Abstract
Monocyte-derived dendritic cells (DCs) are used as immunoadjuvant cells in cancer vaccines and have made great progress. However, an optimal DCs subset is vital for this treatment effect, the current ′gold standard′ cytokine cocktail DCs have a shortcoming in their cytokines secretion, especially IL-12p70, mainly because of the existence of PGE2. Therefore, it is necessary to find an appropriate DCs-based immunotherapeutic protocol. In this study, we compared a novel ′improved′ maturation cytokine cocktail with the current ′gold standard′ maturation cytokine cocktail used for generating standard DCs. The ′improved′ maturation cytokine cocktail DCs showed a higher levels surface markers expression (CD80, CD83, CD86 and HLA-DR), the chemokine receptors CXCR4 and CCR7 and chemokine CCL19, CCL21 and CXCL21, whereas CCR5 expression was reduced. Most importantly, in contrast to ′gold standard′ DCs, which secrete little IL-12p70 and as a result induce mainly Th2 immunity, ′improved′ cytokine cocktail DCs secreted higher levels IL-12p70 and also secreted similar concentration IL-10. To removal of PGE2 from the ′improved′ DCs did increase the IL-12p70 production. In conclusion, we here present the ′improved′ DCs, as an optimal maturation cocktail protocol, can induce high migratory potential, generate immunostimulatory DCs, produce higher levels IL-12p70 with superior capacity to induce Th1 immunity, when compared with the ′gold standard′ DCs.
Introduction
DCs are the most potent and powerful function professional antigen-presenting cells (APCs) for initiating cellular immune responses through the stimulation of naive T cells.Citation1-3 DCs-based immunotherapy against cancer has displayed promising treatment outcome and becomes the centerpiece of clinical trials for active immunotherapy strategies.Citation4-10 In general, most study employed peripheral blood mononuclear cells (PBMCs) or peripheral blood stem cells (PBSCs) monocytes, which are first stimulated to immature DCs before cells are loaded with DNA, total RNA of defined tumor antigen by electroporation, which proves to be a more efficient and frequently used method for Ag loading of DCs when compared to other means.Citation11,12 Such DCs are very efficient in Ag uptake by cell endocytosis. However, they are unstable and immature; that is, co-stimulatory molecules levels and migratory capacity are low and stimulation of T cells is suboptimal.Citation13 To obtain fully matured DCs, additional signals are required. The current ‘gold standard’ DCs used in DCs-based cancer vaccines studies, first reported by Jonuleit et al.,Citation14 produce little the cytokine IL-12p70, which is involved in Th1 cell polarization, may require extra stimulation by toll-like receptor (TLR) signaling.Citation15-17 The use of ‘gold standard’ for ex vivo generation of maturation DCs may impair the efficacy of DCs-based vaccines,Citation18-20 which could be one of the factors responsible for the disappointing results of DCs-based therapeutic strategies. Other maturation cocktails, which include TLR signaling, induced enough IL-12p70 levels, but such DCs have poor migratory potential.Citation21-23 α-type-1 polarized DCs (αDC1), containing rhIL-1β, TNFα, IFNα, IFNγ and poly (I:C), which induced to produce satisfied IL-12p70 and show intermediate migratory capacity.Citation24,25
TLR agonists, as cellular immune adjuvants and immunomodulators of vaccines, attributed to their ability to mediate innate and adaptive immune responses.Citation15,26 Poly (I:C), a TLR3 agonist, could mediate stable mature Th1 immunity responses and produce large amounts of IL-12p70.Citation27 CpG ODN, a TLR9 agonist, can rapidly mature human DCs isolated from human blood,Citation28-31 which is currently being detected as immunotherapeutic agents to induce antitumor activity in clinical trials.Citation30 Previous researches have shown that TLR3 and TLR9 agonists, the 2 signaling pathways, show good synergies in human DCs.Citation32,33
Particularly, we sought to test the hypothesis that a poly(I:C) and CpG ODN-based cytokine cocktail DCs would induce a potent Th1 polarization characterized by higher production of IL-12p70 without an impairment in phenotypical maturity markers, lymph node homing capacity, co-stimulatory, adhesion molecules or immunostimulatory properties. In this study, we concluded that ‘improved’ cytokine cocktail DCs, consisting of TNFα, rh IL-1β, rh IL-6, PGE2, CpG ODN and poly (I:C), could meet the above mentioned requirements.
Results
Electroporated efficacy of DCs
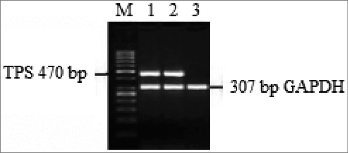
Using this electroporation protocol, we provided a evidence for successful transfection of immature DCs with A549-RNA. PCR results showed a specific band of A549-TPS ().
Expression of surface maturation and migration markers
Immature DCs electroporated with cancer cell line A549-RNA were stimulated with different cytokine cocktails into maturation DCs as demonstrated by the expression levels of CD11c (), HLA-DR (), CD80 (), CD86 (), CD83 (), CXCR4 (), CCR5 () and CCR7 () upon 8 days of maturation. With FACS analysis, the expression of CD11c and HLA-DR were similarly highly expressed on immature and mature DCs. All DCs expressed similar levels CD80 to the same extent and the upregulation of CD80 was minor in ‘improved’ cytokine cocktail DCs. To all types of mature DCs, the surface maturation markers were upregulated. The DCs populations stimulated with ‘improved’ cocktail displayed a much more maturation phenotype, when compared with ‘gold standard’ DCs. Treatment of the DCs with the ‘improved’ cytokine cocktail resulted in the highest CD83 and CD86 surface markers. When PGE2 was removed from the ‘improved’ cytokine cocktail, CD11c, HLA-DR, CD80, CD86 and CD83 surface markers were downregulated in varying degrees when compared with the other 2 maturation groups.
Figure 2. DCs were harvested and analyzed by FACS after labeling with the isotype controls and indicated mAbs. Fifty thousand cells were collected for each analysis. Immature DCs, ′transfected control′ DCs, ′gold standard′ DCs and ′improved′ DCs (with PGE2 or without PGE2) were analyzed with flow cytometry for various markers including CD11c (A), HLA-DR (B), CD80 (C), CD86 (D), CD83 (E), CXCR4 (F), CCR5 (G) and CCR7 (H). Percentages are expressed as mean ± SD of 3 independent experiments. Significant differences are shown (*P < 0.05, **P < 0.01, ***P < 0.001 ANOVA test); Brackets, at least P < 0.01; NS, p > 0.05.
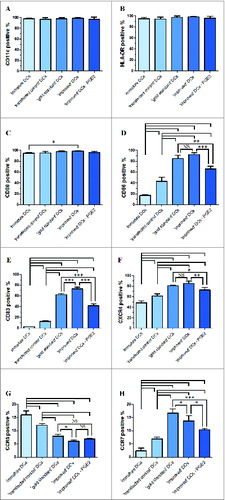
We also analyzed the migratory capacity of the 5 groups DCs populations, ‘improved’ cytokine cocktail stimulation DCs did not upregulate migration marker CCR7 expression level and CXCR4 was slightly upregulated when compared with ‘gold standard’ cytokine cocktail DCs. The omission of PGE2 from the ‘improved’ cytokine cocktail downregulated CCR7 and CXCR4 expression compared with the presence of PGE2. DCs maturation is accompanied by functional upregulation of CCR7 and is associated with downregulation of CCR5. CCR5 expression was detectable on immature DCs. Treatment of the DCs with the ‘improved’ cytokine cocktail resulted in lowest inflammatory chemokine receptor CCR5.
Chemokine and chemokine receptor expression levels
We extracted total RNA with Trizol reagents according to the manufacturer's protocol, and performed RT-PCR using primers for CCR7 and CXCR4. To detect whether CCR7 and CXCR4 mRNA expression attributed to PGE2, we detected their mRNA expression levels after maturation with or without PGE2 in ‘improved’ cytokine cocktail. CCR7 and CXCR4 mRNA expression was concordance with FACS results in these mature DCs. As shown in , mature DCs showed higher mRNA expression for CCR7 and CXCR4 compared with ‘transfected control’ and immature DCs. To the ‘gold standard’ cytokine cocktail, DCs expressed higher levels of CCR7 compared with maturation with ‘improved’ cytokine cocktail, which DCs expressed the highest levels of CXCR4. When the omission of PGE2 from ‘improved’ cytokine cocktail, CCR7 and CXCR4 mRNA expression levels were both downregulated compared with ‘improved’ cytokine cocktail.
Figure 3. The levels of CCR7, CXCR4, CCL19, CCL21 and CXCL12 mRNA and β-actin (internal control) mRNA were determined by RT-PCR. After standardization to yield the same amount of β-actin mRNA, all samples mRNA levels were expressed as fold increase in respect to immature DCs (value = 1). (A) Chemokine receptor expression (CCR7 and CXCR4). (B) Chemokine expression (CCL19, CCL21 and CXCL12). All data represent the mean mRNA level ± SD obtained from 3 independent experiments. Significant differences are shown (*P < 0.05, **P < 0.01, ***P < 0.001 ANOVA test); Brackets, at least P < 0.01; NS, p > 0.05.
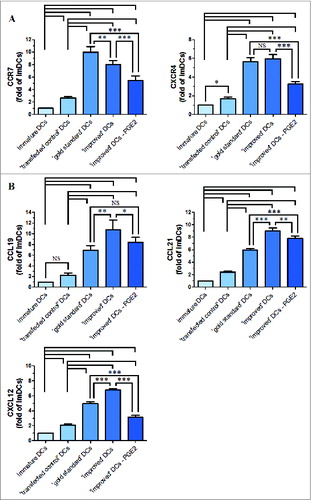
We also investigated the 2 known CCR7 ligands: CCL19 and CCL21, and the one CXCR4 ligand: CXCL12. To this end, the expression of CCL19, CCL21 and CXCL12 were measured by RT-PCR. As shown in , DCs matured with ‘improved’ cytokine cocktail had a higher expression levels to CCL19, CCL21 and CXCL12 than other maturation DCs. When PGE2 was removed from ‘improved’ cytokine cocktail, CCL19, CCL21 and CXCL12 mRNA expression levels were all downregulated compared with ‘improved’ cytokine cocktail, which was consistent with their respective chemokine receptor expression levels.
CCR7 and CXCR4 protein levels expression
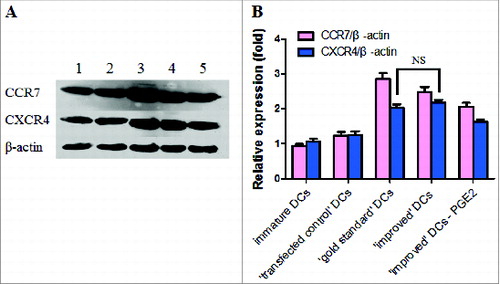
To determine whether the ‘improved’ cytokine upregulated CCR7 and CXCR4 protein expression in mature DCs, CCR7 and CXCR4 protein levels and β-actin (loading control) were detected by western blot (). Mature DCs expressed higher CCR7 and CXCR4 protein levels. The ‘gold standard’ cytokine cocktail DCs augmented CCR7 expression at the protein levels when compared to ‘improved’ cytokine cocktail DCs. ‘Gold standard’ cytokine cocktail DCs and ‘improved’ cytokine DCs expressed similar levels of CXCR4. When PGE2 was removed from the ‘improved’ cytokine cocktail, CCR7 and CXCR4 protein expression levels were both downregulated compared with ‘gold standard’ and ‘improved’ cytokine cocktail.
Figure 4. (A) A representative western blot analysis of CCR7 and CXCR4 protein expression levers in immature DCs and matured DCs is shown. Levels of β-actin were determined and served as loading control. Lane 1, immature DCs; Lane 2, ′transfected control′ DCs; Lane 3, ′gold standard′ DCs; Lane 4, ′improved′ DCs; Lane 5, removal of PGE2 from the ′improved′ DCs. (B) Western blot analysis of CCR7 and CXCR4 protein expression in immature and DCs matured by cytokine cocktail is shown. All results obtained were from 3 independent experiments and are presented as means ±SD. NS, p > 0.05.
DCs cytokine secretion
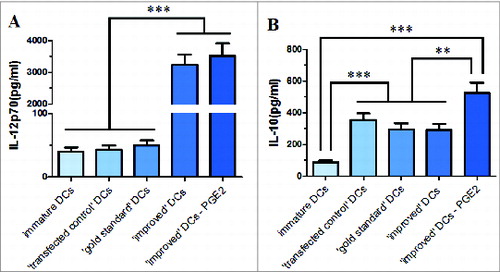
Another vital aspect of DCs function is secretion of IL-12p70 and IL-10 in culture supernatants. There were no differences in the absolute numbers of DCs at the final time of assays among the 5 groups. The IL-12p70 secretion production by maturation DCs with removal of PGE2 from ‘improved’ cytokine cocktail and ‘improved’ cytokine cocktail primed DCs were similarly high (). However, this ‘improved’ cocktails DCs population had a more mature phenotype and migration capability. As it is known to all, immature DCs and ‘gold standard’ cytokine cocktail DCs hardly secreted IL-12p70, which ‘transfected control’ DCs secreted similar levels IL-12p70 with them. We only detected small amounts of IL-10 () in ‘improved’ cytokine cocktail DCs supernatants. When PGE2 was removed from ‘improved’ cytokine cocktail, IL-10 secretion levels were even higher than ‘improved’ cytokine cocktail DCs. There was no significant difference among ‘transfected control’ DCs, ‘gold standard’ cytokine cocktail DCs and ‘improved’ cytokine cocktail DCs 3 groups in IL-10 secretion production.
Figure 5. Inflammatory cytokine secreted by immature or maturing DCs in response to various maturation stimuli and the production of IL-12p70 (A) and IL-10 (B) was determined in the culture supernatants. Data in bars are expressed as means of cytokine concentrations ± SD of 3 independent experiments (*P < 0.05, **P < 0.01, ***P < 0.001 ANOVA test); ⊥ indicates ′or′.
Migration capacity toward spleen in mice vivo
We tracked in vivo migration of injected cells and visualized DCs using a fluorescent marker (CM-DiI). Qualitative analysis of in vivo DCs migration was performed by detection of the total fluorescence-positive cells numbers under a fluorescence microscope, which numbers in each dot plot represent the fluorescence-positive cells. These fluorescence-positive cells formed clusters with lymphoid cells within spleen.
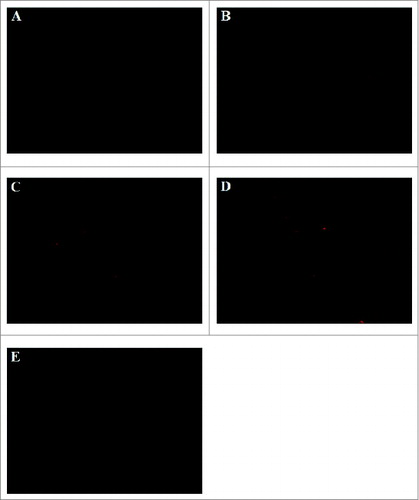
Very few immature DCs were found in the spleen cryosections. ‘Gold standard’ DCs showed a lower migration capability toward spleen as compared to ‘improved’ DCs. For ‘improved’ DCs, numerous fluorescence-positive DCs and fluorescence-positive cells formed clusters were observed in spleen cryosections. ‘Improved’ DCs showed the maximal migration capability, which DCs stimulated with ‘improved’ cytokine cocktail showed enhanced migratory ability to spleen. When remove of PGE2 from the ‘improved’ DCs, cells migratory ability to spleen was lower as compared with ‘improved’ DCs ().
Figure 6. Spleen histological cryosections staining red fluorescence of CM DiI-labeled DCs was detected and images were acquired with Olympus IX51 (Tokyo, Japan) fluorescence microscope using a 100 × objectives. CM DiI-labeled DCs mainly located in periarterial lymphatic sheath and marginal zone. (A) ′immature′ DCs (B) ′transfected control′ DCs; (C) ′gold standard′ DCs; (D) ′improved′ DCs; (E) removal of PGE2 from the ′improved′ DCs.
Discussion
An optimal clinical grade DCs vaccine has to address the following aspects questions, including upregulation of surface maturation and migration markers, cytokine secretion and Th1 polarization.Citation34-37 While innumerable studies have shown the effects of various maturation stimuli on human DCs phenotype and function by comparing immature DCs with mature DCs. For most DCs-based clinical immunotherapy, immature DCs pulsed with tumor antigen peptides were used.Citation38,39 Previous studies have shown that electroporation is a more efficient and frequently applied mean.Citation40-42 In this study, we employed A549 cell line RNA for the loading of DCs and electroporated the APCs in the immature phase when they have the ability to process antigens.
Poly (I:C), a TLR3 agonist, used in our study is pharmaceutical grade, has a uridine ribonucleotide substitution every 12 bp to decrease clinical toxicity, and has been safely administered to patients.Citation43 Overall poly (I:C) was a reasonable maturation stimulus, although poly (I:C) induced a more heterogenous mature phenotype,Citation44,45 lower DCs yieldsCitation46 and survival and in some cases less T cell activation.Citation47 Numerous studies have shown that poly (I:C) produces high quantities of IL-12 and polarizes a strong Th1 immune response. However, these poly (I:C)-based DCs have lower migratory potential.Citation48–50 Therefore, these researchers tried to strengthen the stimulating function of poly (I:C) and applied poly (I:C) combined with TLR7/8 agonist in addition to PGE2 stimulation, which leads to secrete higher levels IL-12p70 and enhance migratory potential of DCs.Citation51–53 CpG ODN, a TLR9 agonist, which can effectively enhance maturation of DCs and strongly mediate antitumor cellular immune responses and antitumor therapeutic effects in mice.Citation32,54 Hoene et al. recent study has shown that monocyte-derived DCs express TLR9 and that their maturation can be triggered directly by CpG ODN.Citation55 Tada et al. reported that human moDCs expressed TLR9 protein and secreted IL-12p70 in reponse to CpG ODN.Citation33 TLR9 shares the same MyD88-mediated intracellular signaling pathway and TLR3-mediated activation of NF-κB and MAP arises by a MyD88-independent way. There is a synergistic manner evidence to suggest that DCs maturation might depend on TLR signaling pathway.Citation32 Thus, our studies explored that addition of poly (I:C) and CpG ODN to ‘gold standard’ cytokine cocktail was based on immumotherapies for cancer. We confirmed a synergistic effect was exhibited on CD83, CD86, CCR7 and CXCR4 expression levels and the synergistic effect was reduced when PGE2 was removed. CCR5 was downregulated in immature DCs during maturation.Citation56,57
IL-12p70 is vital both in promoting Th1 polarization and in improving the ability of T-cell immune responses and IL-10 is a potent suppressor of T-cell function.Citation58,59 Synergism effect between poly (I:C) and CpG ODN for the activation of a macrophage lineage, which resulted in the synergistic induction of IL-12. These studies confirmed further exploration of the combined application of poly (I:C) and CpG ODN as immune adjuvant cells.Citation29 We expected there was a result of IL-12p70 higher levels secretion in the ‘improved’ cytokine cocktail without PGE2, because PGE2 is responsible for the shortage of IL-12p70, but our results indicated that the omission of PGE2 from ‘improved’ cocktail did not produce any noteworthy amounts of IL-12p70, such DCs also displayed a less maturation phenotype when compared with the ‘improved’ DCs. It is clear that PGE2 is necessary for inducing maturation. IL-10 might play a key role to response Th1/Th2 cells in inhibiting IL-12p70. Although IL-10 secreted by ‘improved’ cytokine cocktail maturation DCs is similar to the cytokine secreted by ‘gold standard’ cytokine cocktail DCs, it can be obviously neglected for IL-12p70 levels. The omission of PGE2 from ‘improved’ cocktail enabled IL-12p70 production. Our study supported a theory in which PGE2 based protocols lead to a terminally mature DC, whereas maturation with poly (I:C) and CpG ODN results in DCs which can be stimulated to produce higher levels IL-12p70.
As is known to all, migration process of DCs to secondary lymph organs (SLO) is dependent on CCR7 and its ligands, CCL19 and CCL21,Citation60,61 whereas the characterization of CXCR4 and its ligand CXCL12 in DCs migration potential to SLO seems to be secondary, but not uncorrelated.Citation62 The expression of CCR7 and CXCR4 in DCs eventually results in their migratory capability from peripheral tissues to T-cell zones of the spleen, where higher levels of CCL19, CCL21 and CXCL12 expression exist. In this study, PGE2 was found to be a critical switch factor for the acquisition of migratory capacity. DCs stimulated with ‘gold standard’ and ‘improved’ cocktail expressed higher CCR7, CXCR4 and CXCL12 mRNA and/or protein levels, which were reduced when PGE2 is completely removed from the cytokine cocktail. In addition to, poly (I:C) and CpG ODN positively affected CCL19 and CCL21 mRNA expression, implied that the migratory potential of DCs was promoted by the 2 cytokines. Our results have shown a positive synergistic effect of poly (I:C) and CpG ODN in combination with ‘gold standard’ cytokine cocktail on chemokine receptors and chemokine mRNA and/or protein expression levels.
Previous researches have shown that CM-DiI is safe for the labeling of cells, which it don't alter the cell phenotype or cellular toxicity. CM-DiI has been used to an indicator to observe cells migration potential in vivo.Citation63-65 In our study, CM-DiI labeled- DCs for 24h did not alter the DCs phenotypic or functional characteristics. In vivo of mice, DCs stimulated with ‘improved’ cytokine cocktail demonstrated positive synergistic effect for migration capacity in spleen.
In short, we elaborated in this manuscript a very promising DCs, which maturation cytokine composing of poly (I:C) and CpG ODN in combination with the ‘gold standard’ cytokine cocktail could meet cancer immunotherapy criteria, being the ability to migrate to SLO and IL-12p70 secretion production. We optimized the ‘gold standard’ cytokine cocktail and suggested a novel strategy for generating highly potential DCs vaccines. For their future therapeutic prospects, the in vivo effects will need to be further confirmed in future clinical trials.
Materials and Methods
Reagents
DCs serum-free medium and rh IL-4, rh GM-CSF, rh IL-1β, TNFα and rh IL-6 were all purchased from CellGenix (Peprotech, USA). PGE2 was purchased from Cayman Chemical (Ann Arbor, MI, USA). The CpG ODN 1826 (5′-TCCATGACGTTCCTGACGTT-3′) class B used in the experiment were synthesized by Sangon (Sangon Inc., Shanghai, China). RPMI-1640 medium and Trizol Reagents were obtained from Invitrogen Inc. (Carlsbad, CA, USA). Fetal bovine serum (FBS) was obtained from GIBCO BRL (Grand Island, NY, USA). Poly (I:C) was obtained from Invivogen (Sigma, USA). The human lung cancer cell line A549 was purchased from KeyGEN BioTECH (Nanjing, China). All mAbs used for flow cytometry were purchased from Becton Dickinson (BD, San Jose, USA), except the mAb used for the analysis of CCR7 (R&D systems, Minneapolis, USA). Mouse anti-human β-actin and rabbit anti-human CCR7 and CXCR4 monoclonal antibodies were purchased from Abcam (Cambridge, MA, USA). HRP-conjugated goat anti-mouse IgG and goat anti-Rabbit IgG were obtained from Abcam (Cambridge, MA, USA). Human IL-12p70 and IL-10 ELISA KIT were obtained from R&D Systems (Minneapolis, MN, USA).
A549 cell line cultures
The human lung cancer A549 cells line was grown in a complete RPMI-1640 medium and incubated at 37°C in a humidified incubator with 5% CO2. We digested A549 cell line by trypsin, collected, and washed twice with PBS. Total RNA was extracted using Trizol according to the manufacturer's instructions. Sample concentrations and quality were detected by measuring the OD value at 260 nm, and 280 nm with a NanoDrop® ND-2000 Spectrophotometer (Thermo Scientific, USA). The 260/280 nm ratios of the samples were > 1.8. We detected sample purity and integrity by agarose gel electrophoresis. All samples showed 18S and 28S rRNA bands without obvious degradation products.
Generation and immature of monocyte-derived dendritic cells
PBSCs were obtained from healthy donors mobilized with rh GM-CSF for allogeneic transplantation in accordance with the regulations set by Anhui Medical University ethics committee. Monocyte cells were isolated from leftover PBSCs products after obtaining a consent form. We washed the PBSCs remaining in the apheresis bags 3 times, adjusted to final products of 2–6 ×108 cells. Then PBSCs were isolated by Ficoll density gradient centrifugation, and the enriched PBSCs were adjusted to a final density of 2–3× 106 cells/ml with complete RPMI-1640 medium. Two ml of cell suspension was seeded into 6-well culture plate. We incubated cells for 2 hours in a humidified incubator with 5% CO2 at 37°C. Then we aspirated nonadherent cells, and gently rinsed the remaining cells 3 times with pre-warmed RPMI 1640 medium in order to remove the contaminated lymphocytes. We induced adherent monocytes to differentiate into DCs during a 5-day culture in complete RPMI 1640 medium containing 100 ng/ml rh GM-CSF and 50 ng/ml rh IL-4. A half-of-medium volume was removed and replaced by the same volume of complete RPMI-1640 medium every other day. We harvested immature DCs on days 5–7 of culture.
Electroporation of Immature DCs
At day 5, we washed immature DCs once, suspended the cells in RPMI-1640 medium, regulated to a final cell concentration of 5 × 106 cells/ml and preincubated the cells in a 4-mm gap electroporation cuvette for 5 min on ice. We transferred 30 μg of A549 total RNA to the cuvette and DCs were pulsed using a BTX 830 square-wave electroporator. We adjusted electroporation settings to a monopulse, 300 V, 500 μs. Immature DCs were immediately transferred to 6-well culture plate and resuspended in fresh complete RPMI-1640 medium for 24 h after electroporation.
Maturation of DCs
At day 6, we stimulated the electroporated immature DCs with the ‘gold standard’ cytokine cocktail (rh IL-1β (10 ng/ml), TNFα (10 ng/ml), rh IL-6 (10 ng/ml) and PGE2 (250 ng/ml)) or the ‘improved’ cytokine cocktail (rh IL-1β (10 ng/ml), TNFα (10 ng/ml), rh IL-6 (10 ng/ml), PGE2 (250 ng/ml), CpG ODN (10 μg/ml) and poly (I:C) (20 μg/ml)). We harvested maturation DCs 48 h later and collected the supernatants of cells, then frozen at − 20°C until these cytokines were measured using ELISA and the cells were used for immunologic studies. In this report, the electroporated immature DCs only in the existence of rh GM-CSF (100 ng/ml) and rh IL-4 (50 ng/ml) were defined as ‘transfected control’ DCs.
Electroporation efficacy of A549-RNA
The electroporation efficacy of A549-RNA was detected by assesment of tissue polypeptide specific antigen (TPS) by RT-PCR. We extracted total RNA from the immature DCs, ‘transfected control’ DCs, and A549 using Trizol. Reverse transcription with random hexamer primers was measured with the PrimeScript™ RT Master Mix kit (Takala, Dalian, China) following manufacturer's protocol. Amplification of cDNA was performed on a thermocycler T Gradient (Biometra, Germany) with primer a 5′-GTAGATGCCCCCAAATCTCA-3′ and primer b 5′-CCAAGATTAAAGTCCTCGCC-3′. PCR products were measured by electrophoresis on 1.5% agarose gels and electrophoresis image was photographed.
Flow cytometric analysis of cell surface phenotype
For DCs surface phenotype, we washed the immature DCs and stimulated immature DCs with PBS containing 0.5% BSA and stained by incubation with mAb at 4°C for 30 min. Positive rates of the surface phenotype markers were detected with Isotype IgG intensity as a control. Subsequently, 4-Color immunofluorescence flow cytometry was analyzed using the following panel of mAb: PE-conjugated antihuman CD11c, PE-conjugated antihuman CD80, APC-conjugated antihuman CD83, APC-conjugated antihuman CD86, FITC-conjugated antihuman HLA-DR, FITC-conjugated antihuman CCR5, PE-conjugated antihuman CCR7 and PerCP5.5-conjugated antihuman CXCR4. Fifty thousand cells were measured per sample. Positive cell percentages were analyzed by flow cytometry (BD FACScan using Cell Quest Pro Software, BD Biosciences).
RNA extraction and real-time PCR (RT-PCR)
We extracted immature and mature DCs (3–5×106 cells) total RNA using Trizol. Total RNA was quantitated by A260 measurements (NanoDrop® ND-2000 Spectrophotometer). We designed primers for RT-PCR using DNA sequence information obtained from NCBI. Quantitative real-time PCR was operated in a volume of 20 μL using the ABI 7300 Real Time PCR Detection System (ABI, USA). We used the following primers for quantitative PCR of mRNA expression: for CCR7, sense, 5′- TGG GGA AAC CAA TGA AAA GC −3′, and antisense, 5′- GGA GCA CAA AGA CTC GAA CAA A −3′; for CXCR4, sense, 5′- CAG CAG GTA GCA AAG TGA −3′, and antisense, 5′- CTC GGT GTA GTT ATC TGA AGT −3′; for CCL19, sense, 5′- GCA CCA ATG ATG CTG AAG ACT-3′, and antisense, 5′- CTG GAT GAT GCG TTC TAC CC-3′; for CCL21, sense, 5′- ATG GCT CAG TCA CTG GCT CTG −3′, and antisense, 5′- ATG GCT CAG TCA CTG GCT CTG −3′; for CXCL−12, sense, 5′- ATG AAC GCC AAG GTC GTG G-3′, and antisense, 5′- TCG GCA TGG GCA TCT GT-3′; and for β-actin, sense, 5′- TGG CAA TGA GCG GTT CC −3′, and antisense, 5′- CTT TGC GGA TGT CCA CGT-3′. We normalized relative quantification of mRNA expression with control β-actin and analyzed using the Delta Delta Ct (2−ΔΔCT) method. All PCR products were measured by detection of melting profiles.
Western blots
The DCs were harvested, and the proteins in DCs were extracted and the proteins concentration was detected by Bradford Assay with BSA as a standard. Total proteins (60 μg) were subjected to SDS-PAGE. Proteins were transferred onto a nitrocellulose membrane at 100 mA for 3 h after SDS-PAGE. The membranes were blocked in a solution of 5% nonfat milk in TBST containing 0.05% Tween 20, then cultured with rabbit anti-human CCR7 and CXCR4 (primary antibodies) overnight at 4°C. After washing, we incubated the membranes with goat anti-rabbit HRP-IgG (secondary antibody) for 1 hour at ambient temperature followed by washing with TBS. The target proteins were measured with the ECL system (Millipore, Billerica, MA, USA) and visualized with autoradiography film. After stripping, we reprobed the membranes with anti β-actin Ab (Abcam, Cambridge, MA, USA).
Cytokine measurement by ELISA
We measured IL-10 and IL-12p70 which immature and mature DCs release in the supernatants by specific sandwich ELISA according to the manufacturer's protocol and read optic density at 450 nm using a microplate reader Sunrise Basic (Tecan, Austria).
Animals and husbandry
All experimental animal protocols were approved by Anhui Medical University animal ethics committee. Female BALB/c athymic nude mice (age, 4–5 weeks; weight, 16–18 g), purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China), were used in this study after a 7-d acclimatization period. Mice were placed 5 per polycarbonate cage under controlled temperature (20–25°C) and humidity (40–50% relative humidity) and a 12-h diurnal cycle. Mice received a normal rodent pellet diet and sterile water randomly.
Immunofluorescence
For the in vivo study, lung cancer cell line (A549) was collected and injected subcutaneously into the right lateral axilla region of these athymic nude mice (6 mice per group). After 42 days, we labeled immature and mature DCs with CM-DiI according to the manufacturer's instructions immediately prior to injection. In brief, DCs were co-incubated with 5 μg/ml CM-DiI for 5 min at 37°C and incubated the cells for 15 min at 4°C, washed 3 times with PBS and harvested by blowing lightly. The mice were injected by tail vein with 2 × 106 CM-DiI-labeled DCs in 200 μl RPMI 1640. After 48 h, mice were euthanized and spleen was removed to detect the distribution of CM-DiI-labeled DCs. Spleen was harvested, embedded in optimal cutting temperature compound (Sakura Fine Technical, Tokyo, Japan) and immediately frozen at −80°C. Cryosections were cut using a microtome and examined for presence of fluorescent cells by a fluorescence microscope and the density of the fluorescence-positive DCs in these specimens was assayed.
Statistical analysis
Data from experiments is expressed as mean ±SD. Statistical results were analyzed by ANOVA. Differences were considered statistically significant when P value were less than 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Professor Wang Baolong for his excellent technical and financial assistance and valuable advice.
Funding
This study was funded by grants from the National Natural Science Foundation of China (81172172).
References
- Lanzavecchia A, Sallustoa AF. Regulation of T cell immunity by dendritic cells. Cell 2001; 106:263-6; PMID:11509174; http://dx.doi.org/10.1016/S0092-8674(01)00455-X
- Mellman I, Steiman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell 2001; 106:255-8; PMID:11509172; http://dx.doi.org/10.1016/S0092-8674(01)00449-4
- Takei M, Umeyama A, Shoji N, Hashimoto T. Polyacetylenediols regulate the function of human monocyte-derived dendritic cells. Int Immunopharmacol 2010; 10: 913-21; PMID:20493278; http://dx.doi.org/10.1016/j.intimp.2010.05.002
- Heiser A, Coleman D, Dannull J, Yancey D, Maurice MA, Lallas CD,. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest 2002; 109: 409-17; PMID:11828001; http://dx.doi.org/10.1172/JCI0214364
- O'Rourke MG, Johnson M, Lanagan C, See J, Yang J, Bell JR,. Durable complete clinical responses in a phase I/II trial using an autologous melanoma cell/dendritic cell vaccine. Cancer Immunol Immunother 2003; 52: 387-95; PMID:12682787
- Trepiakas R, Pedersen AE, Met O, Svane IM. Addition of interferon-alpha to a standard maturation cocktail induces CD38 up-regulation and increases dendritic cell function. Vaccine 2009; 27:2213-9; PMID:19428835; http://dx.doi.org/10.1016/j.vaccine.2009.02.015
- Ribas A, Butterfield LH, Glaspy JA, Economou JS. Current developments in cancer vaccines and cellular immunotherapy. J Clin Oncol 2003; 21:2415-32; PMID:12805342; http://dx.doi.org/10.1200/JCO.2003.06.041
- Joo HJ, Kim HS, Choi YS, Kim H, Kim SJ, Moon WK. Detection of prostaglandin E2-induced dendritic cell migration into the lymph nodes of mice using a 1.5 T clinical MR scanner. NMR Biomed 2012; 25:570-9; PMID:22009917; http://dx.doi.org/10.1002/nbm.1774
- Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med 2004; 10: 475-80; PMID:15122249; http://dx.doi.org/10.1038/nm1039
- Walker DG, Laherty R, Tomlinson FH, Chuah T, Schmidt C. Results of a phase I dendritic cell vaccine trial for malignant astrocytoma: potential interaction with adjuvant chemotherapy. J Clin Neurosci 2008; 15:114-21; PMID:18083572; http://dx.doi.org/10.1016/j.jocn.2007.08.007
- Milano F, van Baal JW, Rygiel AM, Bergman JJ, Van Deventer SJ, Kapsenberg ML,. An improved protocol for generation of immuno-potent dendritic cells through direct electroporation of CD14+ monocytes. J Immunol Methods 2007; 321:94-106; PMID:17336322; http://dx.doi.org/10.1016/j.jim.2007.01.004
- Van Camp K, Cools N, Stein B, Van de Velde A, Goossens H, Berneman ZN,. Efficient mRNA electroporation of peripheral blood mononuclear cells to detect memory T cell responses for immunomonitoring purposes. J Immunol Methods. 2010; 354(1–2):1-10; PMID:20138047; http://dx.doi.org/10.1016/j.jim.2010.01.009
- Jongmans W, Tiemessen DM, van Vlodrop IJ, Mulders PF, Oosterwijk E. Th1-polarizing capacity of clinical-grade dendritic cells is triggered by Ribomunyl but is compromised by PGE2: the importance of maturation cocktails. J Immunother 2005; 28:480-7; PMID:16113604; http://dx.doi.org/10.1097/01.cji.0000171290.78495.66
- Jonuleit H, Kühn U, Müller G, Steinbrink K, Paragnik L, Schmitt E,. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol 1997; 27:3135-42; PMID:9464798; http://dx.doi.org/10.1002/eji.1830271209
- Raïch-Regué D, Naranjo-Gómez M, Grau-López L, Ramo C, Pujol-Borrell R, Martínez-Cáceres E,. Differential effects of monophosphoryl lipid A and cytokine cocktail as maturation stimuli of immunogenic and tolerogenic dendritic cells for immunotherapy. Vaccine 2012; 30: 378-87; http://dx.doi.org/10.1016/j.vaccine.2011.10.081
- Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol 2005;6(8):769-76; PMID:15995707; http://dx.doi.org/10.1038/ni1223
- Blander JM, Medzhitov R. On regulation of phagosome maturation and antigen presentation. Nat Immunol 2006;7(10):1029-35; PMID:16985500; http://dx.doi.org/10.1038/ni1006-1029
- Schadendorf D, Ugurel S, Schuler-Thurner B, Nestle FO, Enk A, Bröcker EB,. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol 2006;17(4):563-70; PMID:16418308; http://dx.doi.org/10.1093/annonc/mdj138
- Draube A, Klein-González N, Mattheus S, Brillant C, Hellmich M, Engert A,. Dendritic cell based tumor vaccination in prostate and renal cell cancer: a systematic review and meta-analysis. PLoS One 2011;6(4):e18801; PMID:21533099; http://dx.doi.org/10.1371/journal.pone.0018801
- Engell-Noerregaard L, Hansen TH, Andersen MH, Thor Straten P, Svane IM. Review of clinical studies on dendritic cell-based vaccination of patients with malignant melanoma: assessment of correlation between clinical response and vaccine parameters. Cancer Immunol Immunother 2009;58(1):1-14; PMID:18719915; http://dx.doi.org/10.1007/s00262-008-0568-4
- Peng JC, Thomas R, Nielsen LK. Generation and maturation of dendritic cells for clinical application under serum-free conditions. J Immunother 2005;28(6):599-609; PMID:16224278; http://dx.doi.org/10.1097/01.cji.0000175491.21099.04
- Dauer M, Lam V, Arnold H, Junkmann J, Kiefl R, Bauer C,. Combined use of toll-like receptor agonists and prostaglandin E(2) in the FastDC model: rapid generation of human monocyte-derived dendritic cells capable of migration and IL-12p70 production. J Immunol Methods 2008;337(2):97-105; PMID:18657542; http://dx.doi.org/10.1016/j.jim.2008.07.003
- Ten Brinke A, Karsten ML, Dieker MC, Zwaginga JJ, van Ham SM. The clinicalgrade maturation cocktail monophosphoryl lipid A plus IFN gamma generates monocyte-derived dendritic cells with the capacity to migrate and induce Th1 polarization. Vaccine 2007; 25:7145-52; PMID:17719152; http://dx.doi.org/10.1016/j.vaccine.2007.07.031
- Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML,. alpha-type-I polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res 2004; 64:5934-7; PMID:15342370; http://dx.doi.org/10.1158/0008-5472.CAN-04-1261
- Hansen M, Hjortø GM, Donia M, Met Ö, Larsen NB, Andersen MH,. Comparison of clinical grade type 1 polarized and standard matured dendritic cells for cancer immunotherapy. Vaccine 2013 Jan;31(4):639-46; http://dx.doi.org/10.1016/j.vaccine.2012.11.053
- Mitchell D, Olive C. Regulation of Toll-like receptor-induced chemokine production in murine dendritic cells by mitogen-activated protein kinases. Mol Immunol 2010; 47(11–12):2065-73; PMID:20451253
- Dragicevic A, Dzopalic T, Vasilijic S, Vucevic D, Tomic S, Bozic B,. Signaling through Toll-like receptor 3 and Dectin-1 potentiates the capability of human monocyte-derived dendritic cells to promote T-helper 1 and T-helper 17 immune responses. Cytotherapy 2012; 14:598-607; PMID:22424215; http://dx.doi.org/10.3109/14653249.2012.667873
- Chen W, Chan AS, Dawson AJ, Liang X, Blazar BR, Miller JS. FLT3 ligand administration after hematopoietic cell transplantation increases circulating dendritic cell precursors that can be activated by CpG oligodeoxynucleotides to enhance T-cell and natural killer cell function. Biol Blood Marrow Transplant 2005; 11:23-34; PMID:15625541; http://dx.doi.org/10.1016/j.bbmt.2004.08.004
- Whitmore MM, DeVeer MJ, Edling A, Oates RK, Simons B, Lindner D,. Synergistic activation of innate immunity by double-stranded RNA and CpG DNA promotes enhanced antitumor activity. Cancer Res 2004; 64:5850-60; PMID:15313929; http://dx.doi.org/10.1158/0008-5472.CAN-04-0063
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H. A Toll-like receptor recognizes bacterial DNA. Nature 2000; 408:740-5; PMID:11130078; http://dx.doi.org/10.1038/35047123
- Johannsen A, Genolet R, Legler DF, Luther SA, Luescher IF. Definition of key variables for the induction of optimal NY-ESO-1-specific T cells in HLA transgene mice. J Immunol 2010; 185:3445-55; PMID:20733200; http://dx.doi.org/10.4049/jimmunol.1001397
- Tsujimoto H, Efron PA, Matsumoto T, Ungaro RF, Abouhamze A, Ono S,. Maturation of murine bone marrow-derived dendritic cells with poly(I:C) produces altered TLR-9 expression and response to CpG DNA. Immunol Lett 2006;107(2):155-62; PMID:17011046; http://dx.doi.org/10.1016/j.imlet.2006.09.001
- Tada H, Aiba S, Shibata K, Ohteki T, Takada H. Synergistic effect of Nod1 and Nod2 agonists with toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect Immun 2005;73(12):7967-76; PMID:16299289; http://dx.doi.org/10.1128/IAI.73.12.7967-7976.2005
- Hwang I, Ki D. Receptor-mediated T cell absorption of antigen presenting cell-derived molecules. Front Biosci (Landmark Ed) 2011;16:411-21; PMID:21196178; http://dx.doi.org/10.2741/3695
- Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity 2008;29(3):325-42; PMID:18799141; http://dx.doi.org/10.1016/j.immuni.2008.08.006
- Johnson LA, Jackson DG. Control of dendritic cell trafficking in lymphatics by chemokines. Angiogenesis 2014;17(2):335-45; PMID:24232855; http://dx.doi.org/10.1007/s10456-013-9407-0
- Dudek AM, Martin S, Garg AD, Agostinis P. Immature, Semi-Mature, and Fully Mature Dendritic Cells: Toward a DC-Cancer Cells Interface That Augments Anticancer Immunity. Front Immunol 2013;4:438; PMID:24376443; http://dx.doi.org/10.3389/fimmu.2013.00438
- Garg NK, Dwivedi P, Prabha P, Tyagi RK. RNA pulsed dendritic cells: an approach for cancer immunotherapy. Vaccine 2013 Feb 6;31(8):1141-56; http://dx.doi.org/10.1016/j.vaccine.2012.12.027
- Bae WK, Umeyama A, Chung IJ, Lee JJ, Takei M. Uncarinic acid C plus IFN-γ generates monocyte-derived dendritic cells and induces a potent Th1 polarization with capacity to migrate. Cell Immunol 2010; 266:104-10; PMID:20933226; http://dx.doi.org/10.1016/j.cellimm.2010.09.004
- Van Tendeloo VF, Ponsaerts P, Lardon F, Nijs G, Lenjou M, Van Broeckhoven C,. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood 2001; 98:49-56; PMID:11418462; http://dx.doi.org/10.1182/blood.V98.1.49
- Kyte JA, Kvalheim G, Lislerud K, thor Straten P, Dueland S, Aamdal S,. T cell responses in melanoma patients after vaccination with tumor-mRNA transfected dendritic cells. Cancer Immunol Immunother 2007;56(5):659-75; PMID:16947019; http://dx.doi.org/10.1007/s00262-006-0222-y
- Yu Z, Sun H, Zhang T, Yang T, Long H, Ma B. Specific antitumor effects of tumor vaccine produced by autologous dendritic cells transfected with allogeneic osteosarcoma total RNA through electroporation in rats. Cancer Biol Ther 2009;8(10):973-80; PMID:19287216; http://dx.doi.org/10.4161/cbt.8.10.8281
- Strayer DR, Carter WA, Brodsky I, Cheney P, Peterson D, Salvato P,. A controlled clinical trial with a specifically configured RNA drug, poly(I):poly(C) 12 U, in chronic fatigue syndrome. Clin Infect Dis 1994; 18:S88-95; PMID:8148460; http://dx.doi.org/10.1093/clinids/18.Supplement_1.S88
- Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C,. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009 Jul 6;206(7):1589-602; http://dx.doi.org/10.1084/jem.20090247
- Wilson KL, Xiang SD, Plebanski M. Montanide, Poly I:C and nanoparticle based vaccines promote differential suppressor and effector cell expansion: a study of induction of CD8 T cells to a minimal Plasmodium berghei epitope. Front Microbiol. 2015 Feb 6;6:29
- Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V,. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res 2009;69(5):2000-9; PMID:19244125; http://dx.doi.org/10.1158/0008-5472.CAN-08-2360
- Lee AW, Truong T, Bickham K, Fonteneau JF, Larsson M, Da Silva I., A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: implications for immunotherapy. Vaccine 2002; 20:A8-A22; PMID:12477423; http://dx.doi.org/10.1016/S0264-410X(02)00382-1
- Trepiakas R, Pedersen AE, Met O, Hansen MH, Berntsen A, Svane IM. Comparison of alpha-Type-1 polarizing and standard dendritic cell cytokine cocktail for maturation of therapeutic monocyte-derived dendritic cell preparations from cancer patients. Vaccine 2008;26(23):2824-32; PMID:18450338; http://dx.doi.org/10.1016/j.vaccine.2008.03.054
- Karlsen M, Hovden AO, Vogelsang P, Tysnes BB, Appel S. Bromelain treatment leads to maturation of monocyte-derived dendritic cells but cannot replace PGE2 in a cocktail of IL-1β, IL-6, TNF-α and PGE2. Scand J Immunol 2011; 74:135-43; PMID:21449940; http://dx.doi.org/10.1111/j.1365-3083.2011.02562.x
- Möller I, Michel K, Frech N, Burger M, Pfeifer D, Frommolt P,. Dendritic cell maturation with poly(I:C)-based versus PGE2-based cytokine combinations results in differential functional characteristics relevant to clinical application. J Immunother 2008; 31:506-19; PMID:18463533; http://dx.doi.org/10.1097/CJI.0b013e318177d9e5
- Boullart AC, Aarntzen EH, Verdijk P, Jacobs JF, Schuurhuis DH, Benitez-Ribas D,. Maturation of monocyte-derived dendritic cells with Toll-like receptor 3 and 7/8 ligands combined with prostaglandin E2 results in high interleukin-12 production and cell migration. Cancer Immunol Immunother 2008; 57:1589-97; PMID:18322684; http://dx.doi.org/10.1007/s00262-008-0489-2
- Luft T, Jefford M, Luetjens P, Toy T, Hochrein H, Masterman KA,. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E(2) regulates the migratory capacity of specific DC subsets. Blood 2002; 100:1362-72; PMID:12149219; http://dx.doi.org/10.1182/blood-2001-12-0360
- Kim HJ, Kim HO, Lee K, Baek EJ, Kim HS. Two-step maturation of immature DCs with proinflammatory cytokine cocktail and poly(I:C) enhances migratory and T cell stimulatory capacity. Vaccine 2010;28(16):2877-86; PMID:20156531; http://dx.doi.org/10.1016/j.vaccine.2010.01.061
- Whitmore MM, DeVeer MJ, Edling A, Oates RK, Simons B, Lindner D,. Synergistic activation of innate immunity by double-stranded RNA and CpG DNA promotes enhanced antitumor activity. Cancer Res 2004; 64:5850-60; PMID:15313929; http://dx.doi.org/10.1158/0008-5472.CAN-04-0063
- Hoene V, Peiser M, Wanner R. Human monocyte-derived dendritic cells express TLR9 and react directly to the CpG-A oligonucleotide D19. J Leukoc Biol 2006; 80:1328-36; PMID:17000899; http://dx.doi.org/10.1189/jlb.0106011
- Jung ID, Lee JS, Kim YJ, Jeong YI, Lee CM, Lee MG,. Sphingosine kinase inhibitor suppresses dendritic cell migration by regulating chemokine receptor expression and impairing p38 mitogen-activated protein kinase. Immunology 2007; 121:533-44; PMID:17428311; http://dx.doi.org/10.1111/j.1365-2567.2007.02601.x
- Liu Y, Shi G. Role of G protein-coupled receptors in control of dendritic cell migration. Biomed Res Int 2014;2014:738253; PMID:24734242
- Paustian C, Caspell R, Johnson T, Cohen PA, Shu S, Xu S, Czerniecki BJ, Koski GK. Effect of multiple activation stimuli on the generation of Th1-polarizing dendritic cells. Hum Immunol 2011;72(1):24-31; PMID:20951755; http://dx.doi.org/10.1016/j.humimm.2010.10.004
- Tawab A, Fan Y, Read EJ, Kurlander RJ. Effect of ex vivo culture duration on phenotype and cytokine production by mature dendritic cells derived from peripheral blood monocytes. Transfusion 2009; 49:536-47; PMID:19243546; http://dx.doi.org/10.1111/j.1537-2995.2008.02020.x
- Johnson LA, Jackson DG. Control of dendritic cell trafficking in lymphatics by chemokines. Angiogenesis 2014;17(2):335-45; PMID:24232855; http://dx.doi.org/10.1007/s10456-013-9407-0
- Villablanca EJ, Zhou D, Valentinis B, Negro A, Raccosta L, Mauri L,. Selected natural and synthetic retinoids impair CCR7- and CXCR4-dependent cell migration in vitro and in vivo. J Leukoc Biol 2008; 84:871-9; PMID:18515328; http://dx.doi.org/10.1189/jlb.0108047
- Kabashima K, Shiraishi N, Sugita K, Mori T, Onoue A, Kobayashi M,. CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells. Am J Pathol 2007; 171:1249-57; PMID:17823289; http://dx.doi.org/10.2353/ajpath.2007.070225
- Beem E, Segal MS. Evaluation of stability and sensitivity of cell fluorescent labels when used for cell migration. J Fluoresc 2013; 23:975-87; PMID:23722994; http://dx.doi.org/10.1007/s10895-013-1224-8
- Carrithers MD, Visintin I, Kang SJ, Janeway CA Jr. Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain 2000; 123:1092-101; PMID:10825349; http://dx.doi.org/10.1093/brain/123.6.1092
- Peng Y, Zhang Y, Huang B, Luo Y, Zhang M, Li K,. Survival and migration of pre-induced adult human peripheral blood mononuclear cells in retinal degeneration slow (rds) mice three months after subretinal transplantation. Curr Stem Cell Res Ther 2014; 9:124-33; PMID:24350910; http://dx.doi.org/10.2174/1574888X09666131219115125