Abstract
Over recent decades, the global incidence of hepatitis A virus infection has been reduced by improvements in sanitation infrastructure and through immunization programs. The immunogenicity and field efficacy of the inactivated hepatitis A vaccine (Havrix™, GSK, Belgium) has been demonstrated in clinical trials, population-impact studies as well as in several outbreak settings. However, immunological data in older populations are limited, with only few studies assessing the immune response of this vaccine in adults aged ≥40 years. This retrospective pooled analysis of 4 2-dose primary vaccination studies compared the immunogenicity and safety of the inactivated hepatitis A vaccine in adults aged ≥40 years with subjects aged 20–30 years (control group; N = 80 in each group). Fifteen days after the first vaccine dose, 79.7% (95% CI: 68.8–88.2) and 92.3% (95% CI: 84.0–97.1) of subjects were seropositive in the ≥40 years and control groups, respectively; 97.5% (95% CI: 91.2–99.7) and 97.4% (95% CI: 91.0–99.7), respectively, were seropositive one month after the first dose. All subjects in both groups (95% CIs: 95.4–100 and 95.3–100, respectively) were seropositive one month after the second dose. Safety profiles were similar in both groups. In conclusion, the inactivated hepatitis A vaccine induced similar immune responses in adults aged ≥40 and 20–30 years one month after the first and second dose whereas younger subjects may demonstrate a higher seroconversion rate 15 days after the first dose.
Introduction
Hepatitis A virus (HAV) is a worldwide public health concern with approximately 1.5 million clinically-confirmed cases of HAV infection occurring annually.Citation1-3 In addition, serologic evidence suggests that tens of millions of new HAV infections occur each year.Citation4,5 HAV also causes the second most common vaccine-preventable travel-associated infectious disease.Citation6
HAV exposure in unprotected adults may result in severe and serious symptoms, with the likelihood of associated morbidity and mortality increasing with age.Citation7 Indeed, during a multi-state HAV outbreak in the United States in May 2013, 108 (68%) of 158 reported cases were at least 40 years of age. Of reported cases, 69 (44%) were hospitalized who were all subjects older than 18 years; 72% of these hospitalized subjects were older than 40 years of age.Citation8
HAV infections can therefore be effectively prevented by active immunization with an inactivated hepatitis A vaccine. The first commercially produced inactivated hepatitis A vaccine (Havrix™; GSK, Belgium) was launched in 1992 and is available worldwide for preventing the disease.Citation9 The immune response to the inactivated hepatitis A vaccine is well-documented and the vaccine has been shown to be generally efficacious and well-tolerated in children and adults.Citation9-11
Recent HAV outbreaks in Europe and the United States due to contaminated food have been reported.Citation12-14 Human normal immunoglobulin (HNIG) was the recommended post-exposure prophylaxis against HAV infection for decades.Citation15 However, active immunization with hepatitis A vaccination for HAV outbreak management has been recently recommended as the preferred choice over passive immunization with HNIG by international bodies including The World Health Organization (WHO) and European Center for Disease Prevention and Control (ECDC).Citation16-18 The WHO also recommends hepatitis A vaccination over HNIG for both pre- and post-prophylaxis.Citation16 Countries including Canada and more recently Australia have also issued guidelines advising the use of inactivated hepatitis A vaccine in preference to HNIG during outbreaks.Citation19-23 These guidelines are supported by several well-controlled clinical trials which have demonstrated effective control of HAV outbreaks with the inactivated hepatitis A vaccine.Citation24-31 Nevertheless, while the United Kingdom (UK) recommends HNIG administration within 2 weeks of HAV exposure to specific patient groups - individuals aged ≥50 years, immunocompromised patients or those with chronic liver disease or contraindications for vaccination,Citation32,33 the United States and the Netherlands recommend HNIG for individuals aged ≥40 years or with the same conditions as mentioned above.Citation15,34
Vaccination with hepatitis A vaccine is also recommended for individuals at increased risk for getting HAV infection, e.g., travelers to endemic areas.Citation15 Last-minute travelers are common and several recommendations of the preferred use of active immunization have been issued in countries with low HAV endemicity for such individuals at risk of exposure.Citation6,18
The inactivated hepatitis A vaccine, Havrix™ has been shown to elicit a rapid immune response in children and young adults, within 15 days of first dose administration.Citation9,35,36 However, no study has specifically assessed the effect of age on the immune response in older populations. Furthermore, although hepatitis A outbreaks have occurred in adults over 40 years,Citation13,14,37-39 little is known about the immune response to inactivated hepatitis A vaccine in this age group. We therefore conducted this study to assess the immune response and safety of Havrix™ in an older population aged ≥40 years by pooling data from 4 completed studies (3 published, one unpublished).Citation40,41
Results
This pooled analysis included 160 subjects; 80 aged ≥40 years and 80 matched subjects aged 20–30 years (control group). Of the total 80 subjects, one was taken from study 208109/108,Citation40 3 were taken from study 208109/114,Citation40 66 from study 208109/061Citation41 and 10 from study 208109/123 (unpublished); the same number of matched control subjects were selected from the same studies. The mean age of the subjects was 47 (standard deviation [SD]: ±6.57) years in the ≥40 year group and 24.2 (SD: ± 2.34) years in the control group. There were 46 and 36 females in the ≥40 years and 20–30 years age group, respectively ().
Table 1. Baseline characteristics for the pooled cohorts (total vaccinated cohort)
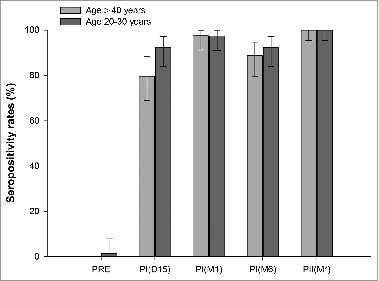
In the ≥40 years age group, seropositivity rates after the first vaccine dose were: 79.7% (95% confidence interval [CI]: 68.8–88.2) at day 15 and 97.5% (95% CI: 91.2–99.7) at one month (). All subjects were seropositive (95% CI: 95.4–100) one month after the second dose. The corresponding values for the control group were: 92.3% (95% CI: 84.0–97.1); 97.4% (95% CI: 91.0–99.7) and 100% (95% CI: 95.3–100), respectively ().
Figure 1. Seropositivity rates at different time points (total vaccinated cohort). Footnote: Control group: subjects aged 20–30 years; PRE: blood sample at pre-vaccination; PI (D15): blood sample 15 days after vaccine dose 1; PI (M1): blood sample one month after vaccine dose 1; PI (M6): blood sample 6 months after vaccine dose 1; PII (M*): blood sample one month after vaccine dose 2; Assay cut-off: 20 mIU/mL.
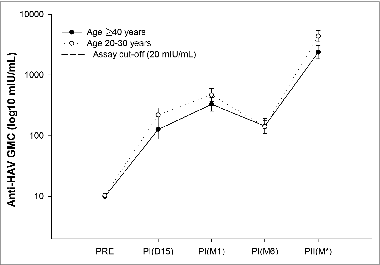
The anti-HAV antibody geometric mean concentrations (GMCs) in the older age group were: 126.5 mIU/mL (95% CI: 88.6–180.7) at day 15 and 329.1 mIU/mL (95% CI: 254.7–425.2) at one month post-dose 1 and 2378.9 mIU/mL (95% CI: 1848.5–3061.5) one month after the second dose. The corresponding values for the control group were: 219.4 mIU/mL (95% CI: 168.0–286.5), 469.2 (95% CI: 365.2–602.7) and 4370.9 mIU/mL (95% CI: 3535.1–5404.3) ().
Figure 2. Anti-HAV GMC following the primary vaccination (total vaccinated cohort). Footnote: Control group: subjects aged 20–30 years; PRE: blood sample at pre-vaccination; PI (D15): blood sample 15 days after vaccine dose 1; PI (M1): blood sample one month after vaccine dose 1; PI (M6): blood sample 6 months after vaccine dose 1; PII (M*): blood sample one month after vaccine dose 2.
During the 4-day post-vaccination follow-up period, 63.8% and 77.5% of subjects reported at least one symptom (solicited and unsolicited, local or general) in the ≥40 year and control groups, respectively. Solicited local and general symptoms were of similar magnitude and frequency in both groups. Pain was the most common solicited local symptom in both groups (71.4% in ≥40 year old subjects and 64.3% in controls) and headache was the most frequently reported solicited general symptom (35.7% each) (). During the 31-day post-vaccination follow-up period, at least one unsolicited symptom was reported for 6.3% and 8.8% of subjects, respectively. No serious adverse events (SAEs) were reported in either group.
Table 2. Incidence of reported solicited local and general symptoms during the 4-day post-vaccination period (total vaccinated cohort)
Discussion
Given the paucity of data available on the immune response of the hepatitis A vaccine compared with HNIG in persons older than 40 years in a post-exposure scenario, physicians are faced with the clinical dilemma of whether to administer the hepatitis A vaccine or HNIG to individuals during HAV outbreaks.Citation7,15 A similar challenge is faced in the pre-exposure scenario for travelers. As older adults from areas of low HAV endemicity are increasingly traveling to areas of high endemicity, there is an increased risk of serious illness following HAV exposure.Citation7 The current pooled analysis was therefore conducted to assess the immunogenicity and safety of Havrix™ in adults aged ≥40 years. The strength of this pooled analysis is that all 4 original studies were conducted in countries with equally low endemicity and a country- and gender-matched control group was included. This pooled analysis is however restricted by the statistical limitations of a post-hoc analysis and a limited sample size.
Using a conservative total serum anti-HAV antibody titer cut-off value of 20 mIU/mL, it was observed that the seropositivity rate at day 15 post-dose 1 was lower in subjects aged ≥40 years, as compared to the younger control group. The seropositivity rates one month after the first vaccine dose were however identical in both groups (). Seropositivity rates of 100% were achieved in both groups one month after the second vaccine dose (). The delay in initial immune response in adults could possibly be due to the aging immune system.Citation42 When compared with the control group, antibody concentrations were lower in subjects aged ≥40 years (with overlapping CIs) (). These findings are in line with those of a previous study conducted by Joines et al (2001), which presented comparable seroconversion rates between subjects aged ≥40 years and <40 years, suggesting that both older and younger subjects respond to the inactivated hepatitis A vaccine to the same extent.Citation43 However, similar to our study, the antibody concentrations in that series were higher in younger adults compared to older adults.Citation43 The lowest antibody concentration required to confer clinical protection has not been established for hepatitis A. However, studies of passively transferred antibodies have shown that even antibody concentrations just above the detection cut-off are associated with an absence of clinical infection; the detection of circulating antibodies is therefore generally considered as a surrogate marker of clinical protection.Citation44–46 The difference in the observed GMC in our study is therefore of limited clinical relevance.
As observed in the current pooled analysis,, vaccination with Havrix™ elicits a more rapid and effective immune response within 15 days of first dosing in both children (age = 2–13 years; seroconversion rate = 96%) and young adults (mean age = 28.4 years; seroconversion rate = 97.7%).Citation35,36 This rapid humoral immune response helps in reducing the duration of hepatitis A outbreaks and is therefore crucial for its management.Citation30 It is also advantageous when immunization is required at short notice, such as when traveling to endemic countries.Citation47 The rapid response to the inactivated hepatitis A vaccine observed in our analysis is consistent with the results from other studies.Citation35,48,49 Indeed, one study of adults intending to travel abroad reported seroconversion as early as day 12 following vaccination; all subjects were seropositive by day 16.Citation48 Another retrospective pooled analysis of 9 clinical trials of the inactivated hepatitis A vaccine (1440 El.U) in 1,694 healthy seronegative adults observed a rapid seroconversion rate of 79% at day 13 which gradually increased to 100% by day 19.Citation49 These findings are indicative of a rapid immune response in a wide age range, including those older than 40 years; the vast majority of vaccinees develop antibodies within 2 weeks of vaccination.Citation46 Thus, given that hepatitis A virus has an average incubation period of 28 days,Citation50 these results indicate that the inactivated hepatitis A vaccine can provide adequate protection to travelers seeking immunity before departing for HAV endemic countries.Citation46,49
Vaccine coverage is another important factor when managing hepatitis A outbreaks. If an adequate number of susceptible individuals are vaccinated with the inactivated hepatitis A vaccine, then community outbreaks can be halted or significantly shortened.Citation24-29 The duration of hepatitis A outbreaks is important in the public health setting where timely intervention has the greatest impact. Indeed, an intervention within 2 weeks of exposure to HAV is recommended for obtaining the greatest reduction in outbreak duration.Citation51 Thus, a rapid humoral immune response, as elicited by the inactivated hepatitis A vaccine, and adequate vaccination coverage of a susceptible population is key in controlling an HAV outbreak.
Before the inactivated hepatitis A vaccine was available, HNIG was recommended for post-exposure prophylaxis against HAV infection.Citation15,52 Post-exposure prophylaxis using hepatitis A vaccine or HNIG or both can now be used to prevent secondary cases in close contacts of hepatitis A cases. HNIG remain recommended in specific situations, for example, the US Advisory Committee on Immunization Practices recommends HNIG (0.02 ml/kg) in addition to vaccination for older adults, immunocompromised individuals and those with chronic liver disease planning to travel to an area of high or intermediate HAV endemicity in 2 weeks or less.Citation15 While guidelines from the Netherlands provide an upper age limit of 40 years for administering hepatitis A vaccine in a post-HAV exposure scenario and the UK recommends HNIG in addition to vaccination in those aged over 50 years,Citation32-34 recommendations by the Canadian and Australian authorities have omitted any such age restrictions.Citation19,22 In a recent study from Australia, no HAV outbreaks were observed among adults aged >40 years who previously received the hepatitis A vaccine, thus justifying the removal of an upper age restriction for vaccination as post-exposure prophylaxis.Citation23
Overall, HAV vaccination as a post-exposure prophylactic measure in subjects >40 years has achieved high success rates in HAV outbreaks.Citation8,31,52 Canada and Australia have recently adapted their guidelines in accordance with the WHO recommendations, advising HAV vaccination in outbreak situations.Citation16,19-23
In conclusion, the immune response and the safety and tolerability profile of 2 doses of Havrix™ in subjects aged ≥40 years is similar to that of younger subjects (aged 20–30 years) one month after the first and second dose in this pooled analysis, whereas younger subjects may demonstrate a more rapid and higher seroconversion 15 days after the first dose. The results of this meta-analysis is supportive of a satisfactory immunogenicity profile in healthy individuals >40 years of age.
Materials and Methods
This post-hoc retrospective pooled analysis was performed on 4 double blind, randomized primary vaccination studies of the inactivated hepatitis A vaccine conducted in 3 countries of low HAV endemicity - Belgium, Finland and Iceland (Studies - 208109/108 [0, 12 months],Citation40 208109/114 [0, 6 months],Citation40 208109/061 [0, 6 months]Citation41 and 208109/123 [0, 6 months; unpublished]). The studies were selected based on the criteria that: 2-dose Havrix™ had been administered to healthy subjects including those aged ≥40 years; anti-HAV antibodies were measured using enzyme linked immunosorbent assay (ELISA); and the availability of the study reports.
There were 194 subjects in study 208109/108,Citation40 120 in 208109/114,Citation40 200 in 208109/061Citation41 and 151 in 208109/123 (unpublished). Of these, 1, 3, 66 and 10 subjects aged 40 years and above, respectively, were included in this pooled analysis. An equal number of country- and gender- matched control group aged between 20–30 years was selected from the same studies. The matching of subjects was first done by calculating the standardized age of the subjects as “age” divided by “standard error for age” among either the ≥40 years study population or control subjects. The subjects in the ≥40 years and control groups were then sorted according to standardized age in ascending order. The lowest standardized age value in the ≥40 years age group was then matched with lowest standardized age value in the control group of the same gender (if available). If the same gender was not available, then gender for matching was ignored. Matching was undertaken for each study, whereby one subject from one study was matched to a control subject from the same study.
All the studies included in this pooled analysis were conducted according to Good Clinical Practice and in accordance with the Declaration of Helsinki. The protocol and associated documents were reviewed and approved by the ethics committees of participating study centers. All subjects provided written informed consent.
In the original studies, all subjects received 2 doses of Havrix™ according to the standard 0, 6 month or an extended 0, 12 month schedule, by intramuscular injection in the deltoid region. Each 1 ml dose of Havrix™ contained at least 1440 El.U HAV (strain HM175). Blood samples were collected at screening (pre-vaccination), on day 15, 1 and 6 months after first vaccine dose; and 1 month after second vaccine dose in both groups. Anti-HAV antibodies were measured using ELISA (Enzymun®, Boehringer-Mannheim).
Solicited local and general symptoms were collected during the 4-day post-vaccination period; unsolicited symptoms were recorded for 30 days post-vaccination and SAEs were recorded throughout the study.
The pooled analysis considered the total vaccinated cohort (TVC), which included all subjects who had received at least one dose of the study vaccine. Immunogenicity analysis based on the TVC included subjects who received at least one dose of the vaccine and for whom immunogenicity data was available. Seropositivity rates (defined as percentage of subjects with anti-HAV antibody titer value ≥20 mIU/mL) and GMCs were calculated with exact 95% CI. The GMC calculations were performed by taking the anti-log of the mean of the log10 concentration transformations. Antibody concentrations below the assay cut-off were given an arbitrary value of half the cut-off for the purpose of GMC calculation.
Disclosure of Potential Conflicts of Interest
All authors are employed by the GSK group of companies. O.V.D.M and M. D. R. declare owning shares from the GSK group of companies.
Trademark Statement
Havrix™ is a trademark of the GSK group of companies. Enzymun® is a registered trademark of Boehringer Mannheim.
Acknowledgments
The authors thank Andrew Trofa for intellectual input during the development of the manuscript. The authors also thank Ramandeep Singh for manuscript writing (GSK group of companies), Julia Donnelly for language editing (on behalf of GSK group of companies) and Ashmita Ravishankar for editorial assistance and publication coordination (GSK group of companies).
Funding
GlaxoSmithKline Biologicals SA was the funding source for the analysis. GlaxoSmithKline Biologicals SA also funded all costs associated with the development and publication of the manuscript. All authors had full access to the data and agreed with the submission of the publication.
References
- World Health Organization, Department of Communicable Disease Surveillance and Response. Hepatitis A. 2000. Available at http://www.who.int/csr/disease/hepatitis/HepatitisA_whocdscsredc2000_7.pdf?ua=1. Accessed on August 14, 2014.
- Franco E, Meleleo C, Serino L, Sorbara D, Zaratti L. Hepatitis A: Epidemiology and prevention in developing countries. World J Hepatol 2012; 4:68-73; PMID:22489258; http://dx.doi.org/10.4254/wjh.v4.i3.68
- Van Herck K, Crasta PD, Messier M, Hardt K, Van Damme P. Seventeen-year antibody persistence in adults primed with two doses of an inactivated hepatitis A vaccine. Hum Vaccin Immunother 2012; 8:323-7; PMID:22327499; http://dx.doi.org/10.4161/hv.18617
- Wasley A, Fiore A, Bell BP. Hepatitis A in the era of vaccination. Epidemiol Rev 2006; 28:101-11; PMID:16775039; http://dx.doi.org/10.1093/epirev/mxj012
- Vaughan G, Goncalves Rossi LM, Forbi JC, de Paula VS, Purdy MA, Xia G, Khudyakov YE. Hepatitis A virus: host interactions, molecular epidemiology and evolution. Infect Genet Evol 2014; 21:227-43; PMID:24200587; http://dx.doi.org/10.1016/j.meegid.2013.10.023
- Wu D, Guo CY. Epidemiology and prevention of hepatitis A in travelers. J Travel Med 2013; 20:394-9; PMID:24165384; http://dx.doi.org/10.1111/jtm.12058
- Genton B, D'Acremont V, Furrer H-J, Hatz C, Louis L. Hepatitis A vaccines and the elderly. Travel Med Infect Dis 2006; 4:303-12; PMID:17098625; http://dx.doi.org/10.1016/j.tmaid.2005.10.002
- Nelson NP, Murphy TV, McMahon BJ. Hepatitis A vaccination for post-exposure prophylaxis in persons aged 40 years and older. Vaccine 2014; 32:2939; PMID:24530928; http://dx.doi.org/10.1016/j.vaccine.2014.01.086
- Van Herck K, Van Damme P. Prevention of hepatitis A by Havrix: a review. Expert Rev Vaccines 2005; 4:459-71; PMID:16117704; http://dx.doi.org/10.1586/14760584.4.4.459
- Innis BL, Snitbhan R, Kunasol P, Laorakpongse T, Poopatanakool W, Kozik CA, Suntayakorn S, Suknuntapong T, Safary A, Tang DB, et al. Protection against hepatitis A by an inactivated vaccine. JAMA 1994; 271:1328-34; PMID:8158817; http://dx.doi.org/10.1001/jama.1994.03510410040030
- Rendi-Wagner P, Korinek M, Winkler B, Kundi M, Kollaritsch H, Wiedermann U. Persistence of seroprotection 10 years after primary hepatitis A vaccination in an unselected study population. Vaccine 2007; 25:927-31; PMID:17005304; http://dx.doi.org/10.1016/j.vaccine.2006.08.044
- Collier MG, Khudyakov YE, Selvage D, Adams-Cameron M, Epson E, Cronquist A, Jervis RH, Lamba K, Kimura AC, Sowadsky R, et al. Outbreak of hepatitis A in the USA associated with frozen pomegranate arils imported from Turkey: an epidemiological case study. Lancet Infect Dis 2014; 14:976-81; PMID:25195178; http://dx.doi.org/10.1016/S1473-3099(14)70883-7
- Nordic Outbreak Investigation Team C. Joint analysis by the Nordic countries of a hepatitis A outbreak, October 2012 to June 2013: frozen strawberries suspected. Euro Surveill 2013; 18; PMID:23870076
- Guzman-Herrador B, Jensvoll L, Einoder-Moreno M, Lange H, Myking S, Nygard K, Stene-Johansen K, Vold L. Ongoing hepatitis A outbreak in Europe 2013 to 2014: imported berry mix cake suspected to be the source of infection in Norway. Euro Surveill 2014; 19; PMID:24762662
- Update: Prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56:1080-4; PMID:17947967
- World Health Organization. Hepatitis A vaccines – July 2012. Available at http://www.who.int/immunization/position_papers/PP_hep_A_july2012_summary.pdf. Accessed on August 14, 2014.
- World Health Organization. The Immunological Basis for Immunization Series. 2011. Available at http://whqlibdoc.who.int/publications/2011/9789241501422_eng.pdf. Accessed on August 14, 2014.
- European Centre for Disease Prevention and Control. Euro Surveill 2006; 11. Available at http://www.eurosurveillance.org/images/dynamic/EQ/v06n01/v06n01.pdf. Accessed on August 14, 2014
- Canada Communicable Disease Report. A review of provin-cial/territorial strategies for Hepatitis A pre- and post-exposureprophylaxis. 2005. Available at http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/05vol31/dr3119b-eng.php. Accessed on August 20, 2014.
- Public Health Agency of Canada. Canadian Immunization Guide. 2012. Available at http://www.phac-aspc.gc.ca/publicat/cig-gci/p04-hepa-eng.php#post. Accessed on August 14, 2014.
- Department of Health, Australian Government. The Australian Immunisation Handbook. 2013. Available at http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/5-1. Accessed on August 17, 2014
- Communicable Diseases Network of Australia. Hepatitis A – series of national guidelines – guidelines for public health units. 2009. Available at http://www.health.gov.au/internet/main/publishing.nsf/Content/cdna-song-hepa.htm. Accessed on August 20, 2014.
- Freeman E, Lawrence G, McAnulty J, Tobin S, MacIntyre CR, Torvaldsen S. Field effectiveness of hepatitis A vaccine and uptake of post exposure prophylaxis following a change to the Australian guidelines. Vaccine 2014; 32:5509-13; PMID:25111168; http://dx.doi.org/10.1016/j.vaccine.2014.07.048
- McMahon BJ, Beller M, Williams J, Schloss M, Tanttila H, Bulkow L. A program to control an outbreak of hepatitis A in Alaska by using an inactivated hepatitis A vaccine. Arch Pediatr Adolesc Med 1996; 150:733-9; PMID:8673200; http://dx.doi.org/10.1001/archpedi.1996.02170320079014
- Prikazsky V, Olear V, Cernoch A, Safary A, Andre FE. Interruption of an outbreak of hepatitis A in two villages by vaccination. J Med Virol 1994; 44:457-9; PMID:7897381; http://dx.doi.org/10.1002/jmv.1890440427
- Craig AS, Sockwell DC, Schaffner W, Moore WL, Jr., Skinner JT, Williams IT, Shaw FE, Shapiro CN, Bell BP. Use of hepatitis A vaccine in a community-wide outbreak of hepatitis A. Clin Infect Dis 1998; 27:531-5; PMID:9770153; http://dx.doi.org/10.1086/514700
- Kaic B, Borcic B, Ljubicic M, Brkic I, Mihaljevic I. Hepatitis A control in a refugee camp by active immunization. Vaccine 2001; 19:3615-9; PMID:11395194; http://dx.doi.org/10.1016/S0264-410X(01)00103-7
- Irwin DJ, Millership S. Control of a community hepatitis A outbreak using hepatitis A vaccine. Commun Dis Public Health 1999; 2:184-7; PMID:10491872
- Bonanni P, Colombai R, Franchi G, Lo Nostro A, Comodo N, Tiscione E. Experience of hepatitis A vaccination during an outbreak in a nursery school of Tuscany, Italy. Epidemiol Infect 1998; 121:377-80; PMID:9825788; http://dx.doi.org/10.1017/S0950268898001411
- Bonanni P, Franzin A, Staderini C, Pitta M, Garofalo G, Cecconi R, Santini MG, Lai P, Innocenti B. Vaccination against hepatitis A during outbreaks starting in schools: what can we learn from experiences in central Italy? Vaccine 2005; 23:2176-80; PMID:15755590; http://dx.doi.org/10.1016/j.vaccine.2005.01.044
- Shen YG, Gu XJ, Zhou JH. Protective effect of inactivated hepatitis A vaccine against the outbreak of hepatitis A in an open rural community. World J Gastroenterol 2008; 14:2771-5; PMID:18461664; http://dx.doi.org/10.3748/wjg.14.2771
- Health Protection Agency. Guidance for the Prevention and Control of Hepatitis A Infection. 2009. Available at http://webarchive.nationalarchives.gov.uk/20140714084352/http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1259152095231. Accessed on Decemeber 17, 2014.
- Health Protection Agency. Immunoglobulin Handbook. 2009. Available at http://webarchive.nationalarchives.gov.uk/20140714084352/http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1194947366684. Accessed on Decemeber 17, 2014.
- National Institute for Public Health and the Environment MoH, Welfare and Sports, Nederlands. LCI-richtlijn Hepatitis A. 2013:1–13. Available at http://www.rivm.nl/Documenten_en_publicaties/Professioneel_Praktisch/Richtlijnen/Infectieziekten/LCI_richtlijnen/LCI_richtlijn_Hepatitis_A. Accessed on August 20, 2014.
- Schmidtke P, Habermehl P, Knuf M, Meyer CU, Sanger R, Zepp F. Cell mediated and antibody immune response to inactivated hepatitis A vaccine. Vaccine 2005; 23:5127-32; PMID:16054733; http://dx.doi.org/10.1016/j.vaccine.2005.06.022
- Findor JA, Canero Velasco MC, Mutti J, Safary A. Response to Hepatitis A Vaccine in Children after a Single Dose with a Booster Administration 6 Months Later. J Travel Med 1996; 3:156-9; PMID:9815444; http://dx.doi.org/10.1111/j.1708-8305.1996.tb00730.x
- Dakic Z, Musa S. Hepatitis A outbreak in Bijeljina, Bosnia and Herzegovina, August 2012 - April 2013. Euro Surveill 2013; 18; PMID:23725978
- Centers for Disease Control and Prevention. Multistate outbreak of hepatitis A virus infections linked to pomegranate seeds from Turkey (Final Update). 2013. Available at http://www.cdc.gov/hepatitis/Outbreaks/2013/A1b-03-31/index.html. Accessed on August 14, 2014.
- Rizzo C, Alfonsi V, Bruni R, Busani L, Ciccaglione A, De Medici D, Di Pasquale S, Equestre M, Escher M, Montano-Remacha M, et al. Ongoing outbreak of hepatitis A in Italy: preliminary report as of 31 May 2013. Euro Surveill 2013; 18; PMID:23870075
- Van Herck K, Van Damme P. Inactivated hepatitis A vaccine-induced antibodies: follow-up and estimates of long-term persistence. J Med Virol 2001; 63:1-7; PMID:11130881; http://dx.doi.org/10.1002/1096-9071(200101)63:1%3c1::AID-JMV1000%3e3.0.CO;2-U
- Briem H, Safary A. Immunogenicity and safety in adults of hepatitis A virus vaccine administered as a single dose with a booster 6 months later. J Med Virol 1994; 44:443-5; PMID:7897378; http://dx.doi.org/10.1002/jmv.1890440424
- Paganelli R, Scala E, Quinti I, Ansotegui IJ. Humoral immunity in aging. Aging (Milan, Italy) 1994; 6:143-50; PMID:7993921
- Joines RW, Blatter M, Abraham B, Xie F, De Clercq N, Baine Y, Reisinger KS, Kuhnen A, Parenti DL. A prospective, randomized, comparative US trial of a combination hepatitis A and B vaccine (Twinrix) with corresponding monovalent vaccines (Havrix and Engerix-B) in adults. Vaccine 2001; 19:4710-9; PMID:11535321; http://dx.doi.org/10.1016/S0264-410X(01)00240-7
- Murphy TV, Stephen M, Bell BP. Hepatitis A vaccines. Vaccines. In: Plotkin SA, Orenstein WA, Offit PA (eds) (6th edition): Elsevier Saunders 2013.
- Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis 2008; 47:401-9; PMID:18558875; http://dx.doi.org/10.1086/589862
- Connor BA. Hepatitis A vaccine in the last-minute traveler. Am J Med 2005; 118(Suppl 10A):58S-62S; PMID:16271543; http://dx.doi.org/10.1016/j.amjmed.2005.07.018
- Wagner G, Lavanchy D, Darioli R, Pecoud A, Brulein V, Safary A, Frei PC. Simultaneous active and passive immunization against hepatitis A studied in a population of travelers. Vaccine 1993; 11:1027-32; PMID:8212822; http://dx.doi.org/10.1016/0264-410X(93)90128-K
- Irwin DJ, Millership S. Antibody responses to Hepatitis A vaccine in healthy adults. Commun Dis Public Health 2001; 4:139-40; PMID:11525004
- Van Damme P, Lievens M, Stoffel M, Nguyen C. Rapid seroconversion rates after first dose of an inactivated hepatitis A vaccine (Havrix 1440™): results of a retrospective analysis. Travel & Epidemics: 3rd European Conference on Travel Medicine, Florence, Italy. 2002: 218. Availbale at http://www.travelmedicine.it/news/ectm3_abstract.pdf. Accessed on August 14, 2014.
- Public health control of hepatitis A: memorandum from a WHO meeting. Bull World Health Organ 1995; 73:15-20; PMID:7704922
- Torner N, Broner S, Martinez A, Tortajada C, Garcia de Olalla P, Barrabeig I, Sala M, Camps N, Minguell S, Alvarez J, et al. Factors associated to duration of hepatitis a outbreaks: implications for control. PLoS One 2012; 7:e31339; PMID:22355358; http://dx.doi.org/10.1371/journal.pone.0031339
- Taliani G, Gaeta GB. Hepatitis A: post-exposure prophylaxis. Vaccine 2003; 21:2234-7; PMID:12744848; http://dx.doi.org/10.1016/S0264-410X(03)00138-5
