Abstract
About 10% adult failed to develop antibody response after primary hepatitis B vaccination, and revaccination may be an option to improve immune response, but the antibody responses to revaccination in adult non-responders have not been fully examined. Adult non-responders to primary 3-dose hepatitis B vaccination were randomly divided into 2 groups and revaccinated with 20 μg hepatitis B vaccine (HepB) derived from Saccharomyces Cerevisiae (HepB-SC) or 20 μg HepB derived from Chinese hamster ovary cells (HepB-CHO), respectively, at 0-, 1-, 6- month. Seroconversion rate and titer of antibody against hepatitis B surface antigen (anti-HBs) was measured one month after the 1st and 3rd revaccination dose. Anti-HBs seroconversion rates significantly increased from 54.98% [95% confidence interval (CI) 48.60%–61.24%] after the 1st revaccination dose to 89.24% (95% CI: 84.74%–92.79%) after the 3rd revaccination dose (P < 0.001), and the geometric mean titer (GMT) of anti-HBs increased from 12.18mIU/ml (95%CI: 7.81–18.98 mIU/ml) to 208.31 mIU/ml (95% CI: 148.87–291.47 mIU/ml) (P = 0.008).Compared with those with anti-HBs titer <2 mIU/ml after primary vaccination, those with antibody titer ≥2 mIU/ml after primary vaccination had higher seroconversion rate after the 1st dose revaccination (38.36% vs. 78.10%, P < 0.001) and after the 3rd dose of revaccination (84.25% vs. 96.19%, P = 0.003), and had higher antibody titer after the 1st dose of revaccination (3.32mIU/ml vs. 74.21mIU/ml, P < 0.0001) and after the 3rd dose of revaccination (145.73mIU/ml vs. 342.34mIU/ml, P = 0.01). Anti-HBs titer was significantly higher in those revaccinated with HepB-CHO than those revaccinated with HepB-SC after the 3rd dose (131.46 mIU/ml vs. 313.38mIU/ml, P = 0.01). Revaccination on adult HepB non-responders increased the immune response to HepB and may confer further protection against hepatitis B virus infection. If possible, revaccination might be an option to HepB non-responders to secure more protection.
Introduction
Over the world, around 350 millions of people were chronically infected with hepatitis B virus (HBV),Citation1 and one quarter of them would die of chronic liver disease, cirrhosis or hepatocellular carcinoma.Citation2 HBV infection is still an endemic disease in China, and the prevalence of hepatitis B surface antigen (HBsAg) was 7.2% among the Chinese population aged 1–59 y and 8–12% in adults above 20 yCitation3
Hepatitis B vaccine (HepB) is the most effective way available to prevent HBV infection, which was recommended to the infants by the Chinese government in 1992. A guideline for adult HepB immunization in China was released by Chinese Center for Disease Control and Prevention (CDC) and Chinese Prevention Medicine Association (CPMA) in 2011, and HepB was recommended to all unvaccinated adults, especially those at high risk for HBV infection.Citation4 The full series of HepB primary vaccination are 3 doses on a 0-, 1-, and 6-month schedule for both infants and adults in China. HepB is available to infants and young children free of charge, but it is vaccinated to adults on a voluntary and self-paid basis, even for the adults at high risk.
Although one full series of hepatitis B vaccination could induce protective antibody among more than 95% infants,Citation5,6 around 5%–10% adults fail to respond or respond poorly to 3 doses of HepB.Citation7,8 In China, the adult non-responding rate after HepB primary vaccination is around 4.7%–14.22% with difference in the dosage, type of HepB, and the participants.Citation9–11 Efforts have been made to enhance immunogenicity among adults including the development of new vaccines,Citation12–14 but the option available now might be to revaccinate with the currently available HepB.Citation15 Although the effectiveness of HepB revaccination has been documented in non-responders, the risk factors associated with the antibody response after HepB revaccination have not been fully understood, which might be due to the limited sample size of non-responders in previous studies.Citation16-18
We conducted this study to examine anti-HBs response after revaccination with different number of dose and with different type of HepB among adult non-responders in China and to identify the risk factors associated with the antibody response.
Results
Demographic characteristics of the subjects
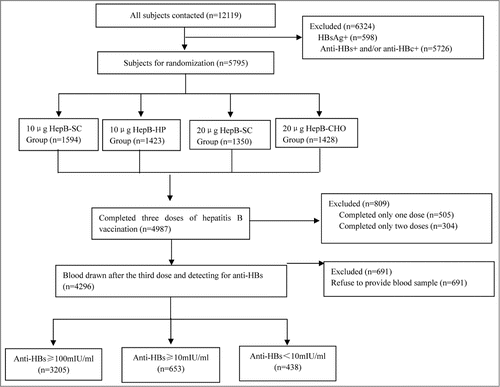
Of the 438 non-responders, there were 187 non-responders loss of contact or declined to participate, with 251 non-responders who were enrolled and completed 3 doses of HepB. There was no difference between the participants and non-participants in gender, anti-HBs titer after primary vaccination or HepB type received in primary vaccination (P = 0.76, 0.09 and 1.00, respectively), while there were more subjects aged <40 years in the non-participants than the participants (44.39% vs. 31.47%, P = 0.02). Among 251 participants, there were 124 males and 127 females with average age of 41.90 y (range: 18–49 years). There were 118 subjects revaccinated with 20 μg HepB derived from Saccharomyces Cerevisiae (HepB-SC) and 133 subjects revaccinated with 20 μg HepB derived from Chinese hamster ovary cells (HepB-CHO). There were no differences in age, gender, HepB type received for primary vaccination, and anti-HBs titer after primary vaccination between the 2 vaccine groups (P = 0.33, 0.86, 0.78 and 0.80, respectively), but there were more overweighed participants revaccinated with HepB-CHO (P = 0.02) ().
Table 1. Demographic characteristics of the adult non-responders receiving different vaccines in revaccination
Seroconversion after HepB revaccination
The anti-HBs seroconversion rate was higher after the 3rd dose of revaccination 89.24% [95% confidence interval (CI): 84.74%–92.79%] than after 1st dose of revaccination 54.98% (95%CI: 48.60%–61.24%) (P < 0.001). The same trends were found in the participants with different gender, different body mass index (BMI) level and in those aged 30–39 y and 40–49 y (P ≤ 0.01), but no significant difference was found in anti-HBs seroconversion between the 1st and 3rd revaccination dose among the participants aged 18–29 y (P = 0.07) ().
Table 2. Anti-HBs seroconversion rate after the first and the third revaccination dose among adult non-responders to primary HepB vaccination
The seroconversion rates after the 1st revaccination dose were 60.17% (95%CI: 50.75%–69.07%) and 50.38% (95%CI: 41.58%–59.16%) among those received HepB-SC and HepB-CHO vaccine, respectively, and the corresponding seroconversion rates increased to 88.14% (95% CI: 80.90%–93.36%) and 90.22% (95%CI: 83.87%–94.69%), respectively, after the 3rd dose of revaccination. The differences in anti-HBs seroconversion rate between the 1st and 3rd dose were statistically significant in both vaccine groups (P < 0.001 for both), but no significant difference was found between the 2 vaccine groups after both the 1st and 3rd revaccination dose (P = 0.12, P = 0.59, respectively) ().
After the 1st revaccination dose, anti-HBs seroconversion rates were 38.36% (95% CI: 30.44%–46.76%) and 78.10% (95%CI: 68.97%–85.58%) among those with low (<2 mIU/ml) and high (≥2 mIU/ml) antibody titer after primary vaccination, and after the 3rd revaccination dose, the seroconversion rates increased to 84.25% (95% CI: 77.31%–89.74%) and 96.19% (95% CI: 90.53%–98.95%) respectively(). The anti-HBs conversion rate was significantly higher after the 3rd revaccination dose than after the 1st revaccination dose among those with low antibody titer after primary vaccination and those with high antibody titer after primary vaccination (P < 0.001 for both). The participants with high anti-HBs titer after primary vaccination achieved significantly higher anti-HBs conversion rate than those with low anti-HBs titer after primary vaccination after both the 1st and 3rd revaccination dose (P < 0.001, P = 0.003, respectively). After adjustment for other factors, only antibody titer after primary vaccination remained significant in multivariable logistic regression with high antibody titer had 5.4 times more likely to achieve seroconversion after 3 doses of revaccination (P = 0.003)().
Table 3. Anti-HBs titer after the first and the third revaccination dose among adult non-responders to primary HepB vaccination
Table 4. Multivariable analyses on factors associated with seroconversion and antibody titer of anti-HBs after 3 doses of revaccination among adult non-responders
Anti-HBs titer after HepB revaccination
The geometric mean titers (GMTs) of anti-HBs were higher after the 3rd revaccination dose (208.31 mIU/ml) than after the 1st revaccination dose (12.18 mIU/ml) (P = 0.008) ().The same trends were found no matter of gender, age and BMI of the participants (P < 0.0001).
The GMTs after the 1st revaccination dose were 15.99mIU/ml (95%CI: 9.31–27.45 mIU/ml) and 9.56 mIU/ml (95%CI: 4.79–19.07 mIU/ml) among those who received HepB-SC and HepB-CHO vaccine respectively, and the GMTs of anti-HBs increased to 131.46 mIU/ml (95%CI: 78.59–219.91 mIU/ml) and 313.38 mIU/ml (95%CI: 202.97–483.86 mIU/ml), respectively, after the 3rd revaccination (). The significantly higher anti-HBs titer was found after the 3rd dose than after the 1st dose in both vaccine groups (P < 0.0001 for both). The titer was significantly higher in the participants revaccinated with HepB-CHO than in those revaccinated with HepB-SC after the 3rd revaccination dose (P = 0.01), but the same trend wasn't found after the 1st revaccination dose (P = 0.26) ().
The GMTs of anti-HBs were 3.32 mIU/ml (95%CI: 1.81–6.08 mIU/ml) and 74.21 mIU/ml (95%CI: 46.49–118.45 mIU/ml) among those with low and high titer level after primary vaccination respectively, but the GMTs significantly increased to 145.73 mIU/ml (95% CI: 88.00–241.34 mIU/ml) and 342.34 mIU/ml (95% CI: 234.02–500.81 mIU/ml), respectively, after the 3rd dose (P < 0.0001 for both) (). In addition, the anti-HBs titers after both the 1st and the 3rd revaccination dose were significantly higher among those with high anti-HBs titer after primary immunization than those with low anti-HBs titer after primary immunization (P < 0.0001, P = 0.01, respectively) ().
After adjustment for other factors, antibody titer after primary vaccination and HepB type received in revaccination remained significant in linear regression (P = 0.004 and 0.002, respectively)().
Discussion
Similar to the findings in clinical trials,Citation17,19-23 in adult non-responders to primary hepatitis B primary vaccination, we found that more than half seroconverted after one dose of revaccination and around 90% seroconverted after 3 doses of revaccination, the high conversion rate further verified that the non-responders to primary vaccination would gain protection after revaccination. In addition, even the antibody levels increase after one and 3 doses of revaccination, the average of antibody level was still low after one dose of revaccination, much below the level of at least 100 mIU/ml to confer sufficient long-lasting protection.Citation19 However, the antibody titer increased dramatically after the 3rd revaccination dose. The higher titer of anti-HBs after HepB vaccination is documented to be associated with the longer duration of protective immunity.Citation24,25 In countries with endemic HBV infection such as China, the pressure of HBV infection is still high for fair long duration of time in the future and it is important to achieve a long-lasting antibody protection against HBV infection. Therefore, 3 doses of revaccination might be more appropriate for any non-responders seeking for revaccination after primary vaccination in China, just as what was suggested by China CDC and CPMA.Citation4
A recent study in Italy found that pre-booster antibody titer higher than 2 mIU/ml might be predictive of an anamnestic response after booster vaccination.38 In our study, the anti-HBs seroconversion rate after HepB revaccination was significantly lower in those whose anti-HBs titer after primary vaccination was < 2 mIU/ml compared with those whose anti-HBs titer after primary vaccination was ≥2 mIU/ml. Our results indicated that a different immune or genetic mechanism about HepB non-response might exist in those whose anti-HBs titer after primary vaccination was very low or undetected. Further studies should be conducted about it.
Two types of HepB including 10 μg and 20 μg are approved to be used in adults by China Food and Drug Administration (CFDA). HepB with higher dosage of antigens is documented to induce higher titer of anti-HBs after primary vaccination,Citation9 which could further induce higher anti-HBs seroconversion rate after revaccination among adult primary vaccination non-responders according to our study. Therefore, 20 μg HepB seems more appropriate to be used in adults rather than 10 μg HepB, especially in those with risk factors of non-response such as elder age and obesity.Citation27,28
HepB-CHO and HepB-SC are 2 types of HepB widely used in China. A higher antibody level after 3-dose revaccination was observed among HepB-CHO group compared with HepB-SC group in our study. The reason for different immunogenicity between these 2 types of HepB has not been fully understood. Previous studies showed that even if the HepB was encoded by the same gene sequence, the immunogenicity differed because of varying molecular size, molecular weight when derived from different expression system.Citation29,30 HepB-CHO has got a glycosylated structure and the composition is more similar to the wild virus,Citation31,32 which might play an important role in the different antibody responses. Although a higher titer of anti-HBs was observed after revaccination with HepB-CHO in our study, the seroconversion rate of anti-HBs after revaccination was similar between these 2 vaccine groups. The 20 μg HepB-CHO used in our study is produced domestically (About US$38 for 3 doses), which is much cheaper than the imported HepB-SC used in our study (About US$57 for 3 doses). So 20 μg HepB-CHO seems to be more cost-effective to be used in revaccination among the adult non-responders.
It is reported some demographic and behavioral factors are associated with non-response to HepB primary vaccination, such as increasing age, male gender, smoking, alcohol use, and obesity.Citation27,28 However, there are only a few studies examined the association between the above factors and the response to HepB revaccination among non-responders. Clemens et al reported that BMI affected the response to one-dose HepB revaccination among HepB non- and low- responders,Citation19 while a study in Sweden failed to detect any significant association of the response rate after revaccination with different vaccination routes, gender, age, or smoking,Citation33 similar to the findings of our study. Therefore, the demographic factors associated with immune response to HBV vaccine remain controversial to date and further studies are needed.
There are some limitations in our study. Among 438 non-responsive adults, only 251 subjects (57.30%) completed 3-dose revaccination and were included in the final analysis, and there were more persons under 40 y old in the lost cohort. However, we did not find correlation between age and anti-HBs response after revaccination, which might decrease the adverse effect of the difference in age composition between the participants and non-participants.
In conclusion, high seroconversion rate and titer of anti-HBs could be achieved after 3-dose HepB revaccination in adult HepB primary vaccination non-responders in China, and 3 doses of revaccination might be an option for those non-responders seeking for revaccination to achieve more protection against HBV infection.
Material and Methods
Screening and enrollment of non-responders
In July 2009, a total of 12,119 adults aged 18–49 years, without history of HBV infection, HepB vaccination, hypertension, and underlying medical conditions were recruited in a program of adult HepB vaccination free of charge after informed consent. All subjects with subclinical infection were excluded before enrollment after laboratory testing on HBsAg, anti-HBs and antibody against hepatitis B core antigen (Anti-HBc). The participants were randomly divided into 4 groups to receive 3-dose HepB primary vaccination on 0-, 1-, 6-month schedule with 4 types of hepatitis B vaccines [10 μg HepB-SC, 10 μg hepatitis B derived from Hansenula Polymorpha (HepB-HP), 20 μg HepB-SC and 20 μg HepB-CHO]. Blood samples were collected one month after the 3rd dose of primary HepB vaccination for anti-HBs testing. Non-responders were defined as those whose anti-HBs titer after HepB primary vaccination was less than 10 mIU/ml and a total of 438 (10.20%) non-responders were identified ().
HepB revaccination and demographic information collection
The available non-responders enrolled were randomly divided into 2 groups on the places they lived. Both groups were revaccinated with additional 3 doses of HepB at 3 months, 4 months and 9 months after the 3rd dose of HepB primary vaccination. One group received 20 µg HepB-SC (GSK, UK) and the other group received 20 µg HepB-CHO (North China Pharmaceutical Co., Ltd., Hebei, China). All participants were interviewed before revaccination by the study staff from local CDC to get their demographic information including age, gender, weight, height, and histories of HBV infection and HepB vaccination.
Specimen collection and laboratory testing
After the 1st and the 3rd dose of revaccination, 3ml blood samples were collected from each participant. Serum was isolated and was frozen at −20□ for testing. The specimens were tested for anti-HBs by Chemiluminescence Microparticle Imunoassay (Abbott, USA) at Shandong Provincial CDC. Samples with anti-HBs≥1000 mIU/ml were diluted for further testing.
Statistical analyses
The seroconversion rates after the 1st and 3rd dose of revaccination across different groups of age, gender, BMI, vaccine received in revaccination, and anti-HBs titer after primary vaccination were assessed with Pearson Chi-square test and between the 1st and 3rd dose of revaccination. The anti-HBs titers after HepB revaccination were log-transformed and were compared among different groups with student t-test or Analysis of Variance wherever appropriate, and with paired t-test between titers after the 1st and 3rd dose of revaccination. Multivariable logistic regression and linear regression models were built to assess the independent contribution from different risk factors to seroconversion or titers of anti-HBs after adjustment for potential confounders. The BMI was categorized into 3 levels (normal: BMI<24 kg/m2; overweighed: 24 kg/m2≤BMI<28 kg/m2; and obese: BMI≥28 kg/m2) based on the national data collected in China.Citation34 The titer of anti-HBs titer after primary vaccination was categorized to low primary titer group (those with anti-HBs <2 mIU/ml after primary vaccination) and high primary titer group (those with anti-HBs titer ≥2 mIU/ml after primary vaccination) as prior report.Citation26 Seroconversion after revaccination was defined as anti-HBs≥10 mIU/ml. All analyses were conducted with SPSS 13.0 and the P value < 0.05 was considered to be statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Ethical Statement
Shandong CDC Ethics Committee approved the protocol and consent forms were signed before starting.
Acknowledgments
We thank our colleagues at Zhangqiu County CDC of Shandong Province for their help in data collection. We also thank Ms. Jing Wang for her assistance with word editing.
Funding
This study was supported by the grants from the Major Project of National Science and Technology (No. 2012ZX100022001, 2013ZX10004902) and from the Shandong Medical and Health Science and Technology Development Programs (No. 2009QZ017, 2014WS0373).
References
- Kane MA. Global programme for control of hepatitis B infection. Vaccine 1995; 13(Supply 1):S47-9; PMID:7571830; http://dx.doi.org/10.1016/0264-410X(95)80050-N
- Chen DS. Hepatitis B vaccination: the key towards elimination and eradication of hepatitis B. J Hepatol 2009; 50(4):805-16; PMID:19231008; http://dx.doi.org/10.1016/j.jhep.2009.01.002
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Epidemiological serosurvey of Hepatitis B in China— Declining HBV prevalence due to Hepatitis B vaccination. Vaccine 2009; 27(47):6550-7; PMID:19729084; http://dx.doi.org/10.1016/j.vaccine.2009.08.048
- Cui FQ; Chinese Prevention Medicine Association; National Immunization Program, Chinese Center for Disease Control and Prevention. Technical guide for adult hepatitis B immunization in China. Zhonghua Liu Xing Bing Xue Za Zhi 2011 Dec; 32(12):1199-203; PMID:22336599
- Greenberg DP. Pediatric experience with recombinant hepatitis B vaccines and relevant safety and immunogenicity studies. Pediatr Infect Dis J 1993; 12(5):438-45; PMID:8327313; http://dx.doi.org/10.1097/00006454-199305000-00037
- Greenberg DP, Vadheim CM, Wong VK, Marcy SM, Partridge S, Greene T, Chiu CY, Margolis HS, Ward JI. Comparative safety and immunogenicity of two recombinant hepatitis B vaccines administered to infants at two, four and six months of age. Pediatr Infect Dis J 1996 Jul; (7):590-6; PMID:8823852; http://dx.doi.org/10.1097/00006454-199607000-00006
- André FE. Summary of safety and efficacy data on a yeast-derived hepatitis B vaccine. Am J Med 1989 Sep 4; 87(3A):14S-20S; PMID:2528292
- Zajac BA, West DJ, McAleer WJ, Scolnick EM. Overview of clinical studies with hepatitis B vaccine made by recombinant DNA. J Infect 1986 Jul; 13(Suppl A):39-45; PMID:2943814
- Zhang W, Han L, Lin C, Wang H, Pang X, Li L, Gao P, Lin H, Gong X, Tang Y, et al. Surface antibody and cytokine response to recombinant Chinese hamster ovary cell (CHO) hepatitis B vaccine. Vaccine 2011 Aug 26; 29(37):6276-82; PMID:21722684; http://dx.doi.org/10.1016/j.vaccine.2011.06.045
- Jiaye L, Bingyu Y, Li Z, Jingjing L, Yi F, Feng J, et al. Comparison on the Antibody Response and Influenced Factors of Hepatitis B Vacine Made by Recombinant Dexyribonucleic Acid Techniques among Adults. Zhong Guo Yi Miao Yu Mian Yi 2013; 19(2):142-146
- Yan BY, Zhang L, Lv JJ, Liu JY, Feng Y, Xu AQ, Chen SY, Gong XH, Cui FQ, Liang XF. Comparison of the antibody response and related influencing factors after primary immunization by 10 μg hepatitis B vaccine made from recombinant DNA techniques in saccharomyces and hansenula polymorpha among adults. Zhong Hua Liu Xing Bing Xue Za Zhi 2012; 33(9):988-989; PMID:23297469
- Jacques P, Moens G, Desomebere I, Dewijngaert J, Leroux-Roels G, Wettendorff M, Thoelen S. The immunogenicity and reactogenicity profile of a candidate hepatitis B vaccine in an adult vaccine non-responder population. Vaccine 2002 Nov 1; 20(31-32):3644-9; PMID:12399191; http://dx.doi.org/10.1016/S0264-410X(02)00397-3
- Surquin M, Tielemans CL, Kulcsár I, Ryba M, Vörös P, Mat O, Treille S, Dhaene M, Stolear JC, Kuriyakose SO, et al. Rapid, enhanced and persistent protection of patients with renal insufficiency by AS02(V)-adjuvanted hepatitis B vaccine. Kidney Int 2010 Feb; 77(3):247-55; PMID:19940840; http://dx.doi.org/10.1038/ki.2009.454
- Chou HY, Lin XZ, Pan WY, Chang CM, Lin TY, Shen HH, Tao MH. Hydrogel-delivered GM-CSF overcomes nonresponsiveness to hepatitis B vaccine through the recruitment and activation of dendritic cells. J Immunol 2010 Nov 1; 185(9): 5468-75; PMID:20889541; http://dx.doi.org/10.4049/jimmunol.1001875
- Roukens AH, Visser LG. Hepatitis B vaccination strategy in vaccine low- and non-responders: a matter of quntity of quality? Human Vaccine 2011; 7:6, 654-657; PMID:21508674; http://dx.doi.org/10.4161/hv.7.1.14924
- Graven DE, Awdeh ZL, Kunches LM, Yunis EJ, Dienstag JL, Werner BG, Polk BF, Syndman DR, Platt R, Crumpacker CS, et al. Nonresponsiveness to hepatitis B vaccine in health care workers. Results of revaccination and genetric typings. Ann Intern Med 1986 Sep; 105(3):356-60; PMID:2943202
- Golwater PN. Randomized, comparative trial of 20 micrograms vs 40 micrograms Engerix B vaccine in hepatitis B vaccine non-responders. Vaccine 1997; 15(4):353-6; PMID:9141204; http://dx.doi.org/10.1016/S0264-410X(96)00202-2
- Kim MJ, Nafziger AN, Harro CD, Keyserling HL, Ramsey KM, Drusano GL, Bertino JS Jr. Revaccination of healthy nonresponders with hepatitis B vaccine and prediction of seroprotection response. Vaccine 2003; 21(11-12):1174-9; PMID:12559795; http://dx.doi.org/10.1016/S0264-410X(02)00626-6
- Clemens R, Sänger R, Kruppenbacher J, Höbel W, Stanbury W, Bock HL, Jilg W. Booster immunization of low- and non-responders after a standard three dose hepatitis B vaccine schedule—results of a post-marketing surveillance. Vaccine 1997; 15(4):349-52; PMID:9141203; http://dx.doi.org/10.1016/S0264-410X(96)00205-8
- Craven DE, Awdeh ZL, Kunches LM, Yunis EJ, Dienstag JL, Werner BG, Polk BF, Syndman DR, Platt R, Crumpacker CS, et al. Nonresponsiveness to hepatitis B vaccine in health care workers. Results of revaccination and genetic typings. Ann Intern Med 1986; 105(3):356-60; PMID:2943202; http://dx.doi.org/10.7326/0003-4819-105-3-356
- Kim MJ, Nafziger AN, Harro CD, Keyserling HL, Ramsey KM, Drusano GL, Bertino JS Jr. Revaccination of healthy nonresponders with hepatitis B vaccine and prediction of seroprotection response. Vaccine 2003 Mar 7; 21(11-12):1174-9; PMID:12559795; http://dx.doi.org/10.1016/S0264-410X(02)00626-6
- Bertino JS Jr, Tirrell P, Greenberg RN, Keyserling HL, Poland GA, Gump D, Kumar ML, Ramsey K. A comparative trial of standard or high-dose S subunit recombinant hepatitis B vaccine versus a vaccine containing S subunit, pre-S1, and pre-S2 particles for revaccination of healthy adult nonresponders. J Infect Dis 1997; 175:678-81; PMID:9041342; http://dx.doi.org/10.1093/infdis/175.3.678
- Weissman JY, Tsuchiyose MM, Tong MJ, Co R, Chin K, Ettenger RB. Lack of response to recombinant hepatitis B vaccine in nonresponders to the plasma vaccine. JAMA 1988; 260(12):1734-8; PMID:2970557; http://dx.doi.org/10.1001/jama.1988.03410120080031
- Gesemann M, Scheiermann N. Quantification of hepatitis B vaccine-induced antibodies as a predictor of anti-HBs persistence. Vaccine 1995 Apr; 13(5):443-7; PMID:7639012; http://dx.doi.org/10.1016/0264-410X(94)00010-K
- Jilg W, Schmidt M, Deinhardt F. Decline of anti-HBs after hepatitis B vaccination and timing of revaccination. Lancet 1990 Jan 20; 335(8682):173-4; PMID:1967468; http://dx.doi.org/10.1016/0140-6736(90)90050-F
- Chiara F, Bartolucci GB, Cattai M, Piazza A, Nicolli A, Buja A, Trevisan A. Hepatitis B vaccination of adolescents: significance of non-protective antibodies. Vaccine 2013 Dec; 32(1):62-8; PMID:24188755; http://dx.doi.org/10.1016/j.vaccine.2013.10.074
- Hadler SC, Margolis HS. Hepatitis B immunization:vaccine types, efficacy and indications for immunization. Curr Clin Top Infect Dis 1992; 12:282-308; PMID:1386520
- Hollinger FB. Factors influencing the immune response to hepatitis B vaccine, booster dose guidelines and vaccine protocol recommendations. Am J Med 1989; 87:36-40; PMID:2528297; http://dx.doi.org/10.1016/0002-9343(89)90530-5
- Zhou W, Bi J, Janson JC, Li Y, Huang Y, Zhang Y, Su Z. Molecular characterization of recombinant Hepatitis B surface antigen from Chinese hamster ovary and Hansenula polymorpha cells by high-performance size exclusion chromatography and multi-angle laser light scattering. J Chromatogr B 2006; 838:71-7; PMID:16757217; http://dx.doi.org/10.1016/j.jchromb.2006.03.064
- Sato Y, Ishikawa N, Takagi T. High-performance size-exclusion chromatography and molar mass measurement by low-angle laser light scattering of recombinant yeast-derived human hepatitis B virus surface antigen vaccine particles. J Chromatogr 1990 May 16; 507:25-31; PMID:2380294; http://dx.doi.org/10.1016/S0021-9673(01)84177-7
- Ren GF, Zhang YM, Ruan WQ, Yang DA, Mei YF, Ping LH, et al. High level expression of hepatitis B virus surface antigen in transformed in transformed Chinese hamster ovary (CHO) cell line and immunogenicity of purified product in mice. Chin J Virol 1987; 3(4):313-20
- Diminisky D, Schirmbeeck R, Reimann J. Comparison between hepatitis B surface anigen (HBsAg) particles derived from mammalian cell (CHO) and yeast cells (Hansenular polymorpha): composition, structure and immunogenicity. Vaccine 1997; 15(6/7):637-47; PMID:9178464; http://dx.doi.org/10.1016/S0264-410X(96)00239-3
- Struve J, Aronsson B, Frenning B, Forsgren M, Weiland O. Seroconversion after additional vaccine doses to non-responders to three doses of intradermally or intramuscularly administered recombinant hepatitis B vaccine. Scand J Infect Dis 1994; 26(4):468-70; PMID:7984980; http://dx.doi.org/10.3109/00365549409008621
- Zhou B, Coorperative Meta-Analysis Group of China Obesity Task Force. Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese population. Zhuanghua Liu Xing Bing Xue Za Zhi 2002 Feb; 23(1):5-10; PMID:12015100