Abstract
Neisseria meningitidis or meningococcus is divided into 12 distinct serogroups of which A, B, C, W, X, and Y are medically most important and cause health problems in different parts of the world. The epidemiology of N. meningitidis is unpredictable over time and across geographic regions. Globally, serogoup A has been prevalent in the African “meningitis belt” whereas serogroup B and C have predominated in Europe. In a paper published earlier in this journalCitation1, an increase in serogroup Y invasive meningococcal disease (IMD) in some European countries was reported based on the epidemiological data for 2010, 2011 and 2012. Here, we report additional data from 30 European countries indicating that high or increased serogroup Y disease levels have continued in 2013 in certain regions of Europe. In the Western and Central Europe, there were no major changes in the proportion of serogroup Y IMD cases in 2013 compared to 2012. In the Scandinavian countries, proportion of serogroup Y disease remained high, ranging from 26% to 51% in 2013. This was in contrast to Baltic, Eastern and most Southern European countries, where the proportion of serogroup Y IMD was low similarly to previous years. For the last 2 decades, the mean age of patients affected by serogroup Y was 41 y for 7 countries from which data was available and 50% of cases were in patients aged 45 to 88 y. The age distribution of serogroup Y was bimodal and did not change significantly despite the increase of the total number and the proportion of serogroup Y IMD in some European regions.
Abbreviations
| IMD | = | Invasive meningococcal disease |
| Men | = | meningococcal |
Introduction
N. meningitidis continues to cause substantial rates of illness, risk of long-term sequelae and death worldwide, and is associated with significant health costs. Six immunologically distinct serogroups of N. meni-ngitidis (A, B, C, W, X, Y) are associated with significant pathogenic potential and cause over 90% of IMD worldwide. Until recently, serogroup Y has been of minor importance in Europe, accounting for approximately 2% or less of reported IMD cases. In 2010–2012, an increase in both the absolute numbers and proportion of serogroup Y was reported in various European countries.Citation1 Here, we present epidemiological data on serogroup Y in Europe in 2013 collected by various national reference laboratories through their locally established surveillance methods.
Results
Proportion of serogroup Y IMD in Europe
In 2013, the epidemiological trend of serogroup Y IMD remained largely static across Europe. Similarly to previous years, the Scandinavian countries reported the highest proportion of serogroup Y IMD reaching over 50% in Sweden. In Eastern and Southeastern European countries, the importance of serogroup Y remained low, accounting for <5% of cases (). In the Central and Western Europe, the percentage of serogroup Y disease varied in most countries between 5 and 10%. In Scotland, a continuous increase in the proportion of serogroup Y IMD was observed during the last 4 years, reaching 17% in 2013. In Switzerland, serogroup Y has become the second most cause of IMD (19%) after serogroup B (55%), while serogroup C appeared to further decrease in 2013.
Figure 1. Absolute numbers of N. meningitis serogroup Y cases in various European countries in 2013 and proportion of serogroup Y IMD in various European countries in 2010–2013. The figure is based on data communicated by the scientists listed in the Acknowledgment or published web pages of national institutes.2 The data for 2013 is compared with data from 2010 to 2012, which has been communicated earlier.1 Color coding refers to 2013 data. Data which were not available to the authors for countries are shown in white. For IMD cases in Luxemburg, no sero-grouping has been carried out in 2013. One case of IMD reported in Romania in 2013 was either caused by serogroup W or Y and the indicated proportion of 2.7% applies if this case was caused by serogroup Y.
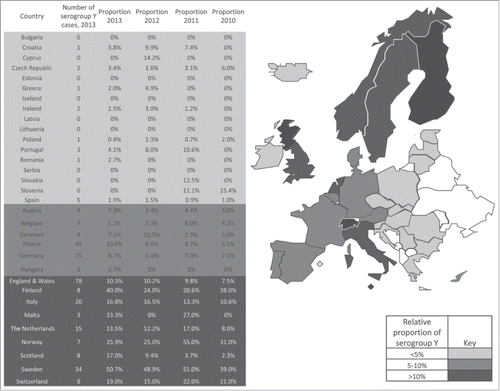
In countries with a small population and /or low IMD incidence, a few cases can cause misleading high rise in the proportion of cases. This was true for Malta, where few cases resulted in a high percentage of serogroup Y IMD in 2013 (33.3%; 3 cases) and in 2011 (27%, 3 cases).Citation3 Thus, exploring data proportions only may result in wrong conclusions.
Age of patients affected by serogroup Y across Europe
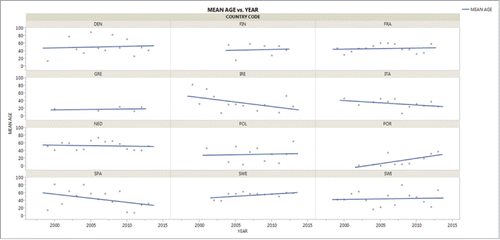
We collected age specific data for serogroup Y IMD from 12 European countries from various geographic regions and with different prevalence for serogroup Y to evaluate the mean age of serogroup Y affected patients in Europe and to study if the mean age has changed during the last 2 decades and especially during the increase of serogroup Y disease in various countries. To avoid bias, countries with very low number of cases, mostly from Eastern and Southeastern parts of the Europe were excluded from this analysis. The regression curve in shows that the majority of serogroup Y IMD cases in most countries occurred in adults. The year-to-year deviation for the mean age was rather high for most of the countries, but was lower for countries with a high number of reported cases (like Sweden and The Netherlands). There was no obvious significant change over 2 decades in the mean age of patients with IMD caused by serogroup Y and an apparent increase like in Portugal or an apparent decrease like in Spain have to be considered carefully due to the low number of IMD cases in these countries. In countries with a strong increase in serogoup Y cases during the last years (like Sweden and Finland) no change in the mean age – neither increase nor decrease – could be found. In Greece (and for certain time spans in Portugal and in Ireland), the mean age of patients seems to be lower than in the other European countries, but this may be an artifact due to the low number of cases and interpretation should be done carefully.
Figure 2. Mean age of patients affected by meningococcal serogroup Y from a variety of European countries between 2000 to 2013.
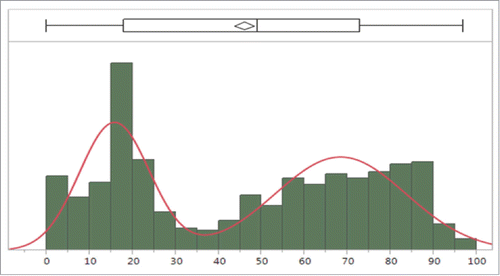
shows the age distribution of all serogroup Y affected cases (594 in total) summed up for 7 countries (Denmark, Greece, Italy, The Netherlands, Poland, Sweden, Switzerland) for a period of 20 y. 50% of all cases occurred in 45–88 year-olds (inner quartile range in ). However, there is a second peak of serogroup Y IMD cases in adolescents/young adults with most cases having been reported between 15 and 20 y of age. The overall mean age for these 7 countries was 41 y (see rhombus in ) for the last 20 y and the median age was 49 y (see vertical line right to the rhombus).
Figure 3. Outlier Box Plot to see the age distribution of serogroup Y affected patients based on 7 European countries (see text). A line through the middle of the diamond would show the mean age, the median age by the vertical line right to the diamond. The inner quartile range representing the 25th and 75th quantiles is indicated by the horizontal box.
European isolates of serogoup Y N. meningitidis were heterogeneous and belonged to several clonal complexes (cc). However, in Greece, the isolates mostly belonged to the clonal complex cc23 and showed the genotypes Y:P1.5–1 (or 5–2,10–4:F4–1:cc23). If only the cc23 isolates considered in other countries, the median age of cases due to MenY/cc23 isolates was 23.3 and 25.6 in 2012 and 2013 in France for example, respectively. These were most frequently of the genotypes Y:P1.5–2,10–1:F4–1(or F5–12):cc23.
Discussion
The increase of MenY disease during the last years has important public health implications and in some European countries requires further close monitoring. Most European countries have recommendations for the use of quadrivalent meningococcal ACWY conjugate vaccine for high or increased risk groups and for travelers. In many European countries, clinical recommendations to use quadrivalent meningococcal conjugate vaccine have been introduced in addition or to replace the monovalent MenC conjugate vaccine as it offers broader protection against more than one meningococcal serogroup. In early 2015, such recommendations are existing in Austria, Czech Republic, Greece, Italy (several regions) Poland, Saxony (federal member state of Germany) and Switzerland, (in alphabetical order). In France, a quadrivalent meningococcal conjugate vaccine was recently recommended for subjects 5–24 y due to shortage in the monovalent MenC conjugate vaccine.Citation4
The proportion of serogroup Y IMD listed in was based on the number of isolates for which sero-grouping has been carried out, representing the vast majority but not all the IMD cases, as some are diagnosed solely by PCR, but not for the total number of IMD cases. Thus, uncertainties remain about the exact proportion. This was e.g. the case for Italy, where 172 IMD cases were reported in 2013, but of which only 117 isolates have been sero-grouped. If the serogroup distribution among those isolates which have not been sero-grouped was significantly different from those which have been sero-grouped, this may have an impact on the proportion of serogroup Y.
Meningococcal epidemiology is unpredictable and incompletely understood and the incidence of IMD is highly variable around the world. It is thought that disease outbreaks occur when new virulent meningococcal linages, the so-called “hyperinvasive lineages,” spread into susceptible human host populations although the details of these interactions are yet to be resolved. Different serogroups have dominated in different parts of the world during different periods. In the present and previous studies, we followed the emergence of serogroup Y IMD in Europe during the past 4 y. Serogroup Y has also been recognized as an agent of pneumonia (not only colonizer of the respiratory tract), but these infections are not included yet in the definition of IMD unless the bacterium spreads to bloodstream. Reported cases of serogroup Y IMD for 1998/99 most often occurred among adults and elderly people.Citation5 For the present study, we collected data on age distribution from a number of European countries from the last 2 decades and examined if changes have occurred over this period of time. In most countries, this was not the case and the mean age remained high. The mean age of serogroup Y cases was independent over time from the total number of IMD cases in the countries and from the total number and the proportion of serogroup Y cases. In Scandinavian countries where a significant increase in both the absolute numbers and the proportion of serogroup Y cases have been reported during the past years, the mean age of patients and the age distribution was similar compared to countries with a low or medium number of cases and/or a low proportion of serogroup Y IMD. Greece was an exception with a mean age much lower compared to other countries. In some other countries like Ireland, Portugal and Spain, values with lower mean age were calculated for some years. However, in these countries, the total number of serogroup Y cases was low and partially gaps were present (no cases reported in certain years) and thus, this data should be interpreted very carefully. There was no clear association in the mean age of patients with certain geographic regions within Europe and no correlation of the mean age for serogroup Y affected patients could be drawn with serogroup C conjugate vaccine uptake during the last 20 y. A high mean age was seen in Central Europe (e.g., Switzerland) which has high vaccine uptake, but also in Scandinavia, where the use of MenC conjugate vaccine has been low.
Interestingly, cc23 isolates were most common among European serogoup Y and were responsible for an increase in cases of meningococcal disease in Maryland, USA since 1990s. Moreover, shifts in major outer membrane proteins such as PorA and FetA with these cc23 isolates were associated with changes of age distribution of cases.Citation6,7 These observations underline the importance of genotyping data when analyzing changing epidemiology of meningococcal disease. The emergence of new clones of serogoup Y in adolescents and young adults may have direct impacts in tailoring vaccination strategies.
In previous studies, the highest proportion of serogroup B cases have been reported to occur in the 1–4 y olds with a small peak in those aged 15–19 years, while the age distribution for serogroup C IMD was more strikingly bimodal with about a quarter of cases occurring in 1–4 y olds and a quarter in 15–19 y olds in the pre-MenC conjugate vaccination era.Citation8 In contrast to these 2 serogroups which have historically accounted for at least 2 thirds of IMD cases in Europe, more than 40% of serogroup Y cases were reported to occur among patients aged 45 y or older, compared with only 11% of serogroup B or C cases in this age group.Citation8 This different age distribution has been detected also in other geographic regions. In Massachusetts, USA, serogroup Y became increasingly predominant in IMD cases in those aged ≥60 years during the past 2 decades, accounting for over 50% of all sero-grouped isolates in that age group after 1995.Citation9
Serogroup W incidence has historically been very low in Europe accounting for only 1–2% of IMD cases each year with the exception of Hajj-related imported cases in 2000 to 2002. IMD caused by serogroup W has increased in England & Wales across all age groups since 2009/10 due to rapid endemic expansion of a single clone belonging to the ST-11 complex (cc11). Notably, in 2013/14, MenW was responsible for 15% of all IMD cases in England & Wales and 12% of patients died.Citation10 The Joint Committee on Vaccination and Immunisation suggested a replacement of the monovalent MenC vaccine with a quadrivalent MenACWY vaccine in the adolescent program, because this should protect children and adults across the population.Citation11 If implemented, such a program would also have an impact on the relatively high number of MenY IMD cases in England & Wales and in Scotland. The JCVI also statedCitation11 that the current MenW clone circulating in the UK is likely to be covered by the MenB vaccine 4CMenBCitation12 when tested pooled serum from vaccinated infants and adolescents against a representative collection of UK MenW strains. Sera from subjects vaccinated with 4CMenB also showed serum bactericidal activity against most of serogroup Y strains tested and isolated from Brazil and European countries.Citation13 However, it is too early to make final conclusions for the use of 4CMenB in order to protect against MenY IMD, because the strain coverage has to be analyzed for each country and for each age group to be vaccinated. This also applies for the second protein-based MenB vaccineCitation14 which is in clinical development and has recently been approved in the US for use in 10–25 y old individuals, but not yet in Europe. Recent development of serogroup B meningococcal vaccines highlights the importance of pharyngeal carriage data, particularly in adolescents and young adults to inform implementation strategies. A study on the carriage prevalence in high risk populations showed that serogroups B and Y were most common in the UK, with carriage up to 6.5% and 5.5% respectively, increasing throughout adolescenceCitation15 and suggesting that immunization to help protect against serogroup Y IMD would be best placed in the early teenage years or before to decrease transmission.
Disclosure of Potential Conflicts of Interest
M.B. and A.P. are employees of Novartis Vaccines and Diagnostics GmbH (NVD), Marburg, Germany, a manufacturer of various meningococcal vaccines. NVD has recently been acquired by GlaxoSmithKline, but the company name NVD will remain for a couple of months. A.S has received assistance to attend scientific meetings and honoraria for lecturing (Baxter, GlaxoSmithKline, Novartis, and Pfizer) and her laboratory has received research funding from GlaxoSmithKline, Novartis, and Pfizer. DP has received support to attend scientific meetings and honoraria for lecturing from GlaxoS-mithKline. M-K.T has acted as a consultant for and received travel support from GlaxoSmithKline, Novartis, and Pfizer, and has undertaken contract research on behalf of the Institut Pasteur, Paris, France, for Novartis, Pfizer, and Sanofi Pasteur. The other authors have no relevant conflict of interest to declare concerning this work. The authors alone are responsible for the views expressed in this publication and do not necessarily represent the decisions, policy, or views of the institutes or companies.
Authors' Contributions
M.B. drafted the outline of the manuscript. All authors were actively involved in reviewing the content and editing the text of the manuscript. All authors read and approved the final version of the manuscript.
Appeal
Readers of this article who can contribute with data regarding European countries which are not listed in are kindly requested to contact M. B.
Acknowledgments
The authors are grateful to many colleagues for support, especially for sharing data, which has not yet been published or has been published in national languages and for the procurement of country-specific disease data.
Data and information were kindly provided (in alphabetical order) with datum specified by: Ioana Alina Anca, Institute for Mother and Child Care “Prof. Dr. Alfred Rusescu” Bucharest, Romania (26. 2. 2015); Lyubomira Bizeva, National Reference Center for Pathogenic Cocci and Diphtheria, Sofia, Bulgaria (18. 11. 2014); Hans Blystad, Norwegian Institut of Public Health, Department of Infectious Disease Epidemiology, Oslo, Norway (24. 10. 2014); Suzana Bukovski, University Hospital for Infectious Diseases, “Dr. Fran Mihaljević”, Zagreb, Croatia (28. 8. 2014); Rosa Cano Portero, Centro Nacional de Epidemiología, Madrid, Spain (11. 10. 2014); Suzanne Cotter, HSE Health Protection Surveillance Center, Dublin, Ireland (13. 11. 2014); Davor Culic, Reference Laboratory for Meningococcus and Haemophilus, Sombor, Serbia (15. 9. 2014); Stéphane Emonet, Switzerland; Hôpitaux Universitaires de Genéve, Services des maladies infectieuses, Genéve, Switzerland (18. 8. 2014); Cecilia Fazio, Istituto Superiore di Sanità, Department of Infectious, Parasitic & Immune-Mediated Diseases, Rome, Italy (10. 11. 2014); Greta Gargasienė, Center for Communicable Diseases and AIDS, Vilnius, Lithuania (11. 8. 2014); Dagmar Gavacova, Public Health Authority of Slovak Republic, Department of Medical Microbiology, Slovak Republic (10. 11. 2014); Thorolfur Gudnason, Center for Health Security and Communicable Disease Control, Directorate of Health, Reykjavik, Iceland (24. 10. 2014); Steen Hoffmann, Statens Serum Institut, Neisseria and Streptococcus Reference Laboratory, København, Denmark (27. 10. 2014); Susanne Jacobsson, Örebro Universit Hospital, Örebro, Sweden (24. 10. 2014); Maria Koliou, Ministry of Health; Unit for Surveillance and Control of Communicable Diseases; Nikosia, Cyprus (24. 10. 2014); Paula Kriz, National Institute for Public Health, Prague, Czech Republic (29. 7. 2014); David Pace, Department of Pediatrics, Mater Dei Hospital, Msida, Malta (14. 11. 2014); Metka Paragi, National Laboratory of Health, Environment and Foodstuffs, Department of Public Health Microbiology, Ljubljana, Slovenia (27. 10. 2014); Rita Peetso, Central Laboratory for Communicable Diseases. Tallinn, Estonia (29. 10. 2014); Larisa Savrasova, Center for Disease Prevention and Control, Department of Infectious Diseases, Risk Analysis and Prevention, Riga, Latvia (28. 11. 2014); Gérard Scheiden, General Directorate of Health, Luxemburg, Luxemburg (12. 8. 2014); Maria João Simões, Instituto Nacional de Saúde Dr. Ricardo Jorge, Lisboa, Portugal (23. 1. 2015); Anna Skoczynska, National Reference Center for Bacterial Meningitis, National Medicines Institute, Warsaw, Poland (26. 8. 2014); Alison Smith-Palmer, Health Protection Scotland, Glasgow, Scotland (29. 7. 2014); Paola Stefanelli, Istituto Superiore di Sanità, Department of Infectious, Parasitic & Immune-mediated Diseases, Rome, Italy (10. 11. 2014); Muhamed-Kheir Taha, Pasteur Institute, Paris, France (30. 10. 2014); Maija Toropainen, National Institute for Health and Welfare, Helsinki, Finland (30. 7. 2014); Georgina Tzanakaki, National School of Public Health, Athens, Greece (29. 10. 2014); Arie van der Ende, Reference Laboratory for Bacterial Meningitis, Department of Medical Microbiology, Amsterdam, The Netherlands (10. 11. 2014); Zsófia Mészner, National Institute of Child Health, Budapest, Hungary (18. 2. 2015).
References
- Bröker M, Bukovski S, Culic D, Jacobsson S, Kolou M, Kuusi, M, Simões MJ, Skozynska Toropainen M, Taha M-K, Tzanakaki G. Meningococcal serogoup Y emergence in Europe. High importance in some European regions in 2012. Hum Vacc Immunother 2014; 10:1725-28; PMID:24608912; http://dx.doi.org/10.4161/hv.28206
- Web pages of national public institutes are not listed here, because many of them are in national languages, but not published in English. Readers interested in the appropriate documents or links are requested to approach the corresponding author
- Bröker M, Jacobsson S, Kuusi M, Pace D, Simões MJ, Skoczynska A, Taha M-K, Toropainen M, Tzanakaki G. Meningococcal serogoup Y in Europe. Update 2011. Hum Vacc Immunother 2012; 8:1907-11; PMID:23032167; http://dx.doi.org/10.4161/hv.21794
- Haut Conseil de la Santé Publique, Jan. 20, 2015. Vaccination contre les infections invasives à méningocoques de sérogroupe C : recommendations du Haut Conseil de la Santé Publique en situation de pénurie de vaccins – Point d'information. http://www.ansm.sante.fr/S-informer/Actualite
- European Centre for Disease Prevention and Control (ECDC). Surveillance of invasive bacterial diseases in Europe 2008/2009. Stockholm, www.ecdc.europa.eu, 2011
- Krauland MG, Dunning Hotopp JC, Riley DR, Daugherty SC, Marsh JW, Messonnier NE, Mayer LW, Tettelin H, Harrison LH. Whole genome sequencing to investigate the emergence of clonal complex 23 Neisseria meningitidis serogroup Y disease in the United States. PLoS One 2012; 7:e35699; PMID:22558202; http://dx.doi.org/10.1371/journal.pone.0035699
- McEllistrem MC, Kolano JA, Pass MA, Caugant DA, Mendelsohn AB, Pacheco AGF, Shutt KA, Razeq J, Harrison LH, and the Maryland Emerging Infections Program. Correlating epidemiologic trends with the genotypes causing meningococcal disease, Maryland. Emerg Infect Dis 2004; 10: 451-56; PMID:15109412
- Noah N, Henderson B. Surveillance of bacterial meningitis in Europe 1998/99. Communicable Disease Surveillance Centre. Europ Bact Mening Surveil Project www.phls.co.uk, 2001
- Peruski AH, Kludt P, Patel RS, DeMaria A. Secular trends in invasive meningococcal disease, Massachusetts, 1998–2011: what happened to invasive disease?. Epidemiol Infect 2014; 142: 2483-90; PMID:25372225; http://dx.doi.org/10.1017/S0950268814000259
- Ladhani SN, Beebeejaum K, Lucidarme J, Campbell H, Gray S, Kaczmarski E, Ramsay ME, Borrow R. Increase in endemic Neisseria meningitidis capsular group W ST-11 complex associated with severe invasive disease in England and Wales. Clin Infect Dis 2015; 60:578-85; PMID:25389259
- Joint Committee on Vaccination and Immunisation. Minutes of the meeting on 4 February 2015. https://app.box.com/s/iddfb4ppwkmtjusir2tc#/s/iddfb4 ppwkmtjusir2tc/1/2199012147/27417264008/1?&_suid=142674966595006959824307533589
- Vernikos G, Medini D. Bexsero© chronicle. Pathog Glob Health 2014; 108: 305-11; PMID:25417906; http://dx.doi.org/10.1179/2047773214Y.0000000162
- Tomei S, Biolchi A, Brunelli B, De Angelis G, Moschioni M, Masignani V, Budroni S, Claus H, Vogel H, Taha M-K, et al. Potential coverage of the Bexsero MenB vaccine on non-B meningococci. XIXth Intl Pathogenic Neisseria Conference (IPNC) October 12-17, 2014, poster no. 30.
- Richmond P, Marsahll H, Nissen M, Jiang Q, Jansen K, Garcés-Sánchez M. Safety, immunogenicity, and tolerability of meningococcal serogroup B bivalent recombinant lipoprotein 2086 vaccine in healthy adolescents. A randomized, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 2012; 12:597-607; PMID:22569484; http://dx.doi.org/10.1016/S1473-3099(12)70087-7
- Jeppesen CA, Snape MD, Robinson H, Gossger N, John TM, Voysey M, Ladhani S, Okike IO, Oeser C, Kent A et al. Meningococcal carriage in adolescents in the United Kingdom to inform timing of an adolescent vaccination strategy. J Infect 2015; 71:43-54; PMID:25709085; http://dx.doi.org/10.1016/j.jinf.2015.02.006
