Abstract
Vaccination directly protects vaccinated individuals, but it also has the potential for indirectly protecting the unvaccinated in a population (herd protection). Unintended negative consequences such as the re-manifestation of infection, mainly expressed as age shifts, result from vaccination programs as well. We discuss the necessary conditions for achieving optimal herd protection (i.e., high quality vaccine-induced immunity, substantial effect on the force of infection, and appropriate vaccine coverage and distribution), as well as the conditions under which age shifts are likely to occur. We show examples to illustrate these effects. Substantial ambiguity in observing and quantifying these indirect vaccine effects makes accurate evaluation troublesome even though the nature of these outcomes may be critical for accurate assessment of the economic value when decision makers are evaluating a novel vaccine for introduction into a particular region or population group. More investigation is needed to identify and develop successful assessment methodologies for precisely analyzing these outcomes.
Abbreviations
| Hib | = | Haemophilus influenza serotype b |
| HPT | = | herd protection threshold |
| HPV | = | Human Papillomavirus |
| ICER | = | incremental cost-effectiveness ratio |
| PCV | = | pneumococcal conjugate vaccine |
| QALY's | = | Quality Adjusted Life Years |
| Rn | = | effective reproduction number |
| R0 | = | reproduction number |
| RVGE | = | rotavirus gastroenteritis |
| S | = | susceptible population |
| SIR | = | Susceptible-Infected-Recovery |
| USA | = | United States of America |
Introduction
Vaccination is a well-recognized way of protecting a population against communicable infections.Citation1,2 Evaluating the total epidemiologic impact vaccination is making on a population is complex. It varies depending on the distinguishing traits of the pathogen, the method of transmission, the characteristics of the vaccine and the target population, and the mixing patterns of social contacts. It is further complicated by the potential of indirect effects, which include additional protection of unvaccinated persons in the population (herd protection) and/or negative effects such as a reappearance of infection that may be manifested under certain conditions.
Therefore, vaccination not only provides direct individual protection, it also provides indirect population effects. Both are assessed (qualified and quantified) with real-life data from retrospective and from well-designed prospective studies, or through modeling exercises.Citation3-10 The objective of this article is to examine these indirect effects of vaccination, to discuss how they are manifested, observed, and measured, and under which conditions they may maximally appear. We report examples from the literature for several different types of infections as illustrations of these effects. Our approach is to stay at the level of epidemiological assessment and avoid moving in the direction of immunological explanations.
But first, we start by explaining the basic concepts involved in the transmission of a pathogen and how it is impacted when a new vaccine is introduced as this helps clarify when and how the indirect effects of a vaccine may occur.
Pathogen transmission
The risk of contracting an infection caused by a pathogen is related to 3 factors: the number of infected subjects in a population who are able to transmit the pathogen; the amount and type of contact between the ones who transmit and the ones who receive the pathogen; and the infectiousness of the pathogen. The latter shows the ease with which a pathogen is transmitted when there is contact between an infectious and a susceptible individual. It is reflected in the speed of an epidemiological disease outbreak.Citation9,11-14
The rate at which susceptible subjects become infected is called the force of infection.Citation9,12,15 It is the expression of the number of infectious subjects (the transmitters of the pathogen) multiplied by a factor that characterizes the effective contact between persons whereby the pathogen is transmitted. That factor is broken down into specific variables, the most important of which is the basic reproduction number (R0). It describes the average number of successful transmissions generated by one infectious individual in a fully susceptible population.Citation13-16
R0 is unique to every type of infection and to the population density of a region.Citation13,15 The higher the R0, the more likely the spread of the pathogen to susceptible subjects.Citation12,13,15 For example, an R0 of 5 means that in a completely susceptible population, 1 infectious case generates 5 other cases. Each of those newly infected cases will generate 5 subsequent cases, and so on.Citation13,14 In reality, the calculation of R0 could only occur after the first infection because only then is the population fully susceptible. When a pathogen enters a population, some individuals in the population become infected and then protected against infection, interrupting the chain of transmission. A pathogen may produce a sub-optimal immune response in some individuals such as immune-compromised persons, Citation17,18 leaving them at higher risk. But once a pathogen has entered a population, the number of susceptible people decreases as the number of infected individuals increases.
Thus, the potential for the spread of a particular infection (the R0) is usually higher than in real-life situations. The actual rate of transmission, the effective reproduction number (Rn), will be lower. Rn is calculated by multiplying R0 by the fraction of the population that is still susceptible at the time Rn is measured.Citation12,14,16 An Rn of 1 is the threshold for invasion of a pathogen into a given population. If Rn is 1, transmission of the pathogen is in equilibrium and we say that the infection process is dynamically stable: it will neither disappear nor will it cause an epidemic even though it will remain endemic.Citation13,15,19 If Rn is <1, the rate of new infections decreases, enabling a build-up of susceptible persons (e.g. by birth).Citation9,12-14,19 When there is an exceptionally low number of susceptible people, it is likely that the infection may disappear because disease transmission is not sustained.Citation9,12-14,19 If Rn is >1, the incidence rate will increase, leading to a new epidemic and a subsequent decline in susceptible subjects.Citation12-14,19 Thus, in a dynamic population Rn changes with time and may lead to cyclic changes in rates of infection or fluctuations in epidemics.
Indirect Effects of Vaccination Programs
Positive indirect effects
What happens when a vaccine is introduced into a population? In the short-term, the number of infections will decline among vaccinated subjects because these individuals will mount an immune response against the antigen to protect themselves (=direct protection). At the same time, the force of infection is also impacted because the vaccine reduces the number of people who are infectious. As a consequence, there is potential for an indirect benefit to be gained through a reduced risk of exposure to the infectious agent or pathogen across the whole population. Vaccination reduces the pool of individuals capable of transmitting the pathogen. Therefore, unvaccinated persons will also benefit from the fact that they are members of the “herd,” producing what is known as herd protection or an indirect benefit to the community.
The benefit of a new vaccine in a community is larger than what is normally expected based on its actual known efficacy.Citation1,2,7-10,12,14-16,20-26 That process of extra or indirect benefit is heavily influenced by a number of specific factors which we will define next. Many studies examining different types of communicable infections (e.g., varicella, polio, rubella, measles, mumps, and diphtheria) have demonstrated herd protection.Citation1,21,23-25,27-47, Citation14,21, Citation23-25, Citation31-46, Citation48-51
But the most spectacular herd protection effects are observed among those normally not considered for vaccination but who have a high potential for being infected by the transmitters of the pathogen. A good example is the effect some pediatric vaccines have on reducing the transmission of pathogens from children/infants to the elderly. In the United Kingdom, the routine use of the pneumococcal conjugate vaccine (PCV7) among infants up to age 2 was shown to reduce the incidence of vaccine-type invasive pneumococcal disease by 81% in adults who had not been vaccinated (≥65 y old).Citation37 Herd protection is also observed (although to a lesser extent) among those at high-risk of infection when the optimal vaccine coverage level is not reached.
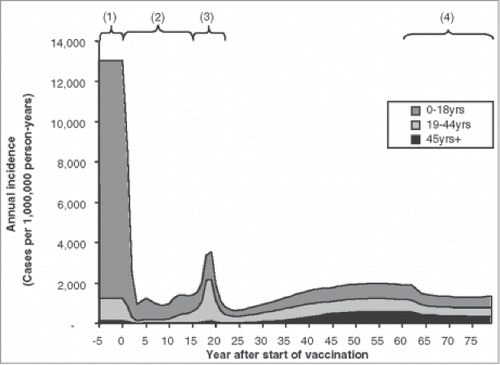
As already mentioned, the transmission of a pathogen is a dynamic process that needs to reach a new equilibrium over time when a vaccine is introduced. The following phases in relation to population-level vaccination have been identified (see ):Citation2,4,9
Pre-vaccination phase (1): the spread of the infection is in equilibrium within the population.
Honeymoon phase (2): at high vaccine coverage levels, the number of susceptible subjects falls to such a low level that sustained endemic transmission is no longer possible (Rn < 1).
Post-honeymoon epidemic (3): the low incidence rate of infection allows susceptibles to accumulate slowly over time until the introduction of an infected individual into this infection-naïve group triggers a new epidemic (Rn > 1).
New equilibrium (4): infection settles back into a new equilibrium with a lower incidence of infection than before vaccination, depending on the characteristics of the vaccine and the disease in question.
Figure 1. Modeled phases of varicella infection after vaccination (used with permission from Brisson et al.Citation2) Brisson M, Edmunds WJ. Med Decis Making, 23(1), pp. 76–82, copyright ©2003 by (SAGE Publications). Reprinted by Permission of SAGE Publications. (1) Pre-vaccination phase; (2) Honeymoon phase (Rn < 1); (3) Post-honeymoon epidemic (Rn > 1); (4) New equilibrium.
Terminology
Another term for herd protection that is frequently used in the literature is herd immunity. This term may cause some confusion although it has been used since 1923.Citation52,53 The problem is that it implies an actual immune response in unvaccinated individuals through exposure to live, attenuated pathogens in the vaccine as they come in contact with vaccinated persons. Some people like using this term because it refers to the secondary protection of unvaccinated individuals due to the immunity of vaccinated persons in the population. Vaccines that truly induce an immune response in unvaccinated persons are rare and not well-documented.
To avoid confusion as to what causes what at the level of immunity in the population, we prefer to use the term herd protection. This is a more general term for the indirect, vaccine-induced benefit to unvaccinated individuals.Citation52 Another term used less frequently in published literature is marginal externality. This is the difference between the marginal individual (direct) benefit and the marginal social benefit (i.e., the total number of illnesses prevented by vaccination).Citation5
When is Herd Protection Observed?
Disease transmission processes have important implications for vaccination programmes as they facilitate or limit the transmission of the pathogen. What we know about herd protection is that a certain level of vaccination coverage in a population must be reached before it manifests itself. This essential level of coverage is what we call the herd protection threshold (HPT). The prevalence of immune individuals in the population must be higher than this threshold in order to attenuate the spread of infection at the population level and produce herd protection.Citation6,9,Citation13,14,Citation19 This essential level of coverage is represented by the following formula: ≥ (1 – s). We can illustrate this calculation in a situation where 20% of the population is susceptible: HPT ≥ (1 – 0.20). In this particular disease situation, ≥80% of the population must be immune (through infection and recovery or through vaccination) to obtain herd protection.
Each type of infection will necessarily have a different HPT, which provides a valuable target for immunisation programmes and influences the critical minimum level of vaccine coverage.Citation6,9, Citation13,14, Citation19 An important assumption is that susceptible and infectious persons mix homogeneously across all relevant sub-groups and across different seasons, which is not always the case in reality. reports the R0 and the HPT for various communicable diseases. These vary by region as well as by the characteristics of a given population and its mixing patterns. In the next sections, we discuss 3 main factors that interact most in obtaining optimal herd protection.
Table 1. Basic reproduction numbers and implied crude HPT for various communicable diseasesCitation13,19
High and maintained vaccine effectiveness
Good vaccine effectiveness is crucial in producing a positive indirect effect or good herd protection from a vaccination program.Citation2 Vaccine effectiveness is the real-life measurement of a vaccine's ability to protect against infection. This is different from vaccine efficacy, which is the capacity of a vaccine to provide protection in a controlled environment like clinical trials.Citation14 Vaccine effectiveness will vary between regions and different (sub)populations,Citation6 and should therefore be taken into account when evaluating the positive indirect effect of a vaccine on a given population. Since vaccines are almost never 100% effective, the critical vaccination coverage level required to protect the population must necessarily increase.Citation6,14
In addition, not all vaccines elicit lifelong, protective immunity (e.g. pertussis, measles, mumps). The waning of immunity reduces the long-term effectiveness and the consequential herd protection benefit.Citation14,54-56 But in such cases, immunity may be augmented by increasing vaccination coverage, and may be resupplied by vaccination boosting or by regular, natural exposure to infection.Citation14,55
Transmission potential decreased with vaccination
A vaccine must substantially reduce the force of infection (the transmission potential of the circulating pathogen) in order to induce herd protection.Citation2,5,Citation10,12,Citation14,15,Citation22,25,Citation61 This occurs when the whole population is at-risk for the infection and contact between infected and susceptible individuals is sufficiently direct and intense. As noted above, the rate at which infection is spread is crucial in understanding the transmission potential of a pathogen: when this rate is very high with a high R0, then vaccination must achieve a correspondingly high uptake in order to assure a decrease in the transmission potential.
This automatically assumes infections in which the reservoir of the pathogen remains within the human species and is communicable (i.e., spread mainly from person-to-person and is not due to contaminated food or water as in hepatitis A).Citation8-10,Citation12,19,Citation22, 25 For example, vaccination for rabies and tetanus are unlikely to produce herd protection because humans are not the primary mode of pathogen transmission. In addition, the different modes of contact (air-borne, food-born, oral, skin or sexual) heavily impact the transmissibility of an infectious agent (e.g. the herd protection of vaccination on a sexually transmitted infection is completely different from a food-borne disease).
To achieve good herd protection, vaccination needs to target the correct reservoir of infection, or the core transmitter of the circulating pathogen.Citation2,9,Citation10,12,Citation15,19,Citation22,25,Citation27-30,Citation51,62,Citation63 For example, a study of hepatitis A vaccination among Israeli toddlers 18–24 months of age resulted in a 95% reduction of infection in all other age groups (ages <1 and ages 5 to >65), even though these toddlers represented <3% of the total population.Citation51
Conversely, if vaccine coverage is low among the main reservoir of infection, then herd protection is compromised even in the presence of an overall high coverage level since the primary group responsible for transmitting the pathogen is not blocked. Modeling scenarios suggest that limited herd protection will be seen against human papillomavirus (HPV) infection if the vaccine coverage among highly sexually active females is low, despite a much higher coverage (>70%) in the general population.Citation54
Appropriate vaccine uptake
Herd protection is highly impacted by vaccination coverage, distribution patterns, and timing.Citation2,13,Citation14,25,Citation26,64,Citation65 We address each of these factors in the following sections.
Coverage levels
Herd protection is best achieved when vaccination coverage is at the higher end.Citation54,66 Extremes of coverage (i.e., no one/very few are vaccinated or almost everyone is vaccinated) will not produce sizable herd protection.Citation2,5,Citation12,66 It should be noted that the coverage levels needed for achieving disease control are not the same as those needed for disease elimination. The latter might be of particular interest to governmental or healthcare authorities in certain situations.
Thus, when very few individuals are immunized, endemic equilibrium is not perturbed by removing a few potentially infectious individuals from a largely susceptible population.Citation5,12 Low coverage levels not only attenuate herd protection, but produce unintended negative consequences that for some diseases could lead to more harm than good.Citation10 For example, a large outbreak of congenital rubella in Greece during 1993 was traced to inconsistent immunization policies resulting in low vaccine coverage rates (<50%).Citation67 In the United States, a major resurgence of measles occurred between 1989 and 1990 among unvaccinated preschool-aged children of ethnic minority groups. The epidemic numbers were at least partly attributable to low coverage rates in a number of cities throughout the early to mid-1980s.Citation68
The other extreme (everyone or almost everyone vaccinated), while it might be useful when disease elimination is the goal, will not produce significant herd protection because it leaves practically no one in the cohort to infect (i.e., the protective benefits are primarily/only to the vaccinated).Citation12,68 Bogaards et al.Citation68 demonstrated through modeling that vaccinating 12-year old girls against HPV at higher coverage rates decreased the positive indirect effects: the percentage of indirectly averted cervical cancer cases decreased from approximately 25% at coverage rates of 50–70% to 10% at coverage levels of 90%.
Distribution
Appropriate distribution patterns, especially targeting the reservoir of infection, are also essential to achieving good herd protection. This is generally more probable when unvaccinated individuals are distributed evenly or at random.Citation14,53,Citation69 In situations where unvaccinated individuals are more likely to be in contact with other unvaccinated individuals than would be expected by chance, clusters or pockets of susceptible individuals may appear.Citation11,14,Citation69-71 A vaccination program that fails to reduce the number of susceptible individuals in these key sub-groups would not be able to produce substantial indirect effects despite a generally high proportion of immune people.Citation19
Factors that play a role in this phenomenon are geographical restrictions (e.g., boarding schools, barracks, prisons)Citation11,14,Citation69-71 or social developments such as “opinion formation,” where individuals with a negative opinion about vaccination are more likely to be in contact with individuals sharing the same opinion (e.g. certain religious groups of tightly-knit communities).Citation69
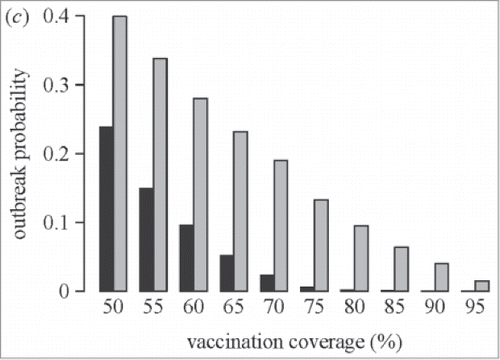
Salathe et al.Citation69 modeled how a simple opinion formation process leads to clusters of unvaccinated individuals, reducing the herd protection and leading to an increase in the probability of a measles outbreak (see ). The effect of clustering on outbreak probabilities was strongest when vaccination coverage was close to the level required to provide herd protection under the assumption of random mixing (i.e., 70% coverage). Thus, while disease outbreaks did not occur in the absence of opinion formation at coverage levels of 90%, opinion formation led to an outbreak frequency that would be expected in a homogeneously vaccinated population at coverage levels of 70%.Citation69
Figure 2. Effect of “clustering” on the outbreak probability of measles (used with permission from Salathe et al.)Citation69 Salathe M, Bonhoeffer S., J R Soc Interface, 5(29), pp. 1505–8, copyright ©2008 by (The Royal Society Publishing). Reprinted by Permission of The Royal Society PublishingBlack bars = probability of measles outbreak without opinion formation Gray bars = probability of measles outbreak with opinion formation.
Clustering leaves certain subpopulations with a higher degree of susceptibility in which infections will spread and cause local outbreaks.Citation11,69,Citation71,72 Some researchers have proposed that this phenomenon may help explain why some countries (e.g., Switzerland) continue to experience relatively large measles outbreaks despite high vaccination coverage levels.Citation69
Results from a study of a measles outbreak in Canada (2007) suggested that minimal changes in the level of aggregation of unvaccinated individuals lead to sustained transmission (>10 generations among unvaccinated individuals dispersed in the population but with a certain level of aggregation), even in highly vaccinated populations.Citation70 Importation of infection from a single infected person can easily cause an outbreak in such an environment.Citation11,69,Citation71
But even in populations with some degree of clustering, if vaccination hits the correct reservoir of infection (i.e., the ones that normally introduce the pathogen into a specific environment), then herd protection is still substantial. In the United States, Samandari et al.Citation27 modeled this phenomenon with an estimated 76% reduction in hepatitis A cases among children 2–18 y old in high incidence states even though coverage rates were much lower (30%).
Timing
The effectiveness of a vaccination program could be affected by the timing of vaccine administration or by individual timeliness in receiving the vaccine. A study in Switzerland evaluated this possibility for vaccination of measles (MCV1 and MCV2). Considering disease susceptibility to count from 6 months of age when maternal antibodies have waned, researchers calculated that 66.5% of an estimated 266 d susceptible to measles among 1-year olds were due to the policy of recommending the MCV1 vaccine to be administered at 12 months of age (despite early uptake among 20% of the infants). Individual delay in vaccination accounted for the other 33.5% of susceptible days. While overall coverage levels were reasonably high among 2-year old children (84.5% of these were up-to-date for measles immunization), delayed administration of the vaccine (e.g. spread-out of vaccine delivery) reduced the estimated effective vaccine coverage to only 48.6%.Citation64
Negative Indirect Effects
Age shifting and rebound effects are unintended consequences that may arise as a result of vaccination, such as an increased emergence/re-emergence of disease incidence or severity. Age shifts are defined as increased disease incidence among unvaccinated age groups. Rebound effects, or the reappearance of disease, occur after a honeymoon period of significantly reduced disease due to vaccination. This is brought about by an accumulation of a new group of susceptible individuals due to vaccination at coverage levels of <100%, until a certain tipping point is reached in which the wild-type pathogen may re-emerge and trigger a new, post-honeymoon epidemic, as mentioned earlier.
These effects may occur quickly after the introduction of a vaccine or with a delay depending on the rate of change and the combination of specific conditions that are traced to different factors. Age shifts are more likely to occur than rebound effects. The latter are more easily simulated in dynamic modeling exercises than are observed in real-life since additional dynamic processes may intervene before any full rebound effect appears. We will discuss these interactions in the sections below.
Reduced impact on natural immunity
In naturally endemic situations, mild infections and exposure to wild-type infections are frequent, leading to immune boosting and a decreased incidence of severe disease.Citation25,58,Citation72 Thus, while vaccination will induce herd protection in the short-term, it could lead to increased rates of infection or disease outbreaks in unvaccinated individuals through the loss of natural boosting mechanisms or by the lack of regular exposure to infection as vaccination reduces circulation of the wild-type pathogen.Citation1,25,Citation72, 73
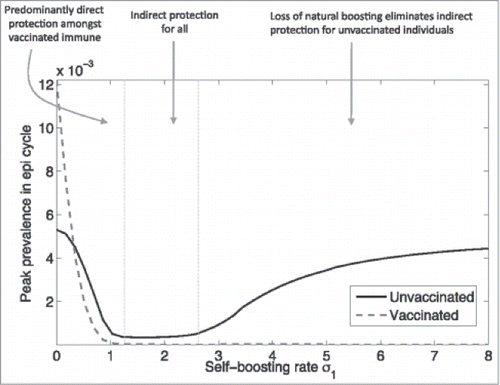
Meanwhile, these negative effects are less likely to occur with high vaccine coverage rates, especially during the first yearCitation10,19,25 (assuming minimal vaccine waning). In such cases, immunity due to natural infection would simply be replaced by vaccine-induced immunity in newly introduced persons (e.g., by birth).Citation19 For example, the lack of boosting from reduced pathogen circulation due to vaccination and vaccine waning have been implicated in increased rates of pertussis infection,Citation1,55-58,60,62 as shows.Citation55
Figure 3. Complex relationship between pertussis vaccination and herd protection (used with permission from Arinaminpathy et al.)Citation55 Reprinted from PNAS USA, 109(49), Arinaminpathy N, Lavine JS, Grenfell BT., Self-boosting vaccines and their implications for herd immunity, pp. 20154–9, Copyright (2012), with permission from PNAS USA.
Serotype replacement or switching
Another effect of large-scale vaccination programs is the emergence of disease serotypes not targeted by the vaccine.Citation1,15,Citation35,37,Citation38,45,Citation73-81 For example, in a large Canadian study over several years (1989–2007), this effect was observed in the increased incidence of severe Haemophilus influenza (blood-stream illness/sepsis) due to serotype replacement after mass vaccination with the serotype b (Hib) vaccine. However, these numbers remained quite limited in the assessment.Citation33
One of the biggest concerns about serotype replacement has been regarding pneumococcal disease, where the emergence of non-7-valent (non-PCV7) pneumococcal vaccine serotypes (1, 3, 7F, 15B/C/F, 10A, 19A, 22F, 33F, and 38) could offset vaccine-induced herd protection.Citation1,25,Citation35,37,Citation45,77-83 The 10- and 13-valent pneumococcal vaccines might allow less replacement disease due to a reduced incidence of all PCV7 serotypes plus several additional serotypes (PCV6+).Citation79,81,Citation82,84,Citation85 Furthermore, increases in the incidence of invasive pneumococcal disease (e.g. bloodstream infections/septicaemia, osteomyelitis, septic arthritis and meningitis)Citation86 from non-PCV7 serotypes have been minor relative to reductions in PCV7-serotype disease,Citation35,38,Citation40,45 relieving some of the concern over this issue.
Upward age shift
An upward shift in the average age of infection has been clearly observed post-vaccination for many different infectious diseases.Citation2,8-10,Citation12,Citation14,19,Citation22,33,Citation46,50,Citation56,58,Citation62,63,Citation67,72,Citation74,87,Citation88 For example, an upward age shift has been witnessed in the incidence of hepatitis A in Spain (),Citation63 the incidence of varicella in the United States,Citation50 and the incidence of rubella in Greece.Citation67
Figure 4. Observed age shift in cases of hepatitis A in Catalonia, Spain (used with permission from Lopalco et al.)Citation63 Reprinted from Vaccine, 19(4–5), Lopalco PL, Salleras L, Barbuti S, et al., Hepatitis A and B in children and adolescents–what can we learn from Puglia (Italy) and Catalonia (Spain)?, pp. 470–474, Copyright (2001), with permission from Elsevier.
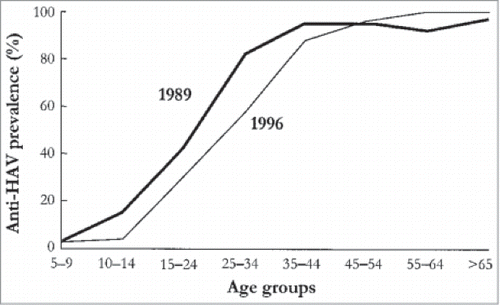
The upward age shift is not necessarily a negative effect unless it leads to an actual increase of disease incidence or severity as compared to pre-vaccination levels. The terminology is important here: if we are referring to an increase in the proportion of infected older age groups due to a sharp decrease in disease incidence among vaccinated cohorts, then this age shift is not accompanied by an absolute increased disease incidence. An example of the latter scenario comes from an epidemiological study conducted in the United States by Wasley et al.,Citation46 which showed that while actual incidence rates had decreased among all age groups post-vaccination, the proportion of adults with hepatitis A was higher than in the pre-vaccination era.Citation50
The mechanism behind this age-shift phenomenon is thought to be caused by waning vaccine-induced immunityCitation53 and a vaccine-induced delay in exposure to infection (or a minimal-to-absent “exogenous boosting” effect), leading individuals to be older when they become infected.Citation2,12,Citation22,67,Citation72, 87 Vaccine coverage also fundamentally influences the outcome: if very high coverage levels are achieved in the first year of vaccination and are maintained (especially among the group who is the reservoir of infection), then an age-shift is unlikely to cause an overall greater disease burden (i.e., the absolute number of cases would likely decrease in all age groups).Citation10,19
In the next paragraphs, we discuss this shift in the average age of infection in greater detail for older and younger age groups.
Incidence or severity in older age groups
Upward age shifting post-vaccination results in higher morbidity and/or mortality if disease severity increases with age (e.g., varicella-zoster virus, polio, hepatitis A and B, mumps, pneumococcal disease, rubella) or if absolute incidence rates increase.Citation2,8,Citation12,14,Citation22,34,Citation41,63,Citation67,75,Citation88-94
Several epidemiological studies have indicated an increasing incidence of pertussis in different countries due to an upward age shift.Citation56,58,Citation60,62 De Vries et al.Citation62 modeled this effect in the Netherlands, in which pertussis vaccination of adolescents decreased the total incidence of disease in the population while causing an increase in absolute numbers of recurrent infections in older age groups.Citation62
Increased varicella incidence among older age groups as a result of vaccination (especially with sub-optimal coverage levels) has raised concerns among researchers since the virus tends to produce more severe consequences as age increases. Complications such as skin super infection, pneumonia, encephalitis and other central nervous system manifestations are common.Citation2,88 Several studies have shown that the proportion of adults relative to children with varicella has increased,Citation50,89,Citation95 although many other studies have reported decreasing incidence rates among most (if not all) age groups.Citation49,50,Citation89,95-102
Early research in the field and results from modeling studies have raised concerns about the possibility of routine varicella vaccination of infants causing an increase in herpes zoster among adults and the elderly.Citation2,87,Citation89,103 However, a number of studies analyzing epidemiological data post-varicella vaccination over the last 15 y in different regions have not been able to confirm this hypothesis and the predictions of modeling exercises.Citation9,96,Citation102,104-123
Evidence has not shown increasing incidence rates of hepatitis A among the elderly post-vaccination, although disease severity is a potential consideration. Exposure to hepatitis A later in life increases the probability of acute disease with more debilitating and long-lasting effects.Citation41,75,Citation91-93 Mortality rates also tend to increase with age (from 0.2% in symptomatic young adults to 3.9% in adults over the age of 80).Citation93 It should be noted that if the only negative effect of vaccination for a particular infection is an increase in disease severity with age, then this effect would need to be modeled to determine if the burden of disease (in terms of costs and/or effects) is actually higher after vaccination.
Incidence or severity in younger age groups
It is also possible that an upward age shift could lead to increased disease incidence or severity among young children via transmission from older age groups in diseases like pertussis, measles, rubella, and Hib.Citation1,33,Citation53,56,Citation67,124
The potential for this effect is illustrated by the results of pertussis vaccination. An epidemiological study done by Guris et al.Citation60 reported fairly stable disease incidence rates in children/infants younger than 5 y of age in the United States during a 7-year period of time (1990–1996). It was postulated that the generally increasing incidence rates of pertussisCitation57 in individuals ≥10 y of ageCitation1,56,Citation60 could lead to disease increases in younger children over the long-term. This is related to a couple of different factors. First, there is an increased risk of transmission to susceptible infants who are too young to be vaccinated (<1 y of age) via siblings, mothers and fathers, since up to 70% of infant infections stem from these familial interactions.Citation1,53,Citation56,58,Citation124 Secondly, there is a risk of less effective trans-placental immunity to infants by mothers with reduced immunity.Citation1
Transfer of pertussis from older to younger age groups are minimized by strategies like “cocooning” (i.e., selective vaccination targeting siblings, parents, grandparents, health care workers, etc.), as well as booster vaccination of adolescents and adults.Citation1,53,Citation56,62,Citation125 While a vaccine-induced immune response does not necessarily guarantee protection against an invading pathogen, serological markers (e.g. antibodies) against infection are nevertheless highly correlated with disease protection.Citation126 In response to the increasing incidence of pertussis (especially in the United States),Citation57 some researchers are advocating the need to universally vaccinate all age groups at frequent intervals.Citation127
In Greece, Panagiotopoulos et al.Citation67 observed an absolute increased incidence of rubella among individuals ≥15 years old in 1986, following over a decade of a country-wide vaccination program. This epidemic was plausibly linked to a subsequent outbreak of congenital rubella in 1993, which was deemed the worst epidemic in Greece since 1950 with 25 serologically confirmed cases, all of which had serious symptoms; 7 deaths also occurred.Citation67 Vaccine coverage rates in this study were <50%, again highlighting the need for adequate uptake to help prevent older age groups from contracting the virus and spreading it to the young.
Thus, both herd protection and age shifts have been observed to result from vaccination programs involving infectious diseases. These effects are oftentimes attenuated by adequate, homogeneous, and consistent vaccination coverage, regular vaccine boosting (in the case of vaccine waning), and vaccination of specific high-risk groups (e.g., cocooning). Thus, when conducting evaluations of a vaccine's impact on a population, herd protection needs to be weighed up against any negative effects, taking into account disease characteristics as well as the country- and population-specific situation.
How to Observe, Quantify, and Model Indirect Effects
The decision regarding the introduction of a new vaccine into a public healthcare program may depend on the expected magnitude of the herd protection as it may impact the economic value of the new vaccine with additional indirect benefits.Citation7,8 In an atmosphere of increasingly stringent criteria for introducing new vaccines into the healthcare system,Citation7 demonstrating herd protection is likely to gain vital importance over time.
Traditionally, indirect vaccine effects have only been assessed after a vaccine has been introduced into a community. But new methodological developments have opened up the possibility of evaluating herd protection beforehand in order to provide decision makers with adequate information from the outset.Citation7 However, not much analytical and empirical work has been done to quantify the magnitude of these vaccination externalities.Citation5
Herd protection is observed and quantified by measuring the registered change in disease incidence among the unvaccinated portion of a partially-vaccinated population (may also be compared with the incidence of a totally unvaccinated population) over a certain period of time, assuming a similar demographic composition and similar regional characteristics ().Citation7,26,Citation128 This may be manifested as a change in disease incidence among unvaccinated persons of the vaccinated cohort that is greater than the actual coverage level or greater than known protective efficacy rates.Citation7, 27 Another manifestation is a reduction in disease incidence in age or gender groups outside (or in addition to) the vaccinated cohort.Citation1,7,Citation27-30,Citation46,51,Citation53, 129
In the United States, a modeled study estimated that hepatitis A vaccination among 2 to 18-year olds could prevent 51% of cases in that age group despite vaccination coverage levels of only 10%.Citation27 PCV-7-related disease decreased by 55% among adults aged ≥50 years due to vaccination of infants 2–18 months of age.Citation30 The incidence of Hib infections among infants too young to be vaccinated (<12 months old) declined when toddlers 15–18 months of age were vaccinated.Citation28 In Sweden, researchers observed reduced rates of pertussis infection among household members of vaccinated individuals (e.g. parents and siblings).Citation29
Measurements of herd protection need to be adjusted by several important and influencing factors such as: the effectiveness and duration of vaccine-induced protection,Citation1 rebound effects like serotype replacement and age shifting (which takes many years to adequately observe),Citation1,53 and behavioral changes in the rate or the type of contact with infected persons due to belief in the protective effects of vaccination.Citation26
In the sections below, we discuss some specific methods used in quantifying changes in disease incidence due to herd protection. It is different for every type of infectious disease and include a variety of outcomes such as the number of hospitalizations and the length of stay, number and/or type of physician visits, mortality rates, differences in costs or Quality-Adjusted Life Years (QALY's), or increased periodicyCitation59 (the length of time between epidemics).
Observational studies
Household trials are used to record the number of infectious disease episodes occurring among vaccinated and unvaccinated members of the same household.Citation4,29 Population surveillance studies (e.g., serological surveysCitation6) are also commonly used to compare the incidence of disease in a given population before and after a vaccination programme is initiated.Citation4,6-8
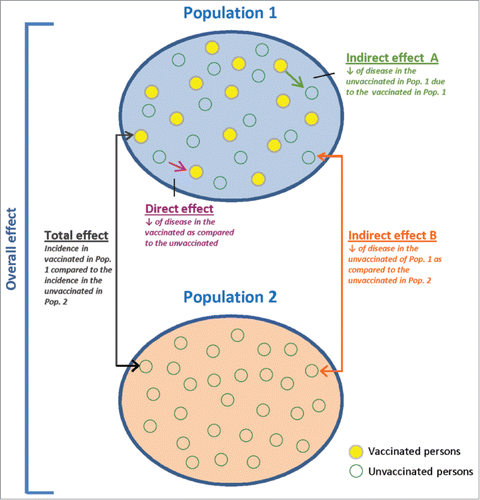
Another type of study is a cluster-randomized trial, which randomizes the entire eligible population of a geographically contiguous area into 2 arms,Citation4,8,Citation26 comparing a partially-vaccinated group (Population 1) with a no-vaccine control group (Population 2).Citation7,8,Citation26 shows the direct protective effect of vaccination that is obtained by comparing the incidence of infection between vaccinated and unvaccinated persons in Population 1.Citation7,8,Citation26 The overall protective effect (direct and indirect) is obtained by comparing the incidence of infection between all individuals in Population 1 and 2.Citation7,8,Citation26 Indirect herd protection is observed by comparing the incidence of disease among unvaccinated persons in Population 1 with the incidence in Population 2.Citation7,8,Citation26
A further development of that approach is the step-wedged cluster randomized design, where cluster regions are randomized and introduced into the study at different time points in order to capture baseline disease fluctuation over time.
Mathematical models
The aforementioned observational evaluation methods have limitations, namely setting-specific variables that are difficult to measure and which differ between settings (e.g. household structure, age distribution, population mixing patterns, infectivity of the disease, susceptibility of individuals, vaccine coverage).Citation4 Consequently, more hypothetical, predictive modeling evaluations have been developed to help fill the gaps in quantifying herd protection.Citation3-5
Dynamic transmission models capture the effect of vaccination on a population that is followed over timeCitation9,10 through a change in the force of infection.Citation2, 3,Citation9,10,Citation12,19,Citation24 All effects, direct and indirect, are tracked and quantified over time through the post-vaccination phases: honeymoon, post-honeymoon epidemic, and post-honeymoon equilibrium.Citation2,4,Citation9 As a consequence, dynamic models have the potential of producing better economic results than typical cohort models because they generally predict more positive outcomes across the whole population rather than limiting the effect to the studied cohort only (both short-term due to a more rapid effect and long-term as the effect of herd protection accumulates over time).Citation4
This is not always the case, however, as when significant rebound effects diminish the protective herd effect. Despite the very real possibility of a target population experiencing one or more of these confounding effects, dynamic models often fail to take them into account. In addition, results from these models are highly dependent on assumptions made about key parameters which are difficult to measure (e.g., probability of a pathogen's transmission).Citation4
Thus, while evaluation methods are necessary in determining the full impact of a vaccination program in a population, there are limitations with every type of assessment tool used. Ideally, information obtained from observational and modeling studies should be compared and then used to validate the results.
Will there always be a rebound effect after herd protection? Or is there a herd without a rebound effect or a rebound effect without herd protection? It should be clear from the previous paragraphs that certain conditions need to be fulfilled before a rebound phenomenon will appear. Meanwhile, a rebound effect without herd protection is unlikely to happen as there needs to be enough susceptibles in the population that remain in contact with each other in order to transmit the pathogen.
Indirect Effects Illustrated
provides a sampling of illustrations detailing the indirect effects of vaccination in 5 infectious disease areas and in different countries. Using the most recent data possible, we have included results from modeling as well as observational studies where available. The results are heterogeneous, differing greatly in the type of outcome reported by study and by region. Nevertheless, these results still provide examples of how vaccination indirectly impacts the population as a whole. Because it takes a much longer observation period to observe clear rebound effects (as they may only appear much later in the process of a vaccine's impact), little data on these effects have been reported in the literature regarding infections for which the vaccine has been recently introduced.
Table 2. Case examples of herd and rebound effects per type of disease and per region
Some modeling exercises for varicella have predicted high rebound effects over time because of restrictions on the assumptions introduced in the dynamic models. It is likely that in real life we will not observe these changes for 2 reasons. First, dynamic models essentially base their analysis on infection and not on disease, whereas in real life it is much more difficult to capture infection than disease; thus we need to obtain a clearer picture about how many of these infections will translate into disease (a number we often do not know). Second, once we observe an increase in disease, clinical practice is much more reactive to changes in management than a dynamic model is set up to demonstrate. To better reflect reality, dynamic-dynamic models should be developed.
Conclusions
Population-level effects (both herd protection and age shifts) have been observed following the implementation of immunization programs. This may have a great impact on measuring the economic value of vaccines and on the implementation of the right vaccine strategy. A variety of articles have been published about certain portions of this subject, but we have here endeavored to synthesize these separate bits and pieces of relevant information into a comprehensive overview of the indirect effects of vaccination. Various elements intersect within this framework and specific methods have been developed that are useful in observing, measuring and quantifying the precise impact of vaccination. But we also pointed out the limitations inherent in estimating the real impact of vaccination.
Through this process, more clarity and definition have been brought to particular concepts and terminology in published literature regarding the indirect impact of vaccination (e.g. the conditions for producing maximal herds protection, what rebound effects are and when they more likely to occur, methods for measuring indirect effects).
This should enable and motivate researchers and modellers to investigate ways of bridging these gaps in data collection and analysis to produce a better picture of these effects and the drivers behind them. It has become clear that greater accuracy, clarity and standardisation in the observation and measurement of the indirect outcomes of vaccination are needed. This will tend to produce a more straightforward and informed decision-making process when evaluating the desirability of incorporating a particular vaccine into a national immunisation program.
Disclosure of Potential Conflicts of Interest
Augustin Terlinden is an employee of Navigha working on behalf of the GSK group of companies and reports consulting fees from the GSK group of companies during the conduct of the study. Carla Lefebvre is an independent research consultant and reports personal fees from the GSK group of companies. Baudouin Standaert is an employee of the GSK group of companies and holds stock in the GSK group of companies.
Trademarks
Rotarix™ is a trade mark of the GSK group of companies. Rotateq™ is a trade mark of Merck and Co. Inc.
Acknowledgments
The authors thank Gregory Collet (Business and Decision Life Sciences, on behalf of GSK Vaccines) for editorial assistance and manuscript coordination.
Funding
GlaxoSmithKline Biologicals SA (Rixensart, Belgium) was the funding source and was involved in all stages of the study and analysis (GSK study identifier: HO-13-14106). GlaxoSmithKline Biologicals SA also took charge of all costs associated with the development and the publication of the present manuscript. All authors had full access to the data. The corresponding author had final responsibility of submitting the manuscript for publication.
References
- Kim TH, Johnstone J, Loeb M. Vaccine herd effect. Scand J Infect Dis 2011; 43: 683-9; PMID:21604922
- Brisson M, Edmunds WJ. Economic evaluation of vaccination programs: the impact of herd-immunity. Med Decis Making 2003; 23: 76-82; PMID:12583457; http://dx.doi.org/10.1177/0272989X02239651
- Kim SY, Goldie SJ. Cost-effectiveness analyses of vaccination programmes: a focused review of modelling approaches. Pharmacoeconomics 2008; 26: 191-215; PMID:18282015; http://dx.doi.org/10.2165/00019053-200826030-00004
- Jit M, Newall AT, Beutels P. Key issues for estimating the impact and cost-effectiveness of seasonal influenza vaccination strategies. Hum Vaccin Immunother 2013; 9: 834-40; PMID:23357859; http://dx.doi.org/10.4161/hv.23637
- Boulier BL, Tejwant SD, Goldfarb RS. Vaccination Externalities. The B.E. Journal of Economic Analysis and Policy 2007; 7; http://dx.doi.org/10.2202/1935-1682.1487
- Plans-Rubio P. Evaluation of the establishment of herd immunity in the population by means of serological surveys and vaccination coverage. Hum Vaccin Immunother 2012; 8: 184-8; PMID:22426372; http://dx.doi.org/10.4161/hv.18444
- Clemens J, Shin S, Ali M. New approaches to the assessment of vaccine herd protection in clinical trials. Lancet Infect Dis 2011; 11: 482-7; PMID:21616458; http://dx.doi.org/10.1016/S1473-3099(10)70318-2
- Smith PG. Concepts of herd protection and immunity. Ninth Global Vaccine Research Forum and Parallel Satellite Symposia; 2009 December 6–9 Bamako, Mali; Keppel Street, London WCIE 7HT, United Kingdom. Procedia Vaccinol 2; 2010. 134-9; http://dx.doi.org/10.1016/j.provac.2010.07.005
- Jit M, Brisson M. Modelling the epidemiology of infectious diseases for decision analysis: a primer. Pharmacoeconomics 2011; 29: 371-86; PMID:21504239; http://dx.doi.org/10.2165/11539960-000000000-00000
- Beutels P, Van Doorslaer E, Van Damme P, Hall J. Methodological issues and new developments in the economic evaluation of vaccines. Expert Rev Vaccines 2003; 2: 649-60; PMID:14711326; http://dx.doi.org/10.1586/14760584.2.5.649
- Donaghy M, Cameron JC, Friederichs V. Increasing incidence of mumps in Scotland: options for reducing transmission. J Clin Virol 2006; 35: 121-9; PMID:16289903; http://dx.doi.org/10.1016/j.jcv.2005.09.009
- Edmunds WJ, Medley GF, Nokes DJ. Evaluating the cost-effectiveness of vaccination programmes: a dynamic perspective. Stat Med 1999; 18: 3263-82; PMID:10602150; http://dx.doi.org/10.1002/(SICI)1097-0258(19991215)18:23%3c3263::AID-SIM315%3e3.0.CO;2-3
- Vynnycky E, White R. An Introduction to Infectious Disease Modelling. Great Clarendon Street, Oxford 0´2 6DP: Oxford University Press; 2011. Section 1.3: Transmission; p. 5-7
- Fine P, Eames K, Heymann DL. “Herd immunity:” a rough guide. Clin Infect Dis 2011; 52: 911-6; PMID:21427399; http://dx.doi.org/10.1093/cid/cir007
- Pitman R, Fisman D, Zaric GS, Postma M, Kretzschmar M, Edmunds J, Brisson M. Dynamic transmission modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–5. Value Health 2012; 15: 828-34; PMID:22999132; http://dx.doi.org/10.1016/j.jval.2012.06.011
- Garnett GP. Role of herd immunity in determining the effect of vaccines against sexually transmitted disease. J Infect Dis 2005; 191 Suppl 1: S97-106; PMID:15627236; http://dx.doi.org/10.1086/425271
- Centers for Disease Control and Prevention. Recommendations of the Advisory Committee on Immunization Practices (ACIP): Use of vaccines and immune globulins in persons with altered immunocompetence. MMWR 1993; 42 (No. RR-5):[inclusive page numbers].
- Moss W, Lederman H. Immunization of the immunocompromised host. Clin Focus Immune Deficiencies: Issues Information Curr Topics 1998; 1: 1-8
- Walker D, Beutels P. WHO guide for standardization of economic evaluations of immunization programmes. CH-1211 Geneva 27, Switzerland: World Health Organization, Department of Immunization, Vaccin Biol; 2008. 100 p. Report No.: WHO/IVB/08.14. Initiative for Vaccine Research (IVR)
- Bauch CT, Anonychuk AM, Van Effelterre T, Pham BZ, Merid MF. Incorporating herd immunity effects into cohort models of vaccine cost-effectiveness. Med Decis Making 2009; 29: 557-69; PMID:19605882; http://dx.doi.org/10.1177/0272989X09334419
- Van Vlaenderen I, Van Bellinghen LA, Meier G, Nautrup BP. An approximation of herd effect due to vaccinating children against seasonal influenza - a potential solution to the incorporation of indirect effects into static models. BMC Infect Dis 2013; 13: 25; PMID:23339290; http://dx.doi.org/10.1186/1471-2334-13-25
- Bos JM, Alphen L, Postma MJ. The use of modeling in the economic evaluation of vaccines. Expert Rev Pharmacoecon Outcomes Res 2002; 2: 443-55; PMID:19807468; http://dx.doi.org/10.1586/14737167.2.5.443
- Trotter CL, Maiden MC. Meningococcal vaccines and herd immunity: lessons learned from serogroup C conjugate vaccination programs. Expert Rev Vaccines 2009; 8: 851-61; PMID:19538112; http://dx.doi.org/10.1586/erv.09.48
- Van Effelterre T, Soriano-Gabarro M, Debrus S, Claire Newbern E, Gray J. A mathematical model of the indirect effects of rotavirus vaccination. Epidemiol Infect 2009; 138: 884-97; PMID:20028612; http://dx.doi.org/10.1017/S0950268809991245
- Rashid H, Khandaker G, Booy R. Vaccination and herd immunity: what more do we know? Curr Opin Infect Dis 2012; 25: 243-9; PMID:22561998; http://dx.doi.org/10.1097/QCO.0b013e328352f727
- Halloran ME, Struchiner CJ, Longini IM, Jr. Study designs for evaluating different efficacy and effectiveness aspects of vaccines. Am J Epidemiol 1997; 146: 789-803; PMID:9384199; http://dx.doi.org/10.1093/oxfordjournals.aje.a009196
- Samandari T, Bell BP, Armstrong GL. Quantifying the impact of hepatitis A immunization in the United States, 1995–2001. Vaccine 2004; 22: 4342-50; PMID:15474727; http://dx.doi.org/10.1016/j.vaccine.2004.04.014
- Wenger JD. Epidemiology of Haemophilus influenzae type b disease and impact of Haemophilus influenzae type b conjugate vaccines in the United States and Canada. Pediatr Infect Dis J 1998; 17: S132-6; PMID:9781746; http://dx.doi.org/10.1097/00006454-199809001-00008
- Trollfors B, Taranger J, Lagergard T, Sundh V, Bryla DA, Schneerson R, Robbins JB. Immunization of children with pertussis toxoid decreases spread of pertussis within the family. Pediatr Infect Dis J 1998; 17: 196-9; PMID:9535245; http://dx.doi.org/10.1097/00006454-199803000-00005
- Lexau CA, Lynfield R, Danila R, Pilishvili T, Facklam R, Farley MM, Harrison LH, Schaffner W, Reingold A, Bennett NM, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. Jama 2005; 294: 2043-51; PMID:16249418; http://dx.doi.org/10.1001/jama.294.16.2043
- Raes M, Strens D, Vergison A, Verghote M, Standaert B. Reduction in pediatric rotavirus-related hospitalizations after universal rotavirus vaccination in Belgium. Pediatr Infect Dis J 2011; 30: e120-5; PMID:21436757; http://dx.doi.org/10.1097/INF.0b013e318214b811
- Berndsen MR, Erlendsdottir H, Gottfredsson M. Evolving epidemiology of invasive Haemophilus infections in the post-vaccination era: results from a long-term population-based study. Clin Microbiol Infect 2011; 18: 918-23; PMID:22070637; http://dx.doi.org/10.1111/j.1469-0691.2011.03700.x
- Adam HJ, Richardson SE, Jamieson FB, Rawte P, Low DE, Fisman DN. Changing epidemiology of invasive Haemophilus influenzae in Ontario, Canada: evidence for herd effects and strain replacement due to Hib vaccination. Vaccine 2010; 28: 4073-8; PMID:20398617; http://dx.doi.org/10.1016/j.vaccine.2010.03.075
- Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003; 348: 1737-46; PMID:12724479; http://dx.doi.org/10.1056/NEJMoa022823
- Hsu HE, Shutt KA, Moore MR, Beall BW, Bennett NM, Craig AS, Farley MM, Jorgensen JH, Lexau CA, Petit S, et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med 2009; 360: 244-56; PMID:19144940; http://dx.doi.org/10.1056/NEJMoa0800836
- Ramsay ME, Andrews NJ, Trotter CL, Kaczmarski EB, Miller E. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. Bmj 2003; 326: 365-6; PMID:12586669; http://dx.doi.org/10.1136/bmj.326.7385.365
- Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 2011; 11: 760-8; PMID:21621466; http://dx.doi.org/10.1016/S1473-3099(11)70090-1
- Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease–United States, 1998–2003. MMWR Morb Mortal Wkly Rep 2005; 54(36): 893-7; PMID:16163262
- Paulke-Korinek M, Kundi M, Rendi-Wagner P, de Martin A, Eder G, Schmidle-Loss B, Vecsei A, Kollaritsch H. Herd immunity after two years of the universal mass vaccination program against rotavirus gastroenteritis in Austria. Vaccine 2011; 29: 2791-6; PMID:21320539; http://dx.doi.org/10.1016/j.vaccine.2011.01.104
- Fitzwater SP, Chandran A, Santosham M, Johnson HL. The worldwide impact of the seven-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2012; 31: 501-8; PMID:22327872; http://dx.doi.org/10.1097/INF.0b013e31824de9f6
- Dominguez A, Oviedo M, Carmona G, Batalla J, Bruguera M, Salleras L, Plasencia A. Impact and effectiveness of a mass hepatitis A vaccination programme of preadolescents seven years after introduction. Vaccine 2008; 26: 1737-41; PMID:18325642; http://dx.doi.org/10.1016/j.vaccine.2008.01.048
- Ong G, Hoon HB, Ong A, Chua LT, Kai CS, Tai GK. A 24-year review on the epidemiology and control of measles in Singapore, 1981–2004. Southeast Asian J Trop Med Public Health 2006; 37: 96-101; PMID:16771219
- Payne DC, Staat MA, Edwards KM, Szilagyi PG, Weinberg GA, Hall CB, Chappell J, Curns AT, Wikswo M, Tate JE, et al. Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US Counties, 2006–2009. Clin Infect Dis 2011; 53: 245-53; PMID:21705316; http://dx.doi.org/10.1093/cid/cir307
- Zlamy M, Kofler S, Orth D, Wurzner R, Heinz-Erian P, Streng A, Prelog M. The impact of Rotavirus mass vaccination on hospitalization rates, nosocomial Rotavirus gastroenteritis and secondary blood stream infections. BMC Infect Dis 2013; 13: 112; PMID:23452879; http://dx.doi.org/10.1186/1471-2334-13-112
- Gil E, Noursadeghi M, Brown JS. The clinical and ecological impact of childhood pneumococcal vaccination. Br J Hosp Med (Lond) 2013; 74: 212-6; PMID:23571392; http://dx.doi.org/10.12968/hmed.2013.74.4.212
- Wasley A, Samandari T, Bell BP. Incidence of hepatitis A in the United States in the era of vaccination. Jama 2005; 294: 194-201; PMID:16014593; http://dx.doi.org/10.1001/jama.294.2.194
- Armstrong GL, Billah K, Rein DB, Hicks KA, Wirth KE, Bell BP. The economics of routine childhood hepatitis A immunization in the United States: the impact of herd immunity. Pediatrics 2007; 119: e22-9; PMID:17200247; http://dx.doi.org/10.1542/peds.2006-1572
- Lenne X, Diez Domingo J, Gil A, Ridao M, Lluch JA, Dervaux B. Economic evaluation of varicella vaccination in Spain: results from a dynamic model. Vaccine 2006; 24: 6980-9; PMID:16860909; http://dx.doi.org/10.1016/j.vaccine.2006.04.051
- Streng A, Grote V, Carr D, Hagemann C, Liese JG. Varicella routine vaccination and the effects on varicella epidemiology - results from the Bavarian Varicella Surveillance Project (BaVariPro), 2006–2011. BMC Infect Dis 2013; 13: 303; PMID:23815523; http://dx.doi.org/10.1186/1471-2334-13-303
- Guris D, Jumaan AO, Mascola L, Watson BM, Zhang JX, Chaves SS, Gargiullo P, Perella D, Civen R, Seward JF. Changing varicella epidemiology in active surveillance sites–United States, 1995–2005. J Infect Dis 2008; 197 Suppl 2: S71-5; PMID:18419413; http://dx.doi.org/10.1086/522156
- Dagan R, Leventhal A, Anis E, Slater P, Ashur Y, Shouval D. Incidence of hepatitis A in Israel following universal immunization of toddlers. Jama 2005; 294: 202-10; PMID:16014594; http://dx.doi.org/10.1001/jama.294.2.202
- Broker M. Indirect effects by meningococcal vaccines: herd protection versus herd immunity. Hum Vaccin 2011; 7: 881-2; PMID:21785283; http://dx.doi.org/10.4161/hv.7.8.16273
- Goncalves G. Herd immunity: recent uses in vaccine assessment. Expert Rev Vaccines 2008; 7: 1493-506; PMID:19053206; http://dx.doi.org/10.1586/14760584.7.10.1493
- Brisson M, van de Velde N, Franco EL, Drolet M, Boily MC. Incremental impact of adding boys to current human papillomavirus vaccination programs: role of herd immunity. J Infect Dis 2011; 204: 372-6; PMID:21742835; http://dx.doi.org/10.1093/infdis/jir285
- Arinaminpathy N, Lavine JS, Grenfell BT. Self-boosting vaccines and their implications for herd immunity. Proc Natl Acad Sci U S A 2012; 109: 20154-9; PMID:23169630; http://dx.doi.org/10.1073/pnas.1209683109
- Coudeville L, Van Rie A, Getsios D, Caro JJ, Crepey P, Nguyen VH. Adult vaccination strategies for the control of pertussis in the United States: an economic evaluation including the dynamic population effects. PLoS One 2009; 4: e6284; PMID:19606227; http://dx.doi.org/10.1371/journal.pone.0006284
- Burns DL, Meade BD, Messionnier NE. Pertussis resurgence: perspectives from the Working Group Meeting on pertussis on the causes, possible paths forward, and gaps in our knowledge. J Infect Dis 2014; 209 Suppl 1: S32-5; PMID:24626870; http://dx.doi.org/10.1093/infdis/jit491
- Aguas R, Goncalves G, Gomes MG. Pertussis: increasing disease as a consequence of reducing transmission. Lancet Infect Dis 2006; 6: 112-7; PMID:16439331; http://dx.doi.org/10.1016/S1473-3099(06)70384-X
- Broutin H, Viboud C, Grenfell BT, Miller MA, Rohani P. Impact of vaccination and birth rate on the epidemiology of pertussis: a comparative study in 64 countries. Proc Biol Sci 2010; 277: 3239-45; PMID:20534609; http://dx.doi.org/10.1098/rspb.2010.0994
- Guris D, Strebel PM, Bardenheier B, Brennan M, Tachdjian R, Finch E, Wharton M, Livengood JR. Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990–1996. Clin Infect Dis 1999; 28: 1230-7; PMID:10451158; http://dx.doi.org/10.1086/514776
- Trotter CL, Edmunds WJ. Reassessing the cost-effectiveness of meningococcal serogroup C conjugate (MCC) vaccines using a transmission dynamic model. Med Decis Making 2006; 26: 38-47; PMID:16495199; http://dx.doi.org/10.1177/0272989X05284109
- de Vries R, Kretzschmar M, Schellekens JF, Versteegh FG, Westra TA, Roord JJ, Postma MJ. Cost-effectiveness of adolescent pertussis vaccination for the Netherlands: using an individual-based dynamic model. PLoS One 2010; 5: e13392; PMID:20976213; http://dx.doi.org/10.1371/journal.pone.0013392
- Lopalco PL, Salleras L, Barbuti S, Germinario C, Bruguera M, Buti M, Dominguez A. Hepatitis A and B in children and adolescents–what can we learn from Puglia (Italy) and Catalonia (Spain)? Vaccine 2001; 19: 470-4; http://dx.doi.org/10.1016/S0264-410X(00)00193-6
- Bielicki JA, Achermann R, Berger C. Timing of measles immunization and effective population vaccine coverage. Pediatrics 2012; 130: e600-6; PMID:22908102; http://dx.doi.org/10.1542/peds.2012-0132
- Atkinson WL, Orenstein WA, Krugman S. The resurgence of measles in the United States, 1989–1990. Annu Rev Med 1992; 43: 451-63; PMID:1580601; http://dx.doi.org/10.1146/annurev.me.43.020192.002315
- Postma MJ, Westra TA, Quilici S, Largeron N. Economic evaluation of vaccines: specificities and future challenges illustrated by recent European examples. Expert Rev Vaccines 2013; 12: 555-65; PMID:23659302; http://dx.doi.org/10.1586/erv.13.36
- Panagiotopoulos T, Antoniadou I, Valassi-Adam E. Increase in congenital rubella occurrence after immunisation in Greece: retrospective survey and systematic review. Bmj 1999; 319: 1462-7; PMID:10582926; http://dx.doi.org/10.1136/bmj.319.7223.1462
- Bogaards JA, Coupe VM, Xiridou M, Meijer CJ, Wallinga J, Berkhof J. Long-term impact of human papillomavirus vaccination on infection rates, cervical abnormalities, and cancer incidence. Epidemiology 2011; 22: 505-15; PMID:21540743; http://dx.doi.org/10.1097/EDE.0b013e31821d107b
- Salathe M, Bonhoeffer S. The effect of opinion clustering on disease outbreaks. J R Soc Interface 2008; 5: 1505-8; PMID:18713723; http://dx.doi.org/10.1098/rsif.2008.0271
- Dallaire F, De Serres G, Tremblay FW, Markowski F, Tipples G. Long-lasting measles outbreak affecting several unrelated networks of unvaccinated persons. J Infect Dis 2009; 200: 1602-5; PMID:19827945; http://dx.doi.org/10.1086/644783
- Sugerman DE, Barskey AE, Delea MG, Ortega-Sanchez IR, Bi D, Ralston KJ, Rota PA, Waters-Montijo K, Lebaron CW. Measles outbreak in a highly vaccinated population, San Diego, 2008: role of the intentionally undervaccinated. Pediatrics 2010; 125: 747-55; PMID:20308208; http://dx.doi.org/10.1542/peds.2009-1653
- Barskey AE, Glasser JW, LeBaron CW. Mumps resurgences in the United States: A historical perspective on unexpected elements. Vaccine 2009; 27: 6186-95; PMID:19815120; http://dx.doi.org/10.1016/j.vaccine.2009.06.109
- Mauskopf J, Talbird S, Standaert B. Categorization of methods used in cost-effectiveness analyses of vaccination programs based on outcomes from dynamic transmission models. Expert Rev Pharmacoecon Outcomes Res 2012; 12: 357-71; PMID:22812559; http://dx.doi.org/10.1586/erp.12.11
- Yi J, Anderson EJ. Rotavirus vaccination: short-term indirect herd protection, long-term uncertainty. Expert Rev Vaccines 2013; 12: 585-7; PMID:23750788; http://dx.doi.org/10.1586/erv.13.43
- Carrion AF, Martin P. Viral hepatitis in the elderly. Am J Gastroenterol 2012; 107: 691-7; PMID:22290404; http://dx.doi.org/10.1038/ajg.2012.7
- Boonacker CW, Broos PH, Sanders EA, Schilder AG, Rovers MM. Cost effectiveness of pneumococcal conjugate vaccination against acute otitis media in children: a review. Pharmacoeconomics 2011; 29: 199-211; PMID:21250759; http://dx.doi.org/10.2165/11584930-000000000-00000
- Pichichero ME, Casey JR. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. Jama 2007; 298: 1772-8; PMID:17940232; http://dx.doi.org/10.1001/jama.298.15.1772
- Pichichero ME, Casey JR. Evolving microbiology and molecular epidemiology of acute otitis media in the pneumococcal conjugate vaccine era. Pediatr Infect Dis J 2007; 26: S12-6; PMID:18049375; http://dx.doi.org/10.1097/INF.0b013e318154b25d
- Rozenbaum MH, Sanders EA, van Hoek AJ, Jansen AG, van der Ende A, van den Dobbelsteen G, Rodenburg GD, Hak E, Postma MJ. Cost effectiveness of pneumococcal vaccination among Dutch infants: economic analysis of the seven valent pneumococcal conjugated vaccine and forecast for the 10 valent and 13 valent vaccines. Bmj 2010; 340: c2509; PMID:20519267; http://dx.doi.org/10.1136/bmj.c2509
- Kyaw MH, Lynfield R, Schaffner W, Craig AS, Hadler J, Reingold A, Thomas AR, Harrison LH, Bennett NM, Farley MM, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med 2006; 354: 1455-63; PMID:16598044; http://dx.doi.org/10.1056/NEJMoa051642
- Munoz-Almagro C, Navarro-Torne A, Pallares R. Epidemiologic and clinical implications of second-generation pneumococcal conjugate vaccines. Curr Infect Dis Rep 2013; 15: 184-90; PMID:23381547; http://dx.doi.org/10.1007/s11908-013-0326-4
- Rozenbaum MH, Boersma C, Postma MJ, Hak E. Observed differences in invasive pneumococcal disease epidemiology after routine infant vaccination. Expert Rev Vaccines 2011; 10: 187-99; PMID:21332268; http://dx.doi.org/10.1586/erv.10.163
- Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011; 378: 1962-73; PMID:21492929; http://dx.doi.org/10.1016/S0140-6736(10)62225-8
- Gounder PP, Bruce MG, Bruden DJ, Singleton RJ, Rudolph K, Hurlburt DA, Hennessy TW, Wenger J. Effect of the 13-Valent Pneumococcal Conjugate Vaccine on Nasopharyngeal Colonization by Streptococcus pneumoniae–Alaska, 2008-2012. J Infect Dis 2013; 209(8):1251-8; PMID:24273178
- Zuccotti G, Mameli C, Daprai L, Garlaschi ML, Dilillo D, Bedogni G, Faccini M, Gramegna M, Torresani E. Serotype distribution and antimicrobial susceptibilities of nasopharyngeal isolates of Streptococcus pneumoniae from healthy children in the 13-valent pneumococcal conjugate vaccine era. Vaccine 32: 527-34; PMID:24342249; http://dx.doi.org/10.1016/j.vaccine.2013.12.003
- Isaacman DJ, Strutton DR, Kalpas EA, Horowicz-Mehler N, Stern LS, Casciano R, Ciuryla V. The impact of indirect (herd) protection on the cost-effectiveness of pneumococcal conjugate vaccine. Clin Ther 2008; 30: 341-57; PMID:18343273; http://dx.doi.org/10.1016/j.clinthera.2008.02.003
- Poletti P, Melegaro A, Ajelli M, Del Fava E, Guzzetta G, Faustini L, Scalia Tomba G, Lopalco P, Rizzo C, Merler S, et al. Perspectives on the impact of varicella immunization on herpes zoster. A model-based evaluation from three European countries. PLoS One 2013; 8: e60732; PMID:23613740; http://dx.doi.org/10.1371/journal.pone.0060732
- Fairley CK, Miller E. Varicella-zoster virus epidemiology–a changing scene? J Infect Dis 1996; 174 Suppl 3: S314-9; PMID:8896538; http://dx.doi.org/10.1093/infdis/174.Supplement_3.S314
- Marin M, Watson TL, Chaves SS, Civen R, Watson BM, Zhang JX, Perella D, Mascola L, Seward JF. Varicella among adults: data from an active surveillance project, 1995–2005. J Infect Dis 2008; 197 Suppl 2: S94-S100; PMID:18419417; http://dx.doi.org/10.1086/522155
- Berge JJ, Drennan DP, Jacobs RJ, Jakins A, Meyerhoff AS, Stubblefield W, Weinberg M. The cost of hepatitis A infections in American adolescents and adults in 1997. Hepatology 2000; 31: 469-73; PMID:10655272; http://dx.doi.org/10.1002/hep.510310229
- Hepatitis A vaccine recommendations. Pediatrics 2007; 120: 189-99; PMID:17606579; http://dx.doi.org/10.1542/peds.2007-1088
- Bonanni P, Boccalini S, Bechini A. Vaccination against hepatitis A in children: A review of the evidence. Ther Clin Risk Manag 2007; 3: 1071-6; PMID:18516263
- Srinivasa Rao AS, Chen MH, Pham BZ, Tricco AC, Gilca V, Duval B, Krahn MD, Bauch CT. Cohort effects in dynamic models and their impact on vaccination programmes: an example from hepatitis A. BMC Infect Dis 2006; 6: 174; PMID:17147828; http://dx.doi.org/10.1186/1471-2334-6-174
- Robinson KA, Baughman W, Rothrock G, Barrett NL, Pass M, Lexau C, Damaske B, Stefonek K, Barnes B, Patterson J, et al. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995–1998: Opportunities for prevention in the conjugate vaccine era. Jama 2001; 285: 1729-35; PMID:11277827; http://dx.doi.org/10.1001/jama.285.13.1729
- Zhou F, Harpaz R, Jumaan AO, Winston CA, Shefer A. Impact of varicella vaccination on health care utilization. Jama 2005; 294: 797-802; PMID:16106004; http://dx.doi.org/10.1001/jama.294.7.797
- Siedler A, Dettmann M. Hospitalization with varicella and shingles before and after introduction of childhood varicella vaccination in Germany. Hum Vaccin Immunother 2014; 10: 3594-600; PMID:25483695; http://dx.doi.org/10.4161/hv.34426
- Tafuri S, Fortunato F, Cappelli MG, Cozza V, Bechini A, Bonanni P, Martinelli D, Prato R. Effectiveness of vaccination against varicella in children under 5 years in Puglia, Italy 2006–2012. Hum Vaccin Immunother 2015; 11: 214-9; PMID:25483538; http://dx.doi.org/10.4161/hv.36153
- Marin M, Meissner HC, Seward JF. Varicella prevention in the United States: a review of successes and challenges. Pediatrics 2008; 122: e744-51; PMID:18762511; http://dx.doi.org/10.1542/peds.2008-0567
- Reynolds MA, Chaves SS, Harpaz R, Lopez AS, Seward JF. The impact of the varicella vaccination program on herpes zoster epidemiology in the United States: a review. J Infect Dis 2008; 197 Suppl 2: S224-7; PMID:18419401; http://dx.doi.org/10.1086/522162
- Schmid DS, Jumaan AO. Impact of varicella vaccine on varicella-zoster virus dynamics. Clin Microbiol Rev 2010; 23: 202-17; PMID:20065330; http://dx.doi.org/10.1128/CMR.00031-09
- Shah SS, Wood SM, Luan X, Ratner AJ. Decline in varicella-related ambulatory visits and hospitalizations in the United States since routine immunization against varicella. Pediatr Infect Dis J 2010; 29: 199-204; PMID:19949362; http://dx.doi.org/10.1097/INF.0b013e3181bbf2a0
- Yih WK, Brooks DR, Lett SM, Jumaan AO, Zhang Z, Clements KM, Seward JF. The incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System (BRFSS) during a period of increasing varicella vaccine coverage, 1998–2003. BMC Public Health 2005; 5: 68; PMID:15960856; http://dx.doi.org/10.1186/1471-2458-5-68
- Brisson M, Edmunds WJ. Impact of model, methodological, and parameter uncertainty in the economic analysis of vaccination programs. Med Decis Making 2006; 26: 434-46; PMID:16997923; http://dx.doi.org/10.1177/0272989X06290485
- Gil-Prieto R, Walter S, Gonzalez-Escalada A, Garcia-Garcia L, Marin-Garcia P, Gil-de-Miguel A. Different vaccination strategies in Spain and its impact on severe varicella and zoster. Vaccine 2014; 32: 277-83; PMID:24275483; http://dx.doi.org/10.1016/j.vaccine.2013.11.008
- Russell ML, Schopflocher DP, Svenson L, Virani SN. Secular trends in the epidemiology of shingles in Alberta. Epidemiol Infect 2007; 135: 908-13; PMID:17291380; http://dx.doi.org/10.1017/S0950268807007893
- Stein M, Cohen R, Bromberg M, Tasher D, Shohat T, Somekh E. Herpes zoster in a partially vaccinated pediatric population in central Israel. Pediatr Infect Dis J 2012; 31: 906-9; PMID:22627868; http://dx.doi.org/10.1097/INF.0b013e31825d33f9
- Tanuseputro P, Zagorski B, Chan KJ, Kwong JC. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine 2011; 29: 8580-4; PMID:21939721; http://dx.doi.org/10.1016/j.vaccine.2011.09.024
- Civen R, Chaves SS, Jumaan A, Wu H, Mascola L, Gargiullo P, Seward JF. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J 2009; 28: 954-9; PMID:19536039; http://dx.doi.org/10.1097/INF.0b013e3181a90b16
- Hales CM, Harpaz R, Joesoef MR, Bialek SR. Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann Intern Med 2013; 159: 739-45; PMID:24297190; http://dx.doi.org/10.7326/0003-4819-159-11-201312030-00006
- Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992–2002. J Infect Dis 2005; 191: 2002-7; PMID:15897984; http://dx.doi.org/10.1086/430325
- Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993–2006: evaluation of impact of varicella vaccination. Clin Infect Dis 2011; 52: 332-40; PMID:21217180; http://dx.doi.org/10.1093/cid/ciq077
- Mullooly JP, Riedlinger K, Chun C, Weinmann S, Houston H. Incidence of herpes zoster, 1997–2002. Epidemiol Infect 2005; 133: 245-53; PMID:15816149; http://dx.doi.org/10.1017/S095026880400281X
- Carville KS, Riddell MA, Kelly HA. A decline in varicella but an uncertain impact on zoster following varicella vaccination in Victoria, Australia. Vaccine 2010; 28: 2532-8; PMID:20117265; http://dx.doi.org/10.1016/j.vaccine.2010.01.036
- Heywood AE, Wang H, Macartney KK, McIntyre P. Varicella and herpes zoster hospitalizations before and after implementation of one-dose varicella vaccination in Australia: an ecological study. Bull World Health Organ 2014; 92: 593-604; PMID:25177074; http://dx.doi.org/10.2471/BLT.13.132142
- Jardine A, Conaty SJ, Vally H. Herpes zoster in Australia: evidence of increase in incidence in adults attribu to varicella immunization? Epidemiol Infect 2011; 139: 658-65; PMID:20727248; http://dx.doi.org/10.1017/S0950268810001949
- Ladhani SN, Slack MP, Andrews NJ, Waight PA, Borrow R, Miller E. Invasive pneumococcal disease after routine pneumococcal conjugate vaccination in children, England and Wales. Emerg Infect Dis 2013; 19: 61-8; PMID:23259937; http://dx.doi.org/10.3201/eid1901.120741
- Weinmann S, Chun C, Schmid DS, Roberts M, Vandermeer M, Riedlinger K, Bialek SR, Marin M. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005–2009. J Infect Dis 2013; 208: 1859-68; PMID:23922376; http://dx.doi.org/10.1093/infdis/jit405
- Chao DY, Chien YZ, Yeh YP, Hsu PS, Lian IB. The incidence of varicella and herpes zoster in Taiwan during a period of increasing varicella vaccine coverage, 2000–2008. Epidemiol Infect 2012; 140: 1131-40; PMID:21906410; http://dx.doi.org/10.1017/S0950268811001786
- Goldman GS. Incidence of herpes zoster among children and adolescents in a community with moderate varicella vaccination coverage. Vaccine 2003; 21: 4243-9; PMID:14505905; http://dx.doi.org/10.1016/S0264-410X(03)00459-6
- Goldman GS. Universal varicella vaccination: efficacy trends and effect on herpes zoster. Int J Toxicol 2005; 24: 205-13; PMID:16126614; http://dx.doi.org/10.1080/10915810591000659
- Kelly HA, Grant KA, Gidding H, Carville KS. Decreased varicella and increased herpes zoster incidence at a sentinel medical deputising service in a setting of increasing varicella vaccine coverage in Victoria, Australia, 1998 to 2012. Euro Surveill 2014; 19
- Patel MS, Gebremariam A, Davis MM. Herpes zoster-related hospitalizations and expenditures before and after introduction of the varicella vaccine in the United States. Infect Control Hosp Epidemiol 2008; 29: 1157-63; PMID:18999945; http://dx.doi.org/10.1086/591975
- Wu PY, Wu HD, Chou TC, Sung FC. Varicella vaccination alters the chronological trends of herpes zoster and varicella. PLoS One 2013; 8: e77709; PMID:24204928; http://dx.doi.org/10.1371/journal.pone.0077709
- Purdy KW, Hay JW, Botteman MF, Ward JI. Evaluation of strategies for use of acellular pertussis vaccine in adolescents and adults: a cost-benefit analysis. Clin Infect Dis 2004; 39: 20-8; PMID:15206048; http://dx.doi.org/10.1086/421091
- Caro JJ, Getsios D, El-Hadi W, Payne K, O'Brien JA. Pertussis immunization of adolescents in the United States: an economic evaluation. Pediatr Infect Dis J 2005; 24: S75-82; PMID:15876932; http://dx.doi.org/10.1097/01.inf.0000160918.72953.51
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol 2010; 17: 1055-65; PMID:20463105; http://dx.doi.org/10.1128/CVI.00131-10
- Cherry JD. Why do pertussis vaccines fail? Pediatrics 2012; 129: 968-70; PMID:22529282; http://dx.doi.org/10.1542/peds.2011-2594
- John TJ, Samuel R. Herd immunity and herd effect: new insights and definitions. Eur J Epidemiol 2000; 16: 601-6; PMID:11078115; http://dx.doi.org/10.1023/A:1007626510002
- Moulton LH, Chung S, Croll J, Reid R, Weatherholtz RC, Santosham M. Estimation of the indirect effect of Haemophilus influenzae type b conjugate vaccine in an American Indian population. Int J Epidemiol 2000; 29: 753-6; PMID:10922355; http://dx.doi.org/10.1093/ije/29.4.753
- Atkins KE, Shim E, Pitzer VE, Galvani AP. Impact of rotavirus vaccination on epidemiological dynamics in England and Wales. Vaccine 2012; 30: 552-64; PMID:22133508; http://dx.doi.org/10.1016/j.vaccine.2011.11.064
- Tu HA, Rozenbaum MH, de Boer PT, Noort AC, Postma MJ. An update of “Cost-effectiveness of rotavirus vaccination in the Netherlands: the results of a Consensus Rotavirus Vaccine model”. BMC Infect Dis 2013; 13: 54; PMID:23363553; http://dx.doi.org/10.1186/1471-2334-13-54
- Rozenbaum MH, Mangen MJ, Giaquinto C, Wilschut JC, Hak E, Postma MJ. Cost-effectiveness of rotavirus vaccination in the Netherlands; the results of a consensus model. BMC Public Health 2011; 11: 462; PMID:21663620; http://dx.doi.org/10.1186/1471-2458-11-462
- Standaert B, Gomez JA, Raes M, Debrus S, Velazquez FR, Postma MJ. Impact of rotavirus vaccination on hospitalisations in Belgium: comparing model predictions with observed data. PLoS One 2013; 8: e53864; PMID:23349754; http://dx.doi.org/10.1371/journal.pone.0053864
- Shim E, Galvani AP. Impact of transmission dynamics on the cost-effectiveness of rotavirus vaccination. Vaccine 2009; 27: 4025-30; PMID:19389452; http://dx.doi.org/10.1016/j.vaccine.2009.04.030
- Aballea S, Millier A, Quilici S, Caroll S, Petrou S, Toumi M. A critical literature review of health economic evaluations of rotavirus vaccination. Hum Vaccin Immunother 2013; 9: 1272-88; PMID:23571226; http://dx.doi.org/10.4161/hv.24253
- Lopman BA, Curns AT, Yen C, Parashar UD. Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis 2011; 204: 980-6; PMID:21878425; http://dx.doi.org/10.1093/infdis/jir492
- Marty R, Roze S, Bresse X, Largeron N, Smith-Palmer J. Estimating the clinical benefits of vaccinating boys and girls against HPV-related diseases in Europe. BMC Cancer 2013; 13: 10; PMID:23298365; http://dx.doi.org/10.1186/1471-2407-13-10
- Sando N, Kofoed K, Zachariae C, Fouchard J. A Reduced National Incidence of Anogenital Warts in Young Danish Men and Women after Introduction of a National Quadrivalent Human Papillomavirus Vaccination Programme for Young Women - An Ecological Study. Acta Derm Venereol 2014; 94(3):288-92.
- Brisson M, Laprise JF, Drolet M, Van de Velde N, Franco EL, Kliewer EV, Ogilvie G, Deeks SL, Boily MC. Comparative cost-effectiveness of the quadrivalent and bivalent human papillomavirus vaccines: a transmission-dynamic modeling study. Vaccine 2013; 31: 3863-71; PMID:23830974; http://dx.doi.org/10.1016/j.vaccine.2013.06.064
- Van de Velde N, Brisson M, Boily MC. Understanding differences in predictions of HPV vaccine effectiveness: A comparative model-based analysis. Vaccine 2010; 28: 5473-84; PMID:20573580; http://dx.doi.org/10.1016/j.vaccine.2010.05.056
- Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis 2007; 13: 28-41; PMID:17370513; http://dx.doi.org/10.3201/eid1301.060438
- Kahn JA, Brown DR, Ding L, Widdice LE, Shew ML, Glynn S, Bernstein DI. Vaccine-type human papillomavirus and evidence of herd protection after vaccine introduction. Pediatrics 2012; 130: e249-56; PMID:22778297; http://dx.doi.org/10.1542/peds.2011-3587
- Bauch CT, Rao AS, Pham BZ, Krahn M, Gilca V, Duval B, Chen MH, Tricco AC. A dynamic model for assessing universal Hepatitis A vaccination in Canada. Vaccine 2007; 25: 1719-26; PMID:17229493; http://dx.doi.org/10.1016/j.vaccine.2006.11.020
- Taranger J, Trollfors B, Bergfors E, Knutsson N, Sundh V, Lagergard T, Lind-Brandberg L, Zackrisson G, White J, Cicirello H, et al. Mass vaccination of children with pertussis toxoid–decreased incidence in both vaccinated and nonvaccinated persons. Clin Infect Dis 2001; 33: 1004-10; PMID:11528572; http://dx.doi.org/10.1086/322639
- Lee GM, Murphy TV, Lett S, Cortese MM, Kretsinger K, Schauer S, Lieu TA. Cost effectiveness of pertussis vaccination in adults. Am J Prev Med 2007; 32: 186-193; PMID:17296470; http://dx.doi.org/10.1016/j.amepre.2006.10.016