Abstract
Adjuvants mainly interact with the innate immune response and are used to enhance the quantity and quality of the downstream adaptive immune response to vaccine antigens. Establishing the safety of a new adjuvant-antigen combination is achieved through rigorous evaluation that begins in the laboratory, and that continues throughout the vaccine life-cycle. The strategy for the evaluation of safety pre-licensure is guided by the disease profile, vaccine indication, and target population, and it is also influenced by available regulatory guidelines. In order to allow meaningful interpretation of clinical data, clinical program methodology should be optimized and standardized, making best use of all available data sources. Post-licensure safety activities are directed by field experience accumulated pre- and post-licensure clinical trial data and spontaneous adverse event reports. Continued evolution of safety evaluation processes that keep pace with advances in vaccine technology and updated communication of the benefit-risk profile is necessary to maintain public confidence in vaccines.
Introduction
The thorough evaluation of safety is a crucial element in the development of any new vaccine. Vaccine manufacturers are responsible for the demonstration of safety prior to licensure of new vaccines for public use, and guidelines for the formal evaluation of vaccine safety have been evolving since the early 20th century.Citation1 The safety evaluation of any new product is performed in conjunction with competent Regulatory Authorities.
Despite the fact that the safety of vaccines compares very favorably with that of other pharmaceutical products,Citation2,3 serious side effects have rarely occurred with vaccines, prompting increasingly rigorous evaluation processes. From a public health standpoint, the benefits of vaccination include not only the prevention of disease outbreaks and epidemics, but also the reduction of morbidity and mortality due to the disease itself in the targeted population. From a health economic perspective there are also direct and indirect benefits related to improved quality of life, productivity and reductions in healthcare costs; and from the individual's point of view, the benefits of vaccination may include prevention of the disease and its complications. However, the perceived benefits of vaccination may be less obvious to the public than its real or perceived risks.Citation4
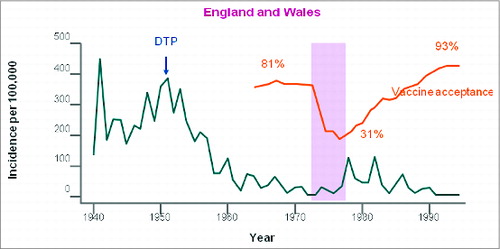
In the past, public perception of a safety risk associated with vaccination has been linked to major decreases in vaccine coverage followed by severe disease outbreaks. For example, in the 1970s, concerns around the safety of whole-cell pertussis vaccines in the United Kingdom led to widespread public distrust of pertussis vaccination with dramatic falls in vaccine coverage, falling to 31% in 1978.Citation5 This was followed by repeated pertussis epidemics on a scale not seen for decades (), with between 17,000 and more than 65,000 pertussis notifications received annually between 1977 and 1983.Citation6 Between 1977 and 1979 alone in England and Wales there were 5000 admissions, and 38 deaths due to pertussis.Citation7 More recently, a preliminary analysis identified that decreased vaccination rates were potentially responsible for the 2015 United States (US) measles outbreak linked to the Disneyland Resort in Anaheim, California.Citation8
Figure 1. Effects of the movement against whole-cell pertussis vaccines on coverage and on disease epidemiology in the United Kingdom and Wales. Reprinted from The Lancet, Vol. 351, Gangarosa EJ, Galazka AM, Wolfe CR, Phillips LM, Gangarosa RE, Miller E, Chen RT, Impact of anti-vaccine movements on pertussis control: the untold story, pages 356–61, Copyright (1998), with permission from Elsevier.
Robust and transparent processes for the assessment of vaccine safety are thus necessary to help to ensure public confidence and continued high uptake of individual vaccines. These processes include continuous dialog with Regulatory Authorities to take into account vaccine-specific and country-specific concerns and requirements.
GSK Vaccines has been manufacturing vaccines for 60 y and produces more than 30 vaccines that are distributed worldwide, many of which contain antigens that require additional components (known as adjuvants) to enhance their immunogenicity; particularly when the antigens are highly purified. Adjuvants (such as aluminum salts) have been used in vaccines since the 1920s.Citation9 Recent advances in our understanding of how the immune system responds to infection now allows adjuvants to be better ‘tailor-made’ to enhance specific components of the immune system, thereby improving their clinical effectiveness. GSK Vaccines has developed a range of proprietary Adjuvant Systems that are used in currently licensed vaccines or in candidate vaccines under development.Citation10 As is the case for any new vaccine, the development of adjuvanted vaccines is accompanied by an exhaustive evaluation of safety which starts in the laboratory, and continues after licensure and throughout the vaccine life cycle. As an expert leader in the field of adjuvant technology and adjuvanted vaccine development, GSK Vaccines is well placed to understand concerns that may arise around the use of new adjuvants and to evaluate and monitor the safety of these vaccines. Here we describe the vaccine safety assessments routinely performed by GSK Vaccines for adjuvanted vaccines.
Vaccines containing adjuvants: key features that guide safety assessments
The first adjuvant used in human vaccines was aluminum salts. Even though the mode of action was not known, aluminum salts were added empirically to vaccines when it was observed that they could increase the antibody response induced by a specific antigen.Citation11 By contrast, modern adjuvants, which may contain several immunoactive components, are selected based on their individual ability to direct and enhance specific immune system functions. Six new adjuvants have been used in licensed vaccines in the last 20 y.9 These include oil-in-water emulsions, which are based on squalene, a naturally occurring oil;Citation12 virosomes, which are spherical phospholipid layers carrying antigen either bound to the surface or encapsulated within the lumen;Citation13 and adjuvant combinations (Adjuvant Systems, AS) such as AS01, AS03 and AS04, which contain several components specifically combined to enhance and modulate the immune responses.Citation14,15
Vaccines destined for use in humans contain carefully selected antigens that are frequently highly purified, as well as other components such as adjuvants, stabilizers, preservatives, and potentially residual trace substances (such as egg proteins, antibiotics, or formaldehyde) left over from the manufacturing process. Safety is rarely assessed specifically on these components alone because they are never administered individually in clinical practice. Rather, safety is assessed on the whole final vaccine product, which is the most clinically relevant evaluation. Each vaccine combination of antigen, adjuvant and excipients is unique, and therefore, each vaccine requires appropriate individual evaluation and characterization in the target population.
Prior to licensure, the disease profile, the vaccine indication (prophylactic, therapeutic), number of doses, route of administration and the population targeted to receive the vaccine (e.g., pediatric age groups, the elderly, immunocom-promised, pregnant women), establish the basis for the safety assessment ().
Figure 2. Factors guiding assessment of adjuvanted vaccine safety:Characteristics of the target population influence initial pre-clinicalevaluations. These features, as well as the results of pre-clinical testing, guide the specific assessment of vaccine safety in clinical trials.
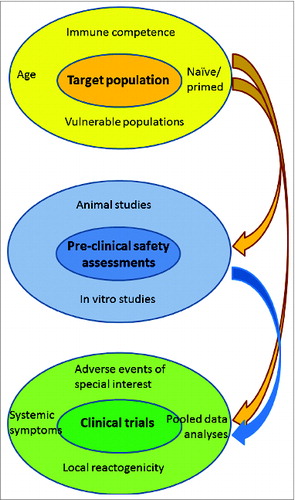
New vaccines are classically tested first in healthy populations (usually adults) and then in the healthy target population (for example, children, adolescents), if this is different. Specific high risk populations (for example, immunocompromised individuals), are usually assessed at a later stage. It is neither feasible nor necessary to test the safety of a vaccine in every possible population, especially those in whom the vaccine will not be used or is contraindicated. However, experience with the vaccine in the ‘real world’ setting after licensure may mean that the assessment of safety in additional populations is necessary ().
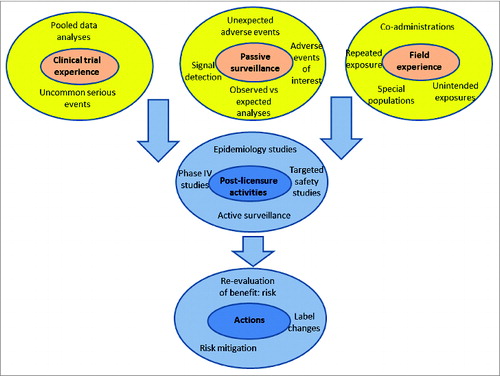
Figure 3. Post-licensure safety assessments are planned based on results of clinical trials, trends identified through spontaneous reports of adverse events after vaccination and field experience with the vaccine.
For example, human papillomavirus (HPV) vaccines are indicated for the prevention of cervical cancer due to HPV in young girls/women; a population that includes women of childbearing age. Pregnant women were excluded from participating in pre-licensure clinical studies because the vaccine is not recommended for use in this population. However, unintended vaccination of women early in pregnancy may occur, and did occur despite precautionary measures to prevent it. Therefore, the evaluation of vaccine safety during pregnancy has been undertaken by manufacturers through pregnancy registries and in post-marketing safety studies.Citation16,17
By contrast, pandemic H1N1 influenza vaccines were recommended for pregnant women who are at high risk for severe influenza. Because of the speed of the pandemic there was not sufficient time to conduct clinical studies in pregnant women, but vaccination was recommended in this group because of the high attendant risk associated with H1N1 infection. Data on the use of H1N1 adjuvanted influenza vaccines in pregnancy was collected, analyzed and acted on during field experience with the vaccine in this population.
The target population intended for vaccination is therefore one of the most important determinants that direct the pre-licensure safety assessment of a new vaccine (). After licensure, safety activities are directed by field experience, accumulated data from clinical trials and through data obtained during commercial use ().
How the characteristics of the target population influence the strategy for safety assessment
The target population drives the evaluation of vaccine safety at many levels. At a cellular level, the ability to respond to an individual vaccine and its reactogenicity and safety profile is influenced by the age of the population: vaccine antigens administered together with adjuvants enhance the activation of innate immune responses in the very young in a manner which promotes T-cell help and development of immune memory.Citation18,19 At the other extreme, the magnitude of humoral and cell-mediated immune responses decreases with age. In the elderly, antibody responses to vaccines are slower and not as strong as those in younger people, and T-cell subpopulations are not very responsive to vaccines, which may impair the ability to achieve protective immunity after vaccination.Citation20,21
At an individual level the assessment of safety may be different in naïve versus primed subjects: for example, pre-existing immunity to a specific antigen may cause lower or higher reactogenicity on re-exposure. The safety evaluation will also be influenced by whether the individual is immune-compromised or has underlying morbidities compared to healthy individuals, and by their physiological status: for example, during pregnancy the maternal immune system is altered to tolerate the semi-allogeneic fetus.Citation22 These alterations include changes in local (decidua) and peripheral immune responses.
At a population level, some age groups may be more susceptible to certain adverse events. For example the occurrence of febrile convulsion peaks at 18 months of age.Citation23 The occurrence of febrile convulsion in this age groups needs careful review to distinguish between a potential risk triggered by fever arising from vaccination, and those occurring coincidentally due to common concurrent infections.Citation24 Pain perception/tolerance also varies with age and gender. Adolescents and young women often report higher frequency and intensity of pain or general symptoms after vaccination than young children or older adults.Citation25,26 Psychogenic events, including vasovagal syncope, panic attacks, and associated symptoms that occur with injectable vaccines and any other injection procedures, are also observed most commonly in adolescents and young women.Citation27
Given the potential variables at a cellular, individual and population level, it is not appropriate to directly extend conclusions regarding the safety profile of one vaccine in a specific population, to another population. Neither is it possible to directly extend conclusions on the safety of one vaccine in a specific population to another vaccine that uses the same adjuvant. Nevertheless, experience with the same adjuvant formulated with other vaccine antigens may be considered as supplementary information for the safety evaluation.
Regulatory aspects relevant to adjuvanted vaccines
Regulatory guidelines for the clinical evaluation of new vaccines are available and are regularly reviewed and updated.Citation28,29 However, because the assessment of vaccine safety is highly product and population specific, these guidelines provide little concrete guidance regarding the safety evaluation. Current guidelines specify that the inclusion of an adjuvant in a vaccine must always be justified. Guidelines from the US Food and Drug Administration (FDA) state that: An adjuvant shall not be introduced into a product unless there is satisfactory evidence that it does not affect adversely the safety or potency of the product.Citation28 Similarly, the European Medicines Agency (EMA) states that: There must be evidence to demonstrate that the benefit in terms of improvement of the immune response has been achieved without an undue increase in local and systemic adverse reactions.Citation30
Adjuvants are not considered to be active ingredients and are not licensed for use alone, but as a specific adjuvant/antigen formulation.Citation31 This is because it is the adjuvanted vaccine formulation in toto, that is tested in clinical trials and that will be administered after licensure.Citation31 There is no a priori regulatory requirement for Phase III studies to demonstrate an incremental benefit of an adjuvanted vs. non-adjuvanted formulation, this because several antigens used in candidate vaccines may not elicit a sufficient immune response if administered alone.Citation31 Safety requirements for vaccine licensure include demonstration that the vaccine is relatively free from harmful effects, and that the evaluation of safety has taken into consideration the character of the product in relation to the condition of the recipient.Citation31 The assessment of safety also implies an evaluation of the benefit-risk ratio: A safe product is one that has reasonable risks, given the magnitude of the benefit expected and the alternatives available. (FDACitation32).
Benefits of adding adjuvants to vaccines
The immune response following exposure to a pathogen (or vaccine) occurs in 2 phases: the initial innate immune response identifies the pathogen and sets off a series of rapid but non-specific pathways that direct the actions of secondary adaptive response. Effectors of the innate immune response include neutrophils, macrophages, monocytes and dendritic cells. Recognition of a pathogen initiates the release of cytokines, complement, and chemokines from the effector cells. These, along with antigen-presenting cells, are responsible for the cross-talk between the innate and adaptive effector cells, and determine the nature and magnitude of the adaptive immune response. Therefore, in most cases the benefit of adding an adjuvant to vaccine arises from its interaction with the innate immune response. By modulating the innate immune response, adjuvants can influence the downstream immune-phenotype, and can contribute to the quality, magnitude and duration of the immune response to the vaccine antigen, and consequently the efficacy of the vaccine.Citation33 This feature of adjuvants is particularly important for vaccines containing highly purified antigens which have poor immunostimulatory capabilities.Citation34 Adjuvants can also therefore improve immunity in populations that respond poorly to unadjuvanted vaccines, including neonates, the elderly and the immunocompromised.
Because the immune response to antigen in the presence of adjuvant is enhanced, a smaller quantity of antigen may be required to induce an effective immune response. This phenomenon is known as ‘antigen sparing’ and allows for the production of more vaccine doses using a fixed quantity of available antigen. Antigen sparing has important implications in settings where shortages of antigen may occur, such as production of influenza vaccines for the global population in the event of a pandemic.Citation35 Adjuvants may also allow extension of vaccine shelf-life by increasing stability, which could also be important in settings of potential vaccine shortage.Citation36,37
Adjuvants are a heterogeneous group of compounds with one or more mechanisms of action, and different adjuvant/antigen combinations will vary in their ability to trigger an immune response. Thus, combinations of adjuvants with antigen may have advantages over a single adjuvant in improving vaccine immunogenicity if the formulation of different compounds can be optimized to induce a synergistic or additive effect to drive the desired immune response.Citation38,39
Approach for evaluating the safety of vaccines containing adjuvants
Each vaccine adjuvant-antigen combination may have a different safety profile and a different benefit-risk ratio (). The benefit-risk ratio may be different for the same vaccines used in a different population that is at higher risk for severe or complicated illness: for example, the potential benefit of influenza vaccination may be greater in the elderly population with comorbidities compared with non-elderly healthy people. Furthermore, the benefit-risk may also change according to the targeted disease: for example, a vaccine against a serious disease with high morbidity/mortality and where no alternative preventive measures are available (such as pandemic influenza), compared to a vaccine against seasonal influenza, a disease that is considered milder, and/or for which other unadjuvanted vaccines are already available.
Figure 4. Factors potentially influencing the influence the benefit-risk profile of adjuvanted vaccines.
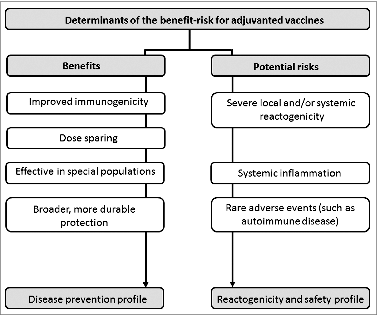
While pooling or extrapolation of data across vaccines or populations is not recommended due to their many inherent differences, information from safety experience obtained with the same adjuvant formulated with another vaccine antigen, or with the same vaccine administered to another population, may be considered as supplementary information in the overall vaccines safety evaluation.
The safety package that supports licensure of a new product is based on pre-clinical and clinical trial data obtained under controlled conditions; that is, in clinical trials where rigorous inclusion and exclusion criteria are applied, where the vaccine cold-chain and storage conditions are strictly monitored, where vaccine administration is performed under conditions controlled in terms of schedule and administration technique, and where subjects undergo systematic safety monitoring.
Pre-clinical safety evaluation
Pre-clinical tests refer to those tests done in vitro or in animal models. These experiments are performed according to recommendations by major regulatory and public health agencies such as the EMA, FDA and World Health Organization (WHO). Pre-clinical studies guide the initial safety evaluation of the vaccine in humans by identifying events of special attention (potential risks) that need to be followed up, and aiding in the planning of studies in humans to identify dose and schedule. Toxicology and safety pharmacology assessment in relevant animal species or in vitro are required before the first-time-in-human clinical studies with a new vaccine. These non-clinical studies guide the safety evaluation of the vaccine in humans by identifying events of special attention that need to be subsequently monitored in clinical studies. In some circumstances, further toxicity testing in animals may occur in parallel with clinical development: for example, reproductive toxicity studies if the vaccine is intended for use during pregnancy.
The potential toxic hazards of a vaccine formulation may be associated with the adjuvant component of the vaccine or the vaccine antigen, and/or the combination of both. Hence, licensing and regulatory authorities have laid out a detailed framework of pre-clinical vaccine testing, recommending that new adjuvants should also be evaluated separately from the vaccines in which they are included. Pre-clinical testing of the adjuvant alone can aid in attributing observed effects to either the antigen or the adjuvant in the final vaccine. To this end a group that receives the adjuvant alone may be included in animal studies to assess any effects of the adjuvant itself.
If a potential safety issue is identified during clinical trials in humans, it may be useful to conduct additional non-clinical research (or re-evaluate safety data from non-clinical studies), to examine potential mechanisms or to look for signals that could have previously gone unrecognized.
Pre-clinical testing has an important role in identifying and potentially elucidating the nature of specific adverse events. The limitations of pre-clinical testing often relate directly to the species used for investigation. This is because disease pathogenesis and immune responses are usually species-specific, and potential safety concerns in animals may not necessarily indicate an issue in humans. Conversely, the absence of safety concerns in an animal model does not rule out an issue in humans. Thus it is only when a new vaccine reaches the clinic that the reactogenicity and safety profile can be definitively explored.Citation40
Various regulatory guidelines for toxicological and pharmacological testing of new vaccines and adjuvants exist.Citation28,30,41,42 However, the approaches vary between authorities and the special challenges associated with the evaluation of adjuvanted vaccines remains at the forefront of ongoing discussions between manufacturers and regulatory agencies.Citation43 In this evolving environment, GSK Vaccines has developed approaches toward non-clinical evaluation that satisfy and expand upon existing regulatory guidelines. For example, pre-clinical assessment of the candidate MAGE-A3 cancer immunotherapeutic which contains the AS15 adjuvant was performed according to EMA and WHO guidelines. However, because multiple doses would be administered in the clinical setting (generally >13 doses being administered in a period longer than 24 months), pre-clinical experiments on repeated dose toxicity were conducted to evaluate the dosing effect as well as the long-term tolerance (including cardiac assessments), adaptive responses and reversibility of effects.Citation44
Mode of action of the adjuvant and implications for safety evaluation
A good understanding of the adjuvant's mode of action can complement safety evaluations and bring valuable insights in the role of the adjuvant in the candidate vaccine's safety profile. For example, characterization of biochemical and hematological markers can identify relationships between adverse events, genetic disposition and individual patient molecular and cellular responses. Identification of molecular responses and the cell populations directly activated by the adjuvant can show whether there is, or is not, a biologically plausible mechanism in which a vaccine or adjuvant could cause a particular adverse event. Much effort may be put into attempting to identify possible associations between a vaccine and the occurrence of a rare but serious adverse event, and unnecessary effort may be able to be avoided if there is a complete lack of biological plausibility for an association.
Clinical safety evaluation
Safety data collected in clinical vaccines trials needs to be based on an appropriate number of subjects and standardized (as far as is possible) to allow meaningful interpretation of the data and comparability between studies using the same vaccine, and ideally with other vaccines ().Citation45-47 Clinical trials need to be appropriately designed in terms of sample size and choice of comparator. It is possible to comprehensively evaluate adverse events that occur at moderate to low frequency events in clinical trials. The EMA recommends that the pre-registration safety database is large enough to detect events occurring at a frequency of between 1/100 and 1/1000 vaccine recipients, with a minimum sample size of 3000.Citation29 Phase III studies are usually large-scale in order to assess safety and efficacy in the relevant population, and often exceed 3000 participants. Larger cohorts are usually needed to identify adverse events that occur at lower frequency. For example, the first licensed tetravalent rhesus-human and reassortant rotavirus vaccine was voluntarily withdrawn because data suggested an increased risk of intussusception in young infants during the week after vaccination.Citation48 The evaluation of this rare event (1:11,000) necessitated some of the largest pre-licensure clinical trials ever conducted. These studies enrolled more than 60,000 children in order to rule out an increased risk of intussusception.Citation49,50 Although no increased risk of intussusception was found in these large trials before licensure, post-licensure data from international settings suggest the possibility of a small increase in the risk of intussusception within 7 d after receiving first dose of rotavirus vaccine (estimated risk in the US of 1 to 3 infants per 100,000 vaccinated).Citation51
Table 1. Safety evaluation during the clinical development program
Comparisons of any adjuvanted vaccine versus saline placebo or unadjuvanted antigen, although preferred for the assessment of safety outcomes, may not be feasible or ethically acceptable. Administration of antigens that are known not to be immunogenic when administered alone is not a useful comparison. Furthermore, when large cohorts are to be enrolled, ethical committees or governing bodies frequently prefer to offer to the control group a licensed vaccine with a well-established safety profile, so that these subjects receive a benefit from study participation. Balanced ratios between vaccine and control groups (1:1 randomization ratio) are preferred for the evaluation of safety outcomes. This is because rare events are more likely to occur in the larger group, making interpretation of the data difficult. Assessment of rare adverse events frequently requires that results from several controlled trials are integrated or pooled, and these trials must therefore be designed in such a way that data pooling is justified. This includes the use of specific statistical methodologies to enable pooling. Ideally, the ratio of vaccine and control recipients in the pooled analysis should be 1:1, but at the very least, a control product exposure that is not less than half that of the candidate vaccine should be maintained.
Many studies utilize Independent Data Monitoring Committees, which are convened to regularly review study data and provide recommendations, including placing the study on hold or stopping the study in the event of a safety concern.
Which data to collect?
Collection of high quality data aims to identify potential adverse events, confirm diagnoses using objective criteria and assess potential relationships with vaccination. Epidemiological information is useful to focus the collection of safety data and aid in the interpretation of safety data during vaccine development. This includes understanding the epidemiology of the disease for which the vaccine is indicated and the characteristics of the population for which it is targeted, including the prevalence of common major comorbidities relative to the general population adjusted for age and sex. Background rates of anticipated events and events of interest may be investigated to permit an assessment of whether rates observed during development or after licensure are as expected, or unexpectedly high. The availability of background adverse events rates is particularly important for uncontrolled clinical trials. Epidemiological data can also inform on the prevalence of risk factors for potential or known adverse reactions associated with the product or the population age, for example, anxiety-related reactions such as vasovagal syncope in adolescents, or the risk of febrile convulsion in infants and young children.
Consideration of these epidemiological data can aid in the identification of adverse events of special interest, which are vaccine and/or population specific and for which close monitoring procedures may be implemented.
Rare adverse events may require specific attention and enhanced data collection methods to ensure that all cases are identified and comprehensively recorded to provide high quality data for interpretation. In addition, aggregating information across studies requires standardized and high quality data collection. To this end, standardized case definitions of a range of adverse events following immunization have been produced by the Brighton Collaboration.Citation52,53 For example, for adjuvanted vaccines there is theoretical concern that the adjuvant may increase the risk of an abnormal immune response which may lead to the development of immune-mediated disease.Citation54,55 Regulatory authorities have therefore introduced special measures for safety assessment of adjuvanted vaccines.Citation30 These include a longer safety follow-up period for these adverse events (typically 12 months) following vaccination.Citation43 A period of 12 months is generally considered a reasonable maximum risk period based on the assumption that autoimmunity after vaccination (should it occur) requires several weeks to develop, but is not likely to occur more than a few months following the last vaccine dose.Citation56 This period is determined based on the likelihood that the development of immune-mediated diseases may require a similar time frame of several weeks (typically 4 to 6 weeks) as that observed for the onset of post-infectious autoimmune diseases: that is, the interval between when an antigenic stimulus is triggered by natural infection, the subsequent generation of an immunologic response to that stimulus, and the onset of signs/symptoms when the disease becomes clinically apparent.Citation24
Vaccine manufacturers actively participate in the conversation on how best to monitor potentially immune mediated diseases. In consultation with experts, GSK Vaccines has developed a paradigm for collection of data relating to autoimmune/neuroinflammatory diseases during clinical trials, which is now implemented in all GSK-sponsored studies evaluating adjuvanted vaccines.Citation56 This approach considers standardized means by which this safety data is obtained to allow its earlier detection and meaningful evaluation.Citation56
Post-licensure safety evaluation
Pre-licensure clinical studies establish the key characteristics of the safety profile of a new vaccine, but these studies have limitations related to their design and sample size. Very rare adverse events cannot be evaluated effectively in controlled clinical trials because of the large numbers or persons required to detect such events. Therefore, it is essential to monitor safety in the larger population exposed after licensure. Rare events may occur temporally with vaccine administration. It is thus important to collect high quality data, and to have a good knowledge of the background incidence of events of interest in the relevant populations, to distinguish events that could be causally associated with the vaccine, from events that are merely coincidental.
Post-registration studies also provide safety information in specific populations that were not included in the pre-registration studies. Some adverse events will predictably increase in these populations relative to what was seen in clinical trials, simply because the background incidence in the specific population is higher than the population as a whole.
Post-licensure epidemiological studies can also address questions not able to be answered prior to licensure or can assess specific adverse events of special interest. These studies can prove challenging to conduct as they require availability of a large vaccinated population, good surveillance mechanisms that are capable of detecting all occurrences of the event, and clear criteria for the diagnosis of the unexpected event under investigation.
In 2010, several retrospective studies suggested an increased risk of narcolepsy among those vaccinated with the AS03-adjuvanted H1N1 pandemic influenza vaccine (Pandemrix™, GSK) and onset of narcolepsy initially in children, and then also in adults. The incidence of narcolepsy in western countries is estimated at 0.74/100,000 person-years.Citation57 Narcolepsy is a complex disease and multiple factors may be involved in its development. The etiology of narcolepsy may include a genetic predisposition strongly associated with an HLA II haplotype (DQB1*0602), and environmental risk factors such as streptococcal infection and viral infections, including H1N1 itself.Citation58,59 Thus, for complex and very rare events such as narcolepsy, further research is required and retrospective studies alone are not sufficient to assess a possible causal association to vaccination. Research should therefore continue into newer sources of data and methods.Citation60,61 Pre-clinical and epidemiological studies are ongoing to further assess the biological plausibility of the observed safety signal, and to better understand the potential contribution of the vaccine and how other factors (i.e., genetic, environmental, circulating infections) may have played a role in the development of narcolepsy. A mimicry-based mechanism could also explain the association between narcolepsy and natural H1N1 influenza infection. For example, a 3-4fold increased incidence of narcolepsy was also observed in 2010 following the H1N1 pandemic wave in China, in a situation of low vaccine coverage (<5 %), with rates of narcolepsy returning to baseline after the pandemic.Citation58
Examples of other rare events that have been described and actively investigated in association with vaccines, include the sevenfold increased risk of Guillain-Barré syndrome (GBS) observed within 6–8 weeks of receiving the A/New Jersey swine vaccine during the United States' 1976 swine flu outbreak.Citation62,63 During the 2009 H1N1 pandemic, GBS was actively monitored as an adverse event of special interest after vaccination, and the available evidence derived from several negative epidemiological studies showed that the benefits of H1N1 vaccines greatly outweighed the potential risks.Citation64-66 Another example is the risk of idiopathic thrombocytopenic purpura shown to be increased in the 6 weeks following the administration of combined measles-mumps-rubella (MMR) vaccination in children, however at a much lower frequency than that observed after natural measles and rubella infection, probably due to the attenuation of the viruses in the MMR vaccines.Citation67,68
As well as epidemiological investigations and post-authorization studies, vaccine safety evaluation includes the continuous review of spontaneous adverse event reports collected post-licensure from worldwide sources that include health care professionals, regulatory authorities, members of the public, and literature sources. These voluntarily reported data are regularly reviewed on several levels: as individual case reports, in quantitative analyses for causality assessment, and systematic reviews of aggregate safety data. These data are used for signal detection and investigation, and can guide the design of targeted studies to assess specific safety endpoints.
Each vaccine license is accompanied by a Risk-Management Plan which outlines the specific strategy for post-marketing evaluation of safety and effectiveness. The benefit-risk profile of each vaccine in specific populations is continually re-assessed as new data become available.
Finally, non-clinical and clinical research may continue after licensure as attempts are made to fully define molecular mechanisms of action, assess factors that influence the quality of the immune response, to validate animal or in vitro models and to find correlations between innate immune parameters and reactogenicity. If a potential safety issue is identified during clinical trials in humans, it may be useful to conduct non-clinical research (or re-evaluate safety data from non-clinical studies), to examine potential mechanisms or to look for signals that could have previously gone unrecognized.
Challenges for evaluating adjuvanted vaccine safety
Adjuvanted vaccines present particular challenges for the assessment of safety. The presence of an adjuvant may increase the theoretical concern for certain adverse effects such as elevated levels of pro-inflammatory and pyrogenic mediators, increased local reactogenicity, breakdown of self-tolerance, as well as unwanted effects due to interactions between antigen and adjuvant-induced mechanisms. There have also been alleged associations between squalene and Gulf War syndrome,Citation69 and aluminum and macrophagic myofasciitis.Citation70 While a link has been demonstrated between vaccination and the presence of granulomas containing aluminum in muscle tissue, no studies in the literature show evidence of a causal association between long-term persistence of aluminum at the injection site, and systemic symptoms or consequences. Although the currently available evidence does not support causal associations between vaccination and the development of immune mediated diseases, the perception of risk remains, and the value placed by the public on scientific evidence can be easily eroded, affecting the acceptance of all vaccines, including adjuvanted vaccines.
Challenges during the pre-clinical phase
Adjuvants can invoke complex immune responses and their precise mode of action is not always known. Appropriate animal models predictive of human responses or disease are infrequently available. This poses challenges not only for accurately identifying the mode of action, but also for assessing potential autoimmunity in an appropriate model.Citation71-73
Challenges during the clinical phase
The use of adjuvants may contribute to the potential for local and/or systemic reactogenicity. Local reactogenicity may increase as a result of increased vascular permeability, cellular infiltration, and fluid accumulation. Symptoms of systemic inflammation may arise if excessive amounts of pro-inflammatory and pyrogenic mediators are released. Combined effect due to interactions between antigen and adjuvant induced mechanisms may increase reactogenicity. Interactions with the innate immune system could theoretically contribute to the break-down of self-tolerance, with induction of auto-reactive cells that could trigger the onset of autoimmune disease. There is also a theoretical risk of adverse immunological responses that may lead to immune-mediated disorders. This could be due to homology of the vaccine antigen to a human protein (antigen mimicry), or non-specific immune enhancement properties of the vaccine adjuvant.Citation46,74 The process of antigen mimicry has frequently been proposed as a potential cause of autoimmunity, and concerns around antigen mimicry have posed obstacles for vaccine design: such as meningococcal serogroup B, where the bacterial polysaccharide resembles molecules present in human neural tissue.Citation75 To date, however, there has not been any reliable scientific evidence to suggest a link between autoimmune disease and vaccination.Citation76
Challenges after licensure
Adverse events of special interest identified during clinical development need to continue to be monitored after licensure. Newly identified potential risks or signals are investigated and actions are taken to mitigate those risks. However, the assessment of rare adverse events may be complex in terms of establishing diagnostic certainty. Furthermore, assessments usually require significant judgment based on available data and information, and a full understanding the possible pathogenic mechanisms and biological plausibility of a possible causal association.
Conclusion
Adjuvants primarily interact with the innate immune response and are rapidly cleared from the injected muscle and draining lymph node. Their rapid, localized and transient effects have been shown to enhance the quality of the downstream adaptive response to improve immunogenicity. Potential benefits include a faster, stronger, broader and more durable immune response, which may or may not be achieved depending on the vaccine and target population. Other potential advantages of adjuvants include antigen sparing, which reduces antigen exposure in the vaccinated individual and allows production of an increased number of doses, and potentially longer shelf life. The availability of new adjuvants and new adjuvant combinations is making possible the development of new vaccines that can help protect against challenging pathogens, and that can improve protection in difficult population groups that typically respond less well to vaccines.
The benefit-risk ratio is both vaccine and population specific, and may be specific to a particular period in time. The evaluation of vaccine safety begins during development in the laboratory and continues and evolves throughout the life of the product in response to study results and post-marketing experiences. Vaccine safety is closely monitored at each stage of development and after licensure so that unexpected events are detected early and measures taken to limit any risk to the target population. The benefit-risk profile is continuously assessed, managed, and communicated by vaccine manufacturers and Regulators throughout the product's lifecycle.
With respect to adjuvanted vaccines, increased reactogenicity, primarily at the injection site, has been consistently observed for adjuvanted vaccines compared with those that are non-adjuvanted. However these local symptoms are generally mild to moderate in intensity and of short duration, and usually do not impact compliance with dosing schedules. Licensed adjuvanted vaccines have been shown to have clinically acceptable benefit-risk profiles, and concerns that adjuvanted vaccines may be associated with an increase in autoimmune disease have been unfounded.Citation77,78
The safety evaluation of an adjuvanted vaccine is ultimately based on the final vaccine (antigen and adjuvant). Collaborations between manufacturers and regulatory authorities aim to develop safety evaluation programs specific to a particular vaccine or population. In recent decades increased efforts have been made by manufacturers and independent groups to standardize methodologies and case definitions of adverse events to allow comparisons and improve assessments of causal associations. Continued evolution of safety evaluation processes that keep pace with advances in vaccine technology and timely communication are essential to maintain public confidence in vaccines, and ensure the public health benefits that high coverage of available vaccines can provide.
Coming years will see licensure of an increasing number of new vaccines containing novel adjuvants, and important experience in the use of these products will be gained. Results of large safety studies and post-marketing surveillance activities currently being undertaken as part of the post-licensure strategies for safety monitoring of adjuvanted vaccines will become available.Citation77 Additional measures that could aid in the interpretation of safety data could include the use of targeted laboratory screening assessment and the use of biomarkers and the storage of banked serum/tissue specimens. These activities remain experimental and under discussion within the scientific community,Citation43 and their utility will be explored in coming years.
Disclosure of Potential Conflicts of Interest
FT, ADiP, and JY were all employed by GSK Vaccines at the time of writing, and hold restricted shares in the company as part of their employee remuneration. NG holds patents, issued by GSK, in the adjuvant systems AS01, AS03, AS04 and AS15; and also holds restricted shares in GSK Vaccines which were issued as part of her remuneration when previously employed by the company.
Authors' Contributions
All authors collaborated in the conception and design of this manuscript. FT and NG actively contributed to the guidelines, definitions and methodology. JY and ADiP ensured that the paper highlighted aspects of adjuvanted vaccine safety assessment of interest for the health care professional community. All authors had full access to the data and the corresponding author was responsible for submission of the publication.
Acknowledgments
Writing services were provided by Joanne Wolter (independent medical writer, Brisbane, Australia); editing and publication co-ordinating services were provided by Veronique Delpire and Mandy Payne (Words &Science, Brussels, Belgium).
Funding
GlaxoSmithKline Biologicals SA was the funding source and funded all costs associated with the development and the publishing of the present manuscript.
References
- Baylor N, Midthun K. Regulation and Testing of Vaccines. In: Plotking SA, Orenstein WA, eds. Vaccines 5th ed. Philadelphia: Saunders; 2004
- Freed GL, Katz SL, Clark SJ. Safety of vaccinations. Miss America, the media, and public health. JAMA J Am Med Assoc 1996; 276:1869-72; http://dx.doi.org/10.1001/jama.1996.03540230019013
- Ward BJ. Vaccine adverse events in the new millennium: is there reason for concern? Bull WHO 2000; 78:205-15; PMID:10743286
- Song G. Understanding public perceptions of benefits and risks of childhood vaccinations in the United States. Risk Anal Off Publ Soc Risk Anal 2014; 34:541-55; http://dx.doi.org/10.1111/risa.12114
- Gangarosa EJ, Galazka AM, Wolfe CR, Phillips LM, Gangarosa RE, Miller E, Chen RT. Impact of anti-vaccine movements on pertussis control: the untold story. Lancet 1998; 351:356-61; PMID:9652634; http://dx.doi.org/10.1016/S0140-6736(97)04334-1
- Whooping cough (pertussis) statistics – Publications – GOV.UK.; [cited 2014 Nov 21]; Available from: https://www.gov.uk/government/publications/whooping-cough-pertussis-statistics
- Amirthalingam G, Gupta S, Campbell H. Pertussis immunisation and control in England and Wales, 1957 to 2012: a historical review. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull 2013; 18:20587; PMID:24084340
- Majumder MS, Cohn EL, Mekaru SR, Huston JE, Brownstein JS. Substandard Vaccination Compliance and the 2015 Measles Outbreak. JAMA Pediatr 2015; 169(5):494-495; PMID:25774618
- Garcon N, Leroux-Roels G, Cheng W. Vaccine adjuvants. In: Understanding Modern Vaccines: Perspectives in Vaccinology. Elsevier; 2011. 89-113
- Garçon N, Chomez P, Van Mechelen M. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines 2007; 6:723-39; PMID:17931153; http://dx.doi.org/10.1586/14760584.6.5.723
- Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol 2009; 9:287-93; PMID:19247370; http://dx.doi.org/10.1038/nri2510
- Fox CB, Haensler J. An update on safety and immunogenicity of vaccines containing emulsion-based adjuvants. Expert Rev Vaccines 2013; 12:747-58; PMID:23885820; http://dx.doi.org/10.1586/14760584.2013.811188
- Moser C, Müller M, Kaeser MD, Weydemann U, Amacker M. Influenza virosomes as vaccine adjuvant and carrier system. Expert Rev Vaccines 2013; 12:779-91; PMID:23885823; http://dx.doi.org/10.1586/14760584.2013.811195
- Garçon N, Vaughn DW, Didierlaurent AM. Development and evaluation of AS03, an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion. Expert Rev Vaccines 2012; 11:349-66; PMID:22380826; http://dx.doi.org/10.1586/erv.11.192
- Garçon N, Morel S, Didierlaurent A, Descamps D, Wettendorff M, Van Mechelen M. Development of an AS04-adjuvanted HPV vaccine with the adjuvant system approach. BioDrugs Clin Immun Biopharm Gene Ther 2011; 25:217-26; PMID:21815697
- Bonanni P, Cohet C, Kjaer SK, Latham NB, Lambert P-H, Reisinger K, Haupt RM. A summary of the post-licensure surveillance initiatives for GARDASIL/SILGARD. Vaccine 2010; 28:4719-30; PMID:20451636; http://dx.doi.org/10.1016/j.vaccine.2010.04.070
- Angelo M-G, Taylor S, Struyf F, Tavares Da Silva F, Arellano F, David M-P, Dubin G, Rosillon D, Baril L. Strategies for continuous evaluation of the benefit-risk profile of HPV-16/18-AS04-adjuvanted vaccine. Expert Rev Vacc 2014; 13:1297-306; http://dx.doi.org/10.1586/14760584.2014.959931
- Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-α induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol Baltim Md 1950 2004; 173:4627-34
- Wood N, Siegrist C-A. Neonatal immunization: where do we stand? Curr Opin Infect Dis 2011; 24:190-5; PMID:21415741; http://dx.doi.org/10.1097/QCO.0b013e328345d563
- Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, Chen S, Towle V, Belshe RB, Fikrig E, et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol Baltim Md 1950 2010; 184:2518-27
- Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol 2007; 211:144-56; PMID:17200946; http://dx.doi.org/10.1002/path.2104
- La Rocca C, Carbone F, Longobardi S, Matarese G. The immunology of pregnancy: Regulatory T cells control maternal immune tolerance toward the fetus. Immunol Lett 2014; 162:41-8; http://dx.doi.org/10.1016/j.imlet.2014.06.013
- Waruiru C, Appleton R. Febrile seizures: an update. Arch Dis Child 2004; 89:751-6; PMID:15269077; http://dx.doi.org/10.1136/adc.2003.028449
- Rowhani-Rahbar A, Fireman B, Lewis E, Nordin J, Naleway A, Jacobsen SJ, Jackson LA, Tse A, Belongia EA, Hambidge SJ, et al. Effect of age on the risk of Fever and seizures following immunization with measles-containing vaccines in children. JAMA Pediatr 2013; 167:1111-7; PMID:24126936; http://dx.doi.org/10.1001/jamapediatrics.2013.2745
- Klein SL, Pekosz A. Sex-based biology and the rational design of influenza vaccination strategies. J Infect Dis 2014; 209 3:S114-9; PMID:24966191; http://dx.doi.org/10.1093/infdis/jiu066
- Herzog C. Influence of parenteral administration routes and additional factors on vaccine safety and immunogenicity: a review of recent literature. Expert Rev Vaccines 2014; 13:399-415; PMID:24512188; http://dx.doi.org/10.1586/14760584.2014.883285
- Braun MM, Patriarca PA, Ellenberg SS. Syncope after immunization. Arch Pediatr Adolesc Med 1997; 151:255-9; PMID:9080932; http://dx.doi.org/10.1001/archpedi.1997.02170400041007
- Food and Drug Administration. Guidance for industry for the evaluation of combination vaccines for preventable diseases: production, testing and clinical studies. Food Drug Admin 1997. [cited 2014 Jul 27]. Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/UCM175909.pdf
- European Medicines Agency. Committee for medicinal products for human use. Guideline on Clinical Evaluation of New Vaccines. London: 2006; [cited 2014 Jul 27]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003870.pdf
- European Medicines Agency. Committee for medicinal products for human use. Guideline on Adjuvants in Vaccines for Human Use. London: 2005; [cited 2014 Jul 27]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003809.pdf
- Gruber M. Regulatory Pathways Supporting Development and Approval of Vaccines formulated with Novel Adjuvant: Regulatory Considerations and Challenges. 2012; [cited 2014 Jul 28]; Available from: http://www.fda.gov/downloads/EmergencyPreparedness/MedicalCountermeasures/UCM292045.pdf
- Report to the FDA Commissioner From the Task Force on Risk Management. Safety of specific products – current risk management for medical products.; [cited 2014 Oct 6]; Available from: http://www.fda.gov/Safety/SafetyofSpecificProducts/ucm180580.htm
- Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity 2010; 33:492-503; PMID:21029960; http://dx.doi.org/10.1016/j.immuni.2010.10.002
- Leroux-Roels G. Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine 2010; 28 3:C25-36; PMID:20713254; http://dx.doi.org/10.1016/j.vaccine.2010.07.021
- WHO | Global pandemic influenza action plan to increase vaccine supply. WHO; [cited 2012 Apr 1]; Available from: http://www.who.int/csr/resources/publications/influenza/WHO_CDS_EPR_GIP_2006_1/en/index.html
- Launay O, Duval X, Fitoussi S, Jilg W, Kerdpanich A, Montellano M, Schwarz TF, Watanveerade V, Wenzel JJ, Zalcman G, et al. Extended antigen sparing potential of AS03-adjuvanted pandemic H1N1 vaccines in children, and immunological equivalence of two formulations of AS03-adjuvanted H1N1 vaccines: results from two randomised trials. BMC Infect Dis 2013; 13:435; PMID:24041010; http://dx.doi.org/10.1186/1471-2334-13-435
- Schijns VEJC, Lavelle EC. Trends in vaccine adjuvants. Expert Rev Vaccines 2011; 10:539-50; PMID:21506650; http://dx.doi.org/10.1586/erv.11.21
- Mount A, Koernig S, Silva A, Drane D, Maraskovsky E, Morelli AB. Combination of adjuvants: the future of vaccine design. Expert Rev Vaccines 2013; 12:733-46; PMID:23885819; http://dx.doi.org/10.1586/14760584.2013.811185
- Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol 2007; 5:505-17; PMID:17558426; http://dx.doi.org/10.1038/nrmicro1681
- Shanks N, Greek R, Greek J. Are animal models predictive for humans? Philos Ethics Humanit Med PEHM 2009; 4:2; http://dx.doi.org/10.1186/1747-5341-4-2
- European Medicines Agency.: Committee for Proprietary Medicinal Products. Note for guidance on preclinical pharmacological and toxicological testing of vaccines London: 1997; [cited 2014 Jul 27]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500004004.pdf
- WHO. World health Organization. Guidelines on the Nonclinical Evaluation of Vaccine Adjuvants and Adjuvanted Vaccines. Switzerland: 2013; [cited 2014 Oct 8]. Available from: http://www.who.int/biologicals/areas/vaccines/ADJUVANTS_Post_ECBS_edited_clean_Guidelines_NCE_Adjuvant_Final_17122013_WEB.pdf
- Mastelic B, Garçon N, Del Giudice G, Golding H, Gruber M, Neels P, Fritzell B. Predictive markers of safety and immunogenicity of adjuvanted vaccines. Biol J Int Assoc Biol Stand 2013; 41:458-68
- Destexhe E, Grosdidier E, Baudson N, Forster R, Gerard C, Garçon N, Segal L. Non-clinical safety evaluation of single and repeated intramuscular administrations of MAGE-A3 Cancer Immunotherapeutic in rabbits and cynomolgus monkeys. 2015; 35:717-28
- Garçon N, Segal L, Tavares F, Van Mechelen M. The safety evaluation of adjuvants during vaccine development: the AS04 experience. Vaccine 2011; 29:4453-9; PMID:21527299; http://dx.doi.org/10.1016/j.vaccine.2011.04.046
- Leroux-Roels G, Bonanni P, Tantawhichien T, Zepp F. Vaccine Development. In, Understanding Modern Vaccines: Perspectives in Vaccinology. Garcon N, Stern PL, Cunningham AL, Stanberry LR, eds. Elsevier B.V; 2011
- Crowe BJ, Xia HA, Berlin JA, Watson DJ, Shi H, Lin SL, Kuebler J, Schriver RC, Santanello NC, Rochester G, et al. Recommendations for safety planning, data collection, evaluation and reporting during drug, biologic and vaccine development: a report of the safety planning, evaluation, and reporting team. Clin Trials Lond Engl 2009; 6:430-40; http://dx.doi.org/10.1177/1740774509344101
- Centers for Disease Control and Prevention (CDC). Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep 1999; 48:1007; PMID:10577495
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11-22; PMID:16394298; http://dx.doi.org/10.1056/NEJMoa052434
- Vesikari T, Itzler R, Karvonen A, Korhonen T, Van Damme P, Behre U, Bona G, Gothefors L, Heaton PM, Dallas M, et al. RotaTeq, a pentavalent rotavirus vaccine: efficacy and safety among infants in Europe. Vaccine 2009; 28:345-51; PMID:19879226; http://dx.doi.org/10.1016/j.vaccine.2009.10.041
- Centers for Disease Control and Prevention. Rotavirus. Vaccine Saf 2014. [cited 2015 Jan 6]; Available from: http://www.cdc.gov/vaccinesafety/vaccines/rotavsb.html
- Bonhoeffer J, Kohl K, Chen R, Duclos P, Heijbel H, Heininger U, Jefferson T, Loupi E. The Brighton Collaboration: addressing the need for standardized case definitions of adverse events following immunization (AEFI). Vaccine 2002; 21:298-302; PMID:12450705; http://dx.doi.org/10.1016/S0264-410X(02)00449-8
- Kohl KS, Magnus M, Ball R, Halsey N, Shadomy S, Farley TA. Applicability, reliability, sensitivity, and specificity of six Brighton Collaboration standardized case definitions for adverse events following immunization. Vaccine 2008; 26:6349-60; PMID:18805456; http://dx.doi.org/10.1016/j.vaccine.2008.09.002
- Beutler B. Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunol Rev 2009; 227:248-63; PMID:19120489; http://dx.doi.org/10.1111/j.1600-065X.2008.00733.x
- Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol 2006; 6:823-35; PMID:17063184; http://dx.doi.org/10.1038/nri1957
- Tavares Da Silva F, De Keyser F, Lambert P-H, Robinson WH, Westhovens R, Sindic C. Optimal approaches to data collection and analysis of potential immune mediated disorders in clinical trials of new vaccines. Vaccine 2013; 31:1870-6; PMID:23391600; http://dx.doi.org/10.1016/j.vaccine.2013.01.042
- Longstreth WT Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep 2007; 30:13-26; PMID:17310860
- Han F, Lin L, Warby SC, Faraco J, Li J, Dong SX, An P, Zhao L, Wang LH, Li QY, et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol 2011; 70:410-7; PMID:21866560; http://dx.doi.org/10.1002/ana.22587
- De la Herrán-Arita AK, Kornum BR, Mahlios J, Jiang W, Lin L, Hou T, Macaubas C, Einen M, Plazzi G, Crowe C, et al. CD4+ T Cell Autoimmunity to Hypocretin/Orexin and Cross-Reactivity to a 2009 H1N1 Influenza A Epitope in Narcolepsy. Sci Transl Med 2013; 5:216ra176; PMID:24353159
- Van der Most R, Van Mechelen M, Destexhe E, Wettendorff M, Hanon E. Narcolepsy and A(H1N1)pdm09 vaccination: shaping the research on the observed signal. Hum Vaccines Immunother 2014; 10:572-6; http://dx.doi.org/10.4161/hv.27412
- European Centre for Disease Prevention and Control. Narcolepsy in association with pandemic influenza vaccination. A multi-country European Epidemiological Investigation. Stockholm: ECDC; 2012
- Langmuir AD. Guillain-Barré syndrome: the swine influenza virus vaccine incident in the United States of America, 1976–77: preliminary communication. J R Soc Med 1979; 72:660-9; PMID:552571
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, Keenlyside RA, Ziegler DW, Retailliau HF, Eddins DL, Bryan JA. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am J Epidemiol 1979; 110:105-23; PMID:463869
- Dodd CN, Romio SA, Black S, Vellozzi C, Andrews N, Sturkenboom M, Zuber P, Hua W, Bonhoeffer J, Buttery J, et al. International collaboration to assess the risk of Guillain Barré Syndrome following Influenza A (H1N1) 2009 monovalent vaccines. Vaccine 2013; 31:4448-58; PMID:23770307; http://dx.doi.org/10.1016/j.vaccine.2013.06.032
- Isai A, Durand J, Le Meur S, Hidalgo-Simon A, Kurz X. Autoimmune disorders after immunisation with Influenza A/H1N1 vaccines with and without adjuvant: EudraVigilance data and literature review. Vaccine 2012; 30:7123-9; PMID:23022149; http://dx.doi.org/10.1016/j.vaccine.2012.09.032
- Romio S, Weibel D, Dieleman JP, Olberg HK, de Vries CS, Sammon C, Andrews N, Svanström H, Mølgaard-Nielsen D, Hviid A, et al. Guillain-Barré syndrome and adjuvanted pandemic influenza A (H1N1) 2009 vaccines: a multinational self-controlled case series in Europe. PloS One 2014; 9:e82222; PMID:24404128; http://dx.doi.org/10.1371/journal.pone.0082222
- Miller E, Waight P, Farrington CP, Andrews N, Stowe J, Taylor B. Idiopathic thrombocytopenic purpura and MMR vaccine. Arch Dis Child 2001; 84:227-9; PMID:11207170; http://dx.doi.org/10.1136/adc.84.3.227
- Sauvé LJ, Scheifele D. Do childhood vaccines cause thrombocytopenia? Paediatr Child Health 2009; 14:31-2; PMID:19436461
- Lippi G, Targher G, Franchini M. Vaccination, squalene and anti-squalene antibodies: facts or fiction? Eur J Intern Med 2010; 21:70-3; PMID:20206873; http://dx.doi.org/10.1016/j.ejim.2009.12.001
- WHO | Statement from the Global Advisory Committee on Vaccine Safety on aluminium-containing vaccines. WHO; [cited 2014 Mar 20]; Available from: http://www.who.int/vaccine_safety/committee/topics/aluminium/statement_112002/en/
- Wraith DC, Goldman M, Lambert P-H. Vaccination and autoimmune disease: what is the evidence? Lancet 2003; 362:1659-66; PMID:14630450; http://dx.doi.org/10.1016/S0140-6736(03)14802-7
- Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol 2007; 120:506-15; PMID:17765751; http://dx.doi.org/10.1016/j.jaci.2007.07.033
- Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain J Neurol 2006; 129:1953-71; http://dx.doi.org/10.1093/brain/awl075
- Offit P, Davis RL, Gust D. Safety of immunizations. In Vaccines. Plotkin SA, Orenstein WA, Offit PA eds. Philadelphia: Elsevier B.V; 2008
- Lo H, Tang CM, Exley RM. Mechanisms of avoidance of host immunity by Neisseria meningitidis and its effect on vaccine development. Lancet Infect Dis 2009; 9:418-27; PMID:19555901; http://dx.doi.org/10.1016/S1473-3099(09)70132-X
- De Martino M, Chiappini E, Galli L. Vaccines and autoimmunity. Int J Immunopathol Pharmacol 2013; 26:283-90; PMID:23755743
- Angelo M-G, Zima J, Tavares Da Silva F, Baril L, Arellano F. Post-licensure safety surveillance for human papillomavirus-16/18-AS04-adjuvanted vaccine: more than 4 years of experience. Pharmacoepidemiol Drug Saf 2014; 23:456-65; PMID:24644078; http://dx.doi.org/10.1002/pds.3593
- Verstraeten T, Descamps D, David M-P, Zahaf T, Hardt K, Izurieta P, Dubin G, Breuer T. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine 2008; 26:6630-8; PMID:18845199; http://dx.doi.org/10.1016/j.vaccine.2008.09.049
