Abstract
A new tuberculosis vaccine is needed to replace or enhance BCG, which induces variable protection against Mycobacterium tuberculosis pulmonary infections in adults. Development of new TB vaccine candidates is severely hampered by the lack of a correlate of immunity, unproven animal models, and limited funding opportunities. One candidate, MVA85A, recently failed to meet its efficacy endpoint goals despite promising early-phase trial data. As a result, some in the field believe we should now shift our focus away from product development and toward a research-oriented approach. Here, we outline our suggestions for this research-oriented strategy including diversification of the candidate pipeline, expanding measurements of immunity, improving pre-clinical animal models, and investing in combination pre-clinical/experimental medicine studies. As with any evolution, this change in strategy comes at a cost but may also represent an opportunity for advancing the field.
Introduction
Tuberculosis (TB) has long been a scourge to public health. In the early 20th century, Albert Calmette and Camille Guérin attenuated a strain of Mycobacterium bovis for use in preventing the spread of Mycobacterium tuberculosis (Mtb). The resulting vaccine, Bacillus Calmette-Guérin (BCG), was first used in human vaccinations in 1921.Citation1 Since that time, BCG has become the most widely used vaccine in the world.Citation2 Throughout its use, BCG has a demonstrated ability to reduce the risk of severe pediatric TB disease, specifically miliary TB and TB meningitis.Citation3,4 However, despite its widespread adoption, BCG has had little impact on the global TB epidemic. A number of studies have shown that BCG is variable in eliciting protection from pulmonary TB infection in different populations.Citation5-7 Further, BCG is not known to protect against latent TB and is not recommended for immunocompromised patients.Citation8,9
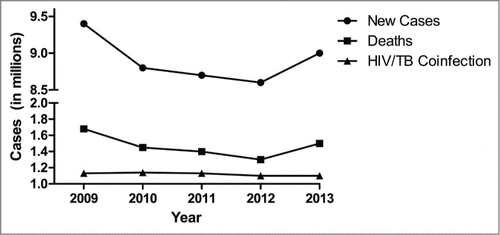
The antibiotics isoniazid and rifampicin were introduced for fighting tuberculosis infection in the 1950′s. Mtb infections and TB disease incidence waned in developed countries where the antibiotics were available,Citation10 and development of a superior TB vaccine was neglected. The global incidence of TB disease took a turn for the worse in the late 1980s and 1990s, mainly due to the spread of HIV infection and resultant immunosuppression of individuals with latent Mtb infection (LTBI), an effect particularly seen in developing countries with high incidences of both HIV and Mtb infection, such as South Africa. The incidence of multiple drug resistant (MDR) strains of TB also began to rise.Citation11,12 As global travel increased, TB infections began to rebound and the World Health Organization declared tuberculosis a global public health emergency in 2005 as a result.Citation13,14 In 2013, 9 million new cases of tuberculosis were reported, and 1.5 million deaths were attributed to TB.Citation15 Both of these numbers represent increases compared to recent years ().Citation15-19 Over 2 billion people throughout the world are infected with Mtb.Citation20 Extremely drug resistant (XDR) tuberculosis has now been diagnosed in 100 countries,Citation15 and incidences of totally drug resistant (TDR) tuberculosis have been reported in India, Iran, and Italy.Citation11,12,21-23
Figure 1. Reported new cases of tuberculosis, deaths attributed to tuberculosis, and HIV/TB coinfection cases by year. Sourced from Global Tuberculosis Reports 2010–2014 by World Health Organization.
Despite numerous advances in science and technology since the introduction of BCG, TB vaccine development faces a number of significant challenges. Perhaps the biggest challenge facing TB vaccine research is the lack of an immune correlate of protection. This correlate of protection represents the composition and magnitude of immune response required for protection from tuberculosis disease. Without a correlate of protection, researchers must rely on the outcomes of large scale clinical studies to demonstrate efficacy. These studies require a large sample size to show statistical significance, which means these studies carry both a high level of expense and a high level of risk. Alternatively, a correlate of protection could be a signal observed in an animal model that is predictive of vaccine-induced protection in humans. However, animal models in TB vaccine research are varied and unproven in that there is yet to be an established link between a vaccine effect observed in animal models and human protection. With these factors combined, the lack of data regarding a correlate of protection or other measures of vaccine efficacy makes it difficult and risky to manage a global pipeline of vaccine candidates.
Despite the lack of a correlate of immunity, there is extensive pre-clinical and clinical evidence to suggest that both CD4+ and CD8+ T cells, along with the Th1 cytokines IFN-γ and TNF, are vital for protection from tuberculosis.Citation24-35 Armed with this knowledge, researchers set out to craft a new generation of TB vaccine candidates. The majority of these new candidates are positioned as a heterologous boost following a BCG prime administered during infancy or young adulthood. Citation7,36 These candidates also are generally focused on promoting strong T-cell immunogenicity. Several of these candidates have transitioned to clinical trials, reporting excellent safety profiles and shown to boost T-cell responses.Citation36
One of the first candidates advanced to a proof-of-concept clinical study is known as MVA85A. Developed as a collaboration between the Oxford Emergent Tuberculosis Consortium and Aeras, the MVA85A candidate is a modified vaccinia Ankara virus encoding the Mtb antigen 85A.Citation37 In preclinical and early phase clinical studies in adults, MVA85A was shown to induce strong CD4+ T cell responses, characterized by IFN-γ and TNF secretion,Citation38,39 and modest protection was observed in animal models.Citation40 MVA85A advanced to a Phase IIb clinical efficacy study, the first efficacy study in infants for tuberculosis since the 1960′s. Nearly 2,800 infants were enrolled into the study, yet the vaccine did not enhance efficacy compared to placebo and immunogenicity was generally poor.Citation41 A second phase II trial for MVA85A was recently completed.Citation42 This trial enrolled 650 HIV+ adults, and was again well-tolerated. The vaccine also elicited antigen 85A-specific T-cell responses. Similar to the infant trial, however, no efficacy against Mtb infection or disease was detected in this trial.
Unfortunately, the historic MVA85A efficacy trials may have provided researchers with more questions than answers. Was the low observed immunogenicity due to a flaw with the candidate, or did changing the patient population from healthy adults to infants or HIV+ adults have the most dramatic effect? Would efficacy have been improved if the magnitude of immune response was higher, or are T-cell responses alone insufficient for immunity to Mtb? Is a vaccine comprised of a single antigen sufficient for inducing protective immunity to an organism as large as Mtb?
Shifting development strategy
Although development of TB vaccine candidates has continued, progress has slowed while researchers consider the impact of the MVA85A studies and develop strategies to address the challenges facing the field. An emerging strategy has been described as a “shift to the left” (STTL). The STTL strategy changes the focus from candidate translational development to an emphasis on preclinical and experimental medicine studies in an effort to elucidate answers critical to moving the field forward. We suggest there are 4 critical points to consider for this STTL strategy: diversification of the candidate pipeline, expanding measurements of immunity, improving animal models, and further investment into preclinical/experimental medicine studies.
Diversification of the candidate pipeline
A recent survey of the current TB vaccine candidates in clinical trials shows 13 total candidates.Citation7,43 Eight of those candidates, representing over 60% of the portfolio, rely on traditional vaccine platforms like protein/adjuvant or viral vectors like MVA or adenovirus for delivery of protein antigens. Furthermore, 6 of those 8 candidates include antigen 85. Only a handful of candidates under clinical investigation use alternative strategies such as attenuated Mtb, recombinant BCG, or whole cell mycobacterial extracts, which may contain lipid, metabolite, and glycoprotein antigens that may be critical for anti-Mtb immunity.
The current portfolio of vaccine candidates in clinical trials highlights a weakness that needs to be addressed in the STTL strategy, but will take time. Heeding evidence generated from previous studies supporting cellular immunity as a key for combating Mtb infection, the majority of candidates stimulate either CD4+ Th1 or CD8+ responses.Citation7 A major problem with the the cellular immunity requirement dogma, however, is that development of vaccines driving other response types has been limited, thus constraining the diversity of candidates in the current clinical pipeline. The similarity in vaccine platforms, antigen cassettes, and induced immunogenicity duplicates effort in the current pipeline without increasing our understanding of alternative and potentially protective immune responses. Further, in an environment with limited funding opportunities, the development of many similar candidates may limit investigation into other platforms and antigens, especially when down-selection is hindered by the lack of a correlate of protection.
Moving forward, the shift in development strategy will focus on testing immune paradigms. A candidate should be able to stand as the best available candidate to stimulate a certain type of immune response, or otherwise demonstrate novelty in its mechanism of action. There are several paradigms of interest that expand the scope of vaccines well beyond traditional cellular immunity. These paradigms include: T-cell responses beyond Th1, antibody immunity, NK cell immunity, innate/adaptive immune system interactions, alternative antigen approaches such as lipids and metabolites, and immune compartmentalization. While investigation into the paradigm itself may provide new information regarding immunity to Mtb, the paradigm will need to be further evaluated for the relevant clinical effect, in this case protection from infection or disease.
Although studies have shown CD4+ Th1 and CD8+ cellular immune responses are significant for fighting Mtb infection, these responses may be insufficient on their own for protection. Recent studies have implicated possible roles for Th17Citation44-46 and Th22Citation47 CD4+ cells in Mtb immunity, as have non-traditional T cells like MAITCitation48,49 and γδ T cells.Citation50 Currently, none of the candidates in the clinical portfolio have been designed to drive those specific responses, and those responses have not been a focus of immunogenicity evaluations or relevant biological effect. The DNA vaccine platform, which is currently not represented in the clinical TB vaccine candidate portfolio, is an example of an emerging technology that could diversify the available candidates. Through the inclusion of molecularly-encoded adjuvants, DNA vaccines may be able to direct immune responses in a particular direction of interest.Citation51
Given the wealth of evidence supporting the role of cellular immunity response to Mtb infection, antibody-based immunity has been largely abandoned in terms of vaccine candidate design. Antibodies, however, may have a number of potential roles in the response to Mtb infection. These roles may include opsonization for antibody-dependent cell-mediated cytotoxicity (ADCC), modulation of the T-cell response, or neutralization of mycobacteria for limiting spread of infection.Citation52 The utility of antibodies is a source of skepticism within the field,Citation53 but remains a paradigm worth investigating in the preclinical and clinical arenas.
Natural killer (NK) cells are another cell type that has been implicated in tuberculosis immunity.Citation54 Though initially thought of as a member of the innate immune system, NK cells have been shown to have memory-like properties. Stimulation of NK cell responses represents a potential avenue for anti-Mtb immunity, but is thus far not represented in the clinical candidate portfolio.
In a related paradigm, evidence is emerging of a greater connection between the adaptive and innate immune systems than previously was understood. Specifically, it appears that the adaptive immune system has a capacity for modulating innate responses, including interactions between innate cells and antibodies, chemokines, and cytokines.Citation55 IL-17, for example, directs inflammation and a greater innate response to infection.Citation56 Although data is still emerging, it appears more and more as though the immune system is an interrelated ecosystem reliant on and affected by multiple interactions rather than a simple branching system.
Another paradigm worth investigating is non-traditional antigens. The current crop of candidates relies heavily on protein-based antigen presentation for immune responses, although whole cell mycobacteria vaccine platforms may allow for recognition of alternate antigens. Roles for lipidsCitation57 and mycobacterial metabolitesCitation48 in triggering anti-Mtb responses have been demonstrated. More work is needed to understand the role of lipids and metabolites in Mtb immunity and their potential as vaccine targets.
Immune compartmentalization is a vaccine strategy that has been under investigation for some time. Essentially, the strategy is founded on the assumption that lung-resident immune memory may be beneficial for combating lung infections such as Mtb. This approach utilizes lung vaccination, such as via aerosol, to harness immune imprinting for targeting cells back to the lungs rather than a typical intramuscular vaccine that relies on circulating lymphocytes migrating to the site of infection. Safety remains a concern, as pulmonary pathology due to vaccine response is seen as a major risk. This strategy has been examined in pre-clinical studiesCitation58,59 and tested in recent clinical trials.Citation60
These paradigms are underrepresented or absent in the current clinical candidate pipeline. Further investigation of these paradigms is warranted, but all will require investment of funds, time, and effort.
Expanding measurements of immunity
Mirroring the candidate pipeline focus on driving Th1 responses, the majority of assays for assessing clinical trial specimens also look almost exclusively at Th1 responses. Although the focus for a desired Th1 response is based on a solid foundation, other immune responses may be necessary for vaccine efficacy. Given how little is understood about the human immune system and the immune response to Mtb infections, a greater emphasis should be placed on measuring responses beyond Th1. Already, assays are being redesigned to incorporate a broader scope, examining Th17 and Th22 responses as well as the role of T cell memory.Citation61 This, however, is only the beginning of the assay development that is necessary to address the paradigms outlined above. While some small scale experiments have proven concepts, assay scale-up and implementation will be critical in order to be cost-effective for evaluating clinical trial specimens. Assays will need to be improved for new cell types, adapted for ex vivo stimulation using lipid and metabolite antigens, and examine tissues beyond peripheral mononuclear blood cells (PBMCs).
One current challenge for measurements of immunity comes from whole-cell vaccine candidates. These candidates typically comprise of the entirety of cellular components from BCG or other mycobacteria. For vaccines of this nature, it is critical to design an assay that differentiates immunogenicity driven by BCG and immunogenicity driven by the vaccine candidate. Answering this challenge will be imperative for the assessment and development of whole-cell vaccine candidates.
Perhaps the best method to broaden our understanding of immunogenicity following vaccination is to examine gene expression through RNA sequencing technology. Samples have already been collected for RNA sequencing in a number of TB vaccine trials, but only a handful of results have been published.Citation62,63 RNA sequencing is a critical method for understanding tuberculosis infection, but the expense of sequencing and interpreting conclusions from the volume of data generated are challenges that must be surmounted.
Improving animal models
Animal models for tuberculosis research and translational development need improvement. The models employed typically do not mimic the course of infection observed in humans. For example, the widely available C57BL/6 and BALB/c mouse strains do not exhibit granuloma formation following infection as typically observed in humans.Citation64 Animal challenge studies also typically employ laboratory strains of Mtb that are quite genetically dissimilar to clinical isolates like the virulent Beijing strain.Citation65 Ten to 20 times more bacteria are being used for challenge than what is thought to cause infections in humans, raising further questions about the ability of current animal challenge experiments to identify vaccine candidates likely to have efficacy in humans.Citation66 Genetic disparities are also a concern, such as the absence of most CD1 subtypes in mice as well as the distribution of Toll-like receptors, and create further uncertainty on how the mouse model may correlate to human infection. In some regards (like granuloma formation), NHPs more closely mimic human infection. NHP models, however, are expensive to use for immunogenicity and challenge studies and also face limitations on facilities, space, and expertise. Additionally, few models have been adopted to mimic latent infection in either mice or NHPs.Citation67,68
One role for an animal challenge model is to predict vaccine efficacy. Clearly, broader efforts are needed to address the deficiencies in the animal models to increase their predictive capabilities. Work is ongoing for the creation of a natural transmission model that may address some of the concerns regarding animal challenge. With this model, animals infected by aerosol would share an air chamber with non-infected animals. The infection would spread at a slower rate, but in a manner more reflective of natural transmission. Though this natural transmission model would be a significant step forward, the model is complicated by increased costs and logistics for housing the greater number of animals in BSL-3 conditions.
Another need is to corroborate animal models against each other or, preferably, to human data through experimental medicine studies (see below). It remains unclear how a protective result in the mouse model relates to the NHP model, and whether or not the induced immunogenicity in mouse correlates to NHPs or humans in terms of composition and magnitude. However, studies in this vein will be necessary, as the NHP model is too expensive to sustain large-scale pre-clinical portfolio management and the mouse model currently has too many uncertainties.
New tools will assist the advancement of animal model research. The introduction of new monitoring tools like PET/CT scans for NHP studiesCitation67 and the IVIS mouseCitation69 allow new opportunities for in vivo monitoring of disease progression. However, despite the potential usefulness of these tools, they remain expensive and are not yet widely available for multi-center studies.
Investing in pre-clinical/experimental medicine studies
As mentioned above, one way to address diversification of the global portfolio is to invest further in preclinical development and experimental medicine studies. This type of investment will be necessary for proving the concept behind a new vaccine candidate. Ideally, a promising vaccine candidate will be tested in an NHP study, examining both immunogenicity and protection. In parallel, the candidate will be tested in a small-scale human experimental medicine study. This study will primarily examine the immune response type and magnitude generated in humans, and allow for comparison back to the NHP model. If the response types match and the NHP model demonstrated protection, there is a better indication of success in a larger-scale clinical trial. Of course, this approach assumes that human and NHP responses to Mtb infection are similar, and that the assays used in the studies are reliable. One major difference from the current product development strategy is that early phase clinical trials typically emphasize safety over immunogenicity with a focus on further clinical development. With the experimental medicine strategy, immunogenicity will play a larger role in determining the advancement of a vaccine candidate. Using the experimental medicine studies to confirm findings in pre-clinical NHP studies will be a central aspect of the change in development strategy. Further clinical studies will then be necessary to verify human protection.
Another opportunity is the creation of a safe human challenge model.Citation70 A human challenge model has been helpful for the advancements being made in the field of malaria vaccines.Citation71-73 Work is underway to create a human challenge model in tuberculosis using BCG,Citation74 yet much work remains to corroborate this model to show how it relates to typical pulmonary infection.
An opportunity worth seizing
The shift in strategy will be costly in terms of both money and time lost for candidate development, but will perhaps lead to more rational selection of candidates for advancement. However, current opportunities exist for the field to respond in an agile manner that will allow new ideas to emerge and be put to the test quickly. The emergence of electroporated DNA vaccines and similar technologies offer such an opportunity. Recent clinical trials for diseases such as HIV and cancer have demonstrated electroporated DNA (EP-DNA) vaccines to be safe, well-tolerated, and highly immunogenic.Citation51,75-77 This research has also indicated that EP-DNA vaccines are capable of inducing broad T-cell responses (both CD4+ and CD8+) as well as antibody responses. The EP-DNA platform also demonstrates versatility in immune response modification not available with most other platforms. The ability to include molecularly-encoded cytokine adjuvants like IL-12, IL-15, and IL-23 offer the possibility to directly modulate the immune response in ways that traditional platforms cannot.Citation51,76,78,79 EP-DNA has also been shown the capability to encode entire immunoglobulin chains.Citation80 Newer technology like Vaccibody opens up possibilities for linking a DNA-encoded antigen cassette to homing molecules that could drive tissue-resident responses.Citation81
DNA vaccine candidates also have a clear path forward to the clinic. Already, 4 DNA vaccine products are licensed for veterinary use,Citation51 and human DNA vaccine trials are underway or completed for HIV,Citation76 HPV,Citation75 influenza,Citation82 a variety of cancers,Citation83-85 and more. In fact, one promising HPV vaccine candidate met its efficacy endpoint goal for preventing HPV-induced cervical cancer.Citation86 Advancing a similarly designed TB vaccine candidate should follow a similar development pathway into the clinic, bypassing many of the regulatory issues that might slow or inhibit clinical development of other novel platforms that have yet to reach the clinic.
The shift in development strategy is also an opportunity for the TB vaccine field to re-evaluate and broaden collaborations. The shift in strategy still requires a foundation of sound science and good decision-making. It will be imperative that collaborators agree on important development goals, and be judicious with available funding. Moving forward, there is an opportunity for greater collaboration with the immunology field as a whole, focusing on utilizing broader expertise in the field to answer specific questions relating to TB. Novel immune mechanisms from the broader immunology field may deepen our understanding of Mtb infection, and in turn lead to new vaccine targets. Similarly, collaborative research into tuberculosis may reveal new discoveries about immune system functions and interactions.
In summation, the TB vaccine field is in a state of transition. While previous portfolio management relied heavily on a strategy of product development, breakthrough discoveries and promising candidates are few. Adoption of a strategy that is more focused on answering core questions and testing new paradigms will be essential for advancement of the field as a whole. While significant time, financial investment, and process development will be necessary for this transition, it is also an opportunity to survey the landscape, forge new collaborations, and propel the field forward.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Oettinger T, Jorgensen M, Ladefoged A, Haslov K, Andersen P. Development of the Mycobacterium bovis BCG vaccine: review of the historical and biochemical evidence for a genealogical tree. Tuber Lung Dis 1999; 79:243-50; PMID:10692993; http://dx.doi.org/10.1054/tuld.1999.0206
- Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D, Pai M. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med 2011; 8:e1001012; PMID:21445325; http://dx.doi.org/10.1371/journal.pmed.1001012
- Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol 1993; 22:1154-8; PMID:8144299; http://dx.doi.org/10.1093/ije/22.6.1154
- Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 2006; 367:1173-80; PMID:16616560; http://dx.doi.org/10.1016/S0140-6736(06)68507-3
- Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat Rev Microbiol 2005; 3:656-62; PMID:16012514; http://dx.doi.org/10.1038/nrmicro1211
- Brandt L, Feino Cunha J, Weinreich Olsen A, Chilima B, Hirsch P, Appelberg R, Andersen P. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect Immun 2002; 70:672-8; PMID:11796598; http://dx.doi.org/10.1128/IAI.70.2.672-678.2002
- Hokey DA, Ginsberg A. The current state of tuberculosis vaccines. Human vaccines & immunotherapeutics 2013; 9:2142-6; PMID:23792698; http://dx.doi.org/10.4161/hv.25427
- Moss WJ, Clements CJ, Halsey NA. Immunization of children at risk of infection with human immunodeficiency virus. Bull World Health Organ 2003; 81:61-70; PMID:12640478
- Talbot EA, Perkins MD, Silva SF, Frothingham R. Disseminated bacille Calmette-Guerin disease after vaccination: case report and review. Clin Infect Dis 1997; 24:1139-46; PMID:9195072; http://dx.doi.org/10.1086/513642
- Malin AS, McAdam KP. Escalating threat from tuberculosis: the third epidemic. Thorax 1995; 50 Suppl 1:S37-42; http://dx.doi.org/10.1136/thx.50.Suppl_1.S37
- Falzon D, Jaramillo E, Schunemann HJ, Arentz M, Bauer M, Bayona J, Blanc L, Caminero JA, Daley CL, Duncombe C, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J 2011; 38:516-28; http://dx.doi.org/10.1183/09031936.00073611
- Zignol M, van Gemert W, Falzon D, Sismanidis C, Glaziou P, Floyd K, Raviglione M. Surveillance of anti-tuberculosis drug resistance in the world: an updated analysis, 2007–2010. Bull World Health Organ 2012; 90:111-9D; PMID:22423162; http://dx.doi.org/10.2471/BLT.11.092585
- WHO declares TB an emergency in Africa. Indian J Med Sci 2005; 59:367-8; PMID:16231441
- Global. WHO declares emergency against AIDS, TB, malaria. AIDS Policy Law 2006; 21:5
- Global Tuberculosis Report 2014. Geneva, Switzerland: World Health Organization, 2014.
- Global Tuberculosis Report 2010. Geneva, Switzerland: World Health Organization, 2010.
- Global Tuberculosis Report 2011. Geneva, Switzerland: World Health Organization, 2011.
- Global Tuberculosis Report 2012. Geneva, Switzerland: World Health Organization, 2012.
- Global Tuberculosis Report 2013. Geneva, Switzerland: World Health Organization, 2013.
- Tufariello JM, Chan J, Flynn JL. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect Dis 2003; 3:578-90; PMID:12954564; http://dx.doi.org/10.1016/S1473-3099(03)00741-2
- Udwadia ZF, Amale RA, Ajbani KK, Rodrigues C. Totally drug-resistant tuberculosis in India. Clin Infect Dis 2012; 54:579-81; PMID:22190562; http://dx.doi.org/10.1093/cid/cir889
- Velayati AA, Farnia P, Masjedi MR, Ibrahim TA, Tabarsi P, Haroun RZ, Kuan HO, Ghanavi J, Varahram M. Totally drug-resistant tuberculosis strains: evidence of adaptation at the cellular level. Eur Respir J 2009; 34:1202-3; http://dx.doi.org/10.1183/09031936.00081909
- Velayati AA, Masjedi MR, Farnia P, Tabarsi P, Ghanavi J, Ziazarifi AH, Hoffner SE. Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in iran. Chest 2009; 136:420-5; PMID:19349380; http://dx.doi.org/10.1378/chest.08-2427
- Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Doffinger R, Bernaudin F, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 1998; 280:1432-5; PMID:9603732; http://dx.doi.org/10.1126/science.280.5368.1432
- Chaisson RE, Martinson NA. Tuberculosis in Africa–combating an HIV-driven crisis. N Engl J Med 2008; 358:1089-92; PMID:18337598; http://dx.doi.org/10.1056/NEJMp0800809
- Chen CY, Huang D, Wang RC, Shen L, Zeng G, Yao S, Shen Y, Halliday L, Fortman J, McAllister M, et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS pathogens 2009; 5:e1000392; PMID:19381260; http://dx.doi.org/10.1371/journal.ppat.1000392
- de Jong R, Altare F, Haagen IA, Elferink DG, Boer T, van Breda Vriesman PJ, Kabel PJ, Draaisma JM, van Dissel JT, Kroon FP, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 1998; 280:1435-8; PMID:9603733; http://dx.doi.org/10.1126/science.280.5368.1435
- Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A 1992; 89:12013-7; PMID:1465432; http://dx.doi.org/10.1073/pnas.89.24.12013
- Jouanguy E, Doffinger R, Dupuis S, Pallier A, Altare F, Casanova JL. IL-12 and IFN-gamma in host defense against mycobacteria and salmonella in mice and men. Curr Opin Immunol 1999; 11:346-51; PMID:10375558; http://dx.doi.org/10.1016/S0952-7915(99)80055-7
- Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 2001; 345:1098-104; PMID:11596589; http://dx.doi.org/10.1056/NEJMoa011110
- Lammas DA, Casanova JL, Kumararatne DS. Clinical consequences of defects in the IL-12-dependent interferon-gamma (IFN-gamma) pathway. Clin Exp Immunol 2000; 121:417-25; PMID:10971505; http://dx.doi.org/10.1046/j.1365-2249.2000.01284.x
- Nunez Martinez O, Ripoll Noiseux C, Carneros Martin JA, Gonzalez Lara V, Gregorio Maranon HG. Reactivation tuberculosis in a patient with anti-TNF-alpha treatment. Am J Gastroenterol 2001; 96:1665-6; PMID:11374737
- Pacheco AG, Durovni B, Cavalcante SC, Lauria LM, Moore RD, Moulton LH, Chaisson RE, Golub JE. AIDS-related tuberculosis in Rio de Janeiro, Brazil. PLoS One 2008; 3:e3132; PMID:18781195; http://dx.doi.org/10.1371/journal.pone.0003132
- Pawlowski A, Jansson M, Skold M, Rottenberg ME, Kallenius G. Tuberculosis and HIV co-infection. PLoS pathogens 2012; 8:e1002464; PMID:22363214; http://dx.doi.org/10.1371/journal.ppat.1002464
- Wangoo A, Sparer T, Brown IN, Snewin VA, Janssen R, Thole J, Cook HT, Shaw RJ, Young DB. Contribution of Th1 and Th2 cells to protection and pathology in experimental models of granulomatous lung disease. J Immunol 2001; 166:3432-9; PMID:11207301; http://dx.doi.org/10.4049/jimmunol.166.5.3432
- Graves A, Hokey D. Tuberculosis vaccines: review of current development trends and future challenges. J Bioterr Biodef 2011:009
- McShane H, Pathan AA, Sander CR, Goonetilleke NP, Fletcher HA, Hill AV. Boosting BCG with MVA85A: the first candidate subunit vaccine for tuberculosis in clinical trials. Tuberculosis (Edinburgh, Scotland) 2005; 85:47-52; PMID:15687027; http://dx.doi.org/10.1016/j.tube.2004.09.015
- Beveridge NE, Price DA, Casazza JP, Pathan AA, Sander CR, Asher TE, Ambrozak DR, Precopio ML, Scheinberg P, Alder NC, et al. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur J Immunol 2007; 37:3089-100; PMID:17948267; http://dx.doi.org/10.1002/eji.200737504
- Hawkridge T, Scriba TJ, Gelderbloem S, Smit E, Tameris M, Moyo S, Lang T, Veldsman A, Hatherill M, Merwe L, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J Infect Dis 2008; 198:544-52; PMID:18582195; http://dx.doi.org/10.1086/590185
- Vordermeier HM, Rhodes SG, Dean G, Goonetilleke N, Huygen K, Hill AV, Hewinson RG, Gilbert SC. Cellular immune responses induced in cattle by heterologous prime-boost vaccination using recombinant viruses and bacille Calmette-Guerin. Immunology 2004; 112:461-70; PMID:15196215; http://dx.doi.org/10.1111/j.1365-2567.2004.01903.x
- Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 2013; 381:1021-8; PMID:23391465; http://dx.doi.org/10.1016/S0140-6736(13)60177-4
- Ndiaye BP, Thienemann F, Ota M, Landry BS, Camara M, Dieye S, Dieye TN, Esmail H, Goliath R, Huygen K, et al. Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV-1: a randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2015; 3(3):190-200; PMID:25726088
- ClinicalTrials.gov. Phase I Trial of DAR-901. National Institutes of Health, 2015.
- da Costa AC, Costa-Junior Ade O, de Oliveira FM, Nogueira SV, Rosa JD, Resende DP, Kipnis A, Junqueira-Kipnis AP. A new recombinant BCG vaccine induces specific Th17 and Th1 effector cells with higher protective efficacy against tuberculosis. PLoS One 2014; 9:e112848; PMID:25398087; http://dx.doi.org/10.1371/journal.pone.0112848
- Doz E, Lombard R, Carreras F, Buzoni-Gatel D, Winter N. Mycobacteria-infected dendritic cells attract neutrophils that produce IL-10 and specifically shut down Th17 CD4 T cells through their IL-10 receptor. J Immunol 2013; 191:3818-26; PMID:23997221; http://dx.doi.org/10.4049/jimmunol.1300527
- Verwaerde C, Debrie AS, Dombu C, Legrand D, Raze D, Lecher S, Betbeder D, Locht C. HBHA vaccination may require both Th1 and Th17 immune responses to protect mice against tuberculosis. Vaccine 2014; 32:6240-50; PMID:25252198; http://dx.doi.org/10.1016/j.vaccine.2014.09.024
- Dhiman R, Venkatasubramanian S, Paidipally P, Barnes PF, Tvinnereim A, Vankayalapati R. Interleukin 22 inhibits intracellular growth of Mycobacterium tuberculosis by enhancing calgranulin A expression. J Infect Dis 2014; 209:578-87; PMID:24041785; http://dx.doi.org/10.1093/infdis/jit495
- Gold MC, Napier RJ, Lewinsohn DM. MR1-restricted mucosal associated invariant T (MAIT) cells in the immune response to Mycobacterium tuberculosis. Immunol Rev 2015; 264:154-66; PMID:25703558; http://dx.doi.org/10.1111/imr.12271
- Sharma PK, Wong EB, Napier RJ, Bishai WR, Ndung'u T, Kasprowicz VO, Lewinsohn DA, Lewinsohn DM, Gold MC. High Expression of CD26 Accurately Identifies Human Bacterial-Reactive MR1-restricted MAIT cells. Immunology 2015; 145(3):84-7 PMID:25752900
- Saitoh T, Yano I, Kumazawa Y, Takimoto H. Pulmonary TCR gammadelta T cells induce the early inflammation of granuloma formation by a glycolipid trehalose 6,6′-dimycolate (TDM) isolated from Mycobacterium tuberculosis. Immunopharmacol Immunotoxicol 2012; 34:815-23; PMID:22963130; http://dx.doi.org/10.3109/08923973.2012.658922
- Flingai S, Czerwonko M, Goodman J, Kudchodkar SB, Muthumani K, Weiner DB. Synthetic DNA vaccines: improved vaccine potency by electroporation and co-delivered genetic adjuvants. Front Immunol 2013; 4:354; PMID:24204366; http://dx.doi.org/10.3389/fimmu.2013.00354
- Achkar JM, Chan J, Casadevall A. Role of B Cells and Antibodies in Acquired Immunity against Mycobacterium tuberculosis. Cold Spring Harb Perspect Med 2014; 5:a018432 PMID:25301934
- Orme IM. Vaccines to prevent tuberculosis infection rather than disease: Physiological and immunological aspects. Tuberculosis (Edinburgh, Scotland) 2014.
- Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Jacobs C, Xavier RJ, van der Meer JW, van Crevel R, Netea MG. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin Immunol 2014; 155:213-9; PMID:25451159; http://dx.doi.org/10.1016/j.clim.2014.10.005
- Ottenhoff TH. New pathways of protective and pathological host defense to mycobacteria. Trends Microbiol 2012; 20:419-28; PMID:22784857; http://dx.doi.org/10.1016/j.tim.2012.06.002
- Zambrano-Zaragoza JF, Romo-Martinez EJ, Duran-Avelar Mde J, Garcia-Magallanes N, Vibanco-Perez N. Th17 cells in autoimmune and infectious diseases. Int J Inflam 2014; 2014:651503; PMID:25152827; http://dx.doi.org/10.1155/2014/651503
- Van Rhijn I, Moody DB. CD1 and mycobacterial lipids activate human T cells. Immunol Rev 2015; 264:138-53; PMID:25703557; http://dx.doi.org/10.1111/imr.12253
- Darrah PA, Bolton DL, Lackner AA, Kaushal D, Aye PP, Mehra S, Blanchard JL, Didier PJ, Roy CJ, Rao SS, et al. Aerosol vaccination with AERAS-402 elicits robust cellular immune responses in the lungs of rhesus macaques but fails to protect against high-dose Mycobacterium tuberculosis challenge. J Immunol 2014; 193:1799-811; PMID:25024382; http://dx.doi.org/10.4049/jimmunol.1400676
- Hokey DA, Wachholder R, Darrah PA, Bolton DL, Barouch DH, Hill K, Dheenadhayalan V, Schwander S, Godin CS, Douoguih M, et al. A nonhuman primate toxicology and immunogenicity study evaluating aerosol delivery of AERAS-402/Ad35 vaccine: Evidence for transient t cell responses in peripheral blood and robust sustained responses in the lungs. Human Vaccin Immunother 2014; 10:2199-210; PMID:25424923; http://dx.doi.org/10.4161/hv.29108
- Satti I, Meyer J, Harris SA, Manjaly Thomas ZR, Griffiths K, Antrobus RD, Rowland R, Ramon RL, Smith M, Sheehan S, et al. Safety and immunogenicity of a candidate tuberculosis vaccine MVA85A delivered by aerosol in BCG-vaccinated healthy adults: a phase 1, double-blind, randomised controlled trial. Lancet Infect Dis 2014; 14:939-46; PMID:25151225; http://dx.doi.org/10.1016/S1473-3099(14)70845-X
- Graves AJ, Padilla MG, Hokey DA. OMIP-022: Comprehensive assessment of antigen-specific human T-cell functionality and memory. Cytometry A 2014; 85:576-9; PMID:24866990; http://dx.doi.org/10.1002/cyto.a.22478
- Sutherland JS, Loxton AG, Haks MC, Kassa D, Ambrose L, Lee JS, Ran L, van Baarle D, Maertzdorf J, Howe R, et al. Differential gene expression of activating Fcgamma receptor classifies active tuberculosis regardless of human immunodeficiency virus status or ethnicity. Clin Microbiol Infect 2014; 20:O230-8; PMID:24205913; http://dx.doi.org/10.1111/1469-0691.12383
- Matsumiya M, Harris SA, Satti I, Stockdale L, Tanner R, O'Shea MK, Tameris M, Mahomed H, Hatherill M, Scriba TJ, et al. Inflammatory and myeloid-associated gene expression before and one day after infant vaccination with MVA85A correlates with induction of a T cell response. BMC Infect Dis 2014; 14:314; PMID:24912498; http://dx.doi.org/10.1186/1471-2334-14-314
- Orme IM, Basaraba RJ. The formation of the granuloma in tuberculosis infection. Semin Immunol 2014; 26:601-9; PMID:25453231; http://dx.doi.org/10.1016/j.smim.2014.09.009
- Niemann S, Supply P. Diversity and evolution of Mycobacterium tuberculosis: moving to whole-genome-based approaches. Cold Spring Harb Perspect Med 2014; 4:a021188; PMID:25190252; http://dx.doi.org/10.1101/cshperspect.a021188
- McShane H, Williams A. A review of preclinical animal models utilised for TB vaccine evaluation in the context of recent human efficacy data. Tuberculosis (Edinburgh, Scotland) 2014; 94:105-10; PMID:24369986; http://dx.doi.org/10.1016/j.tube.2013.11.003
- Flynn JL, Gideon HP, Mattila JT, Lin PL. Immunology studies in non-human primate models of tuberculosis. Immunol Rev 2015; 264:60-73; PMID:25703552; http://dx.doi.org/10.1111/imr.12258
- Shi C, Shi J, Xu Z. A review of murine models of latent tuberculosis infection. Scand J Infect Dis 2011; 43:848-56; PMID:21892898; http://dx.doi.org/10.3109/00365548.2011.603745
- Zelmer A, Carroll P, Andreu N, Hagens K, Mahlo J, Redinger N, Robertson BD, Wiles S, Ward TH, Parish T, et al. A new in vivo model to test anti-tuberculosis drugs using fluorescence imaging. J Antimicrob Chemother 2012; 67:1948-60; PMID:22635525; http://dx.doi.org/10.1093/jac/dks161
- Hokey DA. TB Vaccines: The (Human) Challenge Ahead. Mycobact Dis 2014; 4:e128; http://dx.doi.org/10.4172/2161-1068.1000e128
- Duncan CJ, Draper SJ. Controlled human blood stage malaria infection: current status and potential applications. Am J Trop Med Hyg 2012; 86:561-5; PMID:22492136; http://dx.doi.org/10.4269/ajtmh.2012.11-0504
- Sedegah M, Hollingdale MR, Farooq F, Ganeshan H, Belmonte M, Kim Y, Peters B, Sette A, Huang J, McGrath S, et al. Sterile immunity to malaria after DNA prime/adenovirus boost immunization is associated with effector memory CD8+T cells targeting AMA1 class I epitopes. PLoS One 2014; 9:e106241; PMID:25211344; http://dx.doi.org/10.1371/journal.pone.0106241
- Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 2013; 341:1359-65; PMID:23929949; http://dx.doi.org/10.1126/science.1241800
- Harris SA, Meyer J, Satti I, Marsay L, Poulton ID, Tanner R, Minassian AM, Fletcher HA, McShane H. Evaluation of a human BCG challenge model to assess antimycobacterial immunity induced by BCG and a candidate tuberculosis vaccine, MVA85A, alone and in combination. J Infect Dis 2014; 209:1259-68; PMID:24273174; http://dx.doi.org/10.1093/infdis/jit647
- Bagarazzi ML, Yan J, Morrow MP, Shen X, Parker RL, Lee JC, Giffear M, Pankhong P, Khan AS, Broderick KE, et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med 2012; 4:155ra38; http://dx.doi.org/10.1126/scitranslmed.3004414
- Kalams SA, Parker SD, Elizaga M, Metch B, Edupuganti S, Hural J, De Rosa S, Carter DK, Rybczyk K, Frank I, et al. Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery. J Infect Dis 2013; 208:818-29; PMID:23840043; http://dx.doi.org/10.1093/infdis/jit236
- Villarreal DO, Talbott KT, Choo DK, Shedlock DJ, Weiner DB. Synthetic DNA vaccine strategies against persistent viral infections. Exp Rev Vaccin 2013; 12:537-54; PMID:23659301; http://dx.doi.org/10.1586/erv.13.33
- Li J, Valentin A, Ng S, Beach RK, Alicea C, Bergamaschi C, Felber BK, Pavlakis GN. Differential effects of IL-15 on the generation, maintenance and cytotoxic potential of adaptive cellular responses induced by DNA vaccination. Vaccine 2015; 33:1188-96; PMID:25559187; http://dx.doi.org/10.1016/j.vaccine.2014.12.046
- Williman J, Young S, Buchan G, Slobbe L, Wilson M, Pang P, Austyn J, Preston S, Baird M. DNA fusion vaccines incorporating IL-23 or RANTES for use in immunization against influenza. Vaccine 2008; 26:5153-8; PMID:18456374; http://dx.doi.org/10.1016/j.vaccine.2008.03.084
- Muthumani K, Flingai S, Wise M, Tingey C, Ugen KE, Weiner DB. Optimized and enhanced DNA plasmid vector based in vivo construction of a neutralizing anti-HIV-1 envelope glycoprotein Fab. Hum Vaccin Immunother 2013; 9:2253-62; PMID:24045230; http://dx.doi.org/10.4161/hv.26498
- Oynebraten I, Hinkula J, Fredriksen AB, Bogen B. Increased generation of HIV-1 gp120-reactive CD8+ T cells by a DNA vaccine construct encoding the chemokine CCL3. PLoS One 2014; 9:e104814; PMID:25122197; http://dx.doi.org/10.1371/journal.pone.0104814
- Ledgerwood JE, Hu Z, Gordon IJ, Yamshchikov G, Enama ME, Plummer S, Bailer R, Pearce MB, Tumpey TM, Koup RA, et al. Influenza virus h5 DNA vaccination is immunogenic by intramuscular and intradermal routes in humans. Clin Vaccine Immunol 2012; 19:1792-7; PMID:22956656; http://dx.doi.org/10.1128/CVI.05663-11
- Alam S, McNeel DG. DNA vaccines for the treatment of prostate cancer. Exp Rev Vaccin 2010; 9:731-45; PMID:20624047; http://dx.doi.org/10.1586/erv.10.64
- Diaz CM, Chiappori A, Aurisicchio L, Bagchi A, Clark J, Dubey S, Fridman A, Fabregas JC, Marshall J, Scarselli E, et al. Phase 1 studies of the safety and immunogenicity of electroporated HER2/CEA DNA vaccine followed by adenoviral boost immunization in patients with solid tumors. J Transl Med 2013; 11:62; PMID:23497415; http://dx.doi.org/10.1186/1479-5876-11-62
- Tiriveedhi V, Tucker N, Herndon J, Li L, Sturmoski M, Ellis M, Ma C, Naughton M, Lockhart AC, Gao F, et al. Safety and preliminary evidence of biologic efficacy of a mammaglobin-a DNA vaccine in patients with stable metastatic breast cancer. Clin Cancer Res 2014; 20:5964-75; PMID:25451106; http://dx.doi.org/10.1158/1078-0432.CCR-14-0059
- Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, Moreira ED, Jr., Ngan Y, Petersen LK, Lazcano-Ponce E, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015; 372:711-23; PMID:25693011; http://dx.doi.org/10.1056/NEJMoa1405044
