Abstract
Enterovirus A infections are the primary cause of hand, foot and mouth disease (HFMD) in infants and young children. Although enterovirus 71 (EV-A71) and coxsackievirus A16 (CV-A16) are the predominant causes of HFMD epidemics worldwide, EV-A71 has emerged as a major neurovirulent virus responsible for severe neurological complications and fatal outcomes. HFMD is a serious health threat and economic burden across the Asia-Pacific region. Inactivated EV-A71 vaccines have elicited protection against EV-A71 but not against CV-A16 infections in large efficacy trials. The current development of a bivalent inactivated EV-A71/CV-A16 vaccine is the next step toward that of multivalent HFMD vaccines. These vaccines should ultimately include other prevalent pathogenic coxsackieviruses A (CV-A6 and CV-A10), coxsackieviruses B (B3 and B5) and echovirus 30 that often co-circulate during HFMD epidemics and can cause severe HFMD, aseptic meningitis and acute viral myocarditis. The prospect and challenges for the development of such multivalent vaccines are discussed.
Introduction
Enteroviruses (EVs) have been classified into 4 species EV-A, EV-B, EV-C and EV-D.Citation1 EVs are positive-sense, single-stranded RNA viruses within the Picornaviridae family. They are responsible for a spectrum of various clinical manifestations, including severe neurological complications, and cardiopulmonary diseases in young children.Citation2-7 More than 100 EV serotypes have been identified including polioviruses, coxsackieviruses A (CV-A), coxsackieviruses B (CV-B), echoviruses (E) and numbered enterovirus serotypes (EV). In the past 2 decades, enterovirus A infections have become the primary cause of an increase in the incidence and severity of hand, foot and mouth disease (HFMD) in infants and young children. Both coxsackievirus A16 (CV-A16) and enterovirus 71 (EV-A71) have been the predominant etiologic agents of herpangina (HA) and HFMD epidemics Citation5-9 and . Several other enterovirus serotypes usually detected in sporadic cases or outbreaks of HFMD frequently co-circulate with EV-A71 and CV-A16 in large epidemics. These enteroviruses include coxsackieviruses A CV-A2, CV-A3, CV-A4, CV-A5, CV-A6, CV-A8, CV-A9, CV-A10, CV-A12, CV-A14, coxsackieviruses B CV-B1 to CV-B6 and echoviruses E-4, E-5, E-6, E-7, E-9, E-11, E-18, E-25, E-30 Citation10-69 and . HFMD has become a major health issue and a substantial economic burden throughout the Asia Pacific region.Citation5-7 Following the near complete eradication of poliovirus, EV-A71 has emerged as a major neurotropic virus responsible for severe neurological complications and fatal outcomes. Besides EV-A71, other co-circulating life-threatening enteroviruses such as CV-B3, CV-B5 and E-30 expose children to aseptic meningitis and acute myocarditis.Citation2-4 The recurrence of outbreaks associated with high morbidity and mortality has prompted the World Health Organization in 2009 to declare HFMD a rising menace in Asia.Citation70 The largest population-based HFMD epidemiological survey has recently revealed that the case-severity rate for patients with cardiopulmonary and neurological complications was 1.1% and that the fatality rate for patients with severe disease was 0.03%.Citation9 In the absence of approved antiviral treatment,Citation71 a multivalent prophylactic vaccine against HFMD is urgently needed and the development of an efficacious EV-A71 vaccine in particular has been a national health priority in some Asian countries.Citation72
Table 1. Epidemiology of hand, foot and mouth disease since 2004
Clinical Presentation
Enteroviruses A predominantly infect infants and young children below 5 y of age. Most EV-A71 infectees (71%) remain asymptomatic.Citation5,7 Following a 2–5 day incubation period with a prodrome of fever, malaise, abdominal pain and myalgia, HFMD is typically characterized by a papulovesicular or maculopapular rash, blisters of the hands, soles, and buttocks associated with painful ulcerative lesions of the mouth. HFMD is usually a self-limiting infection and most infected children recover within 2 weeks in the absence of secondary cutaneous infection. However, the virus may be present in the faeces for several weeks after recovery. HFMD is a highly contagious illness which is efficiently propagated to household, day care center and kindergarten contacts by oro-pharyngeal secretions or fecal-oral transmission. EV-A71 outbreaks have been responsible for severe neurological complications including aseptic meningitis, cerebella ataxia, poliomyelitis-like paralysis, Guillain-Barré syndrome, acute brainstem encephalitis, and fulminant neurogenic pulmonary edema/hemorrhage associated with high mortality.Citation73 The annual fatality rate in Taiwan over the last decade has been between 0 to 25%, with an average of 13%.Citation74 Survivors from brainstem encephalitis often suffer from neurological sequelae including long-term motor deficits and cognitive impairment.Citation75 EV-A71 elicits humoral responses, but there is no correlation between neutralizing antibody levels and disease severity indicating that altered cellular responses such as an imbalance in Th1/Th2 and Th17/Treg subset ratios play a significant role in disease outcome and may have potential prognostic value.Citation76-78 However, the presence of EV-A71 neutralizing antibodies was found to be inversely correlated with the number of severe HFMD and the loss of maternal antibodies to be responsible for an increase in severe cases in the 1–2 y age group.Citation34 The presence of EV-A71 neutralizing antibodies did not reduce the incidence of infections caused by non-A71 enteroviruses.Citation79
Coxsackievirus A16 is the other major cause of herpangina and mild HFMD. However, a small number of patients develop neurological complications such as aseptic meningitis, encephalitis, even fatal pneumonia and acute viral myocarditis.Citation8 Coxsackieviruses CV-A6 and CV-A10 have been mainly associated with HA outbreaks and more recently with HFMD epidemics. CV-A6 tends to be a virulent strain which unusually affects both pediatric and adult populations. The virus has been responsible for severe atypical cases of HFMD often requiring hospitalization, characterized by extensive vesiculobullous and erosive cutaneous eruptions, eczema, purpuric lesions and onychomadesis with nail shedding.Citation80,81 CV-A6 infections have also led to fatal encephalitis/encephalopathy and myocarditis.Citation82,83 CV-A10-associated HFMD cases are characterized by high fever, vesicular rashes and oral ulcers Citation63 with occasional meningoencephalitic complications.Citation36
Coxsackieviruses CV-B3 and CV-B5 are both neurotropic and cardiovirulent viruses.Citation2,4 When they co-circulate in HFMD outbreaks, they potentially pose significant health threats to neonates and young infants by exposing them to the risk of acute myocarditis and dilated cardiomyopathy, aseptic meningoencephalitis, interstitial pneumonitis, disseminated intravascular coagulopathy, sequelae and fatal outcomes.Citation2,4 CV-B3 infections are the major cause of myocarditis which can lead to dilated cardiomyopathy, long-term sequelae and fatal outcome.Citation84-86
Echovirus E-30 is a major neurotropic pathogen responsible for worldwide outbreaks of aseptic meningitis and encephalitis.Citation2,87 Neonates and infants are specifically at risk for disseminated CV-B3, CV-B5 and E-30 infections and these 3 enteroviruses have been directly implicated in the pathogenesis of type I diabetes as a result of direct cell damage and autoimmune mimicry.Citation2,88 Herpangina is the most frequent clinical manifestation of CV-A4 and CV-A5 infections.Citation86,89-91
Virology of HFMD-Associated Enteroviruses
Enteroviruses are non-enveloped particles containing a non-segmented, single-stranded, positive sense genomic RNA.Citation1 Their capsids are composed of 60 copies of 4 structural proteins, VP1, VP2, VP3 and VP4. After infection, viral RNA acts as mRNA and its open reading frame is translated into a polyprotein further cleaved by proteolysis into 4 structural proteins (VP). VP1, VP2 and VP3 are displayed on the surface of the virion surface as shown in the EV-A71 and CV-B3 crystal structures.Citation92-94 These subunits expose linear and conformational neutralization epitopes and are responsible for immune responses and host-receptor binding. VP1 contains major neutralization determinants and is used in viral identification and evolutionary analyses. The 5′untranslated region (5′ UTR) RNA contains a type I internal ribosomal site (IRES) that is poorly efficient at initiating viral translation. The efficiency of viral replication and translation depends on the complex interplay of 12 trans-acting host factors (ITAFs) with EV-A71 IRES.Citation95 Like poliovirus, enteroviruses produce empty (E) and full (F) particles in cell culture systems that can be separated and purified using continuous sucrose gradient ultracentrifugation.Citation96 The F-particles like the poliovirus D-antigen have a high content of viral RNA and a full particle structure. In contrast, defective E-particles like the poliovirus C antigen are empty structures virtually devoid of infectious RNA which explains their low infectivity.
Enteroviruses Cell and Tissue Tropism
Enteroviruses use a wide array of cell-surface receptors and cell entry mechanisms. Cell tropism and pathogenicity depend on these receptors and on cellular trans-acting factors.Citation97,98 EV-A71 and CV-A16 but not CV-A6 and CV-A10, use the ubiquitous human scavenger receptor class B member 2 (hSCARB2) and the leukocyte P-selectin glycoprotein ligand 1 (PSGL-1) to infect a wide variety of host cells.Citation99,100 hSCARB2 is capable of viral binding, uncoating and internalization. The inefficiency of L-PSGL1-expressing cells is due to the inability of this receptor to induce viral uncoating.Citation101 hSCARB2 is ubiquitous and likely involved in EV-A71 and CV-A16 systemic and neural cell infections. Annexin-2, sialylated glycans, heparin sulfate and DC-SIGN receptors also play a role in EV-A71 pathogenesis.Citation102-105 Both CV-B3 and CV-B5 use the decay accelerating factor (DAF/CD55) for primary attachment and the coxsackievirus and adenovirus receptor (CAR) as an internalization receptor.Citation98,106 CAR is an integral membrane protein localized to tight junctions highly expressed in developing brain and heart, and mediates CV-B3 and CV-B5 infection of cardiac myocytes as well as neural progenitor and stem cells leading to acute myocarditis and CNS involvement.Citation106-108 Echovirus 30, the most common cause of aseptic meningitis in young infants, binds to the heterodimeric vitronectin αvβ3 receptor controlling neural cell differentiation to induce productive infection of target cells and neuronal cell death through the activation of the TRIO-RhoA signaling pathway.Citation109,110
Epidemiology
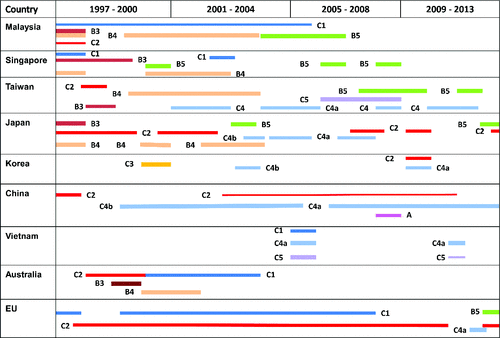
Following the isolation of CV-A16 in South Africa in 1951,Citation111 a CV-A16 outbreak occurred in 1957 in Toronto Citation112 and the new illness was later described in 1960 by Aslop as “hand-foot-and-mouth” disease.Citation113 EV-A71 was isolated in California in 1969 from a patient with encephalitis.Citation114 Since their discoveries, both viruses have been the cause of large life-threatening epidemics throughout the world, in North America, Europe, Australia, and Asia.Citation5-9 In the past decade, cyclic HFMD outbreaks of co-circulating or alternating EV-A71 and CV-A16 infections have become a major health problem in particular in the Asia-Pacific region (). Severe EV-A71 outbreaks were earlier reported in the USA, France, Hungary, Greece, The Netherlands, Norway, and UK.Citation5-7 Large EV-A71 epidemics in Malaysia (1997, 2005 and 2008), Singapore (2006, 2008), Taiwan (1998, 2008), southern Vietnam (2005), Ho Chi Minh City (2011) and Cambodia (2012) were associated with severe neurological complications and fatal outcomes Citation48-50,53,54,59,60,115 and . In the last 5 y, a significant increase in HFMD outbreaks occurred in several provinces of Mainland China.Citation12,116 In response, HFMD was declared a class C notifiable infectious disease by the Ministry of Health of China and a national surveillance system was established in 2008.Citation117 Shanghai experienced a major HFMD outbreak in 2010.Citation34 EV-A71 (54.1%) was the principal causative agent responsible for 86% of severe complications and 100% of fatalities. Several epidemics in Beijing (2013), Shangdong (2009), Shanghai (2011), Shenzhen (2013), Japan (2005–2009), Singapore (2004, 2006, 2008), Taiwan (2005–2008) and Thailand (2012) involved multiple enterovirus serotypes.Citation15,31,36,40,42,47,49,51,52,57 The most prevalent serotypes isolated in Japan between 2005 and 2009 were CV-A2, CV-A4, CV-A6, CV-A16, CV-B3, CV-B5, E-30 and EV-A71 Citation42 whereas in Taiwan the predominant HFMD-associated strains between 2004 and 2010 were CV-A2, CV-A4, CV-A5, CV-A6, CV-A10, CV-A16, CV-B1, CV-B4, EV-A71, E-6, and E-18.Citation52 The dominant serotype varied depending on the outbreak and the country but EV-A71 has always been the pathogen responsible for the majority of severe cases of neurological HFMD and fatal outcomes. Between 2008 and 2014, a total of 10,714,237 HFMD cases of HFMD were caused by EV-A71 (43.4%) and CV-A16 (34.4%). The average morbidity increased from 37.6/100000 in 2008 to 139.6/100000 in 2013. EV-A71 and CV-A16 were responsible for 90.2% and 8.7% of fatal outcomes, respectively.Citation9
However, CV-A16 was the prevalent HFMD-associated enterovirus between EV-A71 epidemics in Changchun (2008), Hebei (2012), Shangdong (2011) provinces in China, India (2009–2010), Japan (2009), Singapore (2004, 2007), Thailand (2010) and Spain (2010).Citation17,24,29,31,40,41,44,47,49,58,67 Although usually mild, CV-A16 infection may lead to neural and muscle cells apoptosis Citation118 and has occasionally been the cause of rhombencephalitis, brain stem encephalitis and acute flaccid paralysis, fatal pneumonitis and fulminant myocarditis with intractable shock.Citation8
Since 2004, the incidence of CV-A6- and CV-A10-associated HFMD epidemics has markedly increased worldwide []. Both viruses cause herpangina and occasionally meningitis, encephalitis and pleurodynia.Citation2 CV-A6 was the first or second most common pathogen in large outbreaks in Cuba,Citation10 China,Citation15,17,21,37,38 India,Citation40 Israel,Citation80 Japan,Citation42,45,46 Singapore,Citation49,50 Taiwan [52.55], Thailand,Citation56-58 Spain,Citation67 UK Citation69 and USA.Citation11 CV-A6 tends to be a virulent strain which unusually affects both pediatric and adult populations. It has been reported that 8.3% of CV-A6-infected patients had high fever and developed meningoencephalitis.Citation37,57 In recent years, CV-A6 and CV-A10 have regularly co-circulated and have been independently high risk factors in HFMD outbreaks in China.Citation13 Dual outbreaks of CV-A6 and CV-A10 occurred in Shenzhen in China (2013),Citation37 Finland (2008),Citation62 France (2010)Citation63 and Spain in 2008 and 2011.Citation65,66 CV-A6 was the prevalent serotype in Finland (71%) and in the 2011 outbreak in Spain (90%) whereas CV-A10 was the predominant pathogen in France (39.9%), Spain in 2008 (45%) and during the onychomadesis outbreak in Valencia (50%)Citation66 ().
Coxsackievirus CV-B3, coxsackievirus CV-B5 and echovirus 30 to a lesser extent have been found to frequently co-circulate with EV-A71 and CV-A16 during HFMD outbreaks around the world (). However, they have rarely been detected together in the same epidemic. Their incidence in multi-serotype HFMD epidemics ranged from 0.3% to 14.7%, 0.7% to 19.0% and 0.6% to 19% for CV-B3, CV-B5, and E-30, respectively. During the 2009 Shangdong epidemic, a significant proportion (78.6%) of patients with CV-B5-associated HFMD suffered from neurological complications.Citation30
Although often detected during HFMD epidemics, coxsackieviruses CV-A4 and CV-A5 are predominantly responsible for herpangina outbreaks.Citation86,89-92 However, CV-A4 was the principal pathogen in the 2004 HFMD epidemics in Taiwan.Citation52
Molecular Epidemiology
There is only one EV-A71 serotype. Based on VP1 gene phylogenetic studies, EV-A71 has now been classified into 6 genotypes, A to F.Citation72,119 Genotype A contains only the prototypic strain BrCr/1970. Genotypes B and C have been further divided into 6 and 5 sub-genotypes, B0-B5 and C1-C5, respectively. The C4 genotype has been further classified into C4a and C4b lineages.Citation5–7 More recently, additional genotypes including the Indian D genotype and 2 African ones (E and F) were identified, illustrating the wide genetic diversity of EV-A71.Citation119 No association could be established between genotype and disease severity.Citation7 EV-A71 epidemics occur throughout the year but usually peak in summer months. However, the seasonal distribution and cyclical patterns (every 2–4 years) of outbreaks vary depending on the year and the country.Citation7 Most countries use different case definitions, sample collections, data analysis and laboratory testing procedures to report HFMD cases, therefore disease burdens are likely underestimated.
Genotype A transiently re-emerged in China in 2008.Citation117 In contrast, genotypes B and C have continued to circulate and co-exist around the world since the 1970s, causing outbreaks with CNS complications and fatal outcomes,Citation7,9 and . The first major EV-A71 outbreak occurred in 1997 in Malaysia where co-circulating neurovirulent B3, B4, C1 and C2 genotypes were responsible for 41 deaths among young children.Citation48 In Singapore, sub-genotypes B5 and C2 caused the largest epidemic in 2008.Citation50 Subgenogroups C2, C4 and B5 with genomic variations have repeatedly appeared during outbreaks in Japan between 1990 and 2013.Citation120 EV-A71 has caused nationwide epidemics in Taiwan with different circulating genotypes and subgenotypes, C2 in 1998, B4 in 200–2001, C4 in 2005, and B5 in 2008 and 2012.Citation121 The C4 subgenotype emerged in 1998 in China with a predominance of the C4b lineage until 2009 followed by the exclusive occurrence of the C4a lineage until now. Between 2008 and 2014, C4 epidemics have been responsible for more than 10 million HFMD cases with a case-severity and case-fatality rates per year of 1.1% and 0.03–0.04%, respectively.Citation9,117 Recently, EV-A71 sub-genotype C5 was observed in severe pandemics associated with high mortality rates that spread through Vietnam in 2005 and 2011, then to Cambodia in 2012.Citation59,60,115
Figure 1. Geographical distribution of enterovirus A71 genotypes and subgenotypes during outbreaks from 1997 to 2013. The figure is an up-dated compilation of tables and data published in refs. Citation6,7,59,60,72,116,137,197. EU includes the Netherlands, Denmark, the United Kingdom, Germany, France and Austria.
Two CV-A16 genotypes (A and B) have been identified.Citation8 The prototypic CV-A16 genotype G-10 is the sole member of genotype A. Genotype B contains 2 subgenotypes B1 and B2 further divided into B1a, B1b, B1c, B2a, B2b and B2c. Genotypes B1a and B1b have been the predominant subgenotypes circulating in Australia and several provinces in China. However, subgenotypes B2a and B2b were identified in Shenzhen from 2005 to 2009.Citation122 A comparative study of the biological properties of 2 clinical isolates revealed that CV-A16 strains of the B genotype may have different pathogenicity.Citation123
CV-A6 strains from the Shenzhen epidemic (2008–2012) were classified into 7 clusters, A to F.Citation36 The predominant strain belonged to genogroup D whereas genogroup C prevailed in other areas of China. A majority of CV-A6 strains isolated in the Shenzhen province were closely related to those detected during the outbreaks in Finland,Citation62 France,Citation63 Spain,Citation65 and Japan.Citation46 CV-A10 phylogenetic trees vary depending on the geography of the epidemic. Co-circulating CV-A10 strains isolated during the epidemic in the Hebei province between 2008 and 2012 were found to segregate into 4 clusters (A–D), the C genotype being further divided into 4 lineages.Citation24 All Chinese CV-A10 segregated into the B and C genotypes, genotype B in Shangdong and genotype C in the other provinces. Another phylogenetic analysis of CV-A10 strains indicated that they cluster into 7 genotypes (A to G).Citation36 The predominant strain associated with HFMD cases in the Shenzhen outbreak between 2008 and 2012 belonged to genogroup C whereas distantly related CV-A10 strains were isolated during epidemics in the Shangdong and Jiangsu provinces (2008–2012), Spain (2008) and France (2010).Citation36 CV-A6 replaced CV-A16 and toppled EV-A71 to become the predominant pathogen in Shenzhen epidemics from 2008 to 2009.Citation37
Five genotypes (A-E) were assigned by phylogenetic analyses to coxsackieviruses CV-B3 and CV-B5.Citation24,124 All Chinese CV-B3 isolates segregated into genotype E but the presence of 2 divergent circulating genotype E strains in Shijiazhuang City (2010–2012) suggests the existence of 2 different lineages. Chinese CV-B3 strains clustered into a genogroup totally different from CV-B3 isolates from other countries.Citation24 The severity of the Linyi epidemic in 2009 was attributed to the introduction of an unusual and distinct CV-B5 lineage.Citation30
Echovirus 30 has been classified into 8 clusters (A–H)Citation23 and the E-30-associated HFMD outbreak in Guanxi (2010) has been attributed to a strain belonging to the H lineage.Citation23
Genotype Switching, Co-Circulation, Co-Infection and Genetic Recombination
Enteroviruses have high mutation rates due to evolutionary pressure and frequent recombination. The EV genome evolves at a rate of 1% to 2% mutation per year contributing to strain diversification.Citation125
EV-A71 epidemics occur cyclically every 2–4 y with changes in the predominant genotype and subgenotype. These epidemics can be the result of infection by a single genotype/subgenotype, the co-circulation of divergent EV-A71 isolates, the emergence of variants or the unpredictable switching of genotypes and sub-genotypesCitation126 (). Multiple serotypes, genotypes and subgenotypes frequently co-circulate during HFMD epidemics, thus facilitating co-infection and genetic recombination that may lead to the generation of new variants with altered tropism, virulence and fitness. Indeed, several mixed EV-A71/CV-A16, EV-A71/CV-B3, CV-A16/CV-A10, CV-A16/CV-B3, CV-A16/CV-B5, CV-A16/CV-A6, CV-A10/CV-A5, CV-A10/CV-A6, CV-A10/CV-B1, CV-A10/E-9 infections have been well documented.Citation29,30,32–34,127,128 The EV-A71 C4 genotype has persisted with progressive drift through time in China.Citation9,117,126 Intra-genotype EV-A71 B shifts from B3 to B4 (1997–2000) and B4 to B5 (2000–2003) have occurred in Malaysia. Sequential inter-genotype shifts from C2 to B4 and subsequently from C4 to B5 were observed in Taiwan.Citation7,121,126 The co-circulation of several EV-A71 genotypes and CV-A16 during HFMD epidemics has been responsible for intra-typic genetic recombination between EV-A71 B and C genotypes in Taiwan and inter-typic recombination between EV-A71 and CV-A16 in China.Citation5,7,14,19,126,129 Another inter-serotypic recombination happened between EV-A71 genotype C2 and CV-A8 to create genotype B4, responsible for outbreaks in Japan and Taiwan in 1998.Citation7,126 The predominant C4a genotype may be a double recombinant virus among EV-A71 genotypes B, C and CV-A16.Citation7,126 Almost each major HFMD outbreak was correlated to genetic variations caused by EV-A71 switches.Citation7 Phylogenetic analyses have revealed that CV-A16 B1a and B1b strains circulating in China were complex recombinant forms containing sequences from multiple EV-A donors.Citation129,130 These co-infection and recombination events have been associated with disease severity. One example is the inter-typic recombination that led to the emergence of new CV-B3/B5 variants responsible for acute myocarditis in children with HFMD.Citation131
Thus, a continuous monitoring of antigenic variation and genetic evolution of HFMD-associated enteroviruses is critical to determine the mosaic composition of epidemics, design vaccines and plan efficacy trials. In this regard, an enhancement of national physician-based sentinel surveillance clinics and the creation of a global surveillance network for enterovirus outbreaks similar to the WHO surveillance system for influenza are urgently needed. An automated alert and response system evaluated in China has shown good sensitivity and specificity in the detection of HFMD outbreaksCitation132 and improved assays to identify multiple pathogens simultaneously are currently being developed.Citation26,133
Hand, Foot and Mouth Disease Vaccines
Clinical and molecular epidemiology data confirm that in the last decade (), CV-A16 and EV-A71 were the most prevalent etiological agents of HFMD and that EV-A71 was the most neurovirulent serotype. CV-A6 and CV-A10 co-circulated in 85% of all epidemics. HFMD-associated coxsackieviruses CV-B3, CV-B5 and echovirus E-30 that were globally detected in a third of the outbreaks remain potential serious threats to neonates and young infants due to their neurotropism and cardiovirulence. Although CV-A4 and CV-A5 were identified in 45% of multiple-serotype epidemics, they are usually associated with HA outbreaks. Thus, an heptavalent vaccine including EV-A71, CV-A16, CV-A6, CV-A10, CV-B3, CV-B5 and E-30 immunogens could be designed to protect against the vast majority of pathogenic HFMD-associated EV serotypes.
EV-A71 vaccines: The first step toward multivalent HFMD vaccines
The development of a vaccine against EV-A71 has been a health priority because of its neurovirulence. It is also the first step toward the development of multivalent HFMD vaccines.Citation134
Recent EV-A71 vaccine development
Several EV-A71 candidate vaccines are still at the pre-clinical stage.Citation72,135,136 Synthetic vaccines based on immunodominant linear neutralization epitopes are safe, cost-effective, but poorly immunogenic even when formulated with Freund's adjuvants. Multi-linear tandem neutralization epitopes expressed in E. coli might be more promising.Citation137 Among EV-A71 subunits, recombinant VP1 subunits produced in different expression systems including Pichia pastoris Citation138 were capable of eliciting good antibody responses and protection in suckling mice when formulated with strong adjuvants.Citation139 VP1 anchored on the surface of baculovirus via a transmembrane domain induced cross-neutralization responses in mice and conferred protection in passive immunization studies.Citation140,141 A plasmid DNA vaccine expressing VP1 was only moderately immunogenic. DNA vaccines do not elicit strong antibody responsesCitation142 and have never been commercialized as human vaccines. Passive transfer of immune sera from mice vaccinated with an adenovirus vector exposing an EV-A71 neutralization epitope on its surface protected suckling mice from live viral challenge but not as efficiently as an inactivated EV-A71 vaccine.Citation143 Recombinant virus-like particles (VLPs) mimic the conformation of authentic native viruses and are safe because devoid of viral genome. Prophylactic VLP-based vaccines against hepatitis B virus and human papillomavirus are currently commercially available. EV-A71 virus-like particles produced in the baculovirus systemCitation138 and in Saccharomyces cerevisiaeCitation144 induced robust neutralization responses in mice as well as potent cellular responses and immune sera conferred protection in neonatal mice against lethal EV-A71 challenge. Their safety, immunogenicity and high-yield production make them attractive candidates for future combination vaccines. However, EV-A71 VLPs elicited only low neutralizing titers in macaques.Citation145 Several immunogens have been evaluated in oral immunization studies. Sera from mice fed with transgenic tomatoes expressing VP1 exhibited neutralizing activity in vitro.Citation146 Oral immunization of maternal mice with VP1 formulated with chitosan,Citation147 recombinant baculovirus displaying VP1 mixed with bilosomes,Citation148 Salmonella Typhimurium and Bifidobacterium longum vectors expressing VP1 Citation149,150 conferred protection to neonatal mice. Although a safe live attenuated EV-A71 would be an ideal and low-cost vaccine, such vaccine exposes to the risk of genetic instability and the possibility of reversion to virulence. An engineered, temperature-sensitive EV-A71 BrCr mutant was shown to be less neurovirulent and to elicit cross-neutralizing antibody responses but it caused mild tremor in cynomolgus monkeys.Citation151 However, the possibility to engineer attenuated high-fidelity-variants of EV-A71 with low pathogenicity could be a promising approach for future live vaccines.Citation152
In a comparative study of prototyptic EV-A71 vaccines produced by different technologies, formalin-inactivated EV-A71 virions adjuvanted in alum were found to be very immunogenic, to elicit strong cross-neutralization titers against different EV-A71 genotypes and subgenotypes in mice and non-human primates and to be the most potent and promising immunogens.Citation72,139,153,154
Formalin-inactivated EV-A71 vaccines and efficacy trials
Based on the promising results of pre-clinical studies and the efficacy of the inactivated poliovirus vaccine (IPV), formalin-inactivated EV-A71 virions were selected for the clinical development of stable and cost-effective monovalent EV-A71 vaccines for regulatory, economic and market acceptability reasons.Citation72 Five inactivated EV-A71 vaccines have been rapidly developed in the past few years.Citation72,136 The Vaccine R&D Center of the National Health Research Institutes (NHRI) of Taiwan produced a B4-based FI-EV-A71 vaccine (EV-A71vac) and launched the first human Phase I clinical trial in adults in 2010. A single vaccine dose of 5 µg or 10 µg was safe and highly immunogenic.Citation155 It elicited 100% seroconversion in naïve volunteers and strong virus neutralizing antibody (VNA) responses (geometric mean titer (GMT) = 210) against the vaccine strain and the B1, B5 and C4a subgenotypes in 85% of the vaccinees.Citation156 In contrast, neutralizing responses against C4b and CV-A16 were weak in 20% of the subjects and 90% of the vaccinees did not develop any VNA against an atypical C2 strain. Inviragen (Takeda Pharmaceuticals Co. Ltd) reports the results of a Phase I trial in adults with an inactivated EV-A71 B2 vaccine. All subjects who received 0.6 ug or 3 ug of vaccine at days 0 and 28 seroconverted and developed VNA GMTs of 323 and 452, respectively.Citation136
Inactivated EV-A71 vaccines based on different C4 isolates were independently developed and evaluated by 3 different Chinese companies, Vigoo Biological Co. Ltd., Sinovac Biotech Ltd., and the Institute of Medical Biology, Chinese Academy of Medical Sciences (CAMS).Citation157-160 The clinical efficacy of these vaccines formulated in alum was assessed in large Phase III trials involving more than 30,000 healthy infants and young children (6 to 35 months of age) who received 2 doses of vaccine 28 d apart or a placebo control. All three vaccines were found to be safe and well tolerated. The most common side-effects observed were induration, erythema and pain at the injection site that resolved within 24–72 hours as well as a grade-3 fever. The rate of seldom-reported serious adverse events (SAEs) in vaccinees was not different from that observed in the control groups and were not causally related to vaccination. The Vigoo's vaccineCitation158 was >90% efficacious against EV-A71-related HFMD and >80% protective against EV-A71-associated serious diseases including herpangina. In the Sinovac's trial, the incidence rate of EV-A71-associated disease was 0.3% vs 2.1% in the control group, corresponding to an 89.3% efficacy.Citation159 In the CAMS' study,Citation160 the seroconversion rate was 100% after 2 vaccinations, with a VNA GMT of 170.6. The vaccine was 97.4% efficacious against EV-A71-related diseases. All C4-based vaccines prevented herpangina and EV-A71-associated hospitalizations. Immune sera from subjects immunized with the Vigoo's and Sinovac's vaccines cross-neutralized the circulating EV-A71 genotypes and subgenotypes B4, B5, C2, and C5 associated with epidemics in recent years.Citation161 Furthermore, pre-existing antibodies due to stealth infections of young children did not interfere with vaccine efficacy against different EV-A71 genotypes.Citation161 However, the vaccines did not protect against CV-A16Citation157,158 and conversely, CV-A16 infection does not interfere with EV-A71 vaccination.Citation162 Interestingly, the VNA titers decreased by half after 6 months but this waning did not affect vaccine efficacy.Citation157 Most importantly, Phase III results suggest that a VNA titer of 1/16 can serve as a correlate of protection against EV-A71-related HFMD.Citation157,158 In spite of differences in vaccine strains and manufacturing processes, C4-based vaccines have shown batch consistency and efficacyCitation163 which should facilitate their licensure and market entry in China if there were no issues regarding vaccine stability, manufacturing capacity and production cost for which information is not yet available.
Development of a bivalent EV-A71/CV-A16 vaccine: The next critical step.
Except for the rare instances when EV-A71 is virtually the only causative agent of HFMD, monovalent EV-A71 vaccines will only protect against a fraction of HFMD cases, in particular if the etiology of HFMD changes over a short period of timeCitation29 and CV-A16 infections become predominant (50%–72%)Citation17,24,29.31,40,41,49,58 and , thus raising the issue of public acceptance of an EV-A71 vaccine. The availability of a bivalent EV-A71/CV-A16 would critically enhance the breadth of protection against HFMD and a combination vaccine is highly desirable. Six conserved, KLH-conjugated VP1 peptides formulated in complete Freund's adjuvants induced neutralizing antibodies against both homologous and heterologous CV-A16 strains.Citation164 The development of chemically-inactivated monovalent CV-A16 vaccines produced in Vero or KMB cells and adjuvanted with alum paved the way toward that of a bivalent vaccine.Citation165–168 Monovalent CV-A16 vaccines have induced neutralizing antibody responses against the vaccine strain and against heterologous CV-A16 isolates.Citation166,167 Furthermore, vaccination conferred full protection to mice lethally challenged with the mouse-adapted strain CV-A16-MAV Citation166 and maternal immunization protected neonatal mice from challenges with a series of circulating CV-A16 isolates.Citation168 Virus-like particles (VLP) produced in Saccharomyces cervisiae have also elicited potent neutralizing responses and passive transfer of immune sera protected neonate mice against lethal CV-A16 challenge.Citation169
Combination of inactivated EV-A71/CV-A16 vaccines formulated in alumCitation170 or with PELC/CpG Citation171 elicited balanced neutralizing responses against both viruses whereas monovalent CV-A16 vaccines did not protect against EV-A71 infection. Furthermore, maternal immunization of mice with the bivalent vaccine protected neonates challenged with the mouse-adapted EV-A71/MVA-N strain and the clinical isolate CV-A16/G08.Citation170 Maternal immunization with a VLP-based bivalent vaccine produced in the baculovirus system and adjuvanted with alum conferred full protection to newborns against lethal challenge either with EV-A71 or CV-A16.Citation172 Crystallographic studies revealed that the EV-A71 BC loop could serve as an ideal insertion site for the display of foreign neutralization epitopes without perturbing the capsid structure,Citation93 thus providing the mean to engineer potential EV-A71/CV-A16 hybrid immunogens. Along a similar concept, antisera raised in mice vaccinated with a novel chimeric EV-A71-based VLP in which the autologous neutralization epitope SP70 had been replaced by that of CV-A16 conferred protection in neonates against lethal challenge in a passive transfer experiment.Citation173 EV-A71 and CV-A16 vaccines produced both as inactivated virions and VLPs were compared for their immunogenicity and protective ability when administered either alone or in combination. Monovalent and bivalent vaccines adjuvanted with alum induced the same level of strain-specific neutralizing antibodies confirming that there is no interference between immunogens in the bivalent vaccine. All bivalent vaccines elicited cross-neutralizing antibodies against 12 EV-A71 and 6 CV-A16 sub-genotypes, respectively. Passive transfer of immune sera conferred protection in newborn mice against lethal challenge with both viruses although bivalent VLPs vaccines were more potent than individual VLPs formulations.Citation174
Multivalent HFMD vaccines: the ultimate need, the ultimate goal
Results obtained with bivalent EV-A71/CV-A16 vaccines serve as proof of concept for a 2-step development of a multivalent vaccine necessary for broad protection against HFMD. Based on epidemiological data, we propose that the first step should be to generate a tetravalent vaccine containing EV-A71, CV-A16, CV-A6 and CV-A10 Citation134,175 to cover the most prevalent HFMD pathogens, then to further incorporate CV-B3, CV-B5 and E-30 immunogens in a heptavalent vaccine to prevent the risks of aseptic meningitis and acute myocarditis associated with these viruses in the course of multi-serotype HFMD epidemics.Citation134 The addition of CV-A4 and CV-A5 components should not be considered at this stage since they are essentially responsible for HA outbreaks.
There is little information on CV-A6 and CV-A10 immunological properties. A bivalent EV-A71/CV-A16 vaccine induced strong humoral responses in mice and rabbits, but immune sera did not neutralize CV-A6 or CV-A10 in an in vitro assay.Citation175 CV-A6 and CV-A10 VLPs were found to be highly immunogenic in mice but only anti-CV-A10 antisera were tested and shown to neutralize CV-A10 infection in vitro. The protective ability of CV-A6 and CV-A10 VLPs needs to be assessed in an appropriate animal model. However, an experimental tetravalent vaccine combining inactivated EV-A71, CV-A16, CV-A6 and CV-A10 was recently found to elicit neutralizing antibody responses in mice against all 4 viruses, indicating that producing such a vaccine is highly feasible (Dr CC. Liu, personal communication).
Since the early attempts to develop a live attenuated temperature-sensitive CV-B3 mutant vaccine against myocarditis in 1997, several recent approaches have been evaluated for their potential to protect against CV-B3 infection. Previous studies have highlighted the importance of both humoral and cellular immunity in preventing CV-B3-induced disease.Citation176 A β-propionolactone-inactivated CV-B3 strain formulated with Quil A matrix or ISCOMs induced neutralizing antibodies and protected mice against myocarditis but was abandoned for technical issues and the lack of interest from the industry.Citation176,177 Several types of either natural or engineered attenuated vaccines have induced protection against experimental myocarditis and pancreatitis but they are prone to antibody-dependent enhancement of disease, reversion to cardiovirulence, and persistent infection in the target tissues.Citation176 Very recently, an attenuated CV-B3 Sabin3-like strain administered orally induced a protective immune response in mice but a limited amount of pancreatic inflammation was still detected in some challenged animals.Citation178 Priming with a DNA vector expressing CV-B3 VP1 followed by 2 VP1 subunit boosts induced neutralizing antibodies and cytotoxic T cells but was only partially protective against live CV-B3 challenge.Citation179 Intranasal co-administration of a chitosan-encapsulated plasmid DNA vector expressing VP1 (chito-pDNA-VP1) and a second chitosan-DNA plasmid producing the high mobility group box 1 protein as an immunostimulant induced both systemic and mucosal immune responses and reduced the viral load and the severity of CV-B3-induced myocarditis.Citation180 Vaccination with CV-B3 VLPs produced in the baculovirus expression system and formulated in complete or incomplete Freund's adjuvant have induced neutralizing antibody titers of 1/320 that conferred incomplete protection upon passive immune serum transfer to mice challenged with a cadiovirulent virus.Citation181 Chromatographically-purified VLPs elicited higher neutralizing antibody titers (1/1100) and an increase in effector-memory T cells. However, VLPs were less immunogenic than a formalin-inactivated CV-B3 vaccine used as positive control and challenge experiments were not performed.Citation182 The immunoprotective ability of a recombinant vesicular stomatitis virus (VSV) vector expressing CV-B3 VP1 was compared to that of a chitosan-pDNA-VP1 vaccine following intranasal administration. The VSV-VP1 vaccine induced significantly higher levels of antigen-specific, systemic and mucosal antibody responses than chitosan-pDNA-VP1 as well as strong polyfunctional T-cell responses and dendritic cell maturation, but was not fully protective against a 50% lethal dose of live CV-B3.Citation183 There is no published information on CV-B5 and E-30 vaccine research activities.
Challenges for a Multivalent HFMD Vaccine Registration
HFMD epidemics will persist for a long time owing to the co-circulation of multiple pathogens, the occurrence of co-infection and recombination, the ever increasing number of travelers and migrants, and the lack of a multivalent vaccine. However, it would be overoptimistic to think that such a vaccine will be available soon because of the numerous challenges faced by its development.
Cross-protective ability of a multivalent vaccine and selection of potential vaccine strains
A multivalent vaccine should ideally protect against all genotypes and subgenotypes of HFMD-associated viruses due to the unpredictability of the composition of epidemics and the emergence of potentially new variants.
With respect to the monovalent EV-A71 vaccine, both C4-based and B4-based vaccines cross-neutralized the current circulating EV-A71 isolates,Citation156-161 but the B4 vaccine poorly neutralized an atyptical C2 strain.Citation156 However, the degree of cross-neutralizing activity of immune responses induced by the C4- and B4-based vaccines against viruses from genotypes D, E and F remains to be evaluated. However, due to its good cross-immunogenicity as well as its large contribution to endemicity and HFMD epidemics, the C4a strain emerges as the best candidate for inclusion in a multivalent vaccine for countries such as China, Taiwan, Singapore, Malaysia, Vietnam and Japan where epidemics are the most frequent. However, only results from international efficacy trials conducted in regions and countries where different epidemic enterovirus A71 circulate will help assess the breadth of the cross-protective ability of a C4a-based vaccine and determine whether additional genotypes/subgenotypes for African countries in particular need to be included in a universal EV-A71 vaccine.
FI-EV-A71 vaccines failed to protect against CV-A16 infections that are predominantly responsible for annual HFMD outbreaks.Citation157-162 It is likely that FI-EV-A71 vaccinations may not significantly reduce the number of clinical cases of HFMD during outbreaks. In this regard, the introduction of a protective bivalent EV-A71/CV-A16 vaccine on the market should markedly reduce the number of HFMD cases.Citation170-174 CV-A16 strains of the B genotype that have elicited both homologous and heterologous protection against genotypes A and B in pre-clinical studies are potential candidates for a multivalent HFMD vaccine.Citation166-168 The selection of CV-A6, CV-A10, CV-B3, CV-B5 and E-30 vaccine strains will have to be based on comprehensive epidemiological information to identify the most prevalent circulating genotype(s) for each enterovirus serotype. Based on current studies, one could propose as vaccine strains clinical isolates from the CV-A6 C/D, CV-A10 B/C, CV-B3 E and E-30 E genotypes. Immunogenicity studies will still be necessary to determine whether like for EV-A71 and CV-A16, vaccine strain candidates induce cross-neutralizing antibody responses against most or all of their respective genotypes. In the absence of broad cross-neutralizing activity, the need for more than a restricted number of representative strains for each EV serotype would be a serious obstacle to the production of multivalent HFMD vaccines. Because of the risk of inter-typic and intra-typic recombination and the possible emergence of new strains with increased virulence, only results from multinational efficacy trials with a multivalent vaccine will reveal if it can elicit broad protection against divergent epidemic viruses in the target age group. Severe HFMD cases would not be suitable as clinical end points due to their low frequency. Selecting herpangina or mild illness would be more appropriate. The efficacy of a multivalent vaccine against coxsakieviruses B and echovirus E-30 infections should also be assessed during aseptic meningitis and acute myocarditis outbreaks. In this regard, the harmonization and standardization of virus strains, immunoassays and rapid diagnostic tools should be established at the national and international levels. A global surveillance network for enterovirus outbreaks and a rapid response system is also urgently needed.
Duration of humoral immunity, role of cellular responses and oral immunization
Phase III trials have unambiguously revealed that humoral immunity is protective against EV-A71 infection. But a significant waning of neutralizing antibody titers during the first 6 months after 2 vaccinations was noticed.Citation157 Importantly, the risk of subneutralizing antibody levels exposing to antibody-dependent enhancement of disease described for EV-A71, CV-A16 and CV-B3 should be prevented.Citation172,176,184 In an early Phase II trial, 773 participants who had received at least 1 dose of EV-A71 vaccine were enrolled to receive a booster dose.Citation185 A 10-fold increase at least in neutralizing antibody responses was induced by the booster injection. The booster dose was very immunogenic and well tolerated. Phase IV clinical trials will determine whether the current schedules and vaccine doses need to be optimized and whether a third immunization at 18–24 months is necessary to ensure long-lasting protection. The development of mucosal vaccines to prevent viral entry in the gastrointestinal tract is attractive, but it may not be necessary since parenteral immunization confers protection. Although live-attenuated viruses would be the best vaccines to induce both systemic and mucosal immunity as well as immune memory, the risk of reversion to virulence remains a major obstacle to their development.
Inducing polyfunctional T-cell responses and broad T-cell memory might be particularly critical for clearing coxsackievirus B infections. The vaccine might require immunogens and adjuvants/delivery systems different from inactivated viruses formulated in alum. Prospective studies should be conducted during EV-A71 and coxsackieviruses epidemics to assess the role of cellular immunity in long-term cross-protection and viral pathogenesis. In addition, longitudinal studies will be necessary to evaluate the role of multivalent vaccines in controlling antigenic shift, virus fitness and the emergence of new virus variants.
Standardized animal models for vaccine potency test
Standardized animal models necessary to understand EV-A pathogenesis and evaluate the potency and consistency of vaccine batches are not yet available.Citation186 Mouse-adapted strains, neonatal suckling mice and immunodeficient animals have been widely used to evaluate the protective efficacy of EV-A71 and CV-A16 vaccine candidates, but they do not mimic human infections.Citation168,186,187 In contrast, cardiac pathogenesis in Balb/c and SWR mice infected with CV-B3 is very similar to that of human patients.Citation177 Macaques develop antibody responses to EV-A71 vaccines similar to those observed in human; however they are not suitable to study neurovirulence and pulmonary edema complications and their use is limited by ethical and economic considerations.Citation186 Transgenic mice carrying the human receptor hSCARB2 Citation188,189 develop HFMD-like skin rashes upon infection with EV-A71 B4 and B5 clinical isolates and severe limb paralysis and death occurred in animals inoculated with a C2 strain.Citation188 The presence of EV-A71 in tissues and CNS was accompanied by the up-regulation of pro-inflammatory mediators (CXCL10, CCL3, TNF-α, and IL-6) and correlated with the recruitment of T lymphocytes and disease severity.Citation188 In addition, passive administration of the monoclonal anti-EV-A71 VP1 neutralizing antibody N3 reduced symptoms induced by EV-A71 B5 infection and protected the transgenic mice against EV-A71 C2-induced severe limb paralysis and death.Citation190 Once standardized, the transgenic mouse model will be useful to assess the cross-protective ability of vaccines against coxsackieviruses A using hSCARB-2 as receptor. However, CV-A6 and CV-A10 do not use this receptor.
Vaccine manufacturing
An ideal multivalent HFMD vaccine should be inexpensive, safe, compatible with large-scale production, easy to administer and acceptable to parents. There is an urgent need to improve and scale-up the current manufacturing processes for inactivated vaccines for broad approval of EV-A71 vaccines by regulatory authorities. Due to intellectual property rights and proprietary technologies, information on the influence of culture medium and production systems on vaccine yields is missing. Both the roller-bottle and cell factory technologies used in producing current clinical lots are easy to operate, although labor intensive. Developing countries could start implementing these technologies first and subsequently optimize the manufacturing processes for large-scale vaccine production. Other technologies could potentially be used in the future to increase virus yields. They include the selection for each virus of optimal cGMP-compliant cell lines, the transfection of more viral receptor genes into host cells or the removal of genes inhibiting viral replication to enhance virus production. Reverse-genetic could also be used to improve virus yields by inserting specific protease cleavage sites to increase virus infectivity.Citation72,135 The development of a multivalent HFMD vaccine remains a challenging task. Although inactivated poliovirus and EV-A71 vaccines have been successfully developed and inactivated CV-A16 candidate vaccines are very promising, there is some indication that VLP-based bivalent vaccines are more immunogenic than a combination of inactivated EV-A71/CV-A16 vaccines.Citation174 It is not clear at the present time whether the production yields for EV-A71 and other HFMD-associated viruses will be sufficient to meet global needs. The selection process for an ideal vaccine strains needs to be addressed in the light of comprehensive epidemiological surveys, the optimal technology to efficiently produce potent and safe immunogens must be defined for each serotype, and clinical trials will have to be conducted for each individual vaccine before combining them. Large-scale production of EV-A71 vaccines will require an improvement of the current manufacturing processes. The use of bioreactors, micro-carriers and perfusion technology could increase cell growth and virus yield by one order of magnitude. To lower the production cost, a simple and efficient downstream chromatographic purification step could be optimized to co-purify immunogenic defective and infectious virus particles.Citation96,135,175 A similar approach could be applied for the production of the other enteroviruses. If the yields of inactivated vaccines could not be improved, VLP-based vaccines are an alternative. They are good and safe immunogens that can be produced at the industrial scale and 2 VLP-based vaccines (HBV, HPV) have been successfully commercialized. One challenge in the development of multivalent HFMD vaccines will be to avoid interference between their various immunogens and between potentially different adjuvants in order to ensure vaccine stability and consistent immunogenicity. However, 2 hexavalent diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b, poliovirus and hepatitis B (DTaP-Hib-IPV-HepB) combination vaccines that contain 10 different immunogens have been successfully licensed and commercialized and contribute to a significant increase in the infant vaccine coverage.
Public health issues
Approximately 50% of neonates have neutralizing anti-EV-A71 antibody titers that decline to be undetectable after 6 months Citation191 while the disease peak occurs between one and 2 y of age. Ideally an HFMD vaccine should target infants before or at 6 months of age. This vaccination schedule will overlap with the administration of other pediatric (DTaP, Hib, IPV, HepB) combination vaccines, Rotavirus and Pneumococcal vaccines depending on the country. Pre-clinical studies and human trials should demonstrate that the co-administration of an inactivated multivalent HFMD vaccine with these vaccines will alter neither its immunogenicity nor the potency of the co-administered vaccine. In this regard, preliminary studies have shown that co-immunization of an inactivated EV-A71 vaccine with Pediacel (Sanofi Pasteur) did not alter antibody responses to individual vaccine components.Citation192 Furthermore, the immunogenicity of an inactivated EV-A71 vaccine was not affected by pre-existing anti-CV-A16 or anti-poliovirus neutralizing antibodies nor by its co-administration with CV-A16 and poliovirus 1,2,3 vaccines.Citation193
Economic issues
HFMD is a significant health and economic burden for the Asia Pacific region in particular for China. An accurate estimation of this burden will require an enhancement of the surveillance system, an improved training of physicians in particular in rural areas and the generalized availability of rapid diagnostic tools. It has been estimated that the average direct and indirect cost was US$129 for an outpatient, $484 for an inpatient, and $1936 for a severe case of HFMD, respectively. The annual economic burden of EV-A71-associated HFMD amounts to US$161–323 million per year.Citation194 This figure is still optimistic since it does not take into account the fact that the number of annual EV-A71 cases is likely underestimated, or the number of HFMD cases caused by other enteroviruses, or the costs linked to sequelae and fatalities. Clinical trials have revealed that the administration of 400 units (1 µg) dose of EV-A71 vaccine twice achieved efficacy.Citation72,157,160 A recent publication Citation135 reports that a 40-liters pilot-scale production batch could yield 50,000 1 µg doses of FI-EV-A71 at a cost of 0.4 US dollar/dose. This translates into 200,000 doses of the lowest C4-vaccine protective dose (0.25 µg) as determined in a Phase III trial Citation72,157 at 0.1 US dollar/dose. Assuming similar potency and yields for the other components of a heptavalent HFMD vaccine, one could speculate that the production cost of the vaccine would be in the range of US$ 1 per dose. It has been forecasted that routine immunization with a 70% efficacious EV-A71 vaccine sold at 25 US dollars/dose would be of great economic value.Citation195 Another recent study reports that immunization with an EV-A71 vaccine would be cost-effective if vaccination costs were ≤US$75 per dose for 90% efficacy.Citation194 With this profit margin and the new emerging vaccine markets in the Asia-Pacific region, the global vaccine companies should become interested in manufacturing monovalent, bivalent and ultimately multivalent HFMD vaccines.
Conclusions
A multivalent HFMD vaccine is an unmet medical need for a life-threatening disease with huge economic burden. Although EV-A71 is responsible for the vast majority of severe complications and fatalities, a monovalent EV-A71 vaccine may not significantly reduce the number of HFMD cases which poses a problem of public acceptance. EV-A71 vaccines will move soon to market in China and a bivalent EV-A71/CV-A16 vaccine should enter Phase I clinical trials in the near future. Based on the current clinical and molecular epidemiology data, there is a strong rationale for including CV-A6 and CV-A10 immunogens in the next tetravalent vaccine. This vaccine would form the core of an ultimate heptavalent HFMD vaccine incorporating CV-B3, CV-B5 and E-30 viral components. The safety and efficacy of poliovirus and EV-A71 vaccines should serve as a rationale for the stream-lined development of a multivalent vaccine based on inactivated viruses. Improvement in manufacturing processes will still be needed to produce cost-effective viral immunogens. The development of a heptavalent HFMD vaccine is feasible but it remains a major undertaking and challenge that will require the cooperation of epidemiologists, policy makers, market analysts, Research and Development Institutes, Governments, and the global vaccine industry. The implementation of EV-A71 vaccination programs might change the landscape of HFMD epidemics by creating a niche for other viruses as a result of selective pressure. Thus, the establishment of a global surveillance system is urgently needed to monitor the safety and long-term immunogenicity of the EV-A71 vaccines, determine whether booster doses are necessary, and detect potential changes in the composition of future HFMD epidemics. Ultimately, the combination of a multivalent HFMD vaccine with Expanded Program on Immunization vaccines would greatly simplify immunization schedules. Such an achievement would be another “invaluable gift to children”Citation196 and a new milestone in the history of combination vaccines.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Pallansch M, Oberste MS, Whitton JL. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In Fields Virology, 6th edn, Fields BN, Knipe DM, Howley PM (eds). Phildelphia, PA: Wolters Kluwer/Lippincott Williams and Wilkins Health, 490-530, 2013.
- Tapparel C. Siegrist F, Petty TJ, Kaiser L. Picornavirus and enterovirus diversity with associated human diseases. Infect Genet Evol 2013; 14:282-93; PMID:23201849; http://dx.doi.org/10.1016/j.meegid.2012.10.016
- Kim KS, Hufnagel G, Chapman NM, Tracy S. The group B coxsackieviruses and myocarditis. Rev Med Virol 2001; 11:355-68; PMID:11746998; http://dx.doi.org/10.1002/rmv.326
- Lee B, Davies HD. Aseptic meningitis. Curr Opin Infect Dis 2007; 20:272-7; PMID:17471037; http://dx.doi.org/10.1097/QCO.0b013e3280ad4672
- Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 2010; 10:778-90; PMID:20961813; http://dx.doi.org/10.1016/S1473-3099(10)70194-8
- McMinn P. Recent advances in the molecular epidemiology and control of human enterovirus 71 infection. Curr Opin Virol 2012; 2:199-205; PMID:22482716; http://dx.doi.org/10.1016/j.coviro.2012.02.009
- Yip CCY, Lau SKP, Woo PCY, Yuen KA. Human enterovirus 71 epidemics: what's next? Emerg Health Threats J 2013; 6:19780; PMID:24119538
- Mao Q, Wang Y, Yao X, Bian L, Wu X, Xu M, Liang Z. Coxsackievirus A16: epidemiology, diagnosis, and vaccine. Hum Vaccin Immunother 2014; 10:360-7; PMID:24231751; http://dx.doi.org/10.4161/hv.27087
- Liu SL, Pan H, Liu P, Amer S, Chan TC, Zhan J, Huo X, Liu Y, Teng Z, Wang L, et al. Comparative epidemiology and virology of fatal and nonfatal cases of hand, foot and mouth disease in mainland China from 2008 to 2014. Rev Med Virol 2015; 25(2):115-28; PMID:25704797; http://dx.doi.org/10.1002/rmv.1827
- Fonseca MC, Sarmiento L, Resik S, Martínez Y, Hung LH, Morier L, Piñón A, Valdéz O, Kourí V, González G. Coxsackievirus A6 and enterovirus 71 causing hand, foot and mouth disease in Cuba, 2011-2013. Arch Virol 2014; 159:2451-5; PMID:24719197; http://dx.doi.org/10.1007/s00705-014-2071-x
- Centers for Disease Control and Prevention (CDC). Notes from the field: severe hand, foot and mouth disease associated with coxsackievirus A6 - Alabama, Connecticut, California, and Nevada, November 2011-February 2012. MMWR Morb Mortal Wkly Rep 2012; 61:213-4; PMID:22456122
- Yang F, Zhang T, Hu Y, Wang X, Du J, Li Y, Sun S, Sun X, Li Z, Jin Q. Survey of enterovirus infections from hand, foot and mouth disease outbreak in China, 2009. Virol J 2011; 8:508-11; PMID:22054534; http://dx.doi.org/10.1186/1743-422X-8-508
- Lu QB, Zhang XA, Wo Y, Xu HM, Li XJ, Wang XJ, Ding SJ, Chen XD, He C, Liu LJ, et al. Circulation of coxsackievirus A10 and A6 in hand, foot and mouth disease in China, 2009-2011. PLoS One 2012; 7(12):e52073; PMID:23272213; http://dx.doi.org/10.1371/journal.pone.0052073
- Liu W, Wu S, Xiong Y, Li T, Wen Z, Yan M, Qin K, Liu Y, Wu J. Co-circulation and genomic recombination of coxsackievirus A16 and enterovirus 71 during a large outbreak of hand, foot and mouth disease in Central China. PLoS One 2014; 9(4):e96051; PMID:24776922; http://dx.doi.org/10.1371/journal.pone.0096051
- Hongyan G, Chengile M, Qiaozhi Y, Wenhao H, Juan L, Lin P, Yanli X, Hongshan W, Xingwang L. Hand, foot and mouth disease caused by coxsackievirus A6, Beijing, 2013. Pediatr Infect Dis J 2014; 33:1302-3; PMID:25037037; http://dx.doi.org/10.1097/INF.0000000000000467
- Han JF, Jiang T, Fan XL, Yang LM, Yu M, Cao RY, Wang JZ, Qin ED, Qin CF. Recombination of human coxsackievirus B5 in hand, foot and mouth disease patients, China. Emerg Infect Dis 2012; 18:351-3; PMID:22305307; http://dx.doi.org/10.3201/eid1802.111524
- Han JF, Xu S, Zhang Y, Zhu SY, Wu DL, Yang XD, Liu H, Sun BX, Wu XY, Qin CF. Hand, foot and mouth disease outbreak caused by coxsackievirus A6, China 2013. J Infect 2014; 69:303-5; PMID:24704297; http://dx.doi.org/10.1016/j.jinf.2014.03.015
- Chen JF, Zhang RS, Ou XH, Chen FM, Sun BC. The role of enterovirus 71 and coxsackievirus A strains in a large outbreak of hand, foot and mouth disease in Changsha, China. Int J Infect Dis 2014; 28:17-25; PMID:25236389; http://dx.doi.org/10.1016/j.ijid.2014.07.024
- Zhang Y, Zhu Z, Yang W, Ren J, Tan X, Wang Y, Mao N, Xu S, Zhu S, Cui A, et al. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand, foot and mouth disease in Fuyang city of China. Virol J 2010; 7:94-102; PMID:20459851; http://dx.doi.org/10.1186/1743-422X-7-94
- He SJ, Han JF, Ding XX, Wang YD, Qin CF. Characterization of enterovirus 71 and coxsackievirus A16 isolated in hand, foot and mouth disease patients in Guandong, 2010. Int J Infect Dis 2013; 17:e1025-30; PMID:23791223; http://dx.doi.org/10.1016/j.ijid.2013.04.003
- Lu J, Zeng H, Zheng H, Yi L, Guo X, Liu L, Sun L, Tan X, Li H, Ke C, Lin J. Hand, foot and mouth disease in Guangdong, China, in 2013: new trends in the continuing epidemic. Clin Microbiol Infect 2014; 20:O442-5; PMID:24428125; http://dx.doi.org/10.1111/1469-0691.12468
- Di B, Zhang Y, Xie H, Li X, Chen C, Ding P, He P, Wang D, Geng J, Luo L, et al. Circulation of coxsackievirus A6 in hand-foot-and-mouth disease in Guangzhou, 2010-2012. Virol J 2014; 11:157-63; PMID:25178398; http://dx.doi.org/10.1186/1743-422X-11-157
- Han JF, Cao RY, Jiang T, Yu M, Liu W, Tian X, Qin ED, Cao WC, Qin CF. Echovirus 30 in EV71-associated hand, foot and mouth disease outbreak, Guanxi, China. J Clin Virol 2011; 50:348-9; PMID:21306942; http://dx.doi.org/10.1016/j.jcv.2011.01.005
- Tian H, Zhang Y, Sun Q, Zhu S, Li X, Pan Z, Xu W, Xu B. Prevalence of multiple enteroviruses associated with hand, foot and mouth disease in Shijiazhuang city, Hebei province, China: outbreaks of coxsackieviruses a10 and b3. PLoS One 2014; 9(1):e84233; PMID:24392117; http://dx.doi.org/10.1371/journal.pone.0084233
- Fan X, Jiang J, Liu Y, Huang X, Wang P, Liu L, Wang J, Chen W, Wu W, Xu B. Detection of human enterovirus 71 and Coxsackievirus A16 in an outbreak of hand, foot and mouth disease in Henan Province, China in 2009. Virus Genes 2013; 46:1-9; PMID:23080402; http://dx.doi.org/10.1007/s11262-012-0814-x
- Guan HY, Yang MJ, Liu LZ, Wang J, Yang GL, Zhang W, Ma XJ, Wang CR. Pathogenic spectrum of enteroviruses associated with hand, foot and mouth disease by a GeXPTM-based multiplex reverse transcription-PCR assay in Jinan, China, 2009-2012. Bing Du Xue Bao 2014; 30:567-71; PMID:25562968
- Zhang X, Wang H, Ding S, Wang X, Chen X, Wo Y, Wang L, Huang D, Liu W, Cao W. Prevalence of enteroviruses in children with and without hand, foot and mouth disease in China. BMC Infect Dis 2013; 13:606-13; PMID:24370001; http://dx.doi.org/10.1186/1471-2334-13-606
- Ni H, Yi B, Yin J, Fang T, He T, Du Y, Wang J, Zhang H, Xie L, Ding Y, et al. Epidemiological and etiological characteristics of hand, foot and mouth disease in Ningbo, China, 2008-2011. J Clin Virol 2012; 54:342-8; PMID:22652041; http://dx.doi.org/10.1016/j.jcv.2012.04.021
- Du J, Wang X, Hu Y, Li Z, Li Y, Sun S, Yang F, Jin Q. Changing aetiology of hand, foot and mouth disease in Linyi, China, 2009-2011. Clin Microbiol Infect 2014; 20:O47-9; PMID:23910234; http://dx.doi.org/10.1111/1469-0691.12301
- Hu YF, Yang F, Du J, Zhang T, Xue Y, Jin Q. Coxsackie B5 associated with neurological hand, foot and mouth disease, China. J Infect 2012; 65:189-91; PMID:22484273; http://dx.doi.org/10.1016/j.jinf.2012.03.021
- Zhang T, Du J, Xue Y, Su H, Yang F, Jin Q. Epidemics and frequent recombination within species in outbreaks of human enteroviruses B-associated hand, foot and mouth disease in Shangdong China in 2010 and 2011. PLoS One 2013; 8(6):e67157; PMID:23840610; http://dx.doi.org/10.1371/journal.pone.0067157
- Han JF, Zhang Y, Hou PQ, Zhu SY, Wu XY, Zhao H, Yu M, Qin CF. Human enterovirus co-infection in severe HFMD patients in China. J Clin Virol 2014; 61:621-2; PMID:25449173; http://dx.doi.org/10.1016/j.jcv.2014.09.005
- Yan XF, Gao S, Xia JF, Ye R, Yu H, Long JE. Epidemic characteristic of hand, foot and mouth disease in Shanghai from 2009 to 2010: Enterovirus 71 subgenotype C4 as the primary causative agent and a high incidence of mixed infections with coxsackievirus A16. Scand J Infect Dis 2012; 44:297-305; PMID:22176514; http://dx.doi.org/10.3109/00365548.2011.634433
- Zeng M, Li YF, Wang XH, Lu GP, Shen HG, Yu H, Zhu QR. Epidemiology of hand, foot and mouth disease in children in Shanghai 2007-2010. Epidemiol Infect 2012; 140:1122-30; PMID:21878145; http://dx.doi.org/10.1017/S0950268811001622
- Xu M, Su L, Cao L, Zhong H, Dong N, Xu J. Enterovirus genotypes causing hand, foot and mouth disease in Shanghai, China: a molecular epidemiological analysis. BMC Infect Dis 2013; 13:489-97; PMID:24148902; http://dx.doi.org/10.1186/1471-2334-13-489
- He YQ, Chen L, Xu WB, Yang H, Wang HZ, Zong WP, Xian HX, Chen HL, Yao XJ, Hu ZL, et al. Emergence, circulation and spatiotemporal phylogenetic analysis of coxsackievirus a6- and coxsackievirus a10-associated hand, foot and mouth disease infections from 2010 to 2012 in Shenzhen, China. J Clin Microbiol 2013; 51:3560-6; PMID:23966496; http://dx.doi.org/10.1128/JCM.01231-13
- Li JL, Yuan J, Yang F, Wu ZQ, Hu YF, Xue Y, Zhou BP, Jin Q. Epidemic characteristics of hand, foot and mouth disease in southern China, 2013: coxsackievirus A6 has emerged as the predominant causative agent. J. Infect 2014; 69:299-303; PMID:24731881; http://dx.doi.org/10.1016/j.jinf.2014.04.001
- Tan X, Li L, Zhang B, Jorba J, Su X, Ji T, Yang D, Lv L, Li J, Xu W. Molecular epidemiology of coxsackievirus A6 associated with outbreaks of hand, foot and mouth disease in Tianjin, China, in 2013. Arch Virol 2015; 160:1097-104; PMID:25680566; http://dx.doi.org/10.1007/s00705-015-2340-3
- Chen L, Mou X, Zhang Q, Li Y, Lin J, Liu F, Yuan L, Tang Y, Xiang C. Detection of human enterovirus 71 and coxsackievirus A16 in children with hand, foot and mouth disease in China. Mol Med Rep 2012; 5:1001-4; PMID:22218731
- Gopalkrishna V, Patil PR, Patil GP, Chitambar SD. Circulation of multiple enterovirus serotypes causing hand, foot and mouth disease in India. J Med Microbiol 2012; 61:420-5; PMID:22052995; http://dx.doi.org/10.1099/jmm.0.036400-0
- Kar BR, Dwibedi B, Kar SK. An outbreak of hand, foot and mouth in Bhubaneswar, Odisha. Indian Pediatr 2013; 50:139-42; PMID:22728634; http://dx.doi.org/10.1007/s13312-013-0033-0
- Lee MS, Tseng FC, Wang JR, Chi CY, Chong P, Su IJ. Challenges to licensure of enterovirus 71 vaccines. PLoS Negl Trop Dis 2012; 6(8):e1737; PMID:22953003; http://dx.doi.org/10.1371/journal.pntd.0001737
- Iwai M, Masaki A, Hasegawa S, Obara M, Horimoto E, Nakamura K, Tanaka Y, Endo K, Tanaka K, Ueda J, et al. Genetic changes of coxsackievirus A16 and enterovirus 71 isolated from hand, foot and mouth disease patients in Toyama, Japan between 1981 and 2007. Jpn J Infect Dis 2009; 62:254-9; PMID:19628900
- Sano T, Saito T, Kondo M, Watanabe S, Onoue Y, Konnai M, Sato Y, Orihara N. Enterovirus detection status of patients with herpangina and hand, foot and mouth disease in epidemic season 2007, Kanagawa Prefecture, Japan. Jpn J Infect Dis 2008; 61:162-3; PMID:18362414
- Miyamoto A, Hirata R, Ishimoto K, Hisatomi M, Wasada R, Akita Y, Ishihara T, Fujimoto T, Eshima N, Hatano Y, et al. An outbreak of hand-foot-and-mouth disease mimicking chicken pox, with a frequent association of onychomadesis in Japan in 2009: a new phenotype caused by coxsackievirus A6. Eur J Dermatol 2014; 24:103-4; PMID:24334231
- Fujimoto T, Iizuka S, Enomoto M, Abe K, Yamashita K, Hanaoka N, Okabe N, Yoshida H, Yasui Y, Kobayashi M, et al. Hand, foot and mouth disease caused by coxsackievirus A6, Japan, 2011. Emerg Infect Dis 2012; 18:337-9; PMID:22304983; http://dx.doi.org/10.3201/eid1802.111147
- Park SH, Lee BH, Baek KA, Cheon D, Yeo S, Park J, Soh J, Cheon H, Yoon K, Choi Y. Enteroviruses isolated from herpangina and hand, foot and mouth disease in Korean children. Virol J 2012; 9:205-10; PMID:22985487; http://dx.doi.org/10.1186/1743-422X-9-205
- Chua KB, Kasri AR. Hand, foot and mouth disease due to enterovirus 71 in Malaysia. Virol Sin 2011; 26:221-8; PMID:21847753; http://dx.doi.org/10.1007/s12250-011-3195-8
- Ang LW, Koh BK, Chan KP, Chua LT, James L, Goh KT. Epidemiology and control of hand, foot and mouth disease in Singapore, 2001-2007. Ann Acad Med Singapore 2009; 38:106-12; PMID:19271036
- Wu Y, Yeo A, Phoon MC, Tan EL, Poh CL, Quak SH, Chow VT. The largest outbreak of hand, foot and mouth disease in Singapore in 2008: the role of enterovirus71 and coxsackievirus A strains. Int J Infect Dis 2010; 14:e1076-81; PMID:20952237; http://dx.doi.org/10.1016/j.ijid.2010.07.006
- Chen KT, Chang HL, Wang ST, Cheng YT, Yang JY. Epidemiologic features of hand-foot-mouth disease and hyperangina caused by enterovirus 71 in Taiwan, 1998-2005. Pediatrics 2007; 120:e245-52
- Lo SH, Huang YC, Huang CG, Tsao KC, Li WC, Hsieh YC, Chiu CH, Lin TY. Clinical and epidemiologic features of coxsackievirus A6 infection in children in northern Taiwan between 2004 and 2009. J Microbiol Immunol Infect 2011; 44:252-7; PMID:21524960; http://dx.doi.org/10.1016/j.jmii.2011.01.031
- Hsu CH, Lu CY, Shao PL, Lee PI, Kao CL, Chung MY, Chang LY, Huang LM. Epidemiologic and clinical features of non-polio enteroviral infections in northern Taiwan in 2008. J Microbiol Immunol Infect 2011; 44:265-73; PMID:21524954; http://dx.doi.org/10.1016/j.jmii.2011.01.029
- Lee MH, Huang LM, Wong WW, Wu TZ, Chiu TF, Chang LY. Molecular diagnosis and clinical presentations of enteroviral infections in Taipei during the 2008 epidemic. J Microbiol Immunol Infect 2011; 44:178-83; PMID:21524611; http://dx.doi.org/10.1016/j.jmii.2011.01.018
- Huang WC, Huang LM, Lu CY, Cheng AL, Chang LY. Atypical hand-foot-mouth disease in children: a hospital-based prospective cohort study. Virol J 2013; 10:209-17; PMID:23800163; http://dx.doi.org/10.1186/1743-422X-10-209
- Puenpa J, Chieochansin T, Linsuwanon P, Korkong S, Thongkomplew S, Vichaiwattana P, Theamboonlers A, Poovorawan Y. et al. Hand, foot and mouth disease caused by coxsackievirus A6, Thailand, 2012. Emerg Infect Dis 2013; 19:641-3; PMID:23631943; http://dx.doi.org/10.3201/eid1904.121666
- Puenpa J, Mauleekoonphairoj J, Linsuwanon P, Suwannakarn K, Chieochansin T, Korkong S, Theamboonlers A, Poovorawan Y. Prevalence and characterization of enterovirus infections among pediatric patients with hand foot mouth disease, herpangina and influenza like illness in Thailand, 2012. PLoS One 2014; 9(6):e98888; PMID:24887237; http://dx.doi.org/10.1371/journal.pone.0098888
- Linsuwanon P, Puenpa J, Huang SW, Wang YF, Mauleekoonphairoj J, Wang JR, Poovorawan Y. Epidemiology and seroepidemiology of human enterovirus 71 among Thai populations. J Biomed Sci 2014; 21:16-28; PMID:24548776; http://dx.doi.org/10.1186/1423-0127-21-16
- Tu PV, Thao NTT, Perera D, Huu TK, Tien NT, Thuong TC, How OM, Cardosa MJ, McMinn PC. Epidemiologic and virologic investigation of hand, foot and mouth disease, southern Vietnam, 2005. Emerg Infect Dis 2007; 13:1733-41; PMID:18217559; http://dx.doi.org/10.3201/eid1307.060999
- Thoa le PK, Chiang PS, Khanh TH, Luo ST, Dan TN, Wang YF, Thuong TC, Chung WY, Hung NT, Wang JR, et al. Genetic and antigenic characterization of enterovirus 71 in Ho Chi Minh City, Vietnam, 2011. PLoS One 2013; 8(7):e69895; PMID:23922846; http://dx.doi.org/10.1371/journal.pone.0069895
- Zander A, Britton PN, Navin T, Horsley E, Tobin S, McAnulty JM. An outbreak of enterovirus 71 in metropolitan Sydney: enhanced surveillance and lessons learnt. Med J Aust 2014; 201:663-6; PMID:25495312; http://dx.doi.org/10.5694/mja14.00014
- Blomqvist S, Klemola P, Kaijalainen S, Paananen A, Simonen ML, Vuorinen T, Roivainen M. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J ClinVirol 2010; 48:49-54
- Mirand A, Henquell C, Archimbaud C, Ughetto S, Antona D, Bailly JL, Peigue-Lafeuille H. Outbreak of hand, foot and mouth disease/herpangina associated with coxsackievirus A6 and A10 infections in 2010, France: a large citywide, prospective observational study. Clin Microbiol Infect 2012; 18:E110-8; PMID:22404077; http://dx.doi.org/10.1111/j.1469-0691.2012.03789.x
- Kapusinszky B, Szomor KN, Farkas A, Takács M, Berencsi G. Detection of non-polioviruses in Hungary 2000-2008 and molecular epidemiology of enterovirus 71, coxsackievirus A16 and echovirus 30. Virus Genes 2010; 40:163-73; PMID:20044791; http://dx.doi.org/10.1007/s11262-009-0440-4
- Bracho MA, Gonzales-Candelas F, Valero A, Córdoba J, Salazar A. Enterovirus co-infections and onychomadesis after hand, foot and mouth disease, Spain, 2008. Emerg Infect Dis 2011; 17:2223-1; PMID:22172227; http://dx.doi.org/10.3201/eid1712.110395
- Davia JL, Bel PH, Ninet VZ, Bracho MA, González-Candelas F, Salazar A, Gobernado M, Bosch IF. Onychomadesis outbreak in Valencia, Spain associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol 2011; 28:1-5; PMID:20553401; http://dx.doi.org/10.1111/j.1525-1470.2010.01161.x
- Cabrerizo M, Tarrago D, Munoz-Almagro C, Del Amo E, Domínguez-Gil M, Eiros JM, López-Miragaya I, Pérez C, Reina J, Otero A, et al. Molecular epidemiology of enterovirus 71, coxsackievirus A16 and A6 associated hand, foot and mouth disease in Spain. Clin Microbiol Infect 2014; 20:O150-6; PMID:24033818; http://dx.doi.org/10.1111/1469-0691.12361
- Montes M, Artieda J, Pineiro LD, Gastesi M, Diez-Nieves I, Cilla G. Hand, foot, and mouth disease outbreak and coxsackievirus A6, northern Spain, 2011. Emerg Infect Dis 2013; 19:676-8; PMID:23751014; http://dx.doi.org/10.3201/eid1904.121589
- Sinclair C, Gaunt E, Simmonds P, Broomfield D, Nwafor N, Wellington L, Templeton K, Willocks L, Schofield O, Harvala H. Atypical hand, foot, and mouth disease associated with coxsackievirus A6 infection, Edinburgh, United Kingdom, January to February 2014. Euro Surveill 2014; 19:20745-51; PMID:24698138
- World Health Organization. Hand, foot and mouth disease a rising menace in Asia. 13 July 2009. Available at http://www.wpro.who.int/mediacentre/news/2009/2009013/en/
- Abzug MJ. The enteroviruses: problems in need of treatment. J Infect 2014; 68 Suppl 1:S108-S114; PMID:24119825; http://dx.doi.org/10.1016/j.jinf.2013.09.020
- Chong P, Liu CC, Chow YH, Chou AH, Klein M. Review of enterovirus 71 vaccines. Clin Infect Dis 2015; 60:797-803; PMID:25352588; http://dx.doi.org/10.1093/cid/ciu852
- McMinn PC. Enterovirus vaccines for an emerging cause of brain-stem encephalitis. N Engl J Med 2014; 370:792-4; PMID:24571750; http://dx.doi.org/10.1056/NEJMp1400601
- Wang SM, Ho TS, Lin HC, Lei HY, Wang JR, Liu CC. Reemerging of enterovirus 71 in Taiwan: the age impact on disease severity. Eur J Microbiol Infect Dis 2012; 31:1219-24; PMID:21983920; http://dx.doi.org/10.1007/s10096-011-1432-6
- Huang MC, Wang SM, Hsu YW, Lin HC, Chi CY, Liu CC. Long-term cognitive and motor deficits after enterovirus 71 brainstem encephalitis in children. Pediatrics 2006; 118:e1785-8; PMID:17116698; http://dx.doi.org/10.1542/peds.2006-1547
- Yang C, Deng C, Wan J, Zhu L, Leng Q. Neutralizing antibody response in the patients with hand, foot and mouth disease to enterovirus 71 and its clinical implications. Virol J 2011; 8:306-11; PMID:21679417; http://dx.doi.org/10.1186/1743-422X-8-306
- Wei R, Xu L, Zhang N, Zhu K, Yang J, Yang C, Deng C, Zhu Z, De Groot AS, Altmeyer R, et al. Elevated antigen-specific Th2 type response is associated with the poor prognosis of hand, foot and mouth disease. Virus Res 2013; 177:62-5; PMID:23886670; http://dx.doi.org/10.1016/j.virusres.2013.07.009
- Li S, Cai C, Feng J, Li X, Wang Y, Yang J, Chen Z. Peripheral T lymphocyte subset imbalances in children with enterovirus 71-induced hand, foot and mouth disease. Virus Res 2014; 180:84-91; PMID:24316007; http://dx.doi.org/10.1016/j.virusres.2013.11.021
- Huang WC, Huang LM, Kao CL, Lu CY, Shao PL, Cheng AL, Fan TY, Chi H, Chang LY. Seroprevalence of enterovirus 71 and no evidence of crossprotection of enterovirus 71 antibody against the other enteroviruses in kindergarten children in Taipei city. J Microbiol Immunol Infect 2012; 45:96-101; PMID:22154997; http://dx.doi.org/10.1016/j.jmii.2011.09.025
- Ben-Chetrit E, Wiener-Well Y, Shulman LM, Cohen MJ, Elinav H, Sofer D, Feldman I, Marva E, Wolf DG. Coxsackievirus A6-related hand, foot and mouth disease: skin manifestations in a cluster of adult patients. J Clin Virol 2014; 59:201-3; PMID:24457116; http://dx.doi.org/10.1016/j.jcv.2013.12.012
- Downing C, Ramirez-Fort MK, Doan HQ, Benoist F, Oberste MS, Khan F, Tyring SK. Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature. J Clin Virol 2014; 60:381-6; PMID:24932735; http://dx.doi.org/10.1016/j.jcv.2014.04.023
- Aizaki K, Tsuru T, Okumura K, Kondo N. [Three pediatric cases of group A coxsackievirus-associated encephalitis/encephalopathy]. No To Hattatsu 2012; 44:397-400; PMID:23012870
- Yang F, Yuan J, Wang X, Li J, Du J, Su H, Zhou B, Jin Q. Severe hand, foot, and mouth disease and coxsackievirus A6-Shenzhen, China. Clin Infect Dis 2014; 59:1504-5; PMID:25091307; http://dx.doi.org/10.1093/cid/ciu624
- Spanakis N, Manolis EN, Tsakris A, Tsiodras S, Panagiotopoulos T, Saroglou G, Legakis NJ. Coxsackievirus B3 sequences in the myocardium of fatal cases in a cluster of acute myocarditis in Greece. J Clin Pathol 2005; 58:357-60; PMID:15790697; http://dx.doi.org/10.1136/jcp.2004.020099
- Chu PY, Ke GM, Chen YS, Lu PL, Chen HL, Lee MS, Chen BC, Huang TS, Li YC, Chou LC, et al. Molecular epidemiology of Coxsackievirus B3. Infect Genet Evol 2010; 10:777-84; PMID:20398802; http://dx.doi.org/10.1016/j.meegid.2010.04.004
- Lee CJ, Huang YC, Yang S, Tsao KC, Chen CJ, Hsieh YC, Chiu CH, Lin TY. Clinical features of coxsackievirus A4, B3 and B4 infections in infants. PLoS One 2014; 9(2):e87391; PMID:24504149; http://dx.doi.org/10.1371/journal.pone.0087391
- Kim HJ, Kang B, Hwang S, Hong J, Kim K, Cheon DS. Epidemics of viral meningitis cause by echovirus 6 and 30 in Korea in 2008. Virol J 2012; 9:38-44; PMID:22336050; http://dx.doi.org/10.1186/1743-422X-9-38
- Hober D, Sauter P. Pathogenesis of type 1 diabetes mellitus: interplay between enterovirus and host. Nat Rev Endocrinol 2010; 6:279-89; PMID:20351698; http://dx.doi.org/; http://dx.doi.org/10.1038/nrendo.2010.27
- Wu PC, Huang LM, Kao CL, Fan TY, Cheng AL, Chang LY. An outbreak of coxsackievirus A16 infection: comparison with other enteroviruses in a preschool in Taipei. J Microbiol Immunol Infect 2010; 43:271-7; PMID:20688286; http://dx.doi.org/10.1016/S1684-1182(10)60043-6
- Miyazawa I, Azegami Y, Kasuo S, Yoshida T, Kobayashi M, Shiraishi T. Prevalence of enterovirus from patients with herpangina and hand, foot and mouth disease in Nagano Prefecture, Japan, 2007. Jpn J Infect Dis 2008; 61:747-8
- Yamashita T, Ito M, Tanigushi A, Sakae K. Prevalence of coxsackievirus A5, A6 and A10 in patients with herpangina in Aichi Prefecture, 2005. Jpn J Infect Dis 2005; 58:390-1; PMID:16377876
- Plevka P, Perera R, Cardosa J, Kuhn RJ, Rossmann MG. Crystal structure of human enterovirus 71. Science 2012; 336:1274-7; PMID:22383808; http://dx.doi.org/10.1126/science.1218713
- Lyu K, Wang GC, He YL, Han JF, Ye Q, Qin CF, Chen R. Crystal structure of enterovirus 71 (EV71) recombinant virus particles provide insights into vaccine design. J Biol Chem 2015; 290:3198-208; PMID:25492868; http://dx.doi.org/10.1074/jbc.M114.624536
- Yoder JD, Cifuente JO, Pan J, Bergelson JM, Hafenstein S. The crystal structure of a coxsackievirus B3-RD variant and a refined 9-angstrom cryo-electron microscopy reconstruction of the virus complexed with decay-accelerating factor (DAF) provide a new footprint of DAF on the virus surface. J Virol 2012; 86:12571-81; PMID:22973031; http://dx.doi.org/10.1128/JVI.01592-12
- Huang PN, Shih SR. Update on enterovirus 71 infection. Curr Opin Virol 2014; 5:98-104; PMID:24727707; http://dx.doi.org/10.1016/j.coviro.2014.03.007
- Liu CC, Guo MS, Lin FH, Hsiao KN, Chang KH, Chou AH, Wang YC, Chen YC, Yang CS, Chong PC. Purification and characterization of enterovirus 71 particles produced from vero cells grown in serum-free microcarrier bioreactor system. PLoS One 2011; 6(5):e20005; PMID:21603631; http://dx.doi.org/10.1371/journal.pone.0020005
- Lin JY, Shih SR. Cell and tissue tropism of enterovirus 71 and other enteroviruses infections. J Biomed Sci 2014; 21:18-23; PMID:24602216; http://dx.doi.org/10.1186/1423-0127-21-18
- Muehlenbachs A, Bhatnagar J, Zaki SR. Tissue tropism, pathology and pathogenesis of enterovirus infection. J Pathol 2015; 235:217-28; PMID:25211036; http://dx.doi.org/10.1002/path.4438
- Nishimura Y, Shimojima M, Tano Y, Miyamura T, Wakita T, Shimizu H. Human P-selectin glycoprotein ligand-1 is a functional receptor for enterovirus 71. Nat Med 2009; 15:794-7; PMID:19543284; http://dx.doi.org/10.1038/nm.1961
- Yamayoshi A, Yamashita Y, Li J, Hanagata N, Minowa T, Takemura T, Koike S. Scavenger receptor B2 is a cellular receptor for enterovirus 71. Nat Med 2009; 15:798-801; PMID:19543282; http://dx.doi.org/10.1038/nm.1992
- Yamayoshi A, Ohka S, Fujii K, Koike S. Functional comparison of SCARB2 and PSGL1 as receptors for enterovirus 71. J Virol 2013; 87:3335-47; PMID:23302872; http://dx.doi.org/10.1128/JVI.02070-12
- Yang SL, Chou YT, Wu CN, Ho MS. Annexin II binds to capsid protein VP1 of enterovirus 71 and enhances viral infectivity. J Virol 2011; 85:11809-20; PMID:21900167; http://dx.doi.org/10.1128/JVI.00297-11
- Tan CW, Poh CL, Sam IC, Chan YF. Enterovirus 71 uses cell surface heparin sulfate glycoaminoglycan as an attachment receptor. J Virol 2013; 87:611-20; PMID:23097443; http://dx.doi.org/10.1128/JVI.02226-12
- Su PY, Liu YT, Chang HY, Huang SW, Wang YF, Yu CK, Wang JR, Chang CF. Cell surface sialylation affects binding of enterovirus 71 to rhabdomyosarcoma and neuroblastoma cells. BMC Microbiol 2012; 12:162-73; PMID:22853823; http://dx.doi.org/10.1186/1471-2180-12-162
- Ren XX, Ma L, Liu QW, Li C, Huang Z, Wu L, Xiong SD, Wang JH, Wang HB. The molecule of DC-SIGN captures enterovirus 71 and confers dendritic cell-mediated viral trans-infection. Virol J 2014; 11:47-55; PMID:24620896; http://dx.doi.org/10.1186/1743-422X-11-47
- Milstone AM, Petrella J, Sanchez MD, Mahmud M, Whitbeck JC, Bergelson JM. Interaction with coxsackievirus and adenovirus receptor, but not with decay-accelerating factor (DAF), induces A-particle formation in a DAF-binding coxsackievirus B3 isolate. J Virol 2005; 79:655-60; PMID:15596863; http://dx.doi.org/10.1128/JVI.79.1.655-660.2005
- Orthopoulos G, Triantafilou K, Triantafilou M. Coxsackie B viruses use multiple receptors to infect human cardiac cells. J Med Virol 2004; 74:291-9; PMID:15332279; http://dx.doi.org/10.1002/jmv.20184
- Tsueng G, Tabor-Godwin JM, Gopal A, Ruller CM, Deline S, An N, Frausto RF, Milner R, Crocker SJ, Whitton JL, et al. Coxsackievirus preferentially replicates and induces cytopathic effect in undifferentiated neural progenitor cells. J Virol 2011; 85:5718-32; PMID:21471247; http://dx.doi.org/10.1128/JVI.02261-10
- Ylipaasto P, Eskelinen M, Salmela K, Hovi T, Roivainen M. Vitronectin receptors, alpha v integrins, are recognized by several non-RGD-containing echoviruses in a continuous laboratory cell line and also in primary human Langerhans' islets and endothelial cells. J Gen Virol 2010; 91:155-65; PMID:19776235; http://dx.doi.org/10.1099/vir.0.012450-0
- Lee JW, Yeo SG, Kang BH, Lee HK, Kim JW, Lee SH, Kim KS, Cheon DS. Echovirus 30 induced neuronal cell death through TRIO-RhoA signaling activation. PLoS One 2012; 7(5):e36656; PMID:22586486; http://dx.doi.org/10.1371/journal.pone.0036656
- Sickles GM, Mutterer M, Feorino P, Plager H. Recently classified types of Coxsackie virus, group A: behavior in tissue culture. Proc Soc Exp Biol Med 1955; 90:529-31; PMID:13273503; http://dx.doi.org/10.3181/00379727-90-22088
- Robinson CR, Doane FW, Rhodes AJ. Report of an outbreak of febrile illness with pharyngeal lesions and exanthem: Toronto, summer 1957; isolation of group A Coxsackie virus. Can Med Ass J 1958; 79:615-21; PMID:13585281
- Alsop J, Flewett TH, Foster JR. “Hand-foot-and-mouth” disease in Birmingham in 1959. Br Med J 1960; 2:1708-11; PMID:13682692; http://dx.doi.org/10.1136/bmj.2.5214.1708
- Schmidt NJ, Lennette EH, Ho HH. An apparently new enterovirus isolated from patients with disease of the central nervous system. J Infect Dis 1974; 129:304-9; PMID:4361245; http://dx.doi.org/10.1093/infdis/129.3.304
- Seiff A. Cambodia unravels cause of mystery illness. Lancet 2012; 380:206; PMID:22826834; http://dx.doi.org/10.1016/S0140-6736(12)61200-8
- Yang F, Ren L, Xiong Z, Li J, Xiao Y, Zhao R, He Y, Bu G, Zhou S, Wang J, et al. Enterovirus 71 outbreaks in the People's Republic of China in 2008. J Clin Microbiol 2009; 47:2351-2; PMID:19439545; http://dx.doi.org/10.1128/JCM.00563-09
- Xing W, Liao Q, Viboud C, Zhang J, Sun J, Wu JT, Chang Z, Liu F, Fang VJ, Zheng Y, et al. Hand, foot and mouth disease in China, 2008-12: an epidemiology study. Lancet Infect Dis 2014; 14:30-8-318; PMID:24485991; http://dx.doi.org/10.1016/S1473-3099(13)70342-6
- Li Z, Yu J, Liu L, Wei Z, Ehrlich ES, Liu G, Li J, Liu X, Wang H, Yu XF, et al. Coxsackievirus A16 infection induces neural cell and non-neural cells apoptosis in vitro. PLoS One 2014; 9(10):e111174; PMID:25350381; http://dx.doi.org/10.1371/journal.pone.0111174
- Bessaud M, Razafindratsimandresy R, Nougairède A, Joffret ML, Deshpande JM, Dubot-Pérès A, Héraud JM, de Lamballerie X, Delpeyroux F, Bailly JL. et al. Molecular comparison and evolutionary analyses of VP1 nucleotide sequences of new African human enterovirus 71 isolates reveal a wide genetic diversity. PLoS One 2014; 9(3):e90624; PMID:24598878; http://dx.doi.org/10.1371/journal.pone.0090624
- Mizuta K, Aoki Y, Matoba Y, Yahagi K, Itagaki T, Katsushima F, Katsushima Y, Ito S, Hongo S, Matsuzaki Y. Molecular epidemiology of enterovirus 71 strains isolated from children in Yamagata, Japan, between 1990 and 2013. J Med Microbiol 2014; 63:1356-62; PMID:25053796; http://dx.doi.org/10.1099/jmm.0.079699-0
- Chia MY, Chiang PS, Chung WY, Luo ST, Lee MS. Epidemiology of enterovirus 71 infections in Taiwan. Pediatr Neonatolo 2014; 55:243-9; PMID:24120535; http://dx.doi.org/10.1016/j.pedneo.2013.07.007
- Zong W, He Y, Yu S, Yang H, Xian H, Liao Y, Hu G. Molecular phylogeny of Coxsackievirus A16 in Shenzhen, China, from 2005 to 2009. J Clin Microbiol 2011; 49:1659-61; PMID:21325543; http://dx.doi.org/10.1128/JCM.00010-11
- Yang E, Zhao H, Zhang Y, Liu J, Liao Y, Wang L, Cui P, Yang L, Liu L, Dong C, et al. A comparative study of the characteristics of two Coxsackie A virus type 16 strains (genotype B). Sci China Life Sci 2012; 55:336-42; PMID:22566090; http://dx.doi.org/10.1007/s11427-012-4313-z
- Baek KA, Yeo SG, Lee BH, Park K, Song J, Yu J, Rheem I, Kim J, Hwang S, Choi Y, et al. Epidemics of enterovirus infection in Chungnam Korea, 2008-2009. Virol J 2011; 8:297-305; PMID:21668960; http://dx.doi.org/10.1186/1743-422X-8-297
- Drake JW, Charlesworth B, Charlesworth D, Cow JF. Rates of spontaneous mutations. Genetics 1998; 148:1667-86; PMID:9560386
- Huang SW, Cheng HL, Hsieh HY, Chang CL, Tsai HP, Kuo PH, Wang SM, Liu CC, Su IJ, Wang JR. Mutations in the non-structural protein region contribute to intra-genotypic evolution of enterovirus 71. J Biomed Sci 2014; 21:33-47; PMID:24766641; http://dx.doi.org/10.1186/1423-0127-21-33
- Yang F, Du J, Hu Y, Wang X, Xue Y, Dong J, Sun L, Li Z, Li Y, Sun S, et al. Enterovirus coinfection during an outbreak of hand, foot and mouth disease in Shangdong, China. Clin Infect Dis 2011; 53:400-1; PMID:21785005; http://dx.doi.org/10.1093/cid/cir346
- Zhang T, Du J, Xue Y, Su H, Yang F, Jin Q. Epidemics and frequent recombination within species in outbreaks of human enterovirus B-associated hand, foot and mouth disease in Shangdong China in 2010 and 2011. PLoS One 2013; 8(6):e67157; PMID:23840610; http://dx.doi.org/10.1371/journal.pone.0067157
- Zhao K, Han X, Wang G, Hu W, Zhang W, Yu XF. Circulating coxsackievirus A16 identified as recombinant type A human enteroviruses, China. Emerg Infect Dis 2011; 17:1537-40; PMID:21801645
- Chen X, Tan X, Li J, Jin Y, Gong L, Hong M, Shi Y, Zhu S, Zhang B, Zhang S, et al. Molecular epidemiology of coxsackievirus A16: Intratype and prevalent intertype recombination identified. PLoS One 2013; 8(12):e82861; PMID:24340064; http://dx.doi.org/10.1371/journal.pone.0082861
- Wu Z, Du J, Zhang T, Xue Y, Yang F, Jin Q. Recombinant human coxsackievirus B3 from children with acute myocarditis in China. J Clin Microbiol 2013; 51:3083-6; PMID:23804378; http://dx.doi.org/10.1128/JCM.00270-13
- Li Z, Lai S, Zhang H, Wang L, Zhou D, Liu J, Lan Y, Ma J, Yu H, Buckeridge DL, et al. Hand, foot and mouth disease in China: evaluating an automated system for detection of outbreaks. Bull World Health Organ 2014; 92:656-63; PMID:25378756; http://dx.doi.org/10.2471/BLT.13.130666
- Chen J, Fu Y, Ju L, Miao X, Shen Y, He L, Wang W, Jin J, Shao L, Sampath R, Ecker DJ, et al. Detection and identification of viral pathogens in patients with hand, foot and mouth disease by multilocus PCR, reverse-transcription PCR and electrospray ionization mass spectrometry. J Clin Virol 2014; 59:115-9; PMID:24365476; http://dx.doi.org/10.1016/j.jcv.2013.11.007
- Klein MH. EV71 vaccines: a first step towards multivalent hand, foot and mouth disease vaccines. Expert Rev Vaccines 2015; 14:337-40; PMID:25536888; http://dx.doi.org/10.1586/14760584.2015.993385
- Chong P, Hsieh SY, Liu CC, Chou AH, Chang JY, Wu SC, Liu SJ, Chow YH, Su IJ, Klein M. Production of EV71 vaccine candidates. Hum Vaccin Immunother 2012; 8:1775-83; PMID:22992566; http://dx.doi.org/10.4161/hv.21739
- Kung YA, Hung CT, Liu YC, Shih SR. Update on the development of enterovirus 71 vaccines. Expert Opin Biol Ther 2014; 14:1455-64; PMID:24989170; http://dx.doi.org/10.1517/14712598.2014.935330
- Li YX, Zhao H, Cao RY, Deng YQ, Han JF, Zhu SY, Ma J, Liu L, Qin ED, Qin CF1. Recombinant tandem multi-linear neutralizing epitopes of human enterovirus 71 elicited protective immunity in mice. Virol J 2014; 11:79-87; PMID:24885030; http://dx.doi.org/10.1186/1743-422X-11-79
- Wang M, Jiang S, Wang Y. Recombinant VP1 protein expressed in Pichia pastoris induces protective immune responses against EV71 in mice. Biochem Biophys Res Commun 2013; 430:387-93; PMID:23159634; http://dx.doi.org/10.1016/j.bbrc.2012.11.035
- Chou AH, Liu CC, Chang JY, Lien SP, Guo MS, Tasi HP, Hsiao KN, Liu SJ, Sia C, Wu SC, et al. Immunological evaluation and comparison of different EV71 vaccine candidates. Clin Dev Immunol 2012; 2012:831282
- Premanand B, Kiener TK, Meng T, Tan YR, Jia Q, Chow VT, Kwang J. Induction of protective immune responses against EV71 in mice by baculovirus encoding a novel expression cassette for capsid protein VP1. Antiviral Res 2012; 95:311-5; PMID:22691220; http://dx.doi.org/10.1016/j.antiviral.2012.05.017
- Kolpe AB, Kiener TK, Grotenbreg GM, Kwang J. Display of enterovirus 71 VP1 on baculovirus as a type II transmembrane protein elicits protective B and T cell responses in immunized mice. Virus Res 2012; 168:64-72; PMID:22728446; http://dx.doi.org/10.1016/j.virusres.2012.06.014
- Tung WS, Bakar SA, Sekawi Z, Rosli R. DNA vaccine constructs against enterovirus 71 elicit immune responses in mice. Genet Vaccines 2007; 5:6-18; PMID:17445254; http://dx.doi.org/10.1186/1479-0556-5-6
- Tian X, Su X, Li X, Li H, Li T, Zhou Z, Zhong T, Zhou R. Protection against enterovirus 71 with neutralizing epitope incorporation within adenovirus type 3 hexon. PLoS One 2012; 7(7):e41381; PMID:22848478; http://dx.doi.org/10.1371/journal.pone.0041381
- Li HY, Han JF, Qin CF, Chen R. Virus-like particles for enterovirus 71 produced from Saccharomyces cerevisiae potently elicits protective immune responses in mice. Vaccine 2013; 31:3281-7; PMID:23726823; http://dx.doi.org/10.1016/j.vaccine.2013.05.019
- Lin YL, Yu CI, Hu YC, Tsai TJ, Kuo YC, Chi WK, Lin AN, Chiang BL. Enterovirus type 71 neutralizing antibodies in the serum of macaque monkeys immunized with EV71 virus-like particles. Vaccine 2012; 30:1305-12; PMID:22214888; http://dx.doi.org/10.1016/j.vaccine.2011.12.081
- Chen HF, Chang MH, Chiang BL, Jeng ST. Oral immunization of mice using transgenic tomato fruit expressing VP1 protein from enterovirus 71. Vaccine 2006; 24:2944-51; PMID:16448730; http://dx.doi.org/10.1016/j.vaccine.2005.12.047
- Zhang F, Hao C, Zhang S, Li A, Zhang Q, Wu W, Liu L, Li C, Liang M, Li X, et al. Oral immunization with recombinant enterovirus 71 VP1 formulated with chitosan protects mice against lethal challenge. Virol J 2014; 11:80-8; PMID:24885121; http://dx.doi.org/10.1186/1743-422X-11-80
- Premanand B, Prabakaran M, Kiener TK, Kwang J. Recombinant baculovirus associated with bilosomes as an oral vaccine candidate against HEV71 infection in mice. PLoS One 2013; 8(2):e55536; PMID:23390538; http://dx.doi.org/10.1371/journal.pone.0055536
- Chiu CH, Chu C, He CC, Lin TY. Protection of neonatal mice from lethal enterovirus 71 infection by maternal immunization with attenuated Salmonella enterica serovar Typhimurium expressing VP1 of enterovirus 71. Microbes Infect 2006; 8:1671-8; PMID:16815726; http://dx.doi.org/10.1016/j.micinf.2006.01.021
- Yu Z, Huana Z, Sao C, Huang Y, Zhang F, Ma G, Chen Z, Zeng Z, Qiwen D, Zeng W. Oral immunization of mice using Bifidobacterium longum expressing VP1 protein from enterovirus 71. Arch Virol 2013; 158:1071-7; PMID:23275129; http://dx.doi.org/10.1007/s00705-012-1589-z
- Arita M, Shimizu H, Nagata N, Ami Y, Suzaki Y, Sata T, Iwasaki T, Miyamura T. Temperature-sensitive mutants of enterovirus 71 show attenuation in cynomolgus monkeys. J Gen Virol 2005; 86:1391-401; PMID:15831951; http://dx.doi.org/10.1099/vir.0.80784-0
- Meng T, Kwang J. Attenuation of human enterovirus 71 high-replication-fidelity variants in AG129 mice. J Virol 2014; 88:5803-15; PMID:24623423; http://dx.doi.org/10.1128/JVI.00289-14
- Bek EJ, Hussain KM, Phuektes P, Kok CC, Gao Q, Cai F, Gao Z, McMinn PC. Formalin-inactivated vaccine provokes cross-protective immunity in a mouse model of human enterovirus 71 infection. Vaccine 2011; 29:4829-38; PMID:21550375; http://dx.doi.org/10.1016/j.vaccine.2011.04.070
- Dong C, Liu L, Zhao H, Wang J, Liao Y, Zhang X, Na R, Liang Y, Wang L, Li Q. Immunoprotection elicited by an enterovirus type 71 experimental inactivated vaccine in mice and rhesus monkeys. Vaccine 2011; 29:6269-75; PMID:21722686; http://dx.doi.org/10.1016/j.vaccine.2011.06.044
- Cheng A, Fung CP, Liu CC, Lin YT, Tsai HY, Chang SC, Chou AH, Chang JY, Jiang RH, Hsieh YC, et al. A phase 1, randomized, open-label study to evaluate the safety and immunogenicity of enterovirus 71 vaccine. Vaccine 2013; 31:2471-6; PMID:23541623; http://dx.doi.org/10.1016/j.vaccine.2013.03.015
- Chou AH, Liu CC, Chang JY, Jiang R, Hsieh YC, Tsao A, Wu CL, Huang JL, Fung CP, Hsieh SM, et al. Formalin-inactivated EV71 vaccine candidate induced cross-neutralizing antibody responses against subgenotypes B1, B4, B5 and C4A in adult volunteers. PLoS One 2013; 8(11):e79783; PMID:24278177; http://dx.doi.org/10.1371/journal.pone.0079783
- Li JX, Mao QY, Liang ZL, Ji H, Zhu FC. Development of enterovirus 71 vaccines: from the lab bench to phase III clinical trials. Expert Rev Vaccines 2014; 13:609-18; PMID:24621093; http://dx.doi.org/10.1586/14760584.2014.897617
- Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H, Zhang YT, Yao X, Chu K, Chen QH, et al. Efficacy, safety, and immunology of an inactivated alum-adjuvanted enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2013; 38:2024-32; http://dx.doi.org/10.1016/S0140-6736(13)61049-1
- Zhu F, Xu W, Xia J, Liang Z, Liu Y, Zhang X, Tan X, Wang L, Mao Q, Wu J, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med 2014; 370:818-28; PMID:24571754; http://dx.doi.org/10.1056/NEJMoa1304923
- Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z, Zhang Y, Li Y, Mao Q, Wang J, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med 2014; 370:829-37; PMID:24571755; http://dx.doi.org/10.1056/NEJMoa1303224
- Mao Q, Cheng T, Zhu F, Li J, Wang Y, Li Y, Gao F, Yang L, Yao X, Shao J, et al. The cross-neutralizing activity of enterovirus 71 subgenotype c4 vaccines in healthy Chinese infants and children. PLoS One 2013; 8(11):e79599; PMID:24260259; http://dx.doi.org/10.1371/journal.pone.0079599
- Wang J, Qi S, Zhang X, Zhang Y, Liu L, Che Y, He Z, Zhao Y, Lu S, Yu W, et al. Coxsackievirus A16 infection does not interfere with the specific immune response induced by an enterovirus 71 inactivated vaccine in Rhesus monkeys. Vaccine 2014; 32:4436-42; PMID:24958699; http://dx.doi.org/10.1016/j.vaccine.2014.06.062
- Chen YJ, Meng FY, Mao QY, Chen YJ, Meng FY, Mao Q, Li JX, Wang H, Liang ZL, Zhang YT, et al. Clinical evaluation for batch consistency of an inactivated enterovirus 71 vaccine in a large-scale phase 3 clinical trial. Hum Vaccin Immunother 2014; 10:1366-72; PMID:24633366; http://dx.doi.org/10.4161/hv.28397
- Shi J, Huang X, Liu Q, Huang Z. Identification of conserved neutralizing linear epitopes within the VP1 protein of coxsackievirus A16. Vaccine 2013; 31:2130-6; PMID:23499595; http://dx.doi.org/10.1016/j.vaccine.2013.02.051
- Chong P, Guo MS, Lin FH, Hsiao KN, Weng SY, Chou AH, Wang JR, Hsieh SY, Su IJ, Liu CC. Immunological and biochemical characterization of coxsackie virus A16 viral particles. PLoS One 2012; 7(11):e499973; PMID:23226233; http://dx.doi.org/10.1371/journal.pone.0049973
- Cai Y, Liu Q, Huang X, Li D, Ku Z, Zhang Y, Huang Z. Active immunization with a Coxsackievirus A16 experimental inactivated vaccine induces neutralizing antibodies and protects mice against lethal infection. Vaccine 2013; 31:2215-21; PMID:23499596; http://dx.doi.org/10.1016/j.vaccine.2013.03.007
- Yang E, Cheng C, Zhang Y, Wang J, Che Y, Pu J, Dong C, Liu L, He Z, Lu S, et al. Comparative study of the immunogenicity in mice and monkeys of an inactivated CA16 vaccine made from a human diploid cell line. Hum Vaccin Immunother 2014; 10:1266-73; PMID:24583556; http://dx.doi.org/10.4161/hv.28083
- Li J, Chang J, Liu X, Yang J, Guo H, Wei W, Zhang W, Yu XF. Protection from lethal challenge in a neonatal mouse model by circulating recombinant form coxsackievirus A16 vaccine candidates. J Gen Virol 2014; 95:1083-93; PMID:24496826; http://dx.doi.org/10.1099/vir.0.063560-0
- Zhao H, Li HY, Han JF, Deng YQ, Li YX, Zhu SY, He YL, Qin ED, Chen R, Qin CF. Virus-like particles produced in Saccharomyces cerevisiae elicit protective immunity against Coxsackievirus A16 in mice. Appl Microbiol Biotechnol 2013; 97:10445-52; PMID:24085395; http://dx.doi.org/10.1007/s00253-013-5257-3
- Cai Y, Ku Z, Liu Q, Leng Q, Huang Z. A combination vaccine comprising of inactivated enterovirus 71 and coxsackievirus A16 elicits balanced protective immunity against both viruses. Vaccine 2014; 32:2406-12; PMID:24657161; http://dx.doi.org/10.1016/j.vaccine.2014.03.012
- Lin CW, Liu CC, Lu TC, Liu SJ, Chow YH, Chong P, Huang MH. Immunogenicity studies of bivalent inactivated virions of EV71/CVA16 formulated with submicron emulsion systems. Biomed Res Int 2014; 2014:670506; PMID:25006583
- Ku Z, Liu Q, Ye X, Cai Y, Wang X, Shi J, Li D, Jin X, An W, Huang Z. A virus-like particle based bivalent vaccine confers dual protection against enterovirus 71 and coxsackievirus A16 infections in mice. Vaccine 2014; 32:4296-303; PMID:24950363; http://dx.doi.org/10.1016/j.vaccine.2014.06.025
- Zhao H, Li HY, Han JF, Deng YQ, Zhu SY, Li XF, Yang HQ, Li YX, Zhang Y, Qin ED, et al. Novel recombinant chimeric virus-like particle is immunogenic and protective against both enterovirus 71 and coxsackievirus A16 in mice. Sci Rep 2015; 5:7878-85; PMID:25597595; http://dx.doi.org/10.1038/srep07878
- Sun S, Jiang L, Liang Z, Mao Q, Su W, Zhang H, Li X, Jin J, Xu L, Zhao D, et al. Evaluation of monovalent and bivalent vaccines against lethal Enterovirus 71 and Coxsackievirus A16 infection in newborn mice. Hum Vaccin Immunother 2014; 10:2885-95; PMID:25483672; http://dx.doi.org/10.4161/hv.29823
- Liu CC, Chow YH, Chong P, Klein M. Prospect and challenges for the development of multivalent vaccines against hand, foot and mouth diseases. Vaccine 2014; 32:6177-82; PMID:25218294; http://dx.doi.org/10.1016/j.vaccine.2014.08.064
- Kim DS, Nam JH. Characterization of attenuated coxsackievirus B3 strains and prospects of their application as live-attenuated vaccines. Expert Opin Biol Ther 2010; 10:179-90; PMID:20088713; http://dx.doi.org/10.1517/14712590903379502
- Fohlman J, Pauksen K, Morein B, Bjare U, Ilbäck NG, Friman G. High yield production of an inactivated coxsackie B3 adjuvant vaccines protective effect against experimental myocarditis. Scand J Infect Dis Suppl 1993; 88:103-8; PMID:8390713
- Jrad-Battikh N, Souli A, Oueslati L, Aouni M, Hober D, Gharbi J, Ben M'hadheb-Gharbi M. Neutralizing activity induced by the attenuated coxsackievirus B3 Sabin3-like strain against CVB3 infection. Curr Microbiol 2014; 68:503-9; PMID:24322405; http://dx.doi.org/10.1007/s00284-013-0498-z
- Lan J, Gao Z, Xiong H, Chuai X, Jin Y, Li J, Xian X, Liu G, Xie L, Zhang Y, et al. Generation of protective immune responses against coxsackievirus B3 challenge by DNA prime-protein boost vaccination, Vaccine 2011; 29:6894-902; PMID:21803097; http://dx.doi.org/10.1016/j.vaccine.2011.07.049
- Wang M, Yue Y, Dong C, Li X, Xu W, Xiong S. Mucosal immunization with high-mobility group box 1 in chitosan enhances DNA vaccine-induced protection against coxsackievirus B3-induced myocarditis. Clin Vaccine Immunol 2013; 20:1743-51; PMID:24027262; http://dx.doi.org/10.1128/CVI.00466-13
- Zhang L, Parham NJ, Zhang F, Aasa-Chapman M, Gould EA, Zhang H. Vaccination with coxsackievirus B3 virus-like particles elicits humoral immune responses and protects mice against myocarditis. Vaccine 2012; 30:2301-08; PMID:22306858; http://dx.doi.org/10.1016/j.vaccine.2012.01.061
- Koho T, Koivunene MR, Oikarinen S, Kummola L, Mäkinen S, Mähönen AJ, Sioofy-Khojine A, Marjomäki V, Kazmertsuk A, Junttila I, et al. Coxsackievirus B3 VLPs purified by ion exchange chromatography elicit strong immune responses in mice. Antiviral Res 2014; 104:93-101; PMID:24485896; http://dx.doi.org/10.1016/j.antiviral.2014.01.013
- Wu F, Fan X, Yue Y, Xiong S, Dong C. A vesicular stomatitis virus-based mucosal vaccine promotes dendritic cell maturation and elicits preferable immune response against coxsackievirus B3 induced viral myocarditis. Vaccine 2014; 32:3917-26; PMID:24874923; http://dx.doi.org/10.1016/j.vaccine.2014.05.052
- Han JF, Cao RY, Deng YQ, Tian X, Jiang T, Qin ED, Qin CF. Antibody-dependent enhancement of infection of enterovirus 71 in vitro and in vivo. Virol J 2011; 8:106-12; PMID:21385398; http://dx.doi.org/10.1186/1743-422X-8-106
- Wang S, Li J, Liang Z, Xiuling L, Qunying M, Fanyue M, Hua W, Yuntao Z, Fan G, Qinghua C, et al. A booster dose of an inactivated enterovirus vaccine in Chinese young children: a randomized, double-blind, placebo controlled clinical trials. J Infect Dis 2014; 210:1073-82; PMID:24625805; http://dx.doi.org/10.1093/infdis/jiu113
- Wang YF, Yu CK. Animal models for enterovirus 71 infection: application and limitations. J Biomed Sci 2014; 21:31-41; PMID:24742252; http://dx.doi.org/10.1186/1423-0127-21-31
- Mao Q, Wang Y, Gao R, Shao J, Yao X, Lang S, Wang C, Mao P, Liang Z, Wang J. A neonatal mouse model of coxsackievirus A16 for vaccine evaluation. J Virol 2012; 86:11967-76; PMID:22951825; http://dx.doi.org/10.1128/JVI.00902-12
- Lin YW, Yu SI, Shao HY, Lin HY, Liu CC, Hsiao KN, Chitra E, Tsou YL, Chang HW, Sia C, et al. Human SCARB2 transgenic mice as an infectious animal model for enterovirus 71. PLoS One 2013; 8(2):e57591; PMID:23451246; http://dx.doi.org/10.1371/journal.pone.0057591
- Fujii K, Nagata N, Sato Y, Ong KC, Wong KT, Yamayoshi S, Shimanuki M, Shitara H, Taya C, Koike S. Transgenic mouse model for the study of enterovirus 71 neuropathogenesis. Proc Natl Acad Sci USA 2013; 110:14753-8; PMID:23959904; http://dx.doi.org/10.1073/pnas.1217563110
- Chang HW, Lin YW, Ho HM, Lin MH, Liu CC, Shao HY, Chong P, Sia C, Chow YH. Protective efficacy of VP1-specific neutralizing antibody associated with a reduction of viral load and pro-inflammatory cytokines in human SCARB2-transgenic mice. PLoS One 2013; 8:269858; PMID:23936115; http://dx.doi.org/10.1371/annotation/e71a42ed-eebf-43c1-b2f5-6ea70e9d8966
- Luo ST, Chiang PS, Chao AS, Liou GY, Lin R, Lin TY, Lee MS. Enterovirus 71 maternal antibodies in infants, Taiwan. Emerg Infect Dis 2009; 15:581-4; PMID:19331737; http://dx.doi.org/10.3201/1504.081550
- Chen CW, Lee YP, Wang YF, Yu CK. Formaldehyde-inactivated human enterovirus 71 vaccine is compatible for co-immunization with a commercial pentavalent vaccine. Vaccine 2011; 29:2772-6; PMID:21315698; http://dx.doi.org/10.1016/j.vaccine.2011.01.094
- Mao Q, Wang Y, Shao J, Ying Z, Gao F, Yao X, Li C, Ye Q, Xu M, Li R, et al. The compatibility of inactivated Enterovirus 71 vaccination with Coxsackievirus A16 and Poliovirus immunizations in humans and animals. Hum Vaccin Immunother 2015; PMID:25715318; http://dx.doi.org/10.1080/21645515.2015.1011975
- Li L, Yin H, An Z, Feng Z. Considerations for developing an immunization strategy with enterovirus 71 vaccine. Vaccine 2015; 33:1107-12; PMID:25444807; http://dx.doi.org/10.1016/j.vaccine.2014.10.081
- Lee BY, Wateska AR, Bailey RR, Tai JH, Bacon KM, Smith KJ. Forecasting the economic value of an enterovirus 71 EV71 vaccine. Vaccine 2010; 28:7731-6; PMID:20923711; http://dx.doi.org/10.1016/j.vaccine.2010.09.065
- Liang Z, Wang J. EV71 vaccine, an invaluable gift for children. Clin Transl Immunol 2014; 3:e28; PMID:25505956; http://dx.doi.org/10.1038/cti.2014.24
- Mirand A, Molet L, Hassel C, Peigue-Lafeuille HC, Rozenberg F, Bailly JL, Henquell CC. Enterovirus A71 subgenotype B5, France, 2013. Emerg Infect Dis 2015; 21:707-9; PMID:25811300; http://dx.doi.org/10.3201/eid2104.141093
