Abstract
Multiple Myeloma (MM) is a plasma cell (PC) malignancy, which despite significant therapeutic advances, is still considered incurable. This is due to the persistence of chemotherapy-resistant minimal residual disease in the patients' bone marrow (BM) after an effective induction therapy. Immunotherapies targeting surface molecules expressed on the bulk of tumor cells and the chemotherapy-resistant, myeloma-propagating cells could play a central role in this clinical setting. We recently described surface molecule CD229 as a potential therapeutic target for MM. In our current study we assessed the expression of CD229 on different PC subtypes and on cells with a myeloma-propagating phenotype in a total of 77 patients with PC dyscrasias independently at 2 different cancer centers. We found that CD229 was strongly and homogeneously overexpressed on the PC of patients with monoclonal gammopathy of undetermined significance (MGUS), smoldering myeloma, MM, and PC leukemia. CD229 was particularly overexpressed on those PC showing an abnormal phenotype such as expression of CD56. Most importantly, CD229 was also highly expressed on those cells in the patients' BM displaying the phenotype of chemotherapy-resistant and myeloma-propagating cells. In conclusion, our combined findings suggest that immunotherapies targeting CD229 will not only be effective for the bulk of tumor cells but will also help to eradicate chemotherapy-resistant cells remaining in the patients' BM after induction treatment. Hopefully, the design of CD229-specific monoclonal antibodies or chimeric antigen receptor-transduced T cells will help to achieve prolonged remissions or even cures in MM patients.
Introduction
Multiple myeloma (MM) is a plasma cell (PC) cancer, which is the result of a malignant transformation of a PC resulting in the secretion of high levels of monoclonal protein with renal failure, bone marrow (BM) insufficiency with cytopenias, osteolytic lesions and hypercalcemia, and immunosuppression with life-threatening infections.Citation1 MM is the second most common hematologic malignancy with about 22,000 new cases in the US alone and 10,000 disease-related deaths per year. The field of myeloma therapy has seen enormous progress in the past decade and as a result median overall survival has increased to approximately 6 years.Citation2 However, MM is still considered an incurable disease and most patients will eventually experience a fatal relapse. This is due to the persistence of chemotherapy-resistant myeloma cellsCitation3-6 in the BM even after destruction of the bulk of tumor cellsCitation7-10 and, accordingly, the disease will become more and more refractory to chemotherapy after each additional line of treatment.
Immunotherapeutic approaches could potentially play an important role in the global treatment concept for myeloma, targeting residual disease after an effective initial therapy. However, an essential first step would be to identify target antigens expressed on the bulk of tumor cells as well as the chemotherapy-resistant and myeloma-propagating cells in the patients' BM. We have recently described surface antigen CD229, which belongs to the SLAM family of receptors, as strongly expressed on the surface of MM cell lines and the malignant plasma cells of myeloma patients.Citation11 However, the number of patients included into our previous analysis was limited, we did not include patients with plasma cell dyscrasias other than MM, and, importantly, we did not evaluate CD229 expression on plasma cell subtypes with myeloma-propagating properties within the BM. The latter issue is of particular importance since it has recently been shown that myeloma-propagating activity is the exclusive property of a cell subpopulation characterized by its ability for bidirectional transition between the dominant CD19-CD138+ PC fraction and a small fraction of pre-PCs expressing a CD19-CD138- phenotype.Citation4 Remarkably, it has been demonstrated that pre-PCs are more quiescent, are enriched in epigenetic regulators, and are up to 300-fold more drug-resistant than the common malignant PCs of myeloma patients.Citation4
In our current study, we analyzed the expression of surface receptor CD229 in a large number of patients with different plasma cell dyscrasias independently at 2 different centers for the treatment of patients with hematologic malignancies. CD229 expression was assessed on different plasma cell subtypes and on cells with a myeloma-propagating phenotype.
Results
Surface molecule CD229 is commonly and strongly overexpressed on the malignant plasma cells of patients with multiple myeloma
Within our current study we, for the first time, examined the expression of CD229 as a target molecule for immunotherapeutic approaches on BM samples of a large group of patients with plasma cell dyscrasias. All patients were admitted for treatment or diagnostic purposes to the University Medical Center Hamburg-Eppendorf (UKE) in Germany or to the Huntsman Cancer Institute (HCI) in Salt Lake City, Utah. After obtaining informed consent, a total of 77 patients with plasma cell dyscrasias were included into the study, 43 of these at UKE and 34 at HCI. In total, 49 patients had had been diagnosed with MM, 7 with smoldering myeloma (SMM), 17 had a monoclonal gammopathy of undetermined significance (MGUS), and 4 patients had a plasma cell leukemia. Of the MM patients 21 were newly diagnosed and untreated and all had active disease with a significant number of plasma cells in their BM.
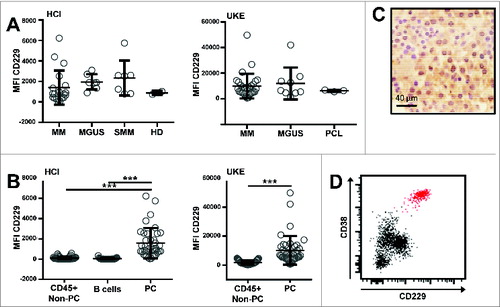
Performing flow cytometry on whole BM samples using the monoclonal antibodies shown in , we observed that CD229 was homogenously expressed on the plasma cells of all our patients irrespective of their individual diagnosis () The homogeneous expression pattern of CD229 on PC was also confirmed by immunohistochemical stains of bone marrow core biopsies (). It is known that CD229 is also expressed on normal leukocytes other than plasma cells such as T and NK cells,Citation11 however, within our patients' bone marrow the malignant plasma cells usually showed higher expression levels of CD229 than normal B cells and leukocytes other than PC ().
Table 1. Monoclonal antibodies used for flow cytometry/immunohistochemistry
Figure 1. CD229 is strongly and homogeneously overexpressed on BM plasma cells. Expression of CD229 was assessed by flow cytometry on whole BM samples, which were collected as parts of routine diagnostic procedures. The study protocol had received approval by the local internal review boards at both institutions. BM samples were analyzed according to standard staining protocols and were stained for 30 minutes using the conjugated monoclonal antibodies shown in . After red blood cell lysis (RBC Lysis/Fixation solution; Biolegend) cells of the HCI cohort were analyzed on a Beckton Dickinson FACSCanto flow cytometer. UKE samples were analyzed with a reduced 5-color panel on a Beckman Coulter Navios flow cytometer. Plotted for both cytometers is Median intensity of arbitrary fluorescent units (MFI). Both cytometers use different analog to digital converters (ADC) and also have different laser and detector configurations, thus providing the relative different values of median intensities. Doublet cells were excluded by using FSC-H/FSC-A characteristics. Plasma cells (PC) were gated according to their CD138 and CD38 expression. Immunohistochemistry was performed on paraffin embedded formalin fixed bone marrow samples according to standard staining procedures. The slides were stained with anti CD229 antibody (clone 249936). (A) At 2 different centers, HCI (left) and UKE (right), BM samples of 7 (HCI) / 10 (UKE) patients with MGUS, 7 (HCI) patients with smoldering myeloma, 19 (HCI) / 30 (UKE) patients with multiple myeloma, 3 (UKE) patients with plasma cell leukemia, and 2 (HCI) healthy donors were analyzed by flow cytometry on CD138+CD38+ PC. (B) CD229 expression levels are shown as median fluorescence intensity (MFI) on CD138+CD38+ PC, CD45+CD19+ B cells, and other CD45+CD138-CD38- non-PC after doublet exclusion with a minimum of 100 acquired events within the respective gate. ***p < 0.001 using student's T test. (C) Positivity of BM plasma cells for CD229 IHC staining is indicated by brown color. Hematoxylin (blue color) was used as a counter stain to highlight nuclei and cytoplasm of haematopoietic cells. (Original magnification 500x). (D) Exemplary dot blot of BM-derived lymphocytes from one MM patient blotted against CD38 and CD229. The CD138+CD38+ PC population is highlighted in red.
Myeloma plasma cells showing a more aberrant phenotype express higher levels of CD229
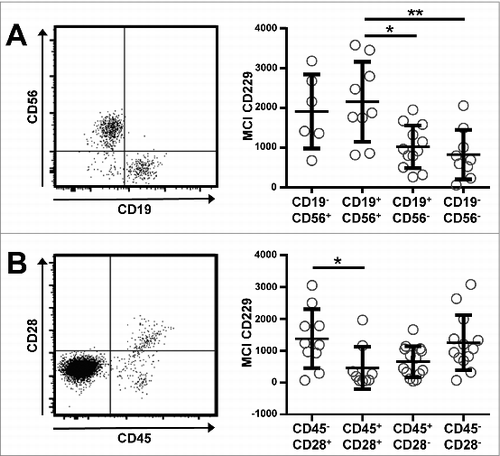
Next, we determined the expression level of CD229 on phenotypically aberrant PC within the BM of patients with all 3 types of plasma cell dyscrasias (N = 17) as defined by the absence or presence of CD19/CD56 and CD45/CD28, respectively. Surprisingly, we found significantly elevated levels of CD229 among those of the patients' plasma cells showing the CD56+ aberrant phenotype (). The same applied to those BM-derived plasma cells showing the aberrant CD45-/CD28+ phenotype versus, for example CD45+/CD28+ PC (). Thus, those myeloma plasma cells showing a more malignant phenotype evidenced increased levels of target molecule CD229.
Figure 2. CD229 is expressed on abnormal plasma cells within the patients' bone marrow. PCs from 17 patients with MM, SMM, or MGUS were grouped according to the expression of (A) CD56 and CD19 or (B) CD28 and CD45 on their BM plasma cells. Exemplary dot blots are shown on the left. Normalized (subtracted from FMO control) median fluorescence intensity (nMFI) of CD229 is shown for the different PC subsets on the right. Only those samples were taken into account where clear populations were detectable and the number of events was greater than 50. Data are presented as mean ± SD. *p < 0.05, **p < 0.01.
CD229 is homogenously expressed on the bulk of myeloma plasma cells and on the majority of chemotherapy-resistant myeloma-propagating cells
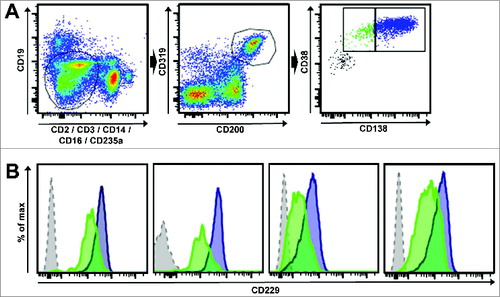
Using multicolor flow cytometry () we next analyzed the expression of CD229 on both, the dominant CD19-CD138+ PC fraction and the comparably small fraction of CD19-CD138- myeloma-propagating pre-PCs.Citation4 Importantly, we found that in all myeloma patients analyzed conventional CD138-positive PC as well as CD138-negative pre-PC myeloma-propagating cells expressed similarly high levels of surface molecule CD229 ().
Figure 3. CD229 is expressed on myeloma-propagating cells including pre-PCs. (A) An exemplary gating scheme for myeloma-propagating cells is shown. After doublet exclusion the gate was set on CD19-, CD2-, CD3-, CD14-, CD16-, CD235a- cells (left) and cells were then gated for CD200+CD319+ (middle). Myeloma-propagating cells (right) were differentiated into CD38+CD138high (blue, PC) and CD38+CD138low/negative (green, pre-PCs) as previously described.Citation4 (B) Histograms show the expression levels of CD229 in 4 different MM patients. The blue histogram represents CD38+CD138high PC and the green histogram shows CD38+CD138low/negative pre-PCs. The gray histogram represents the FMO control gated on CD319+CD200+ cells. Results show that CD138-positive PC as well as CD138-negative pre-PC myeloma-propagating cells expressed similarly high levels of surface molecule CD229.
Discussion
Myeloma therapy has become highly effective and using combinations of conventional chemotherapy and novel agents the vast majority of patients will respond very well to the first lines of treatment.Citation12-16 Unfortunately, cures still remain a rare exception and most patients will eventually experience a chemotherapy-refractory relapse of the disease. Immunotherapy could play an important role in this clinical setting eradicating even chemotherapy-resistant disease from the patients BM and, accordingly, in other cancer types tumor-specific monoclonal antibodies have become essential components of the global therapeutic concept. Very recently, promising clinical results have become available showing the great potential of monoclonal antibodies targeting surface molecules such as CD38 or CS1 in MM.Citation18 However, the number of promising therapeutic targets expressed on the surface of the bulk of myeloma cells as well as the chemotherapy-resistant and myeloma-propagating subpopulation of PC is still very limited.
We have recently described surface receptor CD229 as a potential therapeutic target for MM and applying a murine monoclonal antibody against human CD229 we also found that this antigen can be targeted efficiently via complement-derived cytotoxicity (CDC) and antibody dependent cellular cytotoxicity (ADCC).Citation11 Here, we have shown that CD229 is homogenously expressed on the malignant plasma cells across all plasma cell dyscrasias while it shows much lower levels of expression on the other leukocyte subpopulations present in the patients' bone marrow. We have also shown that CD229 is preferentially expressed on those bone marrow-infiltrating plasma cells showing an abnormal, more “malignant” phenotype as indicated, for example, by expression of CD56. This result would also be supported by our previous observation that PCs from healthy donors show less strong expression of CD229 than PCs from MM patients.Citation11 These combined findings suggest that CD229 represents a promising target for all the different types of plasma cell dyscrasias, e.g. applying a therapeutic monoclonal anti-CD229 antibody or chimeric antigen receptor (CAR)-transduced T cells.
Importantly, we have shown here that CD229 is not only strongly expressed on the bulk of malignant plasma cells but also on the pre-PC carrying the phenotype of chemotherapy-resistant, myeloma-propagating cells. It is a well-known fact that the persistence of chemotherapy-resistant minimal residual disease (MRD) in the bone marrow, even after the achievement of a complete response by induction therapy, will eventually result in relapse and progression of the disease.Citation7-10 It has recently been shown in an elegant study by Chaidos et al. that myeloma-propagating activity is the exclusive property of a cell subpopulation persistent in the patients' bone marrow, which is capable of transitioning back and forth between the dominant CD19-CD138+ PC fraction and a low percentage of pre-PCs expressing a CD19-CD138- phenotype.Citation4 Remarkably, they demonstrated that pre-PCs are more quiescent and are up to 300-fold more drug-resistant than the common malignant PCs of myeloma patients.Citation4 We have shown here for the first time that CD229 is highly and homogeneously expressed on these cells evidencing the phenotype of myeloma-propagating and chemotherapy-resistant cells. Although the role of CD19-CD138- myeloma cells remains somewhat controversial,Citation19 this finding suggests that a myeloma therapy targeting CD229 would not only be able to fight the bulk of tumor cells but would also be capable of eradicating chemotherapy-resistant MRD from the patients bone marrow leading to prolonged remissions or even cures.
A potential issue with the use of CD229 as a therapeutic target is its expression on lymphocytes other than plasma cells.Citation11 In particular, the unintended targeting of normal T and NK cells by CD229-specific CAR T cells may affect strategies for their use as a therapeutic option. However, recent data suggested that the targeting of T cells by cross-reactive CARs may not be as problematic as expected. CD229 belongs to the SLAM family of receptors, which demonstrate varying expression patterns among immune cell subsets.Citation20 This family also includes CS-1, the target of MM-specific monoclonal antibody elotuzumab. Like CD229, CS-1 is also expressed by normal B, T and NK cells. In addition to that, CS-1 is more broadly expressed on healthy tissues than CD229 being present on activated monocytes and granulocytes.Citation21-24 One of the major proposed mechanisms of action of elotuzumab is the induction of CS-1 specific ADCC.Citation22,25 Surprisingly, in those patients receiving elotuzumab no significant targeting of T or NK cells has been observed.Citation26 In addition, NK cells expressing CS-1-specific CARs efficiently targeted myeloma cell lines but again did not appear to be activated by other NK cells.Citation27 Finally, T cells expressing a CD28-based CS-1-specific CAR did not exhibit degranulation or cytotoxicity toward other T cells.Citation28 While this observation of an apparent protection of bystander effector cells remains poorly understood, it may be related to a lower antigen density or the presence of regulatory molecules on normal leukocytes.
In conclusion, we have shown here that surface antigen CD229 is strongly and homogenously overexpressed on the malignant plasma cells of patients across all types of plasma cell dyscrasias including MM. In addition, CD229 is also expressed on the surface of a fraction of cells carrying the phenotype of chemotherapy-resistant and myeloma-propagating cells within the patients' bone marrow. These combined findings suggest that CD229 represents a promising target for anti-myeloma immunotherapies leading to prolonged remissions or even cures in this fatal hematologic malignancy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med 2011; 364(11):1046-60; PMID:21410373; http://dx.doi.org/10.1056/NEJMra1011442.
- Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, Kapoor P, Dingli D, Hayman SR, Leung N, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 2014; 28(5):1122-8; PMID:24157580; http://dx.doi.org/10.1038/leu.2013.313.
- Kim D, Park CY, Medeiros BC, Weissman IL. CD19-CD45 low/- CD38 high/CD138+ plasma cells enrich for human tumorigenic myeloma cells. Leukemia 2012; 26(12):2530-7; PMID:22733078; http://dx.doi.org/10.1038/leu.2012.140.
- Chaidos A, Barnes CP, Cowan G, May PC, Melo V, Hatjiharissi E, Papaioannou M, Harrington H, Doolittle H, Terpos E, et al. Clinical drug resistance linked to interconvertible phenotypic and functional states of tumor-propagating cells in multiple myeloma. Blood 2013; 121(2):318-28; PMID:23169779; http://dx.doi.org/10.1182/blood-2012-06-436220.
- Matsui W, Borrello I, Mitsiades C. Autologous stem cell transplantation and multiple myeloma cancer stem cells. Biol Blood Marrow Transplant 2012; 18(1 Suppl):S27-32; PMID:22226109; http://dx.doi.org/10.1016/j.bbmt.2011.10.036.
- Van Valckenborgh E, Matsui W, Agarwal P, Lub S, Dehui X, De Bruyne E, Menu E, Empsen C, van Grunsven L, Agarwal J, et al. Tumor-initiating capacity of CD138- and CD138+ tumor cells in the 5T33 multiple myeloma model. Leukemia 2012; 26(6):1436-9; PMID:22289925; http://dx.doi.org/10.1038/leu.2011.373.
- Ladetto M, Pagliano G, Ferrero S, Cavallo F, Drandi D, Santo L, Crippa C, De Rosa L, Pregno P, Grasso M, et al. Major tumor shrinking and persistent molecular remissions after consolidation with bortezomib, thalidomide, and dexamethasone in patients with autografted myeloma. J Clin Oncol 2010; 28(12):2077-84; PMID:20308672; http://dx.doi.org/10.1200/JCO.2009.23.7172.
- Rawstron AC, Child JA, de Tute RM, Davies FE, Gregory WM, Bell SE, Szubert AJ, Navarro-Coy N, Drayson MT, Feyler S, et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol 2013; 31(20):2540-7; PMID:23733781; http://dx.doi.org/10.1200/JCO.2012.46.2119.
- Paiva B, Vidriales MB, Cervero J, Mateo G, Perez JJ, Montalban MA, Sureda A, Montejano L, Gutiérrez NC, García de Coca A, et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood 2008; 112(10):4017-23; PMID:18669875; http://dx.doi.org/10.1182/blood-2008-05-159624.
- Ferrero S, Ladetto M, Drandi D, Cavallo F, Genuardi E, Urbano M, Caltagirone S, Grasso M, Rossini F, Guglielmelli T, et al. Long-term results of the GIMEMA VEL-03-096 trial in MM patients receiving VTD consolidation after ASCT: MRD kinetics' impact on survival. Leukemia 2014; 29(3):689-95.
- Atanackovic D, Panse J, Hildebrandt Y, Jadczak A, Kobold S, Cao Y, Templin J, Meyer S, Reinhard H, Bartels K, et al. Surface molecule CD229 as a novel target for the diagnosis and treatment of multiple myeloma. Haematologica 2011; 96(10):1512-20; PMID:21606160; http://dx.doi.org/10.3324/haematol.2010.036814.
- Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Spicka I, Oriol A, Hájek R, Rosiñol L, Siegel DS, Mihaylov GG, et al. Carfilzomib, Lenalidomide, and Dexamethasone for Relapsed Multiple Myeloma. N Engl J Med 2014; 372(2):142-52.
- Bringhen S, Petrucci MT, Larocca A, Conticello C, Rossi D, Magarotto V, Musto P, Boccadifuoco L, Offidani M, Omedé P, et al. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: a multicenter, phase 2 study. Blood 2014; 124(1):63-9; PMID:24855212; http://dx.doi.org/10.1182/blood-2014-03-563759.
- Cavo M, Pantani L, Petrucci MT, Patriarca F, Zamagni E, Donnarumma D, Crippa C, Boccadoro M, Perrone G, Falcone A, et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood 2012; 120(1):9-19; PMID:22498745; http://dx.doi.org/10.1182/blood-2012-02-408898.
- Nooka AK, Kaufman JL, Muppidi S, Langston A, Heffner LT, Gleason C, Casbourne D, Saxe D, Boise LH, Lonial S. Consolidation and maintenance therapy with lenalidomide, bortezomib and dexamethasone (RVD) in high-risk myeloma patients. Leukemia 2014; 28(3):690-3; PMID:24220275; http://dx.doi.org/10.1038/leu.2013.335.
- Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, Pezzatti S, Caravita T, Cerrato C, Ribakovsky E, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med 2014; 371(10):895-905; PMID:25184862; http://dx.doi.org/10.1056/NEJMoa1402888.
- van de Donk NW, Kamps S, Mutis T, Lokhorst HM. Monoclonal antibody-based therapy as a new treatment strategy in multiple myeloma. Leukemia 2012; 26(2):199-213; PMID:21852787; http://dx.doi.org/10.1038/leu.2011.214.
- Ashjian E, Redic K. Multiple myeloma: Updates for pharmacists in the treatment of relapsed and refractory disease. J Oncol Pharm Pract. 2015; 9:e92378.
- Paino T, Sarasquete ME, Paiva B, Krzeminski P, San-Segundo L, Corchete LA, Redondo A, Garayoa M, García-Sanz R, Gutiérrez NC, et al. Phenotypic, genomic and functional characterization reveals no differences between CD138++ and CD138low subpopulations in multiple myeloma cell lines. PLoS One 2014; 9(3):e92378; PMID:24658332; http://dx.doi.org/10.1371/journal.pone.0092378.
- Engel P, Eck MJ, Terhorst C. The SAP and SLAM families in immune responses and X-linked lymphoproliferative disease. Nat Rev Immunol. 2003; 3(10):813-21; PMID:14523387; http://dx.doi.org/10.1038/nri1202.
- Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, Wakeland EK, Germain RN, Schwartzberg PL. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity 2010; 32(2):253-65; PMID:20153220; http://dx.doi.org/10.1016/j.immuni.2010.01.010.
- Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, Huseni M, Powers D, Nanisetti A, Zhang Y, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res 2008; 14(9):2775-84; PMID:18451245; http://dx.doi.org/10.1158/1078-0432.CCR-07-4246.
- Lee JK, Boles KS, Mathew PA. Molecular and functional characterization of a CS1 (CRACC) splice variant expressed in human NK cells that does not contain immunoreceptor tyrosine-based switch motifs. Eur J Immunol 2004; 34(10):2791-9; PMID:15368295; http://dx.doi.org/10.1002/eji.200424917.
- De Salort J, Sintes J, Llinas L, Matesanz-Isabel J, Engel P. Expression of SLAM (CD150) cell-surface receptors on human B-cell subsets: from pro-B to plasma cells. Immunol Lett 2011; 134(2):129-36; PMID:20933013; http://dx.doi.org/10.1016/j.imlet.2010.09.021.
- Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, Lee AI, Podar K, Hideshima T, Rice AG, et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood 2008; 112(4):1329-37; PMID:17906076; http://dx.doi.org/10.1182/blood-2007-08-107292.
- Collins SM, Bakan CE, Swartzel GD, Hofmeister CC, Efebera YA, Kwon H, Starling GC, Ciarlariello D, Bhaskar S, Briercheck EL, et al. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: evidence for augmented NK cell function complementing ADCC. Cancer Immunol Immunother 2013; 62(12):1841-9; PMID:24162108; http://dx.doi.org/10.1007/s00262-013-1493-8.
- Chu J, Deng Y, Benson DM, He S, Hughes T, Zhang J, Peng Y, Mao H, Yi L, Ghoshal K, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia 2014; 28(4):917-27; PMID:24067492; http://dx.doi.org/10.1038/leu.2013.279.
- Chu J, He S, Deng Y, Zhang J, Peng Y, Hughes T, Yi L, Kwon CH, Wang QE, Devine SM, et al. Genetic modification of T cells redirected toward CS1 enhances eradication of myeloma cells. Clin Cancer Res 2014; 20(15):3989-4000; PMID:24677374; http://dx.doi.org/10.1158/1078-0432.CCR-13-2510.
