Abstract
Healthcare workers (HCW) were prioritized for vaccination during the 2009 influenza A(H1N1)pdm09 pandemic. We conducted a clinical trial in October 2009 where 237 HCWs were immunized with a AS03-adjuvanted A(H1N1)pdm09 monovalent vaccine. In the current study, we analyzed the homologous and cross-reactive H1N1 humoral responses using prototype vaccine strains dating back to 1977 by the haemagglutinin inhibition (HI), single radial hemolysis SRH), antibody secreting cell (ASC) and memory B cell (MBC) assays. The cellular responses were assessed by interferon-γ (IFN-γ) ELISPOT and by intracellular staining (ICS) for the Th1 cytokines IFN-γ, interleukin-2 (IL-2) and tumor necrosis factor-α (TNF-α). All assays were performed using blood samples obtained prior to (day 0) and 7, 14 and 21 d post-pandemic vaccination, except for ASC (day 7) and ICS (days 0 and 21). Vaccination elicited rapid HI, SRH and ASC responses against A(H1N1)pdm09 which cross reacted with seasonal H1N1 strains. MBC responses were detected against the homologous and seasonal H1N1 strains before vaccination and were boosted 2 weeks post-vaccination. An increase in cellular responses as determined by IFN-γ ELISPOT and ICS were observed 1–3 weeks after vaccination. Collectively, our data show that the AS03-adjuvanted A(H1N1)pdm09 vaccine induced rapid cellular and humoral responses against the vaccine strain and the response cross-reacted against prototype H1N1 strains dating back to 1977.
Abbreviations:
Introduction
The novel, swine-origin influenza A(H1N1)pdm09 virus was first detected in April 2009 and it caused the first influenza pandemic of the 21st century. The A(H1N1)pdm09 virus was antigenically distinct from the prior seasonal influenza A strains and the majority of the population was immunologically naïve to A(H1N1)pdm09 rendering existing influenza vaccines ineffective against this strain.Citation1-3 New pandemic vaccines were developed against A(H1N1)pdm09 and they induced sero-protective antibody responses 1-2 weeks after administering a single dose in most healthy adults.Citation4 Since 2009, the A(H1N1)pdm09 virus has circulated and has been included in the seasonal trivalent influenza vaccines (TIV) as the H1N1 strain.
Antibody responses are a key mediator of sero-protective immunity induced by influenza vaccines.Citation5 At the start of the pandemic, there were no or little antibody titres against the A(H1N1)pdm09 strain, especially in young adults and children resulting in atypically high rates of severe disease.Citation2,3 However, people over the age of 60 had higher levels of sero-protective immunity, most likely due to having pre-existing, cross-reactive antibodies from prior exposure to A(H1N1)pdm09-like strains in the distant past.Citation6 In this regard, infection with A(H1N1)pdm09 has been shown to activate broadly-cross reactive memory B cells that provided protection even in the absence of pre-existing antibody titres.Citation7 Of interest, recent studies have shown that antibodies specific for the conserved stalk domain of the influenza haemagglutinin were boosted by vaccination and infection with the novel A(H1N1)pdm09 virus and these antibodies have broad cross-reactive neutralizing activity against different group 1 influenza strains.Citation6,8,9 In addition to antibody responses, T cells play a significant role in anti-influenza immunity. A large percentage of T-cell epitopes found in seasonal H1N1 strains in the years preceding the pandemic were conserved in A(H1N1)pdm09, thus were targets of immunological memory.Citation10 A recent report showed that high frequencies of pre-existing T cells to conserved epitopes on A(H1N1)pdm09 virus were found in people that developed less severe disease, suggesting a key role for cellular immunity in anti-A(H1N1)pdm09 responses.Citation11
During the 2009 pandemic, HCWs were prioritized for vaccination in order to maintain the integrity of the healthcare system and to minimize virus transfer to vulnerable patients. In October 2009, we conducted a clinical trial in frontline HCWs to evaluate the safety and immunogenicity of a single dose of a A(H1N1)pdm09 vaccine formulated with the oil-in-water adjuvant AS03. Vaccination commenced 2–3 weeks prior to the peak of pandemic activity. The vaccine was well tolerated and by using the HI assay, we showed that sero-protective responses (titres ≥40) were elicited in a majority of subjects (97%) by 2–3 weeks after vaccination.Citation4 Further studies have shown that influenza vaccines formulated with the oil-in-water adjuvant AS03 to be safe and highly efficacious in children, young adults and the elderly.Citation4,12-20
In the current study, we characterized in detail the homologous and cross-reactive humoral and cellular response in HCW after AS03-adjuvanted A(H1N1)pdm09 vaccination. Our results show that vaccination induced serological (HI and SRH) and B cell (ASC and MBC) responses against A(H1N1)pdm09 and protoype seasonal H1N1 vaccine viruses that prevailed in the years preceding the pandemic. Furthermore, by IFN-γ ELISPOT and intracellular cytokine staining assays, we demonstrate that both homologous and cross-reactive Th1 cytokine responses are elicited in HCWs after vaccination with the AS03-adjuvanted A(H1N1)pdm09 vaccine.
Results
In this study we have evaluated the early homologous and cross reactive immune responses to prototype H1N1 vaccine strains dating back to 1977 after a single low dose of pandemic influenza vaccine adjuvanted with the oil-in-water adjuvant AS03 in frontline HCWs. Blood samples were taken at 4 consecutive time points (day 0, 3, 7 and 14 post-vaccination) to evaluate the dynamics of the homologous and cross-reactive immune response to vaccination ().
Figure 1. The experimental plan. Two hundred and thirty seven healthcare workers were vaccinated with the 2009 A(H1N1)pdm09 monovalent split virus vaccine (3.75 μg HA) formulated with the oil-in-water adjuvant AS03. The figure shows the number of samples (n) analyzed at each sampling day (Day) for each immunological assay and the H1N1 strains against which the assays were performed. Consecutive blood samples were taken from the same subject at 4 time points (day 0 7, 14 and 21 post-vaccination) for HI, SRH, ASC, memory B cell and IFN-γ ELISPOT assays. For ICS, PBMCs were obtained from 8 subjects before vaccination and a separate cohort of 18 subjects on day 21 post-vaccination. The ASC (n = 39) and ICS (n = 8–18) assays were run on fresh PBMCs, whereas memory B cell (n = 16–27), and IFN-γ ELISPOT (n = 9) assays were run on freeze/thawed PBMCs. * Strains used in the SRH assay.
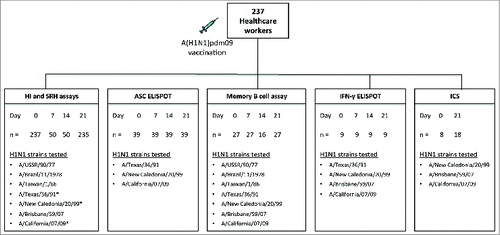
The cross-reactive haemagglutinin inhibition (HI) antibody response after pandemic vaccination
shows the post-vaccination HI response against the homologous A/(H1N1)pdm09-like strain A/California/07/09 (California) and cross-reactivity against 6 seasonal influenza A H1N1 viruses. A sero-protective HI response was defined as an HI titer ≥ 40.Citation21 Prior to vaccination, 13.5% of the subjects had sero-protective HI titres against the homologous California strain with a geometric mean titer (GMT) of 8. Vaccination boosted the California-specific HI response where by day 7, a majority of subjects (78%, GMT = 156) were seroprotected. The HI response continued to increase up to days 14 and 21 post-vaccination with 100% (GMT = 826) and 96% (GMT = 619) of the subjects, respectively having sero-protective HI titres ≥40.
Figure 2. Homologous and cross-reactive haemagglutinin inhibition response after pandemic vaccination. The HI response against the homologous vaccine virus A/California/07/09 and 6 seasonal influenza H1N1 strains A/USSR/90/77, A/Brazil/11/1978, A/Taiwan/1/86, A/Texas/36/91, A/New Caledonia/20/99 and A/Brisbane/59/07 was determined before vaccination (day 0) and at 7, 14 and 21 days after vaccination. The bars show the geometric mean titer with 95% confidence interval and individual responses are presented as symbols. The dotted horizontal line represents a sero-protective HI titer of 40.
The HI assay was used to examine the cross-reactive HI responses against 6 prototype H1N1 vaccine strains; A/USSR/90/77 (USSR), A/Brazil/11/1978 (Brazil), A/Taiwan/1/86 (Taiwan), A/Texas/36/91 (Texas), A/New Caledonia/20/99 (New Caledonia) and A/Brisbane/59/07 (Brisbane). Prior to vaccination, sero-protective HI titres were observed in HCWs against all strains; USSR (26%, GMT = 13), Brazil (26%, GMT = 12), Taiwan (39%, GMT = 24), Texas (56%, GMT = 60), New Caledonia (31%, GMT = 15) and Brisbane (29%, GMT = 14) strains. The post-vaccination HI response peaked on day 14 with 68–94% of subjects having sero-protective titres against USSR (GMT = 116), Brazil (GMT = 145), Taiwan (GMT = 201), Texas (GMT = 796), New Caledonia (GMT = 79), and Brisbane (GMT = 102) strains. Similar HI titres were observed on day 21 post-vaccination with 59–90% subjects having sero-protective titres against USSR (GMT = 84), Brazil (GMT = 110), Taiwan (GMT = 145), Texas (GMT = 653), New Caledonia (GMT = 51) and Brisbane (GMT = 48) strains.
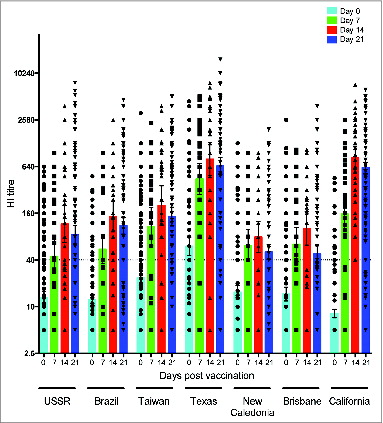
The cross-reactive single radial hemolysis (SRH) response to vaccination
shows the pre- and post-vaccination SRH titres against the homologous California and cross-reactive responses against the New Caledonia and Texas strains. The European Agency for Evaluation of Medicinal Products criterion of protective serological response to influenza vaccines is a SRH titer of ≥25 mm2, which was used as a cut-off for serologic protection. Prior to vaccination, only 4% of the subjects had sero-protective SRH titer of ≥25 mm2 against the Texas strain (GMT = 8), while 24% and 51% of subjects had sero-protective titres against California (GMT = 11) and New Caledonia (GMT = 16) strains, respectively. One week after vaccination, a majority of the subjects (76–84%) had sero-protective SRH titres against New Caledonia (GMT = 35) and California (GMT = 41) strains, while 28% had sero-protective SRH responses against the Texas strain (GMT = 15). The SRH response peaked 2 weeks after vaccination with 92–100% of the vaccinees having sero-protective responses against New Caledonia (GMT = 56) and California (GMT = 73) strains. Lower SRH responses were detected against the Texas strain with only 59% of subjects having sero-protective titres at 2 weeks post vaccination (GMT = 25).
Figure 3. Homologous and cross-reactive single radial hemolysis (SRH) antibody responses after pandemic vaccination. The SRH response against the homologous vaccine strain A/California/7/09 and 2 seasonal influenza strains A/Texas/36/91 and A/New Caledonia/20/99 was determined before vaccination (day 0) and at 7, 14 and 21 days after vaccination. Each symbol represents the SRH titer of one subject, with geometric means and 95% confidence levels indicated. The dotted horizontal line shows the sero-protective SRH titer of 25 mm2.
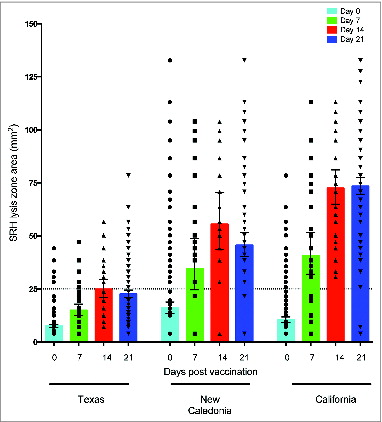
B cellular responses after pandemic vaccination in health care workers
Antibody secreting cell response after vaccination
The humoral response was further characterized by ASC response in peripheral blood mononuclear cells (PBMC) after vaccination. The peak virus-specific ASC response were observed at day 7 post-vaccination (), while no ASC responses were observed before vaccination or at days 14 and 21 after vaccination (data not shown). IgG ASCs dominated the anti-California response (mean = 111 ASC per 1 × 105 PBMC) and was significantly higher than the IgA (P < 0.001) and the IgM (P < 0.0001) ASC responses against the same strain (mean = 45 and 27 ASC per 1 × 105 PBMC, respectively). Similarly, significantly higher (P < 0.0001) IgG ASCs were detected against Texas and New Caledonia strains (mean = 110 and 86 ASC per 1 × 105 PBMC, respectively) compared with corresponding IgM responses against the same strains (mean = 28 and 23 ASC per 1 × 105 PBMC, respectively). In general, weak IgM ASC responses were detected against all 3 H1N1 strains, which may suggest that relatively low ASC responses were elicited against novel epitopes.
Figure 4. The antibody secreting cell response 7 days after pandemic vaccination. The A/Texas/36/91, A/New Caledonia/20/99 and A/California/07/09-specific IgG, IgA and IgM ASC responses were measured by ELISPOT 7 days after vaccination with AS03-adjuvaned pandemic H1N1 vaccine. The bars represent mean numbers of virus-specific ASCs per 100 000 peripheral blood mononuclear cells (PBMC) ± standard error of the mean. Statistical differences are shown by non-parametric Kruskal-Wallis test. ** = P < 0.01, *** = P < 0.001, **** = P < 0.0001.
The memory B cell response after vaccination
Memory B cells (MBC) play a key role in anti-influenza immunity. shows that, prior to vaccination, IgG MBCs were detected against all the influenza A viruses tested with mean frequencies ranging between 114–263 cells per 1 × 106 PBMC and no increase in the response was detected at 7 days after vaccination. The virus-specific IgG MBC frequencies peaked at 14 days after vaccination with the highest responses detected against the Brisbane and California strains (mean 470 and 410 cells per 1 × 106 PBMC, respectively) and the lowest against the Brazil strain (mean 197 cells per 1 × 106 PBMC). A significant increase (P < 0.05) in California-specific IgG MBC frequency was observed at day 14 (mean 410 cells per 1 × 106 PBMC) compared to pre-vaccination (mean 200 cells per 1 × 106 PBMC), while the responses against the other strains were not significantly different. Furthermore, we found a significant correlation between pre-vaccination MBC responses against California and the 6 seasonal H1N1 influenza strains with Spearman correlation coefficients (r) ranging between 0.6 and 0.96 (). A significant correlation was also observed between pre-vaccination MBC frequencies and day 7 HI responses against all viruses except the USSR and Brisbane strains ().
Figure 5. Homologous and cross-reactive memory B cells responses after pandemic vaccination. The pre- and post-vaccination IgG memory B cell responses were determined against the homologous vaccine strain A/California/07/09 and cross-reactive responses against 6 seasonal influenza strains A/USSR/90/77, A/Brazil/11/1978, A/Taiwan/1/86, A/Texas/36/91, A/New Caledonia/20/99 and A/Brisbane/59/07. The vertical bars represent the mean ± standard error of the mean and each symbol represents one subject.
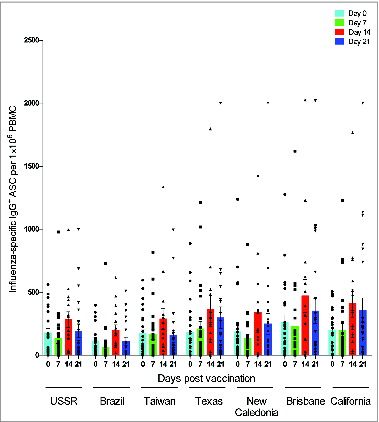
Table 1. Correlation between memory B cell responses to vaccine virus and seasonal H1N1 virus pre- and post-vaccination
Interferon gamma response after vaccination
shows the frequencies of PBMCs secreting IFN-γ in an antigen-specific manner prior to vaccination (day 0) and at 7, 14 and 21 days post-vaccination.
Figure 6. IFN-γ responses against influenza A virus strains after pandemic vaccination. Peripheral blood mononuclear cells (PBMC) obtained from individuals vaccinated with AS03-adjuvanted pandemic H1N1 vaccine were stimulated overnight with split virus antigens from the homologous vaccine virus A/California/7/09 and seasonal H1N1 strains A/Texas/36/91, A/New Caledonia/20/99 and A/Brisbane/59/07 and the IFN-γ response was evaluated by the ELISPOT assay. Each symbol represents one subject and with mean and standard error of the mean indicated.
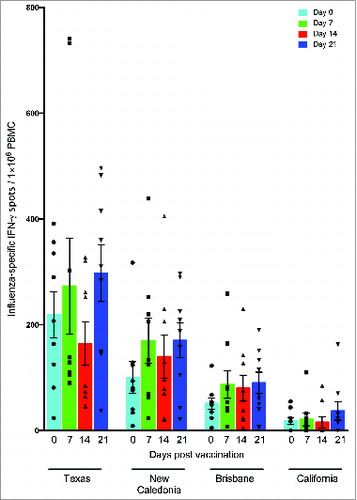
Before vaccination, the highest response was observed against the Texas strain (mean number of IFN-γ+ cells per 1 × 106 PBMC (mean) = 273) followed by the New Caledonia (mean = 100) and the Brisbane (mean = 51) strains, while the weakest pre-vaccination IFN-γ response was detected against the California strain (mean = 18). At 7 days post-vaccination, an increase in IFN-γ response was detected against the Brisbane (mean = 89), New Caledonia (mean = 170) and Texas (mean = 273) strains, although this was not significantly higher than pre vaccination numbers. No significant increases in the IFN-γ response were observed on days 14 and 21 against any strain. Overall, the weakest IFN- γ response was detected against the California strain, however the response at 21 days post-vaccination (mean = 36) was double that observed before vaccination (mean = 18).
Intracellular Th1 cytokine responses after vaccination
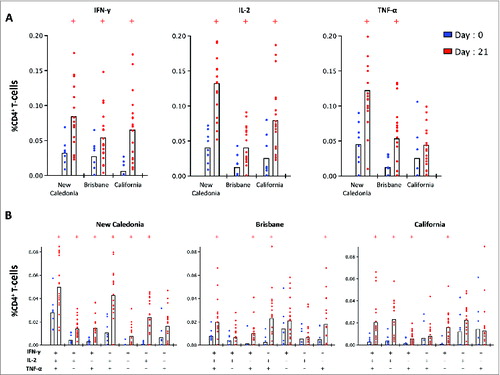
shows frequencies of CD4+ T-cells producing either single (A) or multiple (B) Th1 cytokines against California, New Caledonia and Texas strains before and 21 days after vaccination. Before vaccination (day 0), significantly lower (P < 0.05) IFN-γ responses were detected against California compared with the New Caledonia and Brisbane strains. At 21 days post-vaccination, significantly higher (P < 0.05) IFN-γ levels were detected against all strains compared with pre-vaccination levels. Before vaccination, significantly lower (P < 0.05) frequencies of IL-2 and TNF-α were observed against Brisbane compared with the New Caledonia strain. Vaccination induced a significant increase (P < 0.05) in IL-2 response against all 3 strains compared with pre-vaccination levels. Increased TNF-α responses were also detected after vaccination with significantly higher (P < 0.05) frequencies detected against New Caledonia and Brisbane strains on day 21 compared with day 0. shows the frequency of Th1 cells simultaneously producing one or more cytokine (multi-functional T cells). After vaccination (day 21), significant increases (P < 0.05) in both triple and double cytokine producing cells were detected against all 3 strains compared with pre-vaccinaion levels ().
Figure 7. The single (A) and multi-functional (B) CD4+ T-cell cytokine response before and 21 days after pandemic vaccination. Peripheral blood mononuclear cells (PBMC) were obtained from 8 subjects before vaccination (day 0) and from a separate cohort of 18 subjects on day 21 post-A(H1N1)pdm09 vaccination. PBMCs were stimulated overnight with split virus antigens from A/New Caledonia/20/99, A/Brisbane/59/07 and A/California/07/09 viruses and stained for intracellular cytokines (IFN-γ, IL-2 and TNF-α) and the percentage of single cytokine producing (A) or muli-functioal (B) CD4 T-cells was measured by flow cytometry. +group (day 21) significantly different by Student t test from day 0 (P < 0.05).
Discussion
The 1918 Influenza H1N1 pandemic killed up to 50 million people and H1N1 strains continued to circulate in the human populations until 1957. From 1957 to 1977, H1N1 viruses were not detected in human populations, most likely due to competition from the H2N2 and H3N2 strains. However in 1977, influenza H1N1 re-emerged and circulated as a seasonal virus until the 2009 pandemic.
Phylogenetic analysis of the H1N1 HA gene shows that the A(H1N1)pdm09 strain is highly divergent from the seasonal H1N1 strains, while the seasonal H1N1 strains from 1977 to 2008 are more closely related (Fig. S1). Despite the antigenic divergence, infection with the A(H1N1)pdm09 virus induced broad-cross reactive antibody responses against epitopes that are conserved on the HA of seasonal H1N1 and A(H1N1)pdm09 strains.Citation7,10 Antibody responses directed at common HA epitopes may explain the broad cross-reactivity observed in our cohort following vaccination with the AS03-adjuvanted vaccine. The AS03 adjuvant itself may have contributed to the breath of the cross-reactive response, however the underlying immunological mechanisms for this are not clear. A control group that received a non-adjuvanted A(H1N1)pdm09 vaccine would have shown the benefits of the AS03 adjuvant, however this was not possible as only the AS03 adjuvanted pandemic vaccine was licensed for use in Norway in 2009. We found that 13% of HCW had preexisting HI titres ≥40 to the California strain at baseline, which suggest exposure or subclinical infection with this virus. Almost all HCWs (97%) with preexisting sero-protective HI titres to A(H1N1)pdm09 were under the age of 60, therefore were not exposed to 1918-like H1N1 strains that have been shown to induced cross-reactive antibodies against the A(H1N1)pdm09 virus.Citation2 However, most of our study cohort (60%) had received the trivalent seasonal influenza vaccines in years preceding the 2009 pandemicCitation4 and this may have contributed to the preexisting immunity against both the A(H1N1)pdm09 virus and the seasonal H1N1 strains.Citation2
When stratified by age, older subjects (persons born before 1957) had similar HI and SRH GMTs against A(H1N1)pdm09 and seasonal H1N1 strains at baseline compared with the younger cohort (Fig. S2). This differs from prior reports where higher frequencies of sero-protective antibodies and significantly lower infection rates have been observed in older adults over the age of 60 years.Citation2 Compared with the general public, the potential for exposure or asymptomatic subclinical infection is higher in HCWs and this may explain relatively high baseline sero-protective rates we observed in younger HCWs.
In this study, we evaluated the serological responses by 2 commonly used assays; HI and SRH with contrasting results. While both assays showed that vaccination induced complete protection against A(H1N1)pdm09 by day 14, contrasting results were observed against the Texas strain, with 100% and 59% seroprotection by HI and SRH assays, respectively. Generally there is a good correlation between HI and SRH responses against influenza A virusesCitation22, therefore the discrepancy observed in our study is intriguing. Perhaps this may reflect different sensitivities of serological assays and measurement of different functionalities in the antibodies against different influenza strains, however further work is required to fully understand the measured differences.
The humoral immune response was further characterized by evaluating the influenza-specific ASC and MBC responses. The day 7 post-vaccination ASC response corresponds to the peak plasmablast CD19+CD20−CD27highCD38high response Citation23, and in our study, we observed a significant increase in IgG and IgA ASCs against the pandemic and seasonal H1N1 strains. The rapid ASC response one week after vaccination has been shown to be consistent with a recall response originating from activation of cross-reactive MBCs generated by previous influenza infections and/or vaccination.Citation6,7,24 An important finding in our study is that memory MBCs against A(H1N1)pdm09 strain were detected even before vaccination or widespread circulation of the pandemic virus, at frequencies similar to those observed against recently circulated seasonal H1N1 strains, suggesting cross reaction to conserved epitopes. Furthermore, we observed a significant positive correlation between the pre-vaccination MBC response against A(H1N1)pdm09 and that against the seasonal H1N1 strains (). A significant positive correlation was also observed between the pre-vaccination MBC responses and day 7 HI titres against most of the H1N1 strains tested. Collectively, our results strongly imply and support the suggestion that MBCs targeting A(H1N1)pdm09 exist in the human population and that they arise from prior exposure to seasonal H1N1 strains.Citation6 These A(H1N1)pdm09-specific MBCs have the capacity to rapidly differentiate into ASCs that secrete IgG antibodies after antigen re-encounter and have broad cross-reactivity.Citation25-27 The pre-existing MBCs targeting the A(H1N1)pdm09 virus may at least partly explain the fact that rapid sero-protective responses were elicited in a majority of subjects after only a single dose of the pandemic vaccine.Citation4 Further studies using chimeric virus constructs could evaluate the specificity of the post-vaccination antibody and MBC responses toward the globular head and the relatively well-conserved stalk domains of group 1 HA to confirm that the cross reactivity observed is due to conserved epitopes on the H1 haemagglutinin.Citation28 In this regard, immunization with chimeric virus constructs derived from novel influenza strains was shown to induce broadly cross-reactive HA stalk-specific antibody responses by ELISA and microneutralization assays.Citation29,30
Cellular responses play a significant role in anti-influenza immunity (for a review see ref.Citation31). To assess the vaccine induced T-cell activity, we determined the influenza-specific IFN-γ response by ELISPOT and IFN-γ, IL-2 and TNF-α responses by ICS. Both ICS and ELISPOT analysis showed an increase in Th1 cytokine (IFN-γ, IL-2 and TNF-α) responses against both the A(H1N1)pdm09 and seasonal H1N1 strains after vaccination. Both IFN-γ and TNF-α have powerful anti-influenza virus activity and increased levels may help prevent severe influenza illness.Citation32-34 Furthermore, we observed an increase in homologous and cross-reactive Th1 CD4 T cells simultaneously secreting more than one cytokine (multifunctional T-cells), which are functionally superior to single cytokine producing cells eliciting anti-influenza immunity and conferring protection against lethal influenza infection.Citation35,36 Furthermore, there was an increase in IFN-γ+ IL-2+ TNF-α+ cells post-vaccination, which have a high proliferative potential and are an important target population for anti-A(H1N1)pdm-09 virus activity.Citation37 Interestingly, a very low A(H1N1)pdm09-specific IFN-γ response was observed prior to vaccination by both the ELISPOT and ICS assays compared with responses against the seasonal strains. The higher baseline IFN-γ+ cell frequencies observed against seasonal H1N1 viruses most likely reflects a recall memory T cell response to prior influenza vaccine and/or infection. In our study, the pandemic split virus antigen used for in vitro stimulation consists mainly of A(H1N1)pdm09 HA and NA, which shares only a few T-cell epitopes (12%) with HA and NA of seasonal H1N1 strains Citation10, hence the pre-vaccination response against A(H1N1)pdm09 would mainly be naïve T cells directed toward novel epitopes. Naïve T cells require sustained antigen stimulation over days to produce IFN-γ in vitro Citation38, and this may explain the lack of a pre-vaccination IFN-γ response after overnight stimulation in our ELISPOT and ICS assays.
In conclusion, we have shown that the AS03-adjuvanted A(H1N1)pdm09 vaccine induces both humoral and cellular cross-reactive immune responses in HCW and this may have played a key role in eliciting rapid sero-protective immune responses, which contributed significantly to maintaining the integrity of the healthcare system during the pandemic.Citation4 Our results show that immune responses originally primed by exposure to seasonal strains can be recalled after pandemic vaccination and better understanding of mechanisms that result in cross-reactive immune responses may lead to the development of improved influenza vaccines.
Materials and Methods
Participants and study design
In October 2009, 237 frontline healthcare workers at the Haukeland University Hospital, (Bergen, Norway) were vaccinated with a single dose of the AS03 adjuvanted monovalent split virus vaccine (Pandemrix, GlaxoSmithKline, www.clinicaltrials.gov, NCT01003288).Citation4 All participants provided written informed consent before being included in the study, which was approved by the Regional Ethical committee of Western Norway and the Norwegian Medicines Agency. The inclusion/exclusion criteria for this study are published elsewhere.Citation4 Consecutive blood samples were taken from the same subject at 4 time points (days 0, 7, 14 and 21 post-vaccination) for the serological (HI, SRH), ASC, memory B cell and IFN-γ ELISPOT assays. For the intracellular cytokine staining (ICS) for Th1 cytokines, PBMCs were obtained from 8 subjects before vaccination and a separate cohort of 18 subjects on day 21 post-vaccination. On days 7 and 14, serological responses were determined in only 50 subjects as a part of a kinetic sub-study (). Serum samples were aliquoted and stored at −70°C before use in the serological assays. PBMCs were isolated from a small group of HCWs (n = 39) using Cell preparation Tubes (CPT, BD Biosciences) according to manufacturer's instructions. Fresh lymphocytes were used in the antibody secreting cell ELISPOT assay and for intracellular cytokine staining of CD4+ T-cells. Remaining lymphocytes were frozen at −70°C and prioritized for use in the memory B cell ELISPOT followed by the IFN-γ ELISPOT assays.
Antibody assays
The haemagglutinin inhibition (HI) antibody response was analyzed against the homologous A/(H1N1)pdm09-like strain (A/California/07/09) and against 6 seasonal influenza A (H1N1) strains (A/USSR/90/77, A/Brazil/11/1978, A/Taiwan/1/86, A/Texas/36/91, A/New Caledonia/20/99 and A/Brisbane/59/07) (Fig. S1). Assays were performed in duplicate and the geometric mean titer (GMT) was calculated. The pre- and post-vaccination, influenza-specific HI antibody response was determined by the HI assay using 8 HA units of each virus strain and 0.7% turkey red blood cells, as described earlier.Citation4 HI titers were defined as the reciprocal of the dilution exceeding 50% haemagglutination. Negative titers were assigned a value of 5 for calculation purposes.
The single radial hemolysis (SRH) responses against A/Texas/36/91, A/New Caledonia/20/99 and A/California/07/09 strains were conducted at the University of Siena, Italy, as previously described.Citation39-41
B cell assays
The virus-specific IgG, IgA and IgM antibody secreting cell (ASC) response against A/California/07/09, A/Texas/36/91 and A/New Caledonia/20/99 split virus antigens was determined pre and post-vaccination by ELISPOT assay using fresh PBMCs as described earlier.Citation23 The numbers of IgG, IgA and IgM ASCs were evaluated at 7 days post-vaccination, as this has previously been shown to be the peak response after inactivated influenza vaccination.Citation23
The virus-specific IgG memory B cell (MBC) response against A/California/07/09, A/Brisbane/59/07, A/Texas/36/91 and A/New Caledonia/20/99 split virus antigens and A/USSR/90/77, A/Brazil/11/1978, A/Taiwan/1/86 whole virus was quantified by ELISPOT as described earlier.Citation42 Results are presented as virus-specific IgG MBC cells per 1 × 106 PBMCs.
Interferon gamma ELISPOT assay
The influenza-specific IFN-γ response pre and post-vaccination was examined by using 96 well plates pre-coated with anti-IFN-γ antibodies according to the manufacturer´s instructions (Mabtech AB, Sweden). PBMCs (4 × 105 cells per well) were added in RPMI-1640 medium supplemented with 10% fetal calf serum with negative control (medium alone) and the split virus influenza H1N1 antigens from; A/New Caledonia/20/99, A/Texas/36/91, A/Brisbane/59/07 and A/California/7/09 (X179a). Plates were incubated overnight (37°C, 5% CO2) and developed the following day. The plates were read using an Immunoscan™ reader and associated software (CTL-Europe). The negative control was subtracted from the influenza-specific response.
Intracellular cytokine staining (ICS) for multi-functional CD4+ T cell responses
PBMCs from vaccinated subjects were stimulated overnight with A/California/7/09 (X179a), A/New Caledonia/20/99 and A/Brisbane/59/07 split virus antigens and the cells were stained for intracellular Th1 cytokines IFN-γ, IL-2 and TNF-α and the percentage of single, or multiple cytokine producing CD4+ T-cells were analyzed by flow cytometry as described earlier.Citation43
Statistical assays
Differences in the IFN-γ, ASC and MBC ELISPOT responses were analyzed by non-parametric Kruskal-Wallis test. Correlations between pre-vaccination MBC responses against the A(H1N1)pdm09 virus and seasonal influenza strains were determined by Spearman correlation coefficient analysis. The Kruskal-Wallis and Spearman correlation analysis were performed by GraphPad Prism version 6.00 for Mac (GraphPad software, La Jolla, CA, USA). Differences between intracellular cytokine responses at days 0 and 21 were determined by the student t test and a partial permutation test by using SPICE version 5.1 software, as described earlier.Citation44 P < 0.05 was considered as significant for all statistical tests.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website..
Supplemental_Figures.zip
Download Zip (275.8 KB)Acknowledgments
The split virus influenza antigens were kindly provided by Glaxo Smithkline (Belgium). We thank all the healthcare workers that volunteered for the clinical trial, the clinical staff at the Bergen Clinical Vaccine consortium and staff at the Influenza Center who processed samples.
Funding
The study was funded intramurally by the Influenza Center at the University of Bergen and through funding from the Bergen Clinical Vaccine Consortium. The Influenza Center is funded by the Ministry of Health and Care Services, Norway, the Norwegian Research Council Globvac program (220670/H10), the European Union (Univax 601738 and IMI FLUCOP 115672-3), Helse Vest and the K.G. Jebsen Center for Influenza Vaccines.
References
- Brockwell-Staats C, Webster RG, Webby RJ. Diversity of influenza viruses in swine and the emergence of a novel human pandemic influenza A (H1N1). Influen Other Res Viruses 2009; 3:207-13; PMID:19768134; http://dx.doi.org/10.1111/j.1750-2659.2009.00096.x
- Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, Liu F, Dong L, DeVos JR, Gargiullo PM, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Eng J Med 2009; 361:1945-52; PMID:19745214; http://dx.doi.org/10.1056/NEJMoa0906453
- Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Eng J Med 2009; 360:2605-15; PMID:19423869; http://dx.doi.org/10.1056/NEJMoa0903810
- Madhun AS, Akselsen PE, Sjursen H, Pedersen G, Svindland S, Nostbakken JK, Nilsen M, Mohn K, Jul-Larsen A, Smith I, et al. An adjuvanted pandemic influenza H1N1 vaccine provides early and long term protection in health care workers. Vaccine 2010; 29:266-73; PMID:21034828; http://dx.doi.org/10.1016/j.vaccine.2010.10.038
- Gerhard W. The role of the antibody response in influenza virus infection. Curr Topics Microbiol Immunol 2001; 260:171-90; PMID:11443873
- Li GM, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng NY, Lee JH, Huang M, Qu X, Edupuganti S, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A 2012; 109:9047-52; PMID:22615367; http://dx.doi.org/10.1073/pnas.1118979109
- Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med 2011; 208:181-93; PMID:21220454; http://dx.doi.org/10.1084/jem.20101352
- Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Tan GS, Krause JC, Moran T, Stein CR, et al. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci U S A 2012; 109:2573-8; PMID:22308500; http://dx.doi.org/10.1073/pnas.1200039109
- Thomson CA, Wang Y, Jackson LM, Olson M, Wang W, Liavonchanka A, Keleta L, Silva V, Diederich S, Jones RB, et al. Pandemic H1N1 Influenza Infection and Vaccination in Humans Induces Cross-Protective Antibodies that Target the Hemagglutinin Stem. Front Immunol 2012; 3:87; PMID:22586427; http://dx.doi.org/10.3389/fimmu.2012.00087
- Greenbaum JA, Kotturi MF, Kim Y, Oseroff C, Vaughan K, Salimi N, Vita R, Ponomarenko J, Scheuermann RH, Sette A, et al. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc Natl Acad Sci U S A 2009; 106:20365-70; PMID:19918065; http://dx.doi.org/10.1073/pnas.0911580106
- Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, Bean T, Barclay W, Deeks JJ, Lalvani A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med 2013; 19:1305-12; PMID:24056771; http://dx.doi.org/10.1038/nm.3350
- Ferguson M, Risi G, Davis M, Sheldon E, Baron M, Li P, Madariaga M, Fries L, Godeaux O, Vaughn D. Safety and long-term humoral immune response in adults after vaccination with an H1N1 2009 pandemic influenza vaccine with or without AS03 adjuvant. J Infect Dis 2012; 205:733-44; PMID:22315336; http://dx.doi.org/10.1093/infdis/jir641
- Ikematsu H, Nagai H, Kawashima M, Kawakami Y, Tenjinbaru K, Li P, Walravens K, Gillard P, Roman F. Characterization and long-term persistence of immune response following two doses of an AS03A-adjuvanted H1N1 influenza vaccine in healthy Japanese adults. Hum Vaccin Immunother 2012; 8:260-6; PMID:22426369; http://dx.doi.org/10.4161/hv.18469
- Ikematsu H, Tenjinbaru K, Li P, Madan A, Vaughn D. Evaluation of immune response following one dose of an AS03A-adjuvanted H1N1 2009 pandemic influenza vaccine in Japanese adults 65 years of age or older. Hum Vaccin Immunother 2012; 8:1119-25; PMID:22854661; http://dx.doi.org/10.4161/hv.21081
- Pathirana RD, Bredholt G, Akselsen PE, Pedersen GK, Cox RJ. A(H1N1)pdm09 vaccination of health care workers: improved immune responses in low responders following revaccination. J Infect Dis 2012; 206:1660-9; PMID:22969149; http://dx.doi.org/10.1093/infdis/jis589
- Poder A, Simurka P, Li P, Roy-Ghanta S, Vaughn D. An observer-blind, randomized, multi-center trial assessing long-term safety and immunogenicity of AS03-adjuvanted or unadjuvanted H1N1/2009 influenza vaccines in children 10-17 years of age. Vaccine 2014; 32:1121-9; PMID:24252703; http://dx.doi.org/10.1016/j.vaccine.2013.11.031
- Roy-Ghanta S, Van der Most R, Li P, Vaughn DW. Responses to A(H1N1)pdm09 Influenza Vaccines in Participants Previously Vaccinated With Seasonal Influenza Vaccine: A Randomized, Observer-Blind, Controlled Study. J Infect Dis 2014; 210:1419-30; PMID:24864125; http://dx.doi.org/10.1093/infdis/jiu284
- Saitoh A, Nagai A, Tenjinbaru K, Li P, Vaughn DW, Roman F, Kato T. Safety and persistence of immunological response 6 months after intramuscular vaccination with an AS03-adjuvanted H1N1 2009 influenza vaccine: an open-label, randomized trial in Japanese children aged 6 months to 17 years. Hum Vaccin Immunother 2012; 8:749-58; PMID:22495117; http://dx.doi.org/10.4161/hv.19684
- Van Damme P, Kafeja F, Bambure V, Hanon E, Moris P, Roman F, Gillard P. Long-term persistence of humoral and cellular immune responses induced by an AS03A-adjuvanted H1N1 2009 influenza vaccine: an open-label, randomized study in adults aged 18-60 years and older. Hum Vaccin Immunother 2013; 9:1512-22; PMID:23571166; http://dx.doi.org/10.4161/hv.24504
- Yang WH, Dionne M, Kyle M, Aggarwal N, Li P, Madariaga M, Godeaux O, Vaughn DW. Long-term immunogenicity of an AS03-adjuvanted influenza A(H1N1)pdm09 vaccine in young and elderly adults: an observer-blind, randomized trial. Vaccine 2013; 31:4389-97; PMID:23856331; http://dx.doi.org/10.1016/j.vaccine.2013.07.007
- Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull 1979; 35:69-75; PMID:367490
- Wood JM, Gaines-Das RE, Taylor J, Chakraverty P. Comparison of influenza serological techniques by international collaborative study. Vaccine 1994; 12:167-74; PMID:8147099; http://dx.doi.org/10.1016/0264-410X(94)90056-6
- Cox RJ, Brokstad KA, Zuckerman MA, Wood JM, Haaheim LR, Oxford JS. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine 1994; 12:993-9; PMID:7975853; http://dx.doi.org/10.1016/0264-410X(94)90334-4
- Dormitzer PR, Galli G, Castellino F, Golding H, Khurana S, Del Giudice G, Rappuoli R. Influenza vaccine immunology. Immunol Rev 2011; 239:167-77; PMID:21198671; http://dx.doi.org/10.1111/j.1600-065X.2010.00974.x
- Bende RJ, van Maldegem F, Triesscheijn M, Wormhoudt TA, Guijt R, van Noesel CJ. Germinal centers in human lymph nodes contain reactivated memory B cells. J Exp Med 2007; 204:2655-65; PMID:17938234; http://dx.doi.org/10.1084/jem.20071006
- Berkowska MA, Driessen GJ, Bikos V, Grosserichter-Wagener C, Stamatopoulos K, Cerutti A, He B, Biermann K, Lange JF, van der Burg M, et al. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood 2011; 118:2150-8; PMID:21690558; http://dx.doi.org/10.1182/blood-2011-04-345579
- Purtha WE, Tedder TF, Johnson S, Bhattacharya D, Diamond MS. Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. J Exp Med 2011; 208:2599-606; PMID:22162833; http://dx.doi.org/10.1084/jem.20110740
- Krystal M, Elliott RM, Benz EW, Jr., Young JF, Palese P. Evolution of influenza A and B viruses: conservation of structural features in the hemagglutinin genes. Proc Natl Acad Sci U S A 1982; 79:4800-4; PMID:6956892; http://dx.doi.org/10.1073/pnas.79.15.4800
- Ellebedy AH, Krammer F, Li GM, Miller MS, Chiu C, Wrammert J, Chang CY, Davis CW, McCausland M, Elbein R, et al. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc Natl Acad Sci U S A 2014; 111:13133-8; PMID:25157133; http://dx.doi.org/10.1073/pnas.1414070111
- Nachbagauer R, Wohlbold TJ, Hirsh A, Hai R, Sjursen H, Palese P, Cox RJ, Krammer F. Induction of broadly reactive anti-hemagglutinin stalk antibodies by an H5N1 vaccine in humans. J Virol 2014; 88:13260-8; PMID:25210189; http://dx.doi.org/10.1128/JVI.02133-14
- La Gruta NL, Turner SJ. T cell mediated immunity to influenza: mechanisms of viral control. Trend Immunol 2014; 35:396-402; PMID:25043801; http://dx.doi.org/10.1016/j.it.2014.06.004
- Hill DA, Baron S, Perkins JC, Worthington M, Van Kirk JE, Mills J, Kapikian AZ, Chanock RM. Evaluation of an interferon inducer in viral respiratory disease. Jama 1972; 219:1179-84; PMID:4334380; http://dx.doi.org/10.1001/jama.1972.03190350025006
- Richman DD, Murphy BR, Baron S, Uhlendorf C. Three strains of influenza A virus (H3N2): interferon sensitivity in vitro and interferon production in volunteers. J Clin Microbiol 1976; 3:223-6; PMID:1270590
- Seo SH, Webster RG. Tumor necrosis factor alpha exerts powerful anti-influenza virus effects in lung epithelial cells. J Virol 2002; 76:1071-6; PMID:11773383; http://dx.doi.org/10.1128/JVI.76.3.1071-1076.2002
- Brown DM, Lee S, Garcia-Hernandez Mde L, Swain SL. Multifunctional CD4 cells expressing gamma interferon and perforin mediate protection against lethal influenza virus infection. J Virol 2012; 86:6792-803; PMID:22491469; http://dx.doi.org/10.1128/JVI.07172-11
- Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol 2007; 81:8468-76; PMID:17553885; http://dx.doi.org/10.1128/JVI.00228-07
- Weaver JM, Yang H, Roumanes D, Lee FE, Wu H, Treanor JJ, Mosmann TR. Increase in IFNgamma(-)IL-2(+) cells in recent human CD4 T cell responses to 2009 pandemic H1N1 influenza. PloS One 2013; 8:e57275; PMID:23526940; http://dx.doi.org/10.1371/journal.pone.0057275
- Lai W, Yu M, Huang MN, Okoye F, Keegan AD, Farber DL. Transcriptional control of rapid recall by memory CD4 T cells. J Immunol 2011; 187:133-40; PMID:21642544; http://dx.doi.org/10.4049/jimmunol.1002742
- Groth N, Montomoli E, Gentile C, Manini I, Bugarini R, Podda A. Safety, tolerability and immunogenicity of a mammalian cell-culture-derived influenza vaccine: a sequential Phase I and Phase II clinical trial. Vaccine 2009; 27:786-91; PMID:19027046; http://dx.doi.org/10.1016/j.vaccine.2008.11.003
- Oxford JS, Yetts R, Schild GC. Quantitation and analysis of the specificity of post-immunization antibodies to influenza B viruses using single radial haemolysis. J Hygiene 1982; 88:325-33; PMID:7037949; http://dx.doi.org/10.1017/S0022172400070170
- Schild GC, Pereira MS, Chakraverty P. Single-radial-hemolysis: a new method for the assay of antibody to influenza haemagglutinin. Applications for diagnosis and seroepidemiologic surveillance of influenza. Bull World Health Organ 1975; 52:43-50; PMID:1082381
- Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods 2004; 286:111-22; PMID:15087226; http://dx.doi.org/10.1016/j.jim.2003.12.015
- Pedersen G, Halstensen A, Sjursen H, Naess A, Kristoffersen EK, Cox RJ. Pandemic influenza vaccination elicits influenza-specific CD4+ Th1-cell responses in hypogammaglobulinaemic patients: four case reports. Scand J Immunol 2011; 74:210-8; PMID:21438900; http://dx.doi.org/10.1111/j.1365-3083.2011.02561.x
- Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry Part A2011; 79:167-74; PMID:21265010; http://dx.doi.org/10.1002/cyto.a.21015
