Abstract
Immunization against hepatitis B virus (HBV) has proven to be highly effective and led to significant reduction of new infections worldwide. However, protective immunity measured by anti-HBs titers may decrease to critical levels in the years after basal immunization, particularly in case of exposure to HBV variants different from the vaccine strain. We tested 400 Palestinian children between one and 19 years of age for their anti-HBs titer, challenged the immune memory of those with low or absent anti-HBs with 2 types of hepatitis B vaccines and determined thereafter the anti-HBs titer. At the age of one, 92.2% of the children presented with protective anti-HBs titers (≥10 mIU/ml) with the majority having ≥100 mIU/ml. Protective immunity was still high at ages 2 (87.5%) and 4 (95%), declining by age 5 and 6 (from 69.2% to 66.7%) and down to an average of 39.8% between the ages of 7 and 19. 160 children with a nonprotective or low immune response challenged with either the yeast-derived Engerix-B or the mammalian cell-derived preS1-containing Sci-B-Vac vaccine showed an anamnestic immune response. 92.4% and 85.9% of the children challenged with one dose Sci-B-Vac and Engerix-B presented with anti-HBs titers >100 mIU/ml respectively. Our results reveal that vaccine-induced protective anti-HBs titers against HBV decrease rapidly beyond the age of 6 in Palestinian children, but can be strongly enhanced with a single booster vaccine dose, independent of brand and antigen composition. Our data suggest that a booster vaccine dose against HBV during school years may be useful.
Introduction
The best prevention of hepatitis B virus (HBV) infection is achieved through routine vaccination of infants. The earliest vaccination program against HBV infection was launched 1984 in Taiwan,Citation1 while a worldwide implementation was recommended by WHO in 1992. The global coverage rate with 3 vaccine doses reached 79% by the end of 2012.Citation2 As a consequence, the rate of HBsAg positive children is declining rapidly, especially in highly endemic areas such as Taiwan, China and among native Alaskans,Citation3–5 but also in less endemic areas like Italy.Citation6 Failure of immunoprophylaxis in infants occurs in 10–30% of newborns from HBV positive mothers and is mainly attributed to high viral load of HBV (>107 copies/ml).Citation7 Another reason for breakthrough infection is the selection of vaccine escape mutants in vaccinated individuals.Citation8-10
The first available hepatitis B vaccine was plasma-derived, which was later replaced by the recombinant vaccine currently used worldwide.Citation11 The widely used brands Engerix-B (GlaxoSmithKline, Belgium), RECOMBIVAX HB® (Merck, USA) and many other local brands consist of the small (S) protein of the HBV surface antigen (HBsAg) and are expressed in transformed yeast cell cultures. These vaccines result in comparable protective immunity.Citation12 Beside these popular vaccines, the mammalian cell-derived Sci-B-Vac (SciGen, Israel) was recently introduced in some countries including Israel. In addition to the S protein of the HBV envelope it contains the middle and large surface proteins with preS domains,Citation13 and has been accredited to elicit a more rapid and higher antibody response than the yeast-derived HBV vaccines.Citation14,15 Furthermore, it was able to induce a protective immune response in previous non-responders to the yeast-derived vaccine.Citation16 More recent preliminary findings suggest that it protects passively immunized newborns from HBV positive mothers more effectively than Engerix-B.Citation17
Generally, administration of the vaccine in either form results in protective antibodies in early stages of life, but maintaining the protective immunity among vaccinees in late childhood and adulthood raises issues regarding the introduction of booster HBV vaccine.Citation18-22 In contrast, other studies have shown persistence of immunity for periods of 9–15 years.Citation23-25 Until today, there is no recommendation for booster vaccination with HBV vaccine.Citation26 Nevertheless, mounting evidence suggests clinically silent breakthroughs are more frequent than believed,Citation3,27 and that they often result in occult HBV infection, both soon after birthCitation28,29 as well as later in life.Citation30 The long-term consequences of these seemingly innocuous infections are unknown but the ability to reactivate under severe immunosuppression has been observed.Citation31 A recent observation on new HBV infections in US blood donors suggests that 10 mIU/ml anti-HBs protect even against inapparent infections by the HBV genotype A2, which is the basis for the current vaccines. Protection against heterologous HBV genotypes requires higher titers of anti-HBs exceeding 100 mIU/ml. Citation32 These higher titers, however, are rare in young adults who were vaccinated in early childhood.Citation32
As described in the discussion, studies in various regions of the world found highly divergent seroprotection rates in children of different age groups, who were vaccinated soon after birth. The outcome of the hepatitis B vaccination campaign in Palestine was unknown yet. We initially tested a small group of children born to HBsAg positive/negative mothers for anti-HBs. The observation of low anti-HBs titers among these children led to this comprehensive study. Here, we present the first long-term follow-up of the anti-HBs titers among Palestinian children vaccinated against HBV in early childhood. Furthermore, we present the results of booster vaccination, which we introduced to those children with anti-HBs titers below 100 mIU/ml. Since it was unknown whether children without current protective anti-HBs levels were ever good responders, we included the Sci-B-Vac in the booster as it is presumably more immunogenic than the conventional Engerix-B.Citation11,14-16,33 This was also an opportunity to test whether one dose of Sci-B-Vac would be able to boost the immune memory induced by 3 doses of Engerix-B.
Results
Study population
A sample of 400 children was recruited from different parts of the West Bank districts with the following distribution: Hebron and Bethlehem districts 24%, Jerusalem district 36%, Ramallah 39%. Three children were from Nablus and 3 were from Gaza strip, but currently living in Ramallah. The overall female to male ratio was 1.07.
HBsAg and anti-HBc status in children of HBsAg positive mothers
Twenty-three children were offspring of 10 HBsAg positive mothers. All 23 children tested negative for HBsAg, with the exception of one child (12-year-old); 22 children tested negative for anti-HBc.
Anti-HBs titers of the children before booster injection
The anti-HBs geometric mean titer (GMT) was highest among the one-year-old children (257 mIU/ml) ( and ), which is probably a moderate underestimation as 16 of the 77 one-year-old children presented with anti-HBs >1000 mIU/ml (). Thereafter, anti-HBs GMT declined gradually down to 24 mIU/ml by the age of 5. This initial decrease in the GMT in relation to age was significant (P value <0.0001). GMTs fluctuated later at low levels between 2.0 and 29.5 mIU/ml with large standard deviations (ages 6–19 years, ). Out of the 323 children older than one-year, only 2 presented with anti-HBs >1000 mIU/ml.
Figure 1. Geometric mean titers and 95 % confidence intervals of anti-HBs titers in vaccinated children by age before (squares) and after (filled dots) challenge with hepatitis B vaccine doses. There were no cases post-challenge for age group 1 and only 2 cases for age group 2.
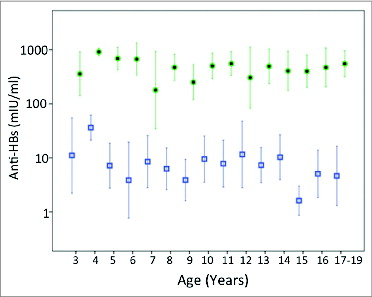
Table 1. Geometric mean titers of anti-HBs by age. Age and total number of children in that age group are indicated in the first and second column. Anti-HBs values <1 mIU/ml were calculated as 1 mIU/ml and those >1000 mIU/ml as 1000 mIU/ml respectively
Table 2. Anti-HBs titer by age. Age and total number of children in that age group are indicated in the first and second column. Anti-HBs titer was categorized in 4 groups, 0-<10; 10-<100; 100-<1000 and >1000 mIU/ml respectively. The number and the percentage (boldface) of children presented with each anti-HBs category are indicated. The percentage of children for each age group with protective anti-HBs antibodies (≥ 10mIU/ml) is shown in the last column
The percentage of protective anti-HBs titer (>10 mIU/ml) was highest among the 4-year-old children (95%), followed by the one-year-olds (92.2%) and the 2-year-olds (87.5%) (). The rate of protective anti-HBs titers was similarly high at ages, 3, 5 and 6 years (69.2, 69.2 and 66.7%) (). Thereafter, the percentage of protective immunity declined and fluctuated between 12.5 and 65.2 % with a mean of 47 % ().
Anti-HBs titers of children after booster injection
Out of 165 children challenged with Sci-B-Vac and 92 challenged with Engerix-B, 94 and 66 returned for testing respectively. Regardless of the vaccine type used for challenge, most children exerted anti-HBs titer >100 mIU/ml after the challenge ( and ). In case of children with non-protective anti-HBs titers and one booster dose, 87.7% of those challenged with Sci-B-Vac and 77.7% of those challenged with Engerix-B produced anti-HBs titers >100 mIU/ml (). The one-dose challenge results were even as high as 97% for Sci-B-Vac and 93.8% of Engerix-B for children with a pre-challenge titer of 10-<100 mIU/ml (). Nevertheless, this difference was not statistically significant (p = 0.736). Only four (2.5%) of the 160 children did not show a clear booster reaction. None of the children challenged with Sci-B-Vac remained in the non-protective anti-HBs titer zone, while this was observed in one single case challenged with Engerix-B. Three doses challenge with either vaccine type (11 with Sci-B-Vac and 6 with Engerix-B) resulted in post-challenge anti-HBs titers >100 mIU/ml in all children. However, ANOVA independent sample test did not show a significant difference in the anti HBs mean titer related to the number of challenge doses (p = 0.287).
Table 3. Anamnestic anti-HBs response after challenge vaccination with either Sci-B-Vac (A) or Engerix-B (B). Anti-HBs titers before challenge are shown in the first column, while that after challenge in the first row. The number and the percentage (in brackets) of children presented with each anti-HBs category after challenge are indicated. All children were vaccinated with 3 doses of Engerix-B in early childhood
There was no trend in post-challenge anti-HBs GMT related to age (; p = 0.392). An average of 241-fold and 124-fold increase in anti-HBs GMT was produced in children following challenge with Sci-B-Vac and Engerix-B respectively, a difference with no statistical significance (p = 0.175). While none of the 160 challenged children had anti-HBs titer above 100, 43% of them presented with titers >1000 mIU/ml thereafter. Thus, the true GMT and the corresponding titer increase may be higher after the booster than indicated in .
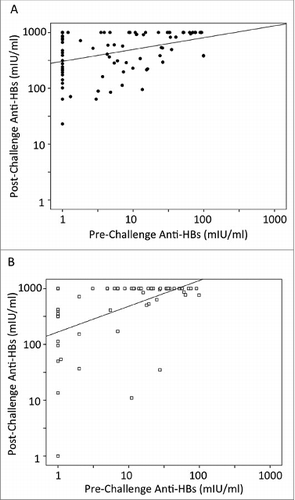
Independent of vaccine type, the one dose challenge resulted in a significant association between post- and pre-challenge anti-HBs titers (p-value = 0.000 for Sci-B-Vac as well as for Engerix-B). Scatter plot analysis of one dose challenges are shown in (Sci-B-Vac) and (Engerix-B). Correlation coefficient was higher for Engerix-B booster (r = 0.491) than that for Sci-B-Vac (r = 0.381).
Figure 2. Scatter plot analysis of anti-HBs responses for either Sci-B-Vac (A) or Engerix-B (B) challenged children. Cases with anti-HBs <1mIU/ml were calculated as 1 mIU/ml and those >1000 mIU/ml as 1000 mIU/ml respectively. The scatter plots represent the one-dose challenged cases (83 Sci-B-Vac and 60 Engerix-B respectively).
ANOVA test showed no significant difference in GMT of anti-HBs pre- and post-challenge between children born to HBsAg positive mothers and those born to negative mothers (p = 0.056 and 0.175 respectively). There were also no significant differences in GMT of anti-HBs between genders pre- and post-challenge (p = 0.283 and 0.366 respectively).
We performed ANOVA analysis on siblings from 27 families to test whether differences in GMT of anti-HBs could be attributed to specific families or family members; the test results both before and after challenge as well as in the fold increase were not significant (p = 0.144, 0.221 and 0.687 respectively).
Discussion
In Palestine, hepatitis B vaccine was introduced into the expanded program of immunization (EPI) in 1992–1993. The current coverage rate is 94.8% throughout Palestine (Ministry of Health, PHIC, Health Status in Palestine 2012, July 2013). The prevalence of HBsAg was found to be 3.5% in the general population and 3.8% in blood donors for the Gaza strip; Citation34 an accurate population based survey for the West bank is not available. According to laboratory records from 2007–2012 of Al-Makassed Islamic Charitable Hospital (MICH), the referral hospital in Palestine, 738 (2.02 %) of 36,512 patients tested positive for HBsAg (data kindly provided by Mr. Sabri Baraghithi, director of the Central Laboratory, MICH, East-Jerusalem, Palestine).
Our data show a clear decline in the mean anti-HBs titer over the first 5–6 years among Palestinians vaccinated as infants with continuously low levels after the age of 6, which is in line with earlier reports from other countries. Seroprotection rates for different age groups in follow-up studies reported from different countries were compared with this study and summarized in . The 90% seroprotection rate detected at the end of the first year of life declines by age in all studies, with considerable differences from region to region (). Highest rates of seroprotection have been reported from Hong Kong and Germany, lowest from Alaska and Micronesia (). These strong regional differences may be explained by genetic or acquired factors, but they may also be caused by methodological and statistical variations.
Table 4. Overview of seroprotection rates against HBV infection in different age groups after vaccination with 3 doses of recombinant hepatitis B vaccines within the first year of life
A strong increase in both, anti-HBs titers and seroprotection rate was observed after a single vaccine challenge dose in the different age groups, which indicates the existence of an anamnestic immune response even after 16 years. The rate of detectable booster reactions was remarkably close to 100% in this study, which is in accordance with earlier reports, Citation23,25,35-39 but in contrast to other studies, where anamnestic response was lower and ranged from 51% to 87% Citation20-22,24. The reason for the lower success of booster injections in the latter studies is not clear, but it may be due to primary undetected vaccination failure. Interestingly, one study reported a slightly less successful response among plasma-derived primary vaccinees when boosted with a recombinant vaccine in comparison to those primarily vaccinated with the recombinant vaccine.Citation38
This is the first report to present results of Sci-B-Vac challenge of waning immunity in children vaccinated with 3 doses of Engerix-B. Remarkably, a single dose of Sci-B-Vac was able to boost the immune system as efficiently as Engerix-B, despite the differences in the antigenic components of the vaccines and the antibody epitopes triggered. Sci-B-Vac does not contain unfolded non-protective epitopes of S protein and has the protective preS1 epitopes of HBV. Both vaccines, however, contain protective S epitopes, which were obviously stimulating the immune response to Sci-B-Vac after the basal immunization with Engerix-B. It should be noted that the anti-HBs assay AxSym uses mammalian cell-derived S protein without preS and, thus, detects preferably protective S antibodies. It has been reported earlier that Sci-B-Vac (also known as Bio-Hep B) causes enhanced antibody responses in low responders to conventional yeast derived vaccine, i.e. Engerix-B, Citation16 and is able to induce preS1 antibodies in newborns.Citation40 Our data does not provide evidence for a significant difference in anti-HBs response attributed to vaccine type (p = 0.736), however, it does not exclude a superior protection efficacy of Sci-B-Vac as recently suggested by Saed et al.Citation17
In contrast to other studies Citation5,21 our results did not suggest that mounting an anamnestic immune response was age dependent (p = 0.392). In line with Zanetti et al.,Citation25 but contrary to McMahon et al.Citation41, anti-HBs immune response in our study was not gender dependent either (p = 0.283, pre-challenge and p = 0.366, post-challenge). Furthermore, we were unable to detect a significant difference in anti-HBs titer between a single dose or 3 booster doses (p = 0.287), contrary to Wang and Lin, Citation22 who concluded that a single booster dose does not induce a sufficient anti-HBs response in adolescents with pre-challenge anti-HBs below 10 mIU/ml.
Although it is generally accepted that the anti-HBs titer declines with increasing age, booster injections are not recommended for the general population even in highly endemic regions and when anti-HBs is <10 mIU/ml.Citation5,18,42,43 Fitzsimons et al.Citation18 argue that the immunological memory for HBsAg outlasts the presence of anti-HBs. Other studies show that T-cell responses exist, even when diagnostic kits fail to detect anti-HBs.Citation44 Other studies on chimpanzees by Ogata et al.Citation45 suggested that the T-cell response alone cannot prevent HBV infection but can mitigate its course and facilitate a rapid recovery. The important role of anti-HBV envelope antibodies in the protection against HBV infection is suggested by the possibility to passively protect even after HBV exposure and by the high rate of HBV reactivation in immune patients who need B cell depletion because of immunological disorders while T cell depletion usually does not lead to reactivation in HBsAg negative patients.Citation46
While public health authorities nowhere consider boosters necessary for the general population, some European countries, e.g. Germany, recommend re-vaccination of people at high risk presented with anti-HBs titer <100 mIU/ml elucidating the goal to prevent not only clinical disease but also inapparent HBV infections.Citation47 Indeed, titers of anti-HBs >100 mIU/ml would be desirable because they protect more reliably against infection with HBV genotypes other than the one in the vaccine.Citation32 Palestine, like other regions in the Middle East, has a predominance of genotype D1Citation48 suggesting that anti-HBs levels above 100 mIU/ml are necessary for protection against clinically inapparent HBV infections.Citation32 In addition, the use of Sci-B-Vac could improve protection by inducing preS1- antibodies Citation40 and is in fact used for newborns in Israel and other countries. Even one dose may prime the immune system against preS1 in case of an exposure to HBV.
Our study has some potential shortcomings: i) A certain degree of underestimation is to be assumed for very high anti-HBs titers because samples with anti-HBs >1000 mIU/ml were not diluted and retested, mainly for economic reasons. This shortcoming however, did not affect our findings as indicated by the clear and strong effect of the booster injections causing >100 fold titer increase in those children with low anti-HBs titers before challenge. ii) The non-probability method of children recruitment could theoretically compromise the representativeness of the study, however there are no implications that this was the case here. The sample size is large; the children tested were from different age groups, social backgrounds (socioeconomic status) and areas, recruited over a time span of 2 years. There are different types of non-probability sampling techniques, of which the one known as snowball or word-of mouth was used in this study. This means we did not have a list of people available but spread the idea of our research via referral procedure through co-workers, friends, relatives, etc., who came from different backgrounds and resided different areas in the West Bank. Indeed, children of any consented parents/guardians could participate without any selection criteria. Lastly, as presented in the results, we looked for possible significance of GMT data among siblings of families. Our results revealed that differences in GMT of anti-HBs before and after challenge could not be attributed to specific families or family members (p = 0.144 and 0.221 respectively). This is an indication that the results presented here are even independent of the relationship between children tested. iii) Only 62% of challenged children were available for anti-HBs testing one month later. There were 2 main reasons for this, either the children were afraid of the blood drawing, mainly those between 2 and 12 years, or they were not reachable. iv) The cohorts receiving Sci-B-Vac or Engerix B were not truly randomized, but rather followed the stratified randomization method. Thus, it cannot yet definitively be concluded whether both vaccines induce equally good titers after primary vaccination with Engerix B.
In conclusion, our data show that the anti-HBs titers are too low in the majority of Palestinian school children and adolescents to dependably protect against inapparent HBV infection and that the titers can be very efficiently increased by one booster dose of the same or another HBsAg-based vaccine. Currently, administration of booster injections after primary vaccination is not recommended. Nevertheless, it is important to pay attention both to how immunity against HBV develops in the long-term, and to which vaccine can best restore protection.
Materials and Methods
Ethical approval
This study was approved by the Al-Quds university ethics committee. At least one parent or guardian signed the informed consent form after explanation of the anticipated research. If the parent or guardian had more than one child participating, he or she signed one consent form for all of them.
Study population
The study population was recruited through snowball non-probability sampling technique (word-of-mouth referral sampling) between January 2011 and December 2012 among families, relatives, and friends of the Virology Research Laboratory team at Al-Quds University and the Central Laboratory team at Al-Makassed Islamic Charitable Hospital (MICH). Ninety-five percent of the one-year-old children were recruited through an UNRWA clinic, Bab Ezzawyeh, East Jerusalem, which we approached with the knowledge that children receive routine check-ups at the age of one including a blood draw. We directed our request to the parents, and obtained an aliquot of 2 ml blood upon consent.
Overall, the 323 children between 2 and 19 years together with 4 children from the one-year-old group came from 135 families having one up to 6 children each. The remaining 73 one-year-olds belonged to 72 other families. 45 families had sibling groups of 3 or more children. All 400 children were in very good health and had received a routine vaccination with 3 doses Engerix-B monovalent vaccine at 0, 1, and 6 months of age.
Sample size calculation
Representative study population size was calculated using the following equation: n = z^2/4E^2. Using 95% confidence level (z = 1.96), and estimation error (E = 5%), this formula gives n = 384.16. We choose the sample size 400 to ensure that the level of confidence does not fall below 95% and the error does not exceed 5%.
HBV serology tests
All HBV serology testing was performed at MICH on the AxSym system (Abbott, Germany) using original Abbott kits for anti-HBs (AUSAB®) and anti-HBc (CORE™). MICH Central Laboratory participates regularly in internationally recognized external quality control (Lab quality, Helsinki, Finland) for HBV serology 3 times per year.
Procedure
The children in our sample were tested for anti-HBs titer; children of HBsAg positive mothers were also tested for anti-HBc. A total of 257 children (2–19 years old) with anti-HBs titers below 100 mIU/ml were subjected to a challenge dose of either Sci-B-Vac (SciGen) or Engerix-B (GSK) administered by intramuscular injection into the deltoid muscle. For the age group 2–10 5 μg Sci-B-Vac (LOT: B0332V1) was used while 10 μg Sci-B-Vac (LOT: B0361V1) was used for ages 11–19 according to manufacturer instructions. The dose for Engerix-B (LOT: AHBVC007BK) was 0.5 ml (10 µg) for the age group 2–10 years and 1 ml (20 µg) for ages 11–19 according to manufacturer instructions. Booster administration was not truly randomized, but rather followed the stratified randomization method to assure the representativeness of all ages in each vaccine group. For this, most of the children tested in 2011 were given Sci-B-Vac, while Engerix-B was given mainly to the group tested in 2012 respectively. In 17 cases, 3 doses of the booster vaccine (11 with Sci-B-Vac and 6 with Engerix-B) were administrated in the traveler vaccination mode at 0, 7, and 21 days. One month after the challenge, 160 children were available for a second check of anti-HBs titer.
Statistical analysis
The age and sex of each child were obtained from the informed consent form. These data were entered to an excel sheet, along with the anti-HBs titer before and, if available, after the challenge vaccination. We did not determine the exact anti-HBs titers >1000 mIU/ml, which exceeded the upper limit of the AxSym anti-HBs assay, In such cases, 1000 mIU/ml was used for statistical analysis instead. Statistical analysis was performed using “R Software” (R development core team, 2011). The one-way analysis of variance (ANOVA) was used to determine whether there were any significant differences between the means of 3 or more independent groups. Correlation coefficient was used to measure the strength of the relationship between pre- and post-challenge anti-HBs titers.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank the following people for their help in recruiting children and drawing blood: The team of the Central Laboratory-MICH, the team of Virology Research Laboratory-Al-Quds University and the UNRWA clinic-Bab Ezzawyeh-Jerusalem. Special thanks to Dr. Elias Habash-UNRWA, West Bank, Nayfeh Ayesh, Pathology laboratory, MICH, Dr. Hatem Khamash, Neonatal Department, MICH, Dr. Iyad Habil, St-John Eye Hospital, Jerusalem, Maysoun Zatari, UNRWA, Hebron, and Mohammad Kurdi, Al-Quds University. Above all, we thank all participating families; without their cooperation this project could not have been completed. Finally we would like to thank Dr. Kae Reynolds from the University of Huddersfield in UK for her critical review of the manuscript.
Funding
This research was supported by German Research Foundation (DFG) grant No. GL595/2-1 to DG and MA.
References
- Chen DS, Hsu NH, Sung JL, Hsu TC, Hsu ST, Kuo YT, Lo KJ, Shih YT. A mass vaccination program in Taiwan against hepatitis B virus infection in infants of hepatitis B surface antigen-carrier mothers. Jama 1987; 257:2597-603; PMID:3573257; http://dx.doi.org/10.1001/jama.1987.03390190075023
- Organization WH. Immunization coverage. 2014: Fact sheet N°378.
- Su WJ, Liu CC, Liu DP, Chen SF, Huang JJ, Chan TC, Chang MH. Effect of age on the incidence of acute hepatitis B after 25 years of a universal newborn hepatitis B immunization program in Taiwan. J Infect Dis 2012; 205:757-62; PMID:22262790; http://dx.doi.org/10.1093/infdis/jir852
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Epidemiological serosurvey of hepatitis B in China-declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009; 27:6550-7; PMID:19729084; http://dx.doi.org/10.1016/j.vaccine.2009.08.048
- McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, Bulkow L, Fiore AE, Bell BP, Hennessy TW. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis 2009; 200:1390-6; PMID:19785526; http://dx.doi.org/10.1086/606119
- Mele A, Tosti ME, Mariano A, Pizzuti R, Ferro A, Borrini B, Zotti C, Lopalco P, Curtale F, Balocchini E, et al. Acute hepatitis B 14 years after the implementation of universal vaccination in Italy: areas of improvement and emerging challenges. Clin Infect Dis 2008; 46:868-75; PMID:18269332; http://dx.doi.org/10.1086/528687
- Ding Y, Sheng Q, Ma L, Dou X. Chronic HBV infection among pregnant women and their infants in Shenyang, China. Virol J 2013; 10:17; PMID:23294983; http://dx.doi.org/10.1186/1743-422X-10-17
- Chang MH. Breakthrough HBV infection in vaccinated children in Taiwan: surveillance for HBV mutants. Antivir Ther 2010; 15:463-9; PMID:20516566; http://dx.doi.org/10.3851/IMP1555
- Carman WF, Zanetti AR, Karayiannis P, Waters J, Manzillo G, Tanzi E, Zuckerman AJ, Thomas HC. Vaccine-induced escape mutant of hepatitis B virus. Lancet 1990; 336:325-9; PMID:1697396; http://dx.doi.org/10.1016/0140-6736(90)91874-A
- Gerlich WH. Breakthrough of hepatitis B virus escape mutants after vaccination and virus reactivation. J Clin Virol 2006; 36(Suppl 1):S18-22; PMID:16831688; http://dx.doi.org/10.1016/S1386-6532(06)80004-1
- Gerlich WH. Prophylactic vaccination against hepatitis B: achievements, challenges and perspectives. Med Microbiol Immunol 2015; 204:39-55; PMID:25523195; http://dx.doi.org/10.1007/s00430-014-0373-y
- Coates T, Wilson R, Patrick G, Andre F, Watson V. Hepatitis B vaccines: assessment of the seroprotective efficacy of two recombinant DNA vaccines. Clin Ther 2001; 23:392-403; PMID:11318074; http://dx.doi.org/10.1016/S0149-2918(01)80044-8
- Shouval D, Ilan Y, Adler R, Deepen R, Panet A, Even-Chen Z, Gorecki M, Gerlich WH. Improved immunogenicity in mice of a mammalian cell-derived recombinant hepatitis B vaccine containing pre-S1 and pre-S2 antigens as compared with conventional yeast-derived vaccines. Vaccine 1994; 12:1453-9; PMID:7533967; http://dx.doi.org/10.1016/0264-410X(94)90155-4
- Shapira MY, Zeira E, Adler R, Shouval D. Rapid seroprotection against hepatitis B following the first dose of a Pre-S1/Pre-S2/S vaccine. J Hepatol 2001; 34:123-7; PMID:11211888; http://dx.doi.org/10.1016/S0168-8278(00)00082-9
- Yerushalmi B, Raz R, Blondheim O, Shumov E, Koren R, Dagan R. Safety and immunogenicity of a novel mammalian cell-derived recombinant hepatitis B vaccine containing Pre-S1 and Pre-S2 antigens in neonates. Pediatr Infect Dis J 1997; 16:587-92; PMID:9194109; http://dx.doi.org/10.1097/00006454-199706000-00009
- Rendi-Wagner P, Shouval D, Genton B, Lurie Y, Rumke H, Boland G, Cerny A, Heim M, Bach D, Schroeder M, et al. Comparative immunogenicity of a PreS/S hepatitis B vaccine in non- and low responders to conventional vaccine. Vaccine 2006; 24:2781-9; PMID:16455169; http://dx.doi.org/10.1016/j.vaccine.2006.01.007
- Saed N. HM, Nijim Y, Farah N, Jamalia J, Nuser T, Ismael K, Ghantous R, Amer J, Safadi R. Sci-B-Vac is superior to Engerix-B for preventing HBV vertical transmission. 49th European Association for the Study of the Liver International Liver Congress (EASL 2014). London, 2014.
- Fitzsimons D, Francois G, Hall A, McMahon B, Meheus A, Zanetti A, Duval B, Jilg W, Böcher WO, Lu SN, et al. Long-term efficacy of hepatitis B vaccine, booster policy, and impact of hepatitis B virus mutants. Vaccine 2005; 23:4158-66; PMID:15964484; http://dx.doi.org/10.1016/j.vaccine.2005.03.017
- Bialek SR, Bower WA, Novak R, Helgenberger L, Auerbach SB, Williams IT, Bell BP. Persistence of protection against hepatitis B virus infection among adolescents vaccinated with recombinant hepatitis B vaccine beginning at birth: a 15-year follow-up study. Pediatr Infect Dis J 2008; 27:881-5; PMID:18756185; http://dx.doi.org/10.1097/INF.0b013e31817702ba
- Hammitt LL, Hennessy TW, Fiore AE, Zanis C, Hummel KB, Dunaway E, Bulkow L, McMahon BJ. Hepatitis B immunity in children vaccinated with recombinant hepatitis B vaccine beginning at birth: a follow-up study at 15 years. Vaccine 2007; 25:6958-64; PMID:17714836; http://dx.doi.org/10.1016/j.vaccine.2007.06.059
- Samandari T, Fiore AE, Negus S, Williams JL, Kuhnert W, McMahon BJ, Bell BP. Differences in response to a hepatitis B vaccine booster dose among Alaskan children and adolescents vaccinated during infancy. Pediatrics 2007; 120:e373-81; PMID:17636112; http://dx.doi.org/10.1542/peds.2007-0131
- Wang LY, Lin HH. Short-term response to a booster dose of hepatitis B vaccine in anti-HBs negative adolescents who had received primary vaccination 16 years ago. Vaccine 2007; 25:7160-7; PMID:17707557; http://dx.doi.org/10.1016/j.vaccine.2007.07.022
- Watson B, West DJ, Chilkatowsky A, Piercy S, Ioli VA. Persistence of immunologic memory for 13 years in recipients of a recombinant hepatitis B vaccine. Vaccine 2001; 19:3164-8; PMID:11312012; http://dx.doi.org/10.1016/S0264-410X(01)00019-6
- Saffar MJ, Rezai MS. Long-term antibody response and immunologic memory in children immunized with hepatitis B vaccine at birth. Indian Pediatr 2004; 41:1232-7; PMID:15623904
- Zanetti AR, Mariano A, Romano L, D'Amelio R, Chironna M, Coppola RC, Cuccia M, Mangione R, Marrone F, Negrone FS, et al. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet 2005; 366:1379-84; PMID:16226616; http://dx.doi.org/10.1016/S0140-6736(05)67568-X
- WHO position paper. World Health Organization. Weekly epidemiological record. No. 40, 2009, 84, 405-420 http://www.who.int/wer/2009/wer8440.pdf
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, Leroux-Roels G, Kuriyakose S, Leyssen M, Jacquet JM. Evidence of protection against clinical and chronic hepatitis B infection 20 years after infant vaccination in a high endemicity region. J Viral Hepat 2011; 18:369-75; PMID:20384962; http://dx.doi.org/10.1111/j.1365-2893.2010.01312.x
- Pande C, Sarin SK, Patra S, Kumar A, Mishra S, Srivastava S, Bhutia K, Gupta E, Mukhopadhyay CK, Dutta AK, et al. Hepatitis B vaccination with or without hepatitis B immunoglobulin at birth to babies born of HBsAg-positive mothers prevents overt HBV transmission but may not prevent occult HBV infection in babies: a randomized controlled trial. J Viral Hepat 2013; 20:801-10; PMID:24168259
- Mu SC, Lin YM, Jow GM, Chen BF. Occult hepatitis B virus infection in hepatitis B vaccinated children in Taiwan. J Hepatol 2009; 50:264-72; PMID:19070923; http://dx.doi.org/10.1016/j.jhep.2008.09.017
- Xu L, Wei Y, Chen T, Lu J, Zhu CL, Ni Z, Huang F, Du J, Sun Z, Qu C. Occult HBV infection in anti-HBs-positive young adults after neonatal HB vaccination. Vaccine 2010; 28:5986-92; PMID:20637763; http://dx.doi.org/10.1016/j.vaccine.2010.06.106
- Feeney SA, McCaughey C, Watt AP, Agnaf MR, McDougall N, Wend UC, Gerlich WH, Coyle PV. Reactivation of occult hepatitis B virus infection following cytotoxic lymphoma therapy in an anti-HBc negative patient. J Med Virol 2013; 85:597-601; PMID:23359331; http://dx.doi.org/10.1002/jmv.23513
- Stramer SL, Wend U, Candotti D, Foster GA, Hollinger FB, Dodd RY, Allain JP, Gerlich W. Nucleic acid testing to detect HBV infection in blood donors. N Engl J Med 2011; 364:236-47; PMID:21247314; http://dx.doi.org/10.1056/NEJMoa1007644
- Shouval D, Roggendorf H, Roggendorf M. Enhanced immune response to hepatitis B vaccination through immunization with a Pre-S1/Pre-S2/S Vaccine. Med Microbiol Immunol 2015; 204:57-68; PMID:25557605; http://dx.doi.org/10.1007/s00430-014-0374-x
- Yassin K, Awad R, Tebi AJ, Queder A, Laaser U. Prevalence and risk factors of HBsAg in Gaza: implications for prevention and control. J Infect 2002; 44:252-6; PMID:12099733; http://dx.doi.org/10.1053/jinf.2001.0998
- Seto D, West DJ, Ioli VA. Persistence of antibody and immunologic memory in children immunized with hepatitis B vaccine at birth. Pediatr Infect Dis J 2002; 21:793-5; PMID:12233715; http://dx.doi.org/10.1097/00006454-200208000-00021
- Zinke M, Kappes R, Kindler K, Paulus-Koschik A, Goering U, Disselhoff J, Soemantri P, Grunert D, Laakmann KH, Gunasekaran R, et al. Immune memory to hepatitis B virus in 4-9-year old children vaccinated in infancy with four doses of hexavalent DTPa-HBV-IPV/Hib vaccine. Hum Vaccin 2009; 5:592-8; PMID:19535920; http://dx.doi.org/10.4161/hv.9051
- Steiner M, Ramakrishnan G, Gartner B, Van Der Meeren O, Jacquet JM, Schuster V. Lasting immune memory against hepatitis B in children after primary immunization with 4 doses of DTPa-HBV-IPV/Hib in the first and 2nd year of life. BMC Infect Dis 2010; 10:9; PMID:20078876; http://dx.doi.org/10.1186/1471-2334-10-9
- Williams IT, Goldstein ST, Tufa J, Tauillii S, Margolis HS, Mahoney FJ. Long term antibody response to hepatitis B vaccination beginning at birth and to subsequent booster vaccination. Pediatr Infect Dis J 2003; 22:157-63; PMID:12586980
- Lee PI, Lee CY, Huang LM, Chang MH. Long-term efficacy of recombinant hepatitis B vaccine and risk of natural infection in infants born to mothers with hepatitis B e antigen. J Pediatr 1995; 126:716-21; PMID:7751994; http://dx.doi.org/10.1016/S0022-3476(95)70398-5
- Hellstrom UB, Madalinski K, Sylvan SP. PreS1 epitope recognition in newborns after vaccination with the third-generation Sci-B-Vac vaccine and their relation to the antibody response to hepatitis B surface antigen. Virol J 2009; 6:7; PMID:19154574; http://dx.doi.org/10.1186/1743-422X-6-7
- McMahon BJ, Bruden DL, Petersen KM, Bulkow LR, Parkinson AJ, Nainan O, Khristova M, Zanis C, Peters H, Margolis HS. Antibody levels and protection after hepatitis B vaccination: results of a 15-year follow-up. Ann Intern Med 2005; 142:333-41; PMID:15738452; http://dx.doi.org/10.7326/0003-4819-142-5-200503010-00008
- Yuen MF, Lim WL, Cheng CC, Lam SK, Lai CL. Twelve-year follow-up of a prospective randomized trial of hepatitis B recombinant DNA yeast vaccine versus plasma-derived vaccine without booster doses in children. Hepatology 1999; 29:924-7; PMID:10051499; http://dx.doi.org/10.1002/hep.510290327
- Alfaleh F, Alshehri S, Alansari S, Aljeffri M, Almazrou Y, Shaffi A, Abdo AA. Long-term protection of hepatitis B vaccine 18 years after vaccination. J Infect 2008; 57:404-9; PMID:18829116; http://dx.doi.org/10.1016/j.jinf.2008.08.008
- Carollo M, Palazzo R, Bianco M, Pandolfi E, Chionne P, Fedele G, Tozzi AE, Carsetti R, Roman∫ L, Ausiello CM. Hepatitis B specific T cell immunity induced by primary vaccination persists independently of the protective serum antibody level. Vaccine 2013; 31:506-13; PMID:23174200; http://dx.doi.org/10.1016/j.vaccine.2012.11.029
- Ogata N, Cote PJ, Zanetti AR, Miller RH, Shapiro M, Gerin J, Purcell RH. Licensed recombinant hepatitis B vaccines protect chimpanzees against infection with the prototype surface gene mutant of hepatitis B virus. Hepatology 1999; 30:779-86; PMID:10462386; http://dx.doi.org/10.1002/hep.510300309
- Di Bisceglie AM, Lok AS, Martin P, Terrault N, Perrillo RP, Hoofnagle JH. Recent US Food and Drug Administration warnings on hepatitis B reactivation with immune-suppressing and anticancer drugs: just the tip of the iceberg? Hepatology 2015; 61:703-11; PMID:25412906; http://dx.doi.org/10.1002/hep.27609
- Harder T, Remschmidt C, Falkenhorst G, Zimmermann R, Hengel H, Ledig T, Oppermann H, Zeuzem S, Wicker S. Background paper to the revised recommendation for hepatitis B vaccination of persons at particular risk and for hepatitis B postexposure prophylaxis in Germany. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013; 56:1565-76; PMID:24170086; http://dx.doi.org/10.1007/s00103-013-1845-8
- Abdelnabi Z, Saleh N, Baraghithi S, Glebe D, Azzeh M. Subgenotypes and mutations in the s and polymerase genes of hepatitis B virus carriers in the west bank, palestine. PLoS One 2014; 9:e113821; PMID:25503289
- Lin YC, Chang MH, Ni YH, Hsu HY, Chen DS. Long-term immunogenicity and efficacy of universal hepatitis B virus vaccination in Taiwan. J Infect Dis 2003; 187:134-8; PMID:12508157; http://dx.doi.org/10.1086/345871
- Poovorawan Y, Theamboonlers A, Hirsch P, Vimolket T, Sinlaparatsamee S, Chaiear K, Siraprapasiri T, Khwanjaipanich S, Owatanapanich S, Chunsuttiwat S. Persistence of antibodies to the surface antigen of the hepatitis B virus (anti-HBs) in children subjected to the Expanded Programme on Immunization (EPI), including hepatitis-B vaccine, in Thailand. Ann Trop Med Parasitol 2000; 94:615-21; PMID:11064763
