Abstract
Routine annual influenza immunization is increasingly recommended in young children. We compared the safety and immunogenicity of vaccination with trivalent inactivated influenza vaccine (TIV) versus MF59-adjuvanted TIV (aTIV) in children who received 2 half or full doses of aTIV or TIV, or non-influenza control vaccine, in an efficacy trial conducted 2 years earlier. 197 healthy children aged 30–96 months were randomized to receive vaccination with aTIV or TIV in 2010. To evaluate responses to the first follow-up seasonal vaccination after priming we excluded children who received influenza vaccine(s) in the 2009 pandemic year leaving 40 children vaccinated with aTIV, 26 children with TIV and 10 children with aTIV after a control vaccine in the parent study. Hemagglutination inhibiting antibodies were assayed on Days 1, 22 and 181. aTIV vaccination produced 6.9 to 8.0-fold higher antibody responses than the reference TIV-TIV regimen against A/H3N2 and B strains, which remained higher 6 months following vaccination. The response to the B/Victoria lineage antigen in the second year's vaccine (the first vaccine contained a B/Yamagata lineage antigen) demonstrated that aTIV primed for an adequate response after a single dose on Day 22 (GMTs 160, 95 to antigens in the 2 lineages, respectively), whereas TIV did not (GMTs 38, 20). Vaccination with aTIV produced slightly higher but acceptable local and systemic reactogenicity compared to TIV-TIV and TIV-aTIV mixed regimens. Within the limitations of a small study, the strong immune responses support the use of aTIV for vaccination in young children.
Abbreviations
| AE | = | adverse event |
| aTIV | = | MF59-adjuvanted trivalent inactivated influenza vaccine |
| CBER | = | Center for Biologics Evaluation & Research |
| CHMP | = | European Committee for Medicinal Products for Human Use |
| FAS | = | full analyses set |
| HI | = | hemagglutination inhibition |
| CI | = | confidence interval |
| LAIV | = | live-attenuated influenza vaccine |
| GMR | = | geometric mean ratio |
| GMT | = | geometric mean titer |
| HI | = | hemagglutination inhibition |
| TIV | = | trivalent inactivated influenza vaccine |
| SAE | = | serious adverse event |
| SD | = | standard deviation |
Introduction
Routine annual influenza immunization of children is recommended in the USA and Finland where nearly half of children who are hospitalized for influenza were previously healthy, without recognized risk factors.Citation1-5 Similarly, 40% of fatal influenza cases in children occur in previously healthy children.Citation6 These characteristics of pediatric influenza support the universal use of influenza vaccine in children at an early age with annual revaccinations.
Although the medical need for routine influenza immunization in children is clear, available vaccines have limitations; trivalent inactivated influenza vaccines (TIV) have less than optimal efficacy and live-attenuated influenza vaccine (LAIV) has restrictions for children under 24 months years of age because of safety concerns.Citation7–9 While TIV may be ∼40–60% efficacious in children under 6 years old, there is no clear evidence for efficacy in the youngest age group, 6–23 month-olds.Citation8,9 LAIV has been shown to be more efficacious than TIV in 2–7 year-olds, but cannot be used in children under 5 years of age who have a history of wheezing, and not at all in children under 24 months of age.Citation7,10
The use of adjuvants such as MF59 can significantly enhance the immune response in previously unvaccinated children, with protective antibodies levels being achieved earlier and lasting longer than with non-adjuvanted TIV.Citation11,12 MF59 also stimulates a broader immune response that may provide for increased efficacy against heterovariant strains.Citation13-15 MF59® (Novartis Vaccines) was the first oil-in-water emulsion adjuvant included in a licensed vaccine, the adjuvanted seasonal TIV (aTIV) indicated for older adults (Fluad® Novartis Vaccines).Citation16,17
We previously demonstrated in a head to head trial in vaccine-naïve 6–71 month old children (conducted principally in the 2007–2008 season) that aTIV had 86% efficacy in preventing laboratory-confirmed influenza while confirming in the same trial the modest efficacy of TIV, at 42%.Citation11 This phase 3b extension study was conducted to assess the safety and immunogenicity of vaccination with aTIV vs. TIV (Agrippal®, Novartis Vaccines) in children from the previous efficacy trial, now at 30– 96 months of age.Citation11 The 2009 H1N1 pandemic prevented us from evaluating the vaccination response in the season immediately following the efficacy trial. All subjects who received either seasonal or monovalent pandemic vaccine in 2009 were excluded in this study analysis, in order to more clearly demonstrate the immune response of re-vaccination in the first follow-up seasonal vaccination after priming. The vaccine strains in 2010–2011 [A/ California/7/2009 (H1N1), A/ Perth/16/2009 (H3N2) and B/Brisbane/60/2008 (Victoria)] were antigenically distinct from those in the priming vaccine in 2008–2009 [A/Brisbane/59/2007 (H1N1), A/Brisbane/10/2007 (H3N2), and B/Florida/4/2006 (Yamagata)], providing us with the opportunity to examine prime to boost responses in young children following a change in B antigens, from B/Yamagata lineage to B/Victoria lineage strain.
Results
Subjects
Fewer subjects were enrolled in the extension study than the originally planned 1970 subjects, due to parental concerns in Finland with narcolepsy associated with an AS03-adjuvanted H1N1 vaccine that was used in several European countries including Finland during the 2009 H1N1 influenza pandemic.Citation18
In total, 197 subjects were enrolled and were categorized into 2 age cohorts, 6 -< 36 months (n = 29) and 36 -< 96 months (n = 168). The younger age group was too small to draw conclusions on the immune response so the age cohorts were combined and results were grouped by vaccine received. Of the 197 subjects that were enrolled and randomized to receive aTIV (n = 135) and TIV (n = 62), 188 completed the study. Reasons for premature withdrawal were withdrawal of consent (N = 4) and loss to follow-up (N = 5) (). To more clearly demonstrate the immune responses to vaccination in follow-up seasonal vaccination after priming, all subjects who received influenza vaccine in 2009, seasonal or monovalent pandemic, were excluded from the immunogenicity and safety analyses presented in this report. Ultimately, 40 children revaccinated with aTIV, 26 children revaccinated with TIV, and 10 control children receiving influenza vaccine for the first time (and were administered aTIV) were included in the analyses for this study ().
The baseline demographic characteristics of the enrolled subjects by priming and subsequent vaccines are presented in . Vaccine groups were comparable with respect to age, body size characteristics, and the majority of study subjects were Caucasian. There was a higher proportion of girls in the children primed with TIV and revaccinated with TIV and those who previously received the control non-influenza vaccines and were vaccinated with aTIV.
Table 1. Study population demographics by priming vaccine in the original efficacy study and vaccination with aTIV or TIV
Immunogenicity analyses
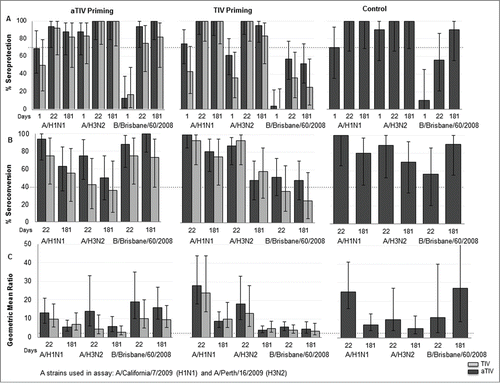
Evaluation of immunogenicity criteria according to European Committee for Medicinal Products for Human Use (CHMP) criteria is presented in . Subjects who were primed with aTIV and TIV and vaccinated with aTIV in the extension study met all 3 CHMP criteria for seroprotection (>70%), seroconversion (>40%) and geometric mean ratio (GMR) (>2.5) against the 3 homologous strains with persistence up to 6 months following vaccination. Subjects who were primed with TIV and vaccinated with aTIV or TIV in the extension study met all 3 CHMP criteria against A/California/7/2009 (A/H1N1) and A/Perth/16/2009 (A/H3N2) and 2 out of 3 criteria (vaccination aTIV) and 1 out of 3 criteria (vaccination TIV) against B/Brisbane/60/2008 (Victoria). Children who were previously vaccinated with non-influenza control vaccine and vaccinated with aTIV met all CHMP criteria except for seroprotection against B/Brisbane/60/2008 (Victoria) at Day 22. When using Center for Biologics Evaluation & Research (CBER) criteria, only the subjects who were primed with aTIV and revaccinated with aTIV met both CBER criteria for seroconversion and seroprotection at Day 22 ().
Figure 2. Homologous antibody responses in children 30-< 96 months old vaccinated with aTIV or TIV, by priming vaccine. Dashed lines indicates Committee for Medicinal products for Human Use (CHMP) criterion; the percentage of subjects achieving seroprotection (HI antibody titer ≥40) should be >70%, the percentage of subjects achieving seroconversion should be ≥40% and geometric mean ratio should be >2.5. None of the subjects were vaccinated with seasonal or pandemic vaccine in 2009. Children vaccinated with non-influenza control during parent study only received aTIV in the extension study.
Percentages of subjects achieving a more stringent cut-off criterion hemagglutination inhibition (HI) titer ≥110 are presented in . In children primed with aTIV, HI titers ≥110 at Day 22 were achieved in 94% (A/H1N1), 100% (A/H3N2) and 69% (B/Brisbane/60/2008) after vaccination with aTIV and 75% (A/H1N1), 100% (A/H3N2) and 58% (B/Brisbane/60/2008) following vaccination with TIV. In TIV-primed children, the proportion achieving HI titres of ≥110 were 91%, 100% and 22% (vaccination aTIV) and 100%, 71% and 0% (vaccination TIV) against A/H1N1, A/H3N2 and B strains, respectively. In children previously vaccinated with non-influenza control and vaccinated with aTIV, 89% achieved a titer ≥110 to A/H1N1, 100% to H3N2 and 33% to B/Brisbane/60/2008 (Victoria lineage).
Figure 3. Percentages of subjects with HI antibody titers ≥110 in children 30-< 96 months old vaccinated with aTIV or TIV, by priming vaccine. None of the subject was vaccinated with seasonal or pandemic vaccine in 2009. Children vaccinated with non-influenza control during parent study only received aTIV in the extension study.
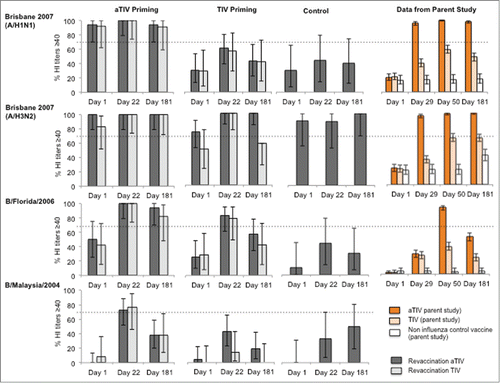
Day 181 HI titers ≥110 were achieved in 24–100% and 8–100% in children after vaccination with aTIV and TIV, respectively ().
Antibody responses by subtype
H1N1
Although subjects who received the 2009 pandemic vaccine were excluded from this analysis, baseline titers in all 3 groups suggested that pandemic exposure had occurred relatively uniformly in all groups, with geometric mean titers (GMTs) in the range of 33 to 59. All groups responded well to their first vaccination against A/California/07/2009 (H1N1) irrespective of having received adjuvanted, non-adjuvanted or no previous influenza vaccine in the earlier study, with 12.6 to 27.9 fold increases in response to aTIV and 9.7 to 24.2 fold increases in response to TIV. Six months later, higher titers, in the range of 1.3 to 1.4 fold higher, were maintained in the aTIV- compared to TIV-vaccinated children ().
Table 2. Geometric mean titers to vaccine strains at Days 1, 22 and 181 in children primed with aTIV or TIV containing A/Brisbane/59/2007 (H1N1), A/Brisbane/10/2007 (H3N2), and B/Florida/4/2006 vaccine and subsequent vaccination in 2010Footnote*
H3N2
Baseline antibody titers were 2.3 to 5.8 fold higher in children who had previously received aTIV compared to children who had been vaccinated originally with TIV. However, better persistence of the antibody-induced response in the aTIV-primed group cannot be inferred because control children who had not previously received influenza vaccine had evidence of baseline H3N2 antibodies at even higher levels, presumably deriving from natural infection. Children primed with aTIV and those who had received control vaccines (and who had evidence of natural infection) responded to aTIV with a 10.4 to 14.3 fold rise to a GMT of ˜2200 at Day 22. Children who had been primed with TIV and then boosted with aTIV exhibited a 17.7 fold rise, though to a lower absolute GMT of 1205 as their baseline titer after TIV priming was lower. The other mixed regimen of aTIV followed by TIV produced an intermediate rise of 4.5 fold to a GMT of 678, while successive TIV vaccinations produced the lowest GMT of 328, albeit as a result of a 13.1 fold rise. aTIV vaccinated children maintained a 2.7 fold higher GMT compared to TIV vaccinated children at 6 months. The GMT of the aTIV-aTIV group (925) was 7.7 fold higher than the TIV-TIV group (120) at that point ().
B
Baseline titers to the Victoria lineage B/Brisbane/60/2008 strain were uniformly low in all groups, including those who had been primed with 2 vaccine doses in 2008–2009, as that formulation contained a B/Yamagata lineage strain. The children who had been primed with TIV and were revaccinated with TIV mounted a suboptimal response against the B/Victoria lineage antigen from GMT 5 (pre-vaccination) to GMT 20 (Day 22 post-vaccination) and GMT 17 (Day 181 post-vaccination). In contrast, aTIV-primed children responded with at least 10-fold rises to the B/Victoria lineage antigen, achieving GMTs of 160 and 95 on Day 22, whether they were revaccinated with adjuvanted or non-adjuvanted vaccine, respectively, with persistence of higher GMTs (135 and 94) up to 6 months following vaccination. The six month GMT of the aTIV-aTIV group was almost 8-fold higher than that of the TIV-TIV group. The responses of the TIV-primed children were intermediate and reached a low level of <40. In unprimed control subjects, who received aTIV in their first influenza vaccination, GMTs at Day 22 and 181 were 74 and 178, respectively. Moderately high responses to the B/Yamagata lineage strain following the initial 2 doses of adjuvanted vaccine indicated priming for the B/Victoria lineage antigen, whereas this was not observed following primary vaccination with TIV. A single vaccination dose of aTIV in the latter subjects compensated to some degree but the cross-lineage titers remained lower than those in children primed with adjuvanted vaccine ().
Safety
Local and systemic adverse reactions across the vaccine groups, by priming vaccine are summarized in (6-<36 months) and (36-<96 months). Overall, reactogenicity of aTIV was slightly higher than for TIV, though not to a marked or clinically unacceptable extent. The majority of children reported at least one reactogenicity sign post-vaccination. Reactions were transient and mostly mild to moderate in severity. In the 6-<36 months cohort, the most common reported local reaction was tenderness with no reports of severe reactions. Most frequent reported systemic reactions were unusual crying and irritability. There was one report of severe fever ≥39.3°C in the youngest age group following vaccination with TIV. In the age group 36-<96 months, the most common reported local reaction was injection site pain reported in 67% to 92% across vaccination groups. There were no reports of severe local reactions. The most commonly reported systemic reaction was fatigue, followed by myalgia. There were 2 reports of severe systemic adverse events (AEs) in subjects receiving aTIV (headache and malaise) and 1 report of severe systemic AE in a subject receiving TIV (myalgia). Fever ≥37.3°C occurred in up to 14% of subjects across groups, with no reports of severe fever.
Table 3. 6-<36 months: Number of subjects experiencing solicited local* and systemic reactions occurring within one week of vaccination, by priming vaccine. Number of local reactions classified as severe are shown in brackets
Table 4. 36-<96 months: Number and percentages of subjects experiencing any and severe solicited local* and systemic reactions occurring within one week of vaccination, by priming vaccine
Spontaneously reported AEs were comparable across vaccine groups, regardless of priming vaccine in the parent study or vaccination with aTIV or TIV. Overall, 59–75% of subjects across groups experienced any unsolicited reaction, of which 17–18% was considered at least possibly related. One subject receiving aTIV experienced a serious adverse event (SAE) (snake bite), considered as not related to study vaccination. There were no deaths and no AEs leading to premature study discontinuation.
In the subpopulation of children that received pandemic AS03-adjuvanted vaccine in 2009, similar trends were observed for immunogenicity and safety outcomes (Tables S1 and S2).
Discussion
Routine influenza vaccination in children, beginning at 6 months of age, is increasingly recommended as children and infants experience the highest rates of seasonal influenza infection and hospitalisation.Citation1-4 We previously showed in a head to head trial in vaccine-naïve 6–71 month old children that aTIV was 86% efficacious in preventing PCR-confirmed influenza, compared with 42% for TIV.Citation11 Moreover, aTIV was efficacious in every age subgroup (96% in 36–71 months olds, 81% in 6–35 months olds, and 75% in 6–23 months olds) at efficacy rates that are in the range expected for a routinely administered childhood vaccine.Citation11 The MF59-adjuvanted vaccine was also well tolerated and in an integrated analysis of 1181 children who received aTIV in clinical trials no pattern of associated serious AEs was demonstrated.Citation16 Because influenza vaccine must be administered annually, if aTIV was to be used in national schedules, data on its immunological advantage as well as its tolerability and safety upon repeated vaccination are needed.
Here we report the results of an extension study of vaccination with aTIV versus TIV in children now aged 30–96 months, whose only influenza vaccination had been in the previous efficacy trial 2 years earlier. The 2009 pandemic prevented us from assessing the vaccination response in the season immediately following the efficacy trial. To examine the immune responses of vaccination in the first follow-up seasonal vaccination after priming, we excluded all subjects who received either seasonal or pandemic vaccination in 2009 from the study analyses. Therefore, only small datasets could be analyzed, which should be taken into consideration in interpreting the results.
The results of this study demonstrate that the aTIV-aTIV sequence produced 6.8-fold and 8-fold higher antibody responses to A/H3N2 and B strains, respectively, than the reference TIV-TIV regimen (referring to the vaccination given in the efficacy study and the current study, respectively), but not to A/H1N1 strain. Also, for A/H3N2 and B strains, primary vaccination with aTIV was found to be more immunogenic than 2 consecutive vaccinations with TIV.
The magnitude of the difference between aTIV-aTIV and TIV-TIV regimen was especially important for influenza B where TIV-TIV provided only a GMT of 20 (Day 22) in the second year while for aTIV-aTIV, the GMT response on Day 22 was 160. For the mixed regimens of aTIV-TIV or TIV-aTIV, the advantage of aTIV in the second year varied with viral subtype, as inferred from elevated baseline GMTs to that viral subtype. For example, the elevated baseline titers and responses to the pandemic H1N1 component in aTIV were similar, irrespective of whether the children had been primed with aTIV, TIV or had had no prior influenza vaccination at all. The uniformly elevated baseline titers in all subjects suggested similar experiences with the pandemic infection in all groups and because the 2008–2009 influenza vaccine contained an H1N1 strain that was so highly divergent from the pandemic H1N1 strain, it provided no additional detectable priming to the naturally-acquired pandemic infection upon H1N1pnd09 immunisation.Citation19,20 Those titers were ∼2-fold higher in the aTIV compared with TIV recipients irrespective of priming history, demonstrating the impact of the adjuvant. However, the sample sizes were small and the confidence intervals between aTIV and TIV overlapped, therefore the difference was not significant.
A similar but more complex pattern was seen in response to the H3N2 antigen against which the aTIV-primed children, and the control children who received aTIV for the first time had similarly elevated baseline and post-vaccination responses to the H3N2 antigen. It is not possible to discriminate whether the elevated baseline titer in the aTIV group may have reflected a declining antibody titer from vaccination 2 years earlier or an intervening H3N2 infection or both. These responses were higher than in the other groups. In the TIV-aTIV children poorer persistence of antibody after priming and, possibly, a lower rate of naturally acquired infection in the intervening year (reflected in a lower baseline GMT) led to an intermediate response to the vaccination with aTIV. In contrast, vaccination with TIV in the aTIV-TIV group led to a lower GMT response, though the group had a similar baseline antibody titer, indicating again, the impact of the adjuvant in the vaccination vaccine. Finally, the TIV-TIV group, whose baseline titers were the lowest, had the lowest response to vaccination.
The response to the B/Victoria lineage antigen in the second year vaccination (while the first vaccine contained a B/Yamagata lineage antigen) showed that aTIV primed with the B/Yamagata lineage antigen boosted the secondary antibody response against the opposing lineage (GMTs 160 for aTIV, 95 for aTIV-TIV), whereas, as noted previously, TIV did not (GMTs 38 for TIV-aTIV and 20 for TIV-TIV).Citation21 However, as cross priming in the other direction was not studied, it would be inappropriate to speculate about cross-lineage responses in general. The cross-lineage priming associated with aTIV, though of great interest, is perhaps moot from a public health perspective, as quadrivalent formulations containing antigens from both B lineages are becoming the new standard for children. We also reconfirmed the phenomenon of original antigenic sin – at least in the direction of stronger responses to a first exposure to B/Yamagata antigen – with stronger responses to the priming B/Yamagata lineage antigen that had been administered 2 years earlier, compared to the response to the more recently administered B/Victoria lineage antigen.Citation22,23
The maintenance of elevated antibodies at high levels for 6 months by the regimen of repeated aTIV administration is of practical importance as annual vaccination campaigns in the Northern Hemisphere now begin in August while the peak month of influenza transmission is in February.Citation24 And in areas of the world where influenza viral transmission is sustained for longer intervals or maintained throughout the calendar year, antibodies induced by a single annual vaccination ideally would persist at protective levels for up to a year.Citation25
The lower absolute levels of HI antibodies to the vaccine-matched and variant B strains, compared with the A subtype responses should be interpreted in the context of the low sensitivity of the HI assay in detecting antibodies to the B vaccine component.Citation26 Compared to HI test results, neutralizing antibody titers elicited by the inactivated vaccine generally are higher and are equally high for B and A components.Citation27,28
Vaccination with aTIV produced slightly higher but acceptable local and systemic reactogenicity compared with repeated TIV vaccination, while the mixed regimens of aTIV-TIV and TIV-aTIV were similar to the “reference” TIV-TIV regimen in terms of the pattern and frequency of local and systemic adverse events. For unsolicited AEs, the pattern and frequency were generally similar between groups. There were no vaccine-related SAEs. Prior to the study, there were concerns of enhanced AEs following revaccination one year following the original study, or when the similar antigenic strains were used in the second round of vaccinations. However, enhanced reactivity was not observed. Thus, within the limited experience of this study with small data sets, the considerably higher antibody titers achieved upon vaccination with aTIV, with a minor increase in reactogenicity, provides a benefit:risk that might be justifiable.Citation29
Higher antibody titers are needed in young children to produce levels of efficacy seen in adults at lower titers of ∼40. In the efficacy study prior to this follow-up, we showed that an HI titer of 110 was correlated with a vaccine efficacy of 50%, and 330 with a clinical efficacy of 80% against the circulating H3N2 strain,Citation12 although this needs to be confirmed in other studies. The demonstration that an HI titer of 40 correlated with 50% protection in children 6–17 years of age supports an age threshold within this range when a majority of children will have been primed – consistent with recommendations in the US that children under 9 years of age need 2 doses in their first season of vaccination in order to prime them.30 Thus the greater reliance on antibodies for protection provided by the inactivated vaccine in young children favors the annual use of aTIV starting at 6 months and through an undefined age, probably in the interval between 6 and 9 years of age.
The impact of influenza vaccination on T-cell immunity in children, and differences elicited by inactivated vs. live attenuated vaccine, are currently important topics in the field of influenza vaccine development. Some investigators have proposed that TIV vaccination of children may prevent the induction of a broad cellular immune response, which could place them at greater risk for pandemic influenza infection, although clinical evidence for this in the 2009 pandemic was unclear.Citation31-38
One limitation of this study was that there was no possibility to investigate the impact of aTIV on cellular immunity, although, in other studies, MF59 is known to stimulate T-cell immunity but not shown to bias the immune response in children toward Th1 or Th2.Citation39-40
Altogether, within the limitations of a small study, the strong immune responses and adequate safety profile of aTIV support the use of such vaccines for annual vaccination in young children, beginning at 6 months of age. Ultimately, a study starting at 6 months of age followed by annual vaccinations using either aTIV or TIV should be designed to prove this assumption.
Methods
Study design
This Phase 3b, randomized, observer-blind, pediatric extension study was performed at 15 sites across Finland from September 2010 until January 2011 (ClinicalTrials.gov identifier: NCT01210898). The protocol was approved by the Ethics Committee of the Pirkanmaa Hospital District, and the study was performed according to the principles of the Declaration of Helsinki and Good Clinical Practice. Before enrollment, written informed consent was obtained from all parents/legal guardians.
Objective
In the previously reported efficacy study (ClinicalTrials.gov: NCT01015885) greater immunogenicity and efficacy were demonstrated for aTIV over split TIV (Influsplit SSW®, GlaxoSmithKline Biologicals) in children aged 6-<72 months in 2008–2009.Citation11 The main objective of this extension study was to evaluate the immunogenicity following vaccination with aTIV or subunit TIV administered as age-dependent full or half doses in 2010–2011 according to the criteria defined by the European CHMP and US. CBER.Citation41,42 All subjects who received either seasonal or monovalent pandemic vaccine in 2009 were excluded from the analyses. Additional objectives were to assess antibody persistence at 6 months following vaccination and to evaluate the reactogenicity and safety profiles for each vaccine. SAEs were collected up to 1 year postvaccination.
Of those subjects receiving pandemic vaccination in 2009 (AS03-adjuvanted containing vaccine Pandemrix®, GSK), safety and immunogenicity against A/H1N1 strain were added as supplementary data
Subjects
Eligible study participants were healthy children who had received 2 doses of at least 1 of the study vaccines in the previous parent trial and whose parents/legal guardians had given prior written informed consent. Principal exclusion criteria included any history or ongoing (chronic) illness likely to interfere with the results; hypersensitivity to vaccine components; receipt of any other vaccine <2 weeks (for inactivated vaccines) or 4 weeks (for live vaccines) prior to enrollment in this study or who were planning to receive any vaccine <4 weeks from the study vaccine; participation in any clinical trial with another investigational product 30 days prior to the first study visit or intention to participate in another clinical study at any time during the conduct of this study; antibiotics <6 days before vaccination; axillary temperature ≥37.3°C within 3 days prior to the first visit, and receipt of blood, blood products and/or plasma derivative or any parenteral immunoglobulin preparation in the previous 12 weeks.
Study procedures
In the original efficacy study, subjects were primed in 2008–09 with 2 age-appropriate doses of TIV, aTIV or noninfluenza control vaccine (Control) vaccines (meningococcal C conjugate [Menjugate®, Novartis Vaccines] or tickborne encephalitis vaccines [Encepur Children®, Novartis Vaccines]). In this study, aTIV- and TIV-primed children were re-randomized to be revaccinated with either aTIV or TIV. Children aged <36 months were randomized 1:1 to receive half a dose (0.25mL) of TIV or aTIV; children ≥36 months of age were randomized 1:1:1 to receive a full dose (0.5mL) of TIV, or a half or full dose of aTIV. Influenza vaccine-naïve control subjects previously vaccinated with control noninfluenza received half a dose of aTIV (children <36 months), or were randomized 1:1 to receive half or full dose of aTIV (children aged ≥36 months). Because the numbers of subjects in these dose subgroups were so small, they were combined within each treatment arm for this paper.
Blood samples (5 mL per sample) were obtained for immunogenicity analyses at baseline (Day 1, prevaccination), 3 weeks after vaccination (Day 22), and approximately 6 months (Day 181) after immunization.
Vaccines
aTIV and TIV each contained 15 μg of hemagglutinin antigen from each of the 3 World Health Organization recommended influenza strains for the 2010–2011 influenza season in the northern hemisphere: A/ California/7/2009 (H1N1), A/ Perth/16/2009 (H3N2) and B/Brisbane/60/2008(Victoria).
A standard dose of the adjuvant MF59, as used in one 0.5-mL dose of the commercially available seasonal influenza vaccine Fluad, contains 9.75 mg squalene, 1.175 mg polysorbate 80, 1.175 mg sorbitan trioleate, 0.66 mg sodium citrate, 0.04 mg citric acid, and water for injection. Vaccines were supplied in monodose, prefilled syringes filled with 0.5 mL with a pediatric mark at 0.25mL for administration in subjects <36 months. The vaccine formulations were prepared and administered by unblinded study personnel, who otherwise did not participate in evaluation of the subjects during the study. The vaccines were administered intramuscularly in the deltoid muscle preferably of the non-dominant arm.
Immunogenicity assessment
Blood samples taken for immunologic assays were tested at the Novartis Vaccines Clinical Serology Laboratory in Marburg, Germany, for HI antibody responses to strains contained in the vaccine (vide supra). HI antibody responses on Days 1, 22, and 181 were expressed as GMTs, GMRs of the postvaccination to prevaccination titer (Day 22/Day 1 titer and Day 181/Day 1 titer); seroprotection rates were defined as the percentage of subjects with HI titers ≥40, and seroconversion rates were defined as the percentage of subjects per group achieving at least a 4-fold increase in HI titer from a seropositive prevaccination titer (≥10) or a rise from <10 to ≥40 in those who were originally seronegative. Percentages of subjects with HI titers ≥110 were also calculated, as this cut-off titer was shown in one analysis to predict a 50% clinical protective level in young children, compared with the conventional HI titer of ≥1:40 associated with 50% protection of young adults.Citation12
Safety assessment
Subjects were monitored for 30 minutes after each vaccination for possible immediate adverse reactions. Parents/legal guardians were unaware of the study vaccine administered to their children and were instructed to complete diary cards to record specified local and systemic reactions for 7 days, starting on the day of vaccination. Solicited local reactions included ecchymosis, erythema, induration, swelling and tenderness (children <36 months) or ecchymosis, erythema, induration, swelling and pain at injection site (children ≥36 months). Solicited systemic reactions were sleepiness, diarrhea, vomiting, irritability, change in eating habits, shivering, and unusual crying (children <36 months of age), or chills, malaise, myalgia, arthralgia, headache and fatigue (children ≥36 months of age). Other indicators of reactogenicity were fever (axillary temperature ≥ 37.3°C) and use of analgesics/antipyretic medication. Reports of any AEs were recorded from Day 1 to Day 22, SAEs and AEs leading to study withdrawal were recorded throughout the study period. The severity of AEs was categorized as mild, moderate, or severe, if they resulted in no limitation, some limitation, or inability to perform normal daily activities, respectively. Assessments of the causal relationship of unsolicited AEs to the vaccination were classified by the investigator as not related, possibly related, or probably related.
Statistical analyses
CHMP has not established influenza vaccine immunogenicity criteria for children so those used for young adults were referenced: the percentage of subjects achieving seroconversion for HI antibody should be >40%; the percentage of subjects achieving HI antibody titer ≥40% should be >70% and the GMR should be >2.5.Citation42 Immunogenicity was also analyzed using CBER criteria: the lower bound of the 2-sided 95% confidence interval (CI) for the percent of subjects achieving seroconversion for HI antibody should be ≥40% (seroconversion criterion); and the lower bound of the 2-sided 95% CI for the percent of subjects achieving an HI antibody titer ≥40 should be ≥70% (seroprotection criterion).Citation41 Percentages of subjects with HI titers ≥110 were also calculated. Log10-transformed antibody titers were modeled using analysis of variance for each strain, and GMTs, GMRs, and corresponding 2-sided 95% CI were calculated. Safety data were evaluated descriptively and expressed as the percentage or number of subjects with AEs in each group. Immunogenicity analyses were conducted on the full analyses set (FAS), which consisted of subjects who received the study vaccine and provided at least one evaluable serum sample before and after vaccination. Safety was analyzed for all subjects exposed to study vaccines who provided at least 1 safety data point.
Disclosure of Potential Conflicts of Interest
T.V. has received consulting fees or honoraria, travel support, and fees for participation in review activities from Novartis Vaccines and Diagnostics, Inc.. T.V. has also received consultancy fees from GlaxoSmithKline PLC, Merck & Co., Inc.., MedImmune LLC, SanofiPasteur MSD, and Novartis Vaccines and Diagnostics, Inc.. A.F. has no conflicts of interest to disclose. A.A., T.T. and R.C. are or were employees of Novartis Vaccines and Diagnostics, Inc. during the time of the study.
Authos' Contributions
All authors participated in the conception, design and implementation of the trials. All authors were involved in the interpretation of analyzed data and the decision to submit for publication.
Supplemental_Tables.zip
Download Zip (38.2 KB)Acknowledgments
The authors are grateful to all the volunteers who participated in the clinical trial, and thank Patricia de Groot (independent Medical Writer, CtrlP), Shanthi Voorn and Keith Veitch for providing editorial assistance in the preparation of this manuscript.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Pan American Health Organization Immunization in the Americas. 2013 Summary. PAHO. Washington DC 2013, 1–12.
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, Iskander JK, Wortley PM, Shay DK, Bresee JS, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR 2010;59(RR-8):1–62; PMID:20689501
- Ropero-Alvarez AM, Kurtis HJ, Danovaro-Holliday MC, Ruiz-Matus C, Andrus JK. Expansion of seasonal influenza vaccination in the Americas. BMC Pub Health 2009;9:361; http://dx.doi.org/10.1186/1471-2458-9-361
- Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng PY, Steiner C, Abedi GR, Anderson LJ, Brammer L, Shay DK. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infectious Dis 2012;54(10):1427–36; http://dx.doi.org/10.1093/cid/cis211
- Centers for Disease Control and Prevention. 2010–2011 Influenza Season Summery. http://www.cdc.gov/flu/weekly/weeklyarchives2010–2011/10–11summary.html. Accessed 22 April 2014.
- Wong KK, Jain S, Blanton L, Dhara R, Brammer L, Fry AM, Finelli L. Influenza-associated pediatric deaths in the United States, 2004–2012. Pediatrics 2013;132(5):796–804; PMID:24167165; http://dx.doi.org/10.1542/peds.2013-1493
- Ambrose CS, Wu X, Knuf M, Wutzler P. The efficacy of intranasal live attenuated influenza vaccine in children 2 through 17 years of age: a meta-analysis of 8 randomized controlled studies. Vaccine 2012;30(5):886–92; PMID:22155144; http://dx.doi.org/10.1016/j.vaccine.2011.11.104
- DiazGranados CA, Denis M, Plotkin S. Seasonal influenza vaccine efficacy and its determinants in children and non-elderly adults: a systematic review with meta-analyses of controlled trials. Vaccine 2012;31(1):49–57; PMID:23142300; http://dx.doi.org/10.1016/j.vaccine.2012.10.084
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012;12(1):36–44; PMID:22032844; http://dx.doi.org/10.1016/S1473-3099(11)70295-X
- Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, Kemble G, Connor EM; CAIV-T Comparative Efficacy Study Group. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med 2007;356(7):685–96; PMID:17301299; http://dx.doi.org/10.1056/NEJMoa065368
- Vesikari T, Knuf M, Wutzler P, Karvonen A, Kieninger-Baum D, Schmitt HJ, Baehner F, Borkowski A, Tsai TF, Clemens R. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med 2011;365(15):1406–16; PMID:21995388; http://dx.doi.org/10.1056/NEJMoa1010331
- Black S, Nicolay U, Vesikari T, Knuf M, Del Giudice G, Della Cioppa G, Tsai T, Clemens R, Rappuoli R. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J 2011;30(12):1081–5; PMID:21983214; http://dx.doi.org/10.1097/INF.0b013e3182367662
- Ansaldi F, Zancolli M, Durando P, Montomoli E, Sticchi L, Del Giudice G, Icardi G. Antibody response against heterogeneous circulating influenza virus strains elicited by MF59- and non-adjuvanted vaccines during seasons with good or partial matching between vaccine strain and clinical isolates. Vaccine 2010;28(25):4123–9; PMID:20433807; http://dx.doi.org/10.1016/j.vaccine.2010.04.030
- O'Hagan DT, Rappuoli R, De Gregorio E, Tsai T, Del Giudice G. MF59 adjuvant: the best insurance against influenza strain diversity. Exp Rev Vacc 2011;10(4):447–62; http://dx.doi.org/10.1586/erv.11.23
- Vesikari T, Forsten A, Borkowski A, Gaitatzis N, Banzhoff A, Clemens R. Homologous and heterologous antibody responses to a one-year booster dose of an MF59((R)) adjuvanted A/H5N1 pre-pandemic influenza vaccine in pediatric subjects. Hum Vaccin Immunother 2012;8(7):921–8; PMID:22777094; http://dx.doi.org/10.4161/hv.20248
- Black S, Della Cioppa G, Malfroot A, Nacci P, Nicolay U, Pellegrini M, Sokal E, Vertruyen A. Safety of MF59-adjuvanted versus non-adjuvanted influenza vaccines in children and adolescents: an integrated analysis. Vaccine 2010;28(45):7331–6; PMID:20813217; http://dx.doi.org/10.1016/j.vaccine.2010.08.075
- Pellegrini M, Nicolay U, Lindert K, Groth N, Della Cioppa G. MF59-adjuvanted versus non-adjuvanted influenza vaccines: integrated analysis from a large safety database. Vaccine 2009;27(49):6959–65; PMID:19751689; http://dx.doi.org/10.1016/j.vaccine.2009.08.101
- Nohynek H, Jokinen J, Partinen M, Vaarala O, Kirjavainen T, Sundman J, Himanen SL, Hublin C, Julkunen I, Olsén P, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PloS One 2012;7(3):e33536; PMID:22470453; http://dx.doi.org/10.1371/journal.pone.0033536
- Melon K, Partinen M, Tynell J, Sillanpää M, Himanen S-L, Saarenpää-Heikkilä O, Hublin C, Olsen P, Ilonen J, Nohynek H, et al. No serological Evidence of Influenza A H1N1pdm09 Virus Infection as a Contributing Factor in Childhood Narcolepsy After Pandemrix Vaccination Campaign in Finland. Plos One 2013; 8(8):e68402; PMID:23950869; http://dx.doi.org/10.1371/journal.pone.0068402
- Lyytikäinen O, Kuusi M, Toikkanen M, Virtanen M, Rönkkö E, Strengell M, et al. The surveillance results of the second wave of 2009 pandemic influenza A (H1N1) in Finland, November 2010-April 2011. Lääkärilehti 2011; 40:2925–2930 (English Summary)
- Walter EB, Neuzil KM, Zhu Y, Fairchok MP, Gagliano ME, Monto AS, Englund JA. Influenza vaccine immunogenicity in 6- to 23-month-old children: are identical antigens necessary for priming? Pediatrics 2006;118(3):e570–8; PMID:16950948; http://dx.doi.org/10.1542/peds.2006-0198
- Skowronski DM, Hamelin ME, Janjua NZ, De Serres G, Gardy JL, Rheaume C, Bouhy X, Boivin G. Cross-lineage influenza B and heterologous influenza A antibody responses in vaccinated mice: immunologic interactions and B/Yamagata dominance. PloS One 2012;7(6):e38929; PMID:22745690; http://dx.doi.org/10.1371/journal.pone.0038929
- Skowronski DM, Hottes TS, De Serres G, Ward BJ, Janjua NZ, Sabaiduc S, Chan T, Petric M. Influenza Beta/Victoria antigen induces strong recall of Beta/Yamagata but lower Beta/Victoria response in children primed with two doses of Beta/Yamagata. Pediatr Infect Dis J 2011;30(10):833–9; PMID:21857263; http://dx.doi.org/10.1097/INF.0b013e31822db4dc
- Center of Disease Control and Prevention (CDC): Key facts about seasonal flu vaccine. http://www.cdc.gov/flu/protect/keyfacts.htm
- Durando P, Iudici R, Alicino C, Alberti M, de Florentis D, Ansaldi F, Icardi G. Adjuvants and alternative routes of administration towards the development of the ideal influenza vaccine. Hum Vacc 2011; 7 Suppl: 29–40; http://dx.doi.org/10.4161/hv.7.0.14560
- Katz JM, Hancock K, Xu X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Exp Ref Anti-Infect Ther. 2011;9(6):669–83; PMID:21692672
- Katz J, Kancock K, Veguilla V, Zhong W, Lu XH, Sun H, et al. Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR 2009; 58(19): 521–4; PMID:19478718
- Montomoli E, Capecchi B, Hoschler K. Correlates of protection against influenza. In: Influenza vaccines for the future, 2nd edition. Rappuoli R, del Giudice G (eds). 2011. Springer, Basel
- Banzhoff A, Stoddard J. Effective influenza vaccines for children. A critical unmet medical need and a public health priority. Hum Vaccin Immunother. 2012; 8(3): 398–402; PMID:22327501; http://dx.doi.org/10.4161/hv.18561
- Ng S, Fang VJ, Ip DK, Chan KH, Leung GM, Peiris JS, Cowling BJ. Estimation of the association between antibody titers and protection against confirmed influenza virus infection in children. J Infect Dis. 2013;208(8):1320–4; PMID:23908481; http://dx.doi.org/10.1093/infdis/jit372
- Bodewes R, Fraaij PL, Geelhoed-Mieras MM, van Baalen CA, Tiddens HA, van Rossum AM van der Klis FR, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. Annual vaccination against influenza virus hampers development of virus-specific CD8+ T cell immunity in children. J Virol 2011; 85(22): 11995–20000; PMID:21880755; http://dx.doi.org/10.1128/JVI.05213-11
- Bodewes R, Kreijtz RH, Rimmelzwaan GF. Yearly influenza vaccinations: a double-edged sword? Lancet Infect Dis 2009; 9(12):784–8; PMID:19879807; http://dx.doi.org/10.1016/S1473-3099(09)70263-4
- Bodewes R, Kreijtz K, Hillaire ML, Geelhoed-Mieras MM, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. Vaccination with whole inactivated virus vaccine affects the induction of heterosubtypic immunity against influenza virus A/H5N1 and immunodominance of virus-specific CD8+ T-cell responses in mice. J Gen Virol 2010; 91 (7): 1743–53; PMID:20335492; http://dx.doi.org/10.1099/vir.0.020784-0
- Sridhar S, Begom S, Bermingham A, Hoscler K, Adamson W, Carman W, Bean T, Barclay W, Deeks JJ, Lalvani A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med 2013; 19(10):1305–12; PMID:24056771; http://dx.doi.org/10.1038/nm.3350
- Kreijtz JH, de Mutsert G, van Baalen CA, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. Cross-recognition of avian H5N1 influenza virus by human cytotoxic T-lymphocyte populations directed to human influenza A virus. J Virol 2008;82(11):1598–606; http://dx.doi.org/10.1128/JVI.02694-07
- van de Sandt CE, Kreijtz JH, de Mutsert G, Geelhoed-Mieras MM, Hillaire ML, Vogelzang van Trierum SE, Osterhaus AD, Fouchier RA, Rimmelzwaan GF. Human cytotoxic T lymphocytes directed to seasonal influenza A viruses cross-react with the newly emerging H7N9 virus. J Virol 2014; 88(3):1684–93; PMID:24257602; http://dx.doi.org/10.1128/JVI.02843-13
- Lee LY, Ha do LA, Simmons C, de Jong MD, Chau NV, Schumacher R, Peng YC, McMichael AJ, Farrar JJ, Smith GL, et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest 2008; 118(10):3478–90; PMID:18802496
- Quiñones-Parra S, Grant E, Loh L, Nguyen TH, Campbell KA, Tong SY, Miller A, Doherty PC, Vijaykrishna D, Rossjohn J, et al. Pre-existing CD8+ T-cell immunity to the H7N9 influenza A virus varies across ethnicities. Proc Natl Acad Sci USA 2014;111(3):1049–54; http://dx.doi.org/10.1073/pnas.1322229111
- Coulter A, Wong TY, Drane D, Bates J, Macfarlan R, Cox J. Studies on experimental adjuvanted influenza vaccines: comparison of immune stimulating complexes (Iscoms) and oil-in-water vaccines. Vaccine 1998; 16(11–12): 1243–53; PMID:9682385; http://dx.doi.org/10.1016/S0264-410X(98)80125-4
- Zedda L, Forleo-Neto E, Vertruyen A, Raes M, Marchant A, Jansen W, Clouting H, Arora A, Beatty ME, Galli G, et al. Dissecting the Immune Response to MF59-adjuvanted and Nonadjuvanted Seasonal Influenza Vaccines in Children Less Than Three Years of Age. Pediatr Infect Dis J 2015; 34(1):73–8; PMID:25037034; http://dx.doi.org/10.1097/INF.0000000000000465
- FDA Food and Drug Administration. Guidance for Industry: Clinical Data Needed to Support the Licensure of Seasonal Inactivated Influenza Vaccines. May 2007. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091985.pdf. Accessed 22 April 2014
- The Committee for Proprietary Medicinal Products (CHMP). Note for guidance on hamonisation of requirements for influenza vaccines. CHMP/ BWP/214/96. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf Accessed 22 April 2014