate significant correlations between the percentage of Treg and cellular proliferation or between Treg and IL-2 concentrations (p > 0.05).
Post-vaccination
The cellular immune responses to the influenza vaccine were measured at day 8 by cytokine secretion of PBMC and T lymphocyte proliferation. Post-vaccination cellular proliferation was not significantly different between fatigued and non-fatigued cancer survivors (). Interferon gamma (IFN-γ), interleukin 4 (IL-4), and interleukin 5 (IL-5) production were not significantly different between both patient groups upon stimulation with the 3 individual influenza strains ().
There is no significant correlation (p > 0.05) between pre-existing strain-specific antibody titres or cellular proliferation and post-vaccination strain-specific antibody titres or cellular proliferation, neither in fatigued, nor in non-fatigued patients (data not shown).
Discussion
We believe that we are the first to explore both the humoral and cellular immune responses upon influenza vaccination in patients suffering from severe postcancer fatigue. The hypothesized immunological imbalance in postcancer fatigue was confirmed by our data, as patients suffering from postcancer fatigue show several significant differences in immunological reactivity at baseline, compared to non-fatigued patients.
Severe fatigued cancer survivors are able to develop sufficient antibody levels and adequate cellular immune reactions after a single shot of seasonal influenza vaccine, which is similar to non-fatigued cancer survivors. Both the humoral and cellular immune responses to influenza vaccination were also comparable in one of our previous studies comparing chronic fatigue syndrome patients and healthy controls.Citation25 However, interestingly, compared to non-fatigued patients, patients suffering from postcancer fatigue showed significant differences in pre-vaccination cellular immune responses.
We observed that, in comparison to non-fatigued patients, the T cell capacity to proliferate was significantly diminished at baseline in fatigued patients upon stimulation with virus-strain H3N2 and for strain H1N1. To study this finding in more detail, we investigated 2 immunological mechanisms generally known to suppress T cell immunity. First, the baseline production of the immunosuppressive cytokine IL-10, which has the capacity to inhibit T cell proliferation,Citation26 was measured. However, baseline IL-10 production was comparable in fatigued and non-fatigued patients. Secondly, since Treg are known to negatively modify T cell responses, we examined pre-vaccination Treg percentages.Citation27 Indeed, Treg percentages were significantly increased at baseline in postcancer fatigue patients, compared to non-fatigued controls, and could not be explained by a difference in the number of lymphocytes between both patient groups (data not shown). This finding could explain the diminished cellular proliferation in fatigued patients compared to non-fatigued patients before vaccination.
Consistent with the increase in Treg, the baseline production of IL-2, which drives T cell growth,Citation28 is decreased in fatigued compared to non-fatigued patients. The reduction in IL-2 levels is likely the result of IL-2 consumption by Treg,Citation29 thereby driving their expansion.
We hypothesized that impaired HSP90α functioning in patients suffering from postcancer fatigue resulted in Treg accumulation. Treg accumulation might lead to the development of functional somatic syndromes, like chronic fatigue syndrome.Citation30 HSP90 is highly up regulated during cell stress,Citation31 which occurs as a result of the tumor or its treatment, and HSP90 is involved in controlling the functions of FoxP3+ Treg.Citation32 The difference in Treg was not caused by HSP90 because fatigued and non-fatigued patients did not differ in baseline HSP90α concentrations in serum.
The influenza virus slightly changes its antigenic makeup every season, but still viral proteins are identical between the different viral strains (shared epitopes). At baseline the reactivity against these shared antigens is measured since the novel epitopes on the vaccination strains are not yet exposed to the immune system. Apparently, the Tregs were able to inhibit the response to these shared antigens. Both patient groups showed a normal level of proliferation when stimulated with the new viral strains. This indicates that naïve T cells were equally responsive toward those novel viral antigens. This was confirmed by the IL-2 production, which did not significantly differ between fatigued and non-fatigued patients (data not shown). We conclude that patients suffering from postcancer fatigue expand the percentage of Treg after vaccination. Seemingly, the naïve T cell response is normal between the 2 groups but the memory response is hampered in the severe fatigue patient group. This would imply that patients suffering from postcancer fatigue have to invest more energy in handling immunity against pathogens that are encountered earlier in live. This leads to less energy available for other tasks of the organism, explaining their fatigue. In contrast, cancer survivors who are able to handle the immune response to recurrent immunological challenges more efficiently may suffer less from fatigue.
In conclusion, assumed abnormalities in immune responses in postcancer fatigue patients were demonstrated for a memory immunological response. We show comparable humoral and cellular immune reactions to influenza vaccination in fatigued and non-fatigued cancer survivors indicating a normal naïve T cell response in both groups. Therefore, influenza vaccination is favorable for patients suffering from postcancer fatigue and should be recommended when indicated.
Patients and Methods
Participants
Fifteen severely fatigued and 12 non-fatigued cancer survivors were included in this study. From a previous influenza study in cancer patients with similar sample size and using identical measurement techniques, we know that our group sizes are sufficient to detect significant differences in immunological parameters.Citation34 Fatigue severity was determined according to the CIS-fatigue.Citation3,4 The CIS-fatigue was shown to be sensitive to detect changes and was used in previous research to assess fatigue in cancer patients.Citation11,18,35,36 A score of ≥35 on the CIS-fatigue is an indicator for severe fatigue. Cancer survivors suffering from severe fatigue who were referred for CBT to the Expert Center for Chronic Fatigue of the Radboud University Medical Center (Radboudumc) were asked for participation. Non-fatigued patients (score of <27 on the CIS-fatigue) were recruited from the outpatient clinics of Medical Oncology and Radiation Oncology of the Radboudumc. All participants were between 18 and 60 y old, used no corticosteroids during the previous 2 weeks, did not have an immune disorder or allergy to chicken eggs, and had no symptoms of influenza or an influenza-like illness on the day of the vaccination. For each patient, the oncological treatment history was retrieved from the medical chart. The study was approved by the local ethics committee of the Radboudumc and all participants provided a written informed consent.
Vaccination and blood collection
All participants visited the outpatient clinic of Medical Oncology of the Radboudumc between September 2010 and January 2011 and were intramuscularly vaccinated with a single dose of the inactivated trivalent split influenza vaccine (VaxigripR, Sanofi Pasteur MSD, Hoofddorp, the Netherlands), which contained inactivated, split virion of the 3 influenza strains (A/H3N2/Perth/16/2009, A/H1N1/California/7/2009, and B/Brisbane/60/2008).
PBMC were harvested at baseline (day 1) and 7 d post-vaccination (day 8). PBMC were collected from heparinized venous blood by density-gradient centrifugation on lymphoprepTM (Axis-Shield PoC AS, Oslo, Norway) and cells were frozen using a cryo 1°C freezing container (Nalgene, Rochester, New York, USA) in freezing medium (49% X-VIVO15 (Lonza, Verviers, Belgium), 1% human serum (Sanquin blood bank, Nijmegen, the Netherlands), 40% human serum albumin (Albuman; Sanquin blood bank), and 10% DMSO (Cryo Sure; Wak Chemie Medical GMBH, Steinbach, Germany)). After 24h in a freezing container at −80°C, PBMC were stored in liquid nitrogen until analysis.
Serum was collected at day 1 and 21 d post-vaccination (day 22).Citation34 Serum samples were stored at −80°C until analysis.
Humoral immune response
Humoral immune reactions to the influenza vaccine were studied in serum by the HI antibody test as described previously.Citation37 Virus antibody responses were assessed at day 1 and day 22 for the 3 individual virus-strains of the vaccine (A/H3N2/Perth/16/2009, A/H1N1/California/01/2010, and B/Florida/004/2006). Seroprotection represents an antibody titer of 1:40 or higher.Citation36,37 Participants were considered fully protected if the titres to all 3 virus-strains reached the criteria for seroprotection, partially protected when having protective titres to only one or 2 virus-strains, and non-protected when titres to all 3 virus-strains were below 1:40. Post-vaccination seroresponse represents an at least fold 4- increase in antibody titer.Citation37
Cellular immune response
Cellular immune reactions were studied by T lymphocyte proliferation and cytokine secretion of PBMC collected at day 1 and 8, the presence of Treg at day 1, and the concentration of HSP90α at baseline.
Cells were simultaneously thawed. For the analysis of lymphocyte proliferation and cytokine secretion, 1.5 × 105 PBMC were added per well of a 96-wells plate in culture medium (RPMI 1640 (Cambrex Bio Science, Verviers, Belgium) supplemented with 4% human serum albumin). In the proliferation assay, PBMC were incubated with 1μg/ml PHA and a 1:10 dilution of the individual virus-strains (A/H3N2/Perth/25/11/2008, A/H1N1/California/01/2010, and B/Florida/05/11/2008). Supernatant was collected to measure cytokine production after 48 hours of culture. The Th1/Th2 11plex kit (eBioscience, San Diego, CA) was used according to the manufacturer's protocol to measure levels of IL-10 at day 1 and IFN-γ, IL-4, and IL-5 at day 8. After four days of culture, 1 µCi Citation3[H]-thymidine (MP Biomedicals, Santa Ana, California, USA) was added to each well for 8 hours of incubation to measure T lymphocyte proliferation.
Multi-color flow cytometric analyses were performed on unstimulated PBMC harvested at day 1 according to the manufacturer's protocol. Cells were stained for surface antigens anti-CD4/FITC (Beckman Coulter, Woerden, the Netherlands), anti-CD25/PE-Cy7 (BD Biosciences, Breda, the Netherlands), and anti-CD127/PE (BD Bioscience), fixed and permeabilized prior to staining with anti-FoxP3/APC (clone PCH101, eBioscience). Treg were defined as CD4+CD25+CD127-FoxP3+ cells and calculated as a percentage of all CD4+ cells. Data were analyzed using FlowJo software (Treestar).
HSP90α concentrations were detected in serum collected at baseline, using a colorimetric, immunometric enzyme immunoassay kit according to the manufacturer's protocol (Enzo Life Sciences, Antwerpen, Belgium).
Statistics
Statistical analyses were performed using PASW for Windows®, version 18.0.2 (Armonk, New York, USA) and Prism, version 4.0 (San Diego, California, USA). Independent samples t tests were performed to assess differences in numerical variables at day 1, 8, and 22. Paired t tests were used to assess changes in numerical variables from pre- to post-vaccination. Chi-square tests were performed to compare groups on categorical variables. Differences were considered statistically significant at p < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all patients for participating in this study; all physicians for referring patients; colleagues from the outpatient clinic of Medical Oncology for vaccination and blood collection; colleagues from the Department of Tumor Immunology for assistance with PBMC isolation, experiment performance, and helpful advice.
Funding
This work was supported by Pharmachild [FP7] and Paul Speth Fund.
References
- Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol 2000; 18:743-53; PMID:10673515.
- Servaes P, Verhagen C, Bleijenberg G. Fatigue in cancer patients during and after treatment: prevalence, correlates and interventions. Eur J Cancer 2002; 38:27-43; PMID:11750837; http://dx.doi.org/10.1016/S0959-8049(01)00332-X.
- Dittner AJ, Wessely SC, Brown RG. The assessment of fatigue: a practical guide for clinicians and researchers. J Psychosom Res 2004; 56:157-70; PMID:15016573; http://dx.doi.org/10.1016/S0022-3999(03)00371-4.
- Vercoulen JH, Swanink CM, Fennis JF, Galama JM, van der Meer JW, Bleijenberg G. Dimensional assessment of chronic fatigue syndrome. J Psychosom Res 1994; 38:383-92; PMID:7965927; http://dx.doi.org/10.1016/0022-3999(94)90099-X.
- Irvine D, Vincent L, Graydon JE, Bubela N, Thompson L. The prevalence and correlates of fatigue in patients receiving treatment with chemotherapy and radiotherapy. A comparison with the fatigue experienced by healthy individuals. Cancer Nurs 1994; 17:367-78; PMID:7954384; http://dx.doi.org/10.1097/00002820-199410000-00001.
- Smets EM, Garssen B, Schuster-Uitterhoeve AL, de Haes JC. Fatigue in cancer patients. Br J Cancer 1993; 68:220-4; PMID:8347475; http://dx.doi.org/10.1038/bjc.1993.319.
- Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer 2006; 106:751-8; PMID:16400678; http://dx.doi.org/10.1002/cncr.21671.
- Hjermstad MJ, Fossa SD, Oldervoll L, Holte H, Jacobsen AB, Loge JH. Fatigue in long-term Hodgkin's Disease survivors: a follow-up study. J Clin Oncol 2005; 23:6587-95; PMID:16170166; http://dx.doi.org/10.1200/JCO.2005.09.936.
- Nieboer P, Buijs C, Rodenhuis S, Seynaeve C, Beex LV, van der Wall E, Richel DJ, Nooij MA, Voest EE, Hupperets P, et al. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol 2005; 23:8296-304; PMID:16219926; http://dx.doi.org/10.1200/JCO.2005.10.167.
- Servaes P, Gielissen MF, Verhagen S, Bleijenberg G. The course of severe fatigue in disease-free breast cancer patients: a longitudinal study. Psychooncology 2007; 16:787-95; PMID:17086555; http://dx.doi.org/10.1002/pon.1120.
- Servaes P, Verhagen S, Schreuder HW, Veth RP, Bleijenberg G. Fatigue after treatment for malignant and benign bone and soft tissue tumors. J Pain Symptom Manage 2003; 26:1113-22; PMID:14654263; http://dx.doi.org/10.1016/j.jpainsymman.2003.03.001.
- Bartsch HH, Weis J, Moser MT. Cancer-related fatigue in patients attending oncological rehabilitation programs: prevalence, patterns and predictors. Onkologie 2003; 26:51-7; PMID:12624518; http://dx.doi.org/10.1159/000069864.
- Dimeo F, Schmittel A, Fietz T, Schwartz S, Kohler P, Boning D, Thiel E. Physical performance, depression, immune status and fatigue in patients with hematological malignancies after treatment. Ann Oncol 2004; 15:1237-42; PMID:15277264; http://dx.doi.org/10.1093/annonc/mdh314.
- Gielissen MF, Schattenberg AV, Verhagen CA, Rinkes MJ, Bremmers ME, Bleijenberg G. Experience of severe fatigue in long-term survivors of stem cell transplantation. Bone Marrow Transplant 2007; 39:595-603; PMID:17369868; http://dx.doi.org/10.1038/sj.bmt.1705624.
- Okuyama T, Akechi T, Kugaya A, Okamura H, Shima Y, Maruguchi M, Hosaka T, Uchitomi Y. Development and validation of the cancer fatigue scale: a brief, three-dimensional, self-rating scale for assessment of fatigue in cancer patients. J Pain Symptom Manage 2000; 19:5-14; PMID:10687321; http://dx.doi.org/10.1016/S0885-3924(99)00138-4.
- Servaes P, van der Werf S, Prins J, Verhagen S, Bleijenberg G. Fatigue in disease-free cancer patients compared with fatigue in patients with chronic fatigue syndrome. Support Care Cancer 2001; 9:11-7; PMID:11147137; http://dx.doi.org/10.1007/s005200000165.
- Woo B, Dibble SL, Piper BF, Keating SB, Weiss MC. Differences in fatigue by treatment methods in women with breast cancer. Oncol Nurs Forum 1998; 25:915-20; PMID:9644708.
- Gielissen MF, Verhagen S, Witjes F, Bleijenberg G. Effects of cognitive behavior therapy in severely fatigued disease-free cancer patients compared with patients waiting for cognitive behavior therapy: a randomized controlled trial. J Clin Oncol 2006; 24:4882-7; PMID:17050873; http://dx.doi.org/10.1200/JCO.2006.06.8270.
- Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist 2007; 12 Suppl 1:22-34; PMID:17573453; http://dx.doi.org/10.1634/theoncologist.12-S1-22.
- Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun 2007; 21:413-27; PMID:17178209; http://dx.doi.org/10.1016/j.bbi.2006.11.004.
- Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med 2002; 64:604-11; PMID:12140350; http://dx.doi.org/10.1097/00006842-200207000-00010.
- Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav Immun 2003; 17 Suppl 1:S112-8; PMID:12615196; http://dx.doi.org/10.1016/S0889-1591(02)00077-6.
- Gaab J, Rohleder N, Heitz V, Engert V, Schad T, Schurmeyer TH, Ehlert U. Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology 2005; 30:188-98; PMID:15471616; http://dx.doi.org/10.1016/j.psyneuen.2004.06.008.
- Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, Pearce BD, Landry J, Glover S, McDaniel JS, et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry 2001; 158:1252-7; PMID:11481159; http://dx.doi.org/10.1176/appi.ajp.158.8.1252.
- Prinsen H, de Vries IJ, Torensma R, Pots JM, Mulder SF, van Herpen CM, Elving LD, Bleijenberg G, Stelma FF, van Laarhoven HW. Humoral and cellular immune responses after influenza vaccination in patients with chronic fatigue syndrome. BMC immunol 2012; 13:71; PMID:23244635; http://dx.doi.org/10.1186/1471-2172-13-71.
- Taga K, Tosato G. IL-10 inhibits human T cell proliferation and IL-2 production. J Immunol 1992; 148:1143-8; PMID:1737931.
- Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med 2001; 193:1295-302; PMID:11390436; http://dx.doi.org/10.1084/jem.193.11.1295.
- Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol 2011; 23:598-604; PMID:21889323; http://dx.doi.org/10.1016/j.coi.2011.08.003.
- Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 2012; 12:180-90; PMID:22343569.
- Pukhal'skii AL, Shmarina GV, Aleshkin VA. [Regulatory T-cells: modern approaches to optimization of their numbers]. Vestn Ross Akad Med Nauk 2011:24-33; PMID:21950132.
- Csermely P, Schnaider T, Soti C, Prohaszka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther 1998; 79:129-68; PMID:9749880; http://dx.doi.org/10.1016/S0163-7258(98)00013-8.
- de Zoeten EF, Wang L, Butler K, Beier UH, Akimova T, Sai H, Bradner JE, Mazitschek R, Kozikowski AP, Matthias P, et al. Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3(+) T-regulatory cells. Mol Cell Biol 2011; 31:2066-78; PMID:21444725; http://dx.doi.org/10.1128/MCB.05155-11.
- Mulder SF, Jacobs JF, Olde Nordkamp MA, Galama JM, Desar IM, Torensma R, Teerenstra S, Mulders PF, Vissers KC, Punt CJ, et al. Cancer patients treated with sunitinib or sorafenib have sufficient antibody and cellular immune responses to warrant influenza vaccination. Clin Cancer Res 2011; 17:4541-9; PMID:21712444; http://dx.doi.org/10.1158/1078-0432.CCR-11-0253.
- Gielissen MF, Verhagen CA, Bleijenberg G. Cognitive behaviour therapy for fatigued cancer survivors: long-term follow-up. Br J Cancer 2007; 97:612-8; PMID:17653075; http://dx.doi.org/10.1038/sj.bjc.6603899.
- Servaes P, Verhagen S, Bleijenberg G. Determinants of chronic fatigue in disease-free breast cancer patients: a cross-sectional study. Ann Oncol 2002; 13:589-98; PMID:12056710; http://dx.doi.org/10.1093/annonc/mdf082.
- de Jong JC, Palache AM, Beyer WE, Rimmelzwaan GF, Boon AC, Osterhaus AD. Haemagglutination-inhibiting antibody to influenza virus. Dev Biol (Basel) 2003; 115:63-73; PMID:15088777.
- Pollyea DA, Brown JM, Horning SJ. Utility of influenza vaccination for oncology patients. J Clin Oncol 2010; 28:2481-90; PMID:20385981; http://dx.doi.org/10.1200/JCO.2009.26.6908.
