Abstract
All currently available Streptococcus pneumoniae (Spn) vaccines have limitations due to their capsular serotype composition. Both the 23-valent Spn polysaccharide vaccine (PPV) and 7, 10, or 13-valent Spn conjugate vaccines (PCV-7, 10, -13) are serotype-based vaccines and therefore they elicit only serotype-specific immunity. Emergence of replacement Spn strains expressing other serotypes has consistently occurred following introduction of capsular serotype based Spn vaccines. Furthermore, capsular polysaccharide vaccines are less effective in protection against non-bacteremic pneumonia and acute otitis media (AOM) than against invasive pneumococcal disease (IPD). These shortcomings of capsular polysaccharide-based Spn vaccines have created high interest in development of non-serotype specific protein-based vaccines that could be effective in preventing both IPD and non-IPD infections. This review discusses the progress to date on development of Spn protein vaccine candidates that are highly conserved by all Spn strains, are highly conserved, exhibit maximal antigenicity and minimal reactogenicity to replace or complement the current capsule-based vaccines. Key to development of a protein based Spn vaccine is an understanding of Spn pathogenesis. Based on pathogenesis, a protein-based Spn vaccine should include one or more ingredients that reduce NP colonization below a pathogenic inoculum. Elimination of all Spn colonization may not be achievable or even advisable. The level of expression of a target protein antigen during pathogenesis is another key to the success of protein based vaccines.. As with virtually all currently licensed vaccines, production of a serum antibody response in response to protein based vaccines is anticipated to provide protection from Spn infections. A significant advantage that protein vaccine formulations can offer over capsule based vaccination is their potential benefits associated with natural priming and boosting to all strains of Spn. One of the most universal and comprehensive approaches of identifying novel vaccine candidates is the investigation of human sera from different disease stages of natural infections. Antigens that are robustly reactive in preliminary human serum screening constitute a pathogen-specific antigenome. This strategy has identified a number of Spn protein vaccine candidates that are moving forward in human clinical trials.
Introduction
Streptococcus pneumoniae (Spn), an encapsulated gram-positive diplococcus, is responsible for a wide spectrum of infections in children and adults including invasive (meningitis, bacteremia and bacteremic pneumonia) and non-invasive infections (non-bacteremic pneumonia, otitis media, sinusitis and conjunctivitis).Citation1 Invasive Spn infections (IPD) are a major cause of morbidity and mortality.Citation2-5 It was estimated in 2008 that 333000–529000 children aged < 5 years died of Spn infections worldwide.Citation6
The capsular polysaccharide has been considered the most important virulence factor and target for vaccines to prevent Spn infections.Citation7 To date, 94 distinct capsular serotypes have been identified based on polysaccharide composition, including most recently reported serotypes 11E, 20A/B.Citation8-11 Two types of Spn vaccines are presently used for prevention of Spn infections: a 23-valent Spn polysaccharide vaccine (PPV-23) and 7, 10, or 13-valent Spn conjugate vaccines (PCV-7, 10, -13). PPV-23 includes serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9F, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F. PPV-23 is widely recommended as a single dose for person ≥65 years old and for persons aged 2–65 years with high risk for Spn diseases. However, because PPV-23 is poorly immunogenic in children < 2 years of age, fails at any age to generate an immune memory anamnestic response upon revaccination, and does not generate herd immunity, alternative capsular-protein conjugate vaccines were introduced in year 2000.Citation6 PCV-7, PCV-10 and PCV-13 are currently licensed for prevention of pneumococcal infections caused by the serotypes included in the vaccines in children from 6 weeks to 5 years of age. PCV-7 includes serotypes 4, 6B, 9V, 14, 18C, 19F, 23F. PCV-10 includes serotypes PCV7+ 1, 5, 7F, 23F and PCV-13 includes PCV10 + 3, 6A, 19A.Citation12 PCV-7 is gradually being removed from the market in developed countries.Citation1,6
All currently available Spn vaccines have limitations due to their capsular serotype composition (). Both PPV and PCVs are serotype-based vaccines and therefore they elicit only serotype-specific immunity. Emergence of replacement Spn strains expressing other serotypes has consistently occurred following introduction of capsular serotype based Spn vaccines.Citation9,13,14 For instance, after introduction of PCV-7, IPD and acute otitis media (AOM) cases caused by serotype 19A dramatically increased, including emergence of a strain that was resistant to all antibiotics approved for children.Citation9,15,16 Furthermore, capsular polysaccharide vaccines are less effective in protection against non-bacteremic pneumonia and AOM than against IPD.Citation3,17 There has been only a marginal decline in overall AOM rate in child populations since introduction of PCV13. However serotype specific reductions in AOM caused by Spn strains expressing the 6 new serotypes included in PCV13 have not been demonstrated. Such a study is ongoing with results expected in 2016 (Pichichero et al, unpublished). These shortcomings of capsular polysaccharide-based Spn vaccines has created high interest in development of non-serotype specific protein-based vaccines that could be effective in preventing both IPD and non-IPD infections.Citation3,5,18 Therefore, research scientists from academia and industry have been seeking Spn protein vaccine candidates that are universally expressed by all Spn strains, are highly conserved, exhibit maximal antigenicity and minimal reactogenicity to replace or complement the current capsule-based vaccines.Citation3,5,19,20 This review discusses the progress to date.
Table 1. Limitations of current licensed pneumococcal vaccines
Key to Development of a Protein Based Spn Vaccine is an Understanding of Spn Pathogenesis
Spn is a commensal of the nasopharynx (NP) and only becomes pathogenic when changes in the NP, typically caused by viral upper respiratory infections, allow the organism to achieve a pathogenic inoculum that overcomes innate and adaptive host defense.Citation21-23 Spn carriage is most prevalent in vulnerable populations such as young children, immunocompromised adults, and the elderly.Citation24,25 In children under 5 years of age, Spn can be detected in the NP from 10% up to 90% of children with the variation mostly attributable to the frequency of NP sampling, and study population.Citation26 The burden of non-invasive and invasive Spn disease correlates with the NP carriage rate.Citation24,27,28 Children in developing countries generally have a higher carriage rate and density of organisms than in developed countries. Spn colonization is an immunizing event that leads to the generation of antigen specific antibodies and cellular immunity, and is therefore beneficial for the host.Citation29-33 However, colonization is also a prerequisite for Spn diseases.Citation24 The strategy currently guiding vaccination with polysaccharide-based conjugate vaccines seeks to prevent Spn disease by completely eliminating colonization.Citation34–36 This has been an overwhelming success with respect to the Spn serotypes included in the vaccines.
Based on pathogenesis, a protein-based Spn vaccine should include one or more ingredients that reduce NP colonization below a pathogenic inoculum. Elimination of all Spn colonization may not be achievable or even advisable. Therefore, the challenge before vaccinologists is to define the NP carriage threshold below which perturbations by viral co-infections will not enable Spn to make the transition to invasive pathogen.
Expression of Proteins During Pathogenesis
The level of expression of a target protein antigen during pathogenesis is key to the success of protein based vaccines. It is therefore imperative to have a detailed expression analysis of candidate vaccine antigenic components during both healthy and disease conditions. Unlike constitutively-expressed capsular antigens, the expression of any given Spn protein may vary considerably during colonization compared to when the organism is locally or systemically invasive. An adequate and functional mucosal immune response to protein antigens requires expression of the target antigen by Spn in order for the response to be protective.
Host Inflammatory and Bacterial Virulence Factors
From the standpoint of modern vaccination strategies it is imperative to comprehensively understand the host inflammatory and bacterial virulence factors in the polymicrobial NP environment. Spn synergistically or antagonistically interacts with other upper respiratory bacteria and viruses and the commensal flora in the NP environment. The dynamics of this interaction modulates the transition of Spn from a commensal to a disease phenotype.Citation37-41
Bacterial-viral interaction plays a critical role in pathogenesis of upper and lower respiratory infections. Spn infection almost always follows a viral upper respiratory infection (URI). Therefore animal co-infection models have been used to study co-pathogenesis of Spn with viruses such as human influenza virus,Citation42-47 respiratory syncytial virus,Citation48 adenovirus,Citation49,50 metapneumovirus,Citation51 parainfluenza virus.Citation52
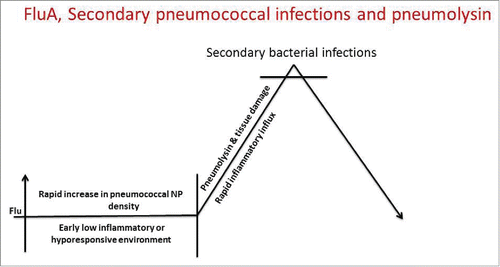
The most well-investigated bacterial-viral interaction is the synergism between influenza virus and Spn Citation23 and the interaction has been shown to be strongly associated with Spn dissemination from the NP and subsequent local or systemic invasion.Citation22,53,54 Influenza dampens host innate immunity, compromising innate immune surveillance and allows Spn to rapidly increase in inoculum in the NP, a prerequisite for invasion.Citation54-57 The prolonged low inflammatory environment during a viral URI permits a subsequent rapid increase in the Spn population, followed by pneumolysin-mediated tissue damage and a robust pro-inflammatory response that sets the stage for pathogenesis ( ).
In the human, apart from well described Spn-viral pathogenesis synergy, other potential determinants of Spn carriage transition to pathogen include host genetics,Citation58 environmental exposures, e. g. tobacco smoke and air particulates,Citation59,60 antibiotic use, and variation in the microbiome.Citation61 Spn has an antagonistic relationship with Staphylococcus aureus in the NP.Citation62 Vaccination induced decreases in Spn colonization have been associated with an increased incidence of S. aureus-induced carriage in children.Citation63 An interaction of Spn with Haemophilus influenza (Hi) has also been shown in humans whereby the latter organism outcompetes Spn except when Spn expresses the 19A serotype capsule.Citation64 Also, co-colonization with Spn and Hi enhances serum antibody responses against Spn antigens, modulating pathogen specific adaptive immunity. In contrast, the presence of Spn does not change Hi specific immunity.Citation38 These data suggest an antagonistic relationship in humans between Spn and Hi in the NP. Introduction of Spn protein vaccines therefore will require a comprehensive examination of alterations in the balance among Spn, Hi, S. aureus and likely Moraxella catarrhalis.
Innate Immunity Especially in Early Life
The fundamental role for the innate immune system in sensing vaccines and adjuvants and in programming protective adaptive immune responses has been increasingly recognized.Citation65 It is now clear that activation of innate immunity by particular adjuvants plays a major role in determining the magnitude and quality of the adaptive immune response following vaccination.Citation65 Data from mouse models suggest that infant mice are more susceptible to Spn colonization than adult mice, as evidenced by higher densities and longer durations of colonization.Citation66 Macrophages are an indispensable innate effector cell subset for carriage clearance, and neonatal macrophages have been shown to produce significantly less cytokines like IL-8/KC, IL-1α, TNF-α and IFN-γ.Citation66 These are important inflammatory mediators involved in immune defense against Spn. In contrast, neonatal macrophages have been found to produce elevated levels of the anti-inflammatory cytokine IL-10 compared to adults.Citation66
In humans, a number of studies have highlighted that compromised TLR dependent signaling leading to low pro-inflammatory responses in early life. The overall pattern of response deficiency has been reported among infants (). Neonatal innate immune cells including monocytes, plasmacytoid dendritic cells, and conventional dendritic cells produce less interleukin (IL)-12p70 and type I interferon, and similar or higher levels of IL-1β, IL-6, IL-23, and IL-10 than adult cells when stimulated by the same pattern recognition receptor (PRR) stimulants.Citation67 In addition to compromised cytokine responses, neonatal cells have also been shown to exhibit relatively limited ability to produce multiple cytokines in polyfunctional responses to PRR stimulation.Citation68 Perinatal IL-10 production decreases in concert with increasing pro-inflammatory responses (such as TNF-α and IL-1β) over the first few years of life while neonatal antiviral cytokine responses (type I IFN) reach adult levels within the first month after birth.Citation69 Underlying patterns of immune ontogeny lead to windows of vulnerability to different types of infection during different stages of development. Human genetic and environmental factors may also have a profound impact on the magnitude and quality of these responses.Citation70 Innate immune ontogeny in early life could significantly be influenced by environment, overall microbial burden and genetic factors like polymorphisms in key innate effector genes. Knowledge of innate immunity in different age groups and different populations informs on questions of anticipating different results following Spn protein based vaccination. Populations in developing countries, elderly and young children with poor innate responses may demonstrate a less robust adaptive response than populations in developed countries and older children and adults. Alternative adjuvants may be needed.Citation71
Adaptive Immunity: Assessment of Antigen Specific Functional Immune Response
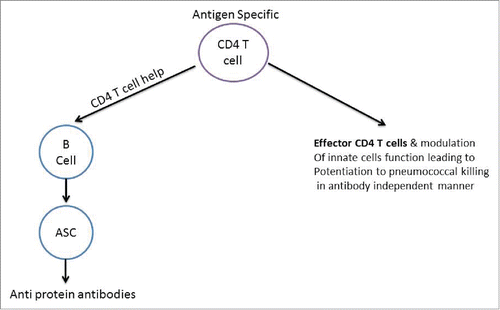
As with virtually all currently licensed vaccines, production of a serum antibody response in response to protein based vaccines is anticipated to provide protection from Spn infections.Citation72 Moreover, a correlate of protection in IgG antibody levels should be sought that can provide a signature of immunity. An independent mucosal IgA response to systemic vaccination can occur but usually sustained immune memory at the mucosal level is not observed. Protection from Spn infections following protein based vaccination therefore likely will be mediated by serum antibody with transudation of IgG to mucosal sites such as the middle ear, sinuses, eye and lung. However, antigen specific CD4 T cells can also contribute to Spn clearance in an antibody independent manner Citation73 so those cellular responses will also require characterization.
It has been well documented that specific anti-Spn antibody levels induced by polysaccharide-based vaccines correlate well with opsonophagocytic/bactericidal antibodies.Citation74,75 Anti-capsular opsonophagocytic assay (OPA) constitutes the main tool for evaluating the functional immune response as a correlate of protection against Spn diseases.Citation76 Unlike capsular opsonophagocytic antibodies, protein based vaccines are hypothesized to have different protective mechanisms against Spn carriage and invasiveness. Anti-adhesin antibodies are expected to reduce carriage density in the NP. We would not expect that blocking one Spn adhesin would eliminate carriage entirely since Spn expresses multiple adhesins and the surface expression of adhesins occupies far less physical space on the surface of Spn compared to the polysaccharide capsule. A multi-epitope approach involving a number of adhesins in one formulation could potentially block NP carriage to a greater extent. We endorse this particular approach as a next generation vaccination strategy.
Protein based Spn vaccines will surely target infants an especially vulnerable population. Though human neonates are capable of mounting T-cell responses, CD4 T-cell responses in early life have a predisposition to T helper-2 (Th2).Citation77,78 Th1 responses are less well developed and reach adult levels only after about 2 years of ageCitation77,78 (). Epigenetic programming is potentially an important factor in the differentiation of naïve and memory T cells, and a precise understanding of the involvement of epigenetic factors in immune ontogeny will have lasting implications for health and disease.Citation68,79 A higher proportion and homing of Tregs in neonatal lymph nodes also forms an important immunosuppressive response leading to the persistence of microbial flora in mucosal areas.Citation80 This is a strategy the host adapts in order to retain commensal bacteria at mucosal surfaces in order to prevent the colonization and persistence of unwanted bacteria in these environments. It also helps the host to prevent inflammation-induced pathology. Although these immune effector and regulatory responses are developed in early life, their balance is continually altered with age.Citation81
A growing body of evidence in mouse models of NP colonization suggests an indispensable contribution of Th17 cells in host defense. Prominently, B cell deficient mice were able to clear bacterial colonization, demonstrating the importance of CD4 T cell subsets in mouse colonization model.Citation73,82 In addition to defense against colonization, substantial evidence of their involvement in lung defense has also been found in both experimental mouse models and human studies in the context of Spn.Citation32,82-84 In our ongoing work, we found infant mice elicit a less robust natural Th17 response after Spn colonization, and this deficiency potentially reduces their ability to fight subsequent Spn lung infection (unpublished data). Therefore, the Th17 CD4+ T cell subset has emerged as a crucial component of the host response against Spn and will need to be studied as part of Spn protein-based vaccines.
Genocea (Cambridge, MA, USA) is developing a Spn whole cell-based vaccine that targets stimulation of Th17 cells. Th17 cells are characterized by the production of the signature cytokines IL-17A and IL-22.Citation85 While IL-17A enhances the recruitment and function of innate effector cells, IL-22 is an important mediator for epithelial proliferation, repair and integrity at mucosal lining.Citation83,86,87 The balance of IL-17A+/IL-22+ could be instrumental in an infection or immunization setting as IL-17A+/IL-22+ double positive cells have been found to be of more profound biological significance than IL-17A+ single positive cells.Citation88 While IL-17A+ cells form an important protective host component, IL-17A-induced immunopathology has been reported in the context of infection and autoimmunity.Citation89 Therefore, immunization strategies aiming at eliciting Th17 responses involving both IL-17A and IL-22 could be of more clinical significance. Ontogenic factors in early life that are involved in the differentiation of antigen specific Th17 subsets are required to be better understood in order to design next-generation Th17 based vaccination strategies against microbial pathogens including Spn.
With regard to infant vaccination, in our prospective studies we have seen little or no antigen specific or global CD4 T cell IL-17A response in children during the first year of life and only moderate responses in children age 1–2 years. A number of previous reports using mouse cells have demonstrated that although neonatal cells can have an apparently normal innate inflammatory microenvironment that supports Th17 differentiation, they still produce less IL-17A.Citation90–92 It is therefore plausible that intrinsic early life CD4 T cell defects may limit acquisition of the Th17 phenotype. In a recent study, PBMCs from Bangladesh young children were found to produce levels of IL-17A equivalent to adults. More interestingly, their overall IL-17A responses were higher than the response from Swedish children, and, furthermore, overall IL-17A responses from Swedish children were lower than in Swedish adults.Citation93 These observations suggest that young children in a developing country (Bangladesh) differ from young children in a developed country (Sweden) and such differences may impact successful vaccination. It may be that different microbial burdens and divergent immune priming in different geographies leads to differential Th17 responses.Citation78,94 Understanding the epigenetic changes in these developing and developed country populations driving these responses could help develop adjuvants to enhance immune priming in early life.
Importance of Natural Priming and Boosting in Different Populations/Geographies
Spn colonization in the NP is an immunizing event that elicits serum and mucosal antibody responses and cellular immunity that contributes to host protection against subsequent colonization episodes.Citation32,33 However, the immunity induced by natural colonization may not persist as long as vaccine induced immunity. PPV and PCV vaccination strategies are based on complete elimination of carriage in the NP and subsequent low cross reactivity of vaccine serotypes with non-vaccine serotypes.Citation95
A significant advantage that protein vaccine formulations can offer over capsule based vaccination is their potential benefits associated with natural priming and boosting to all strains of Spn. Protein based vaccines are not expected to eliminate NP carriage; rather protein-based vaccines will prevent increased in Spn carriage density below disease threshold. Thus, protein-based vaccines which may elicit stronger priming may also elicit stronger boosting of vaccine induced memory responses to help maintain stable protective immunity over the long term. However, this effect may not manifest the same way in resource poor countries with a high Spn bacterial burden. It has been reported that persistently high antigenic exposure might lead to immune hyporesponsiveness resulting in low vaccine efficacy, since antigen specific T cells can lose their function and become anergic in response to persistently high antigenic exposure.Citation96 Protein based antigens are based on eliciting antigen specific CD4 T cell responses that help B cells to differentiate into antibody secreting cells (). A loss of T cell function would therefore lead to poor B cell responses. Vaccine development and deployment strategies will therefore require a more comprehensive understanding of various factors that might influence vaccine responses in a particular population/geography.
Animal Models
Animals including mouse,Citation97-100 rat,Citation101 chinchilla,Citation102 rabbit,Citation103 monkeys,Citation104 ferret,Citation105 and gerbil Citation106 have been employed for studying pathogenesis of Spn diseases, assessing host response to Spn carriage and infections, identifying virulence genes, and testing the efficacy of vaccines against Spn disease.Citation107,108 Due to low cost, ease of genetic manipulation and availability of ex vivo reagents for mechanism studies, the mouse has become the model of choice for IPD.Citation108 The chinchilla was historically the preferred model for otitis media (OM) due to its large bullae which makes it easier for both inoculation of Spn and collection of middle ear fluid (MEF) samples, however, in recent years, the mouse has become the preferred model for OM research.Citation108,109
Antigenome Approach to Identify Protein Vaccine Candidates
One of the most universal and comprehensive approaches of identifying novel vaccine candidates is the investigation of human sera from different disease stages of natural infections. Antigens that are robustly reactive in preliminary human serum screening constitute a pathogen-specific antigenome.Citation110 The antigens are subsequently characterized, individually cloned and purified. The immunogenicity and protective efficacy of these antigens is tested in a number of animal disease models in order to ascertain the possibility of using them as potential vaccine candidates. This is especially important since a number of microbial antigens could be reactive in human sera due to their phylogenetic differences. In addition, a number of these antigens might not be surface expressed, which is necessary for vaccine candidates apart from secreted toxins like pneumolysin. The protein antigens that are currently being investigated have the potential to address the needs of both children and the elderly by being exceptionally conserved among all serotypes and highly immunogenic.Citation31,111-114
Spn Protein Vaccine Components
In a 10 year long prospective study in children age 6–36 months old, we have made a comprehensive investigation of 5 Spn proteins considered potential vaccine candidates. Three of the antigens have transitioned to clinical trials in single- and multi-component vaccine formulations.
Histidine triad proteins PhtD and PhtE belong to a well-conserved Pht protein family expressed by Spn.Citation111 They are surface exposed proteins characterized mainly by a histidine triad motif.Citation111 In animal models, both of these proteins have been shown to elicit protection against sepsis, pneumonia and colonization.Citation113,115,116 We have demonstrated their role as Spn adhesins that are required for attachment to NP and lung epithelial cells.Citation112 Spn attachment to NP epithelium is a first step in establishing carriage and potential for pathogenesis and attachment to lung epithelia must occur for Spn to cause pneumonia.Citation24 Among the Pht family proteins (PhtD, PhtE, PhtA, PhtB), PhtD has been shown to display the least variability, and is expressed by all Spn strains screened thus far, while PhtE has been PhtD is expressed by 100% of Spn strains studied to date whereas has been found to be expressed by 97% of strains Citation111 (). All 5 Pht proteins elicit serum antibody that is cross-reactive to some degree.
Table 2. Characteristic components of Spn Protein Vaccine Candidates
PcpA is a highly prevalent protein antigen of Spn. In mouse models we and others have demonstrated a predominant role for PcpA in lung defense with very little or no involvement in NP colonization.Citation117 Higher manganese levels in the NP environment of the mouse have been discussed as a negative regulator of PcpA expression.Citation117 However, our clinical studies have shown that PcpA elicits robust serum antibody responses during asymptomatic colonization in young childrenCitation31,114 and that during a viral URI the concentration of manganese drops to undetectable in many children due to dilution occurring with rhinorrhea (Kaur and Pichichero, unpublished results ().
Pneumolysin (Ply), a 53 kDa toxin, is a key virulence factor of Spn that is expressed by virtually all clinically relevant serotypes.Citation118 Its detoxified derivative is a leading component of Spn protein based vaccines currently being developed. GlaxoSmithKline has detoxified Ply chemically and Sanofi Pasteur has detoxified Ply by genetic substitution.Citation119,120 At sublytic concentrations, Ply activates host innate immune responses including TLR4 and the NLRP3 inflammasome.Citation121-123 However, at higher concentrations Ply is toxic to host cells and is associated with immunopathology.Citation124 Therefore, increas-ed expression of Ply in the host is considered to be associated with Spn pathogenesisCitation125 (). This supports the observation that an increase in density of Spn in the NP is a precursor to disease. Therefore, vaccination strategies that do not completely eliminate carriage but reduce its density to sub optimal level should carefully orchestrate the inflammatory environment in NP such that it would favor the host and harness the beneficial aspects of Ply.
We contend that candidate vaccine antigens should be evaluated for elicitation of a naturally-induced immune response in the target population(s) intended for vaccination. Proof that a vaccine candidate stimulates serum and mucosal antibody and B and T cell memory responses in young children would indicate the potential for natural priming of the immune system for a vaccine response and natural boosting of vaccine-induced responses. Therefore in a prospective clinical study we evaluated PhtD, PhtE, PcpA, LytB and Ply for their potential to stimulate naturally induced antibodies in 6–30 month old children. We found NP colonization was an immunizing event for PhtD, PhtE, PcpA, and Ply but much less so for LytB.Citation31 Elicited serum antibodies were higher in children that were colonized with Spn but rose in all children most likely indicating that NP colonization events occurred but were not detected due to the program of every 3 month prospective samplings.Citation126 PcpA IgG serum titers were the highest followed by PhtD and PhtE titers were equivalent followed by Ply and lastly LytB.Citation126 Subsequently we tested the 5 antigens for their capacity to stimulate antigen specific memory CD4 T and B cell responses and found the same rank-order of potency (PcpA > PhtD = PhtE > Ply > LytB).Citation127
Based in the results generated by our group and other considerations Sanofi Pasteur transitioned to clinical trials in humans with 3 protein candidates–PhtD, PcpA and PlyD1 (genetically detoxified Ply) for monovalent, bivalent and trivalent protein based vaccines. GlaxoSmithKline has ongoing clinical trials in humans using vaccines containing PhtD and Ply (chemically detoxified).
Mucosal vs. Serum Antibody; Transudate in the NP and MEF
Spn colonization is a necessary pre-requisite for Spn infections. NP carriage of Spn is mostly asymptomatic.Citation17 Spn carriage as an immunizing event elicits serum and mucosal antibody response to Spn protein vaccine candidates. A robust systemic immune response against protein antigens results in transudation of serum antibodies to mucosal sites such as NP and middle ear, which constitutes a major defense component. We have recently shown a correlation of higher mucosal antibody titers against PhtD, PcpA and PlyD1 with a reduction in acute otitis media caused by Spn.Citation128 In addition to serum transudate antibodies at mucosal sites, active upper respiratory mucosal tissues such as tonsils and adenoids are mucosal inductive secondary lymphoid sites and contributors to local antibody production in the NP. A number of Spn protein antigens have been shown to elicit robust mucosal responses in an infection or ex-vivo stimulation setting.Citation129
Numerous previous studies show serum antibodies to Spn proteins rise following Spn carriage,Citation31,38,130–132 however the naturally acquired antibody levels in serum are not directly associated with reduced subsequent carriage.Citation132 Holmlund E et al (2006)Citation130 reported an increase in serum antibody concentrations to Spn surface adhesin A and pneumolysin in infants who were colonized with Spn. Simell B et al showed that children with prior positive NP cultures for Spn had significantly higher serum anti-CbpA and anti-PhtD IgG.Citation131 Prevaes SM et al reported that colonization with Spn induced serum IgG against 14 Spn proteins.Citation132 Our group reported that colonization elicits serum IgG and IgA responses to PhtD, PcpA, and PlyD1 Citation38,133 More recently we established a correlation between sera antibody titers to these proteins and protection of occurrence of Spn AOM (Unpublished data).
Mucosal immunity plays a critical role in control of Spn locally invasive infections such as pneumonia, AOM and sinusitis.Citation134 Spn colonization stimulates mucosal antibody levels in nasal secretions to PhtD, PcpA and PlyD1 proteins.Citation38,135 We have shown that higher mucosal antibody levels in the NP to PhtD, PcpA and PlyD1 correlates with reduced risk of AOM in young children caused by Spn.Citation135 We also showed that antibody levels in the NP correlated with serum antibody levels.Citation135
For a vaccine to prevent AOM, we contend that special attention should be given to studies in the infection prone child who has an immune response that is generally deficient compared to non-otitis prone children. We have shown that children who are prone to otitis media have lower antibody levels to PhtD, PhtE, PcpA, LytB and Ply after Spn colonization and AOM. However, among the 5 proteins PcpA stood out as more immunogenic than the others.Citation38,135,136
Functional Antibodies to Spn Protein Candidates
Protein based Spn vaccines currently under evaluation lack a robustly validated functional assay as a correlate of their efficacy. In the context of protein-based vaccines, there is a need for a reliable assay to assess the efficacy of antibodies.
We have applied a flow cytometry based assay to evaluate the functional efficacy of human anti-adhesin PhtD, PhtE and PcpA antibodies in reducing Spn adherence in vitro.Citation112,137 Unlike traditional CFU-based methods, this assay is highly sensitive and robustly reproducible. We are further evaluating the role of aforementioned antigen specific antibodies for their ability to induce opsonophagocytic/bactericidal response and developing assays that can be used as surrogate functionality assays in conjunction with the established adherence assay.
Animal Studies
In order to support our clinical findings, we have also tested the efficacy of PhtD, PhtE, PcpA and Ply vaccine candidates in a number of mouse infection models. Our mouse studies have highlighted the role of PhtD and PhtE in Spn NP colonization.Citation113 In our laboratory we have been studying the effect of vaccination with adhesion proteins of Spn in an Spn-Flu A coinfection model and we have established a carriage density threshold in the NP that is required for local and systemic invasion. Moreover, we found that vaccination with PhtD reduces the density of Spn carriage sufficiently to provide protection in an invasive Spn-Influenza A coinfection model (Khan and Pichichero: unpublished data).
In a mouse co-infection model, co-colonization with H. influenzae results in a rapid depletion of Spn from the NP within 3 days post-inoculation and complete clearance by 2 weeks.Citation57 This is in contrast to Spn colonization without H. influenza that remains stable for 3–4 weeks.Citation57 Based on our ongoing work involving the role of PhtD in a complex Spn-influenza A co-infection model, it is plausible that even PhtD-induced reduction in Spn carriage inocula in the NP would confer indirect benefits by bringing down the level of Ply in the local NP environment (Khan and Pichichero, unpublished data). Because young children are at the greatest risk of Spn infections and a primary target population for vaccination,Citation20 a preferred animal model for vaccine development should be an infant mouse as it more closely mimics the maturing immunological state of young children. Therefore recently an infant murine vaccination model has been developedCitation98 and used to evaluate protection from pneumonia by pneumococcal protein vaccine candidates PhtD, PcpA and Ply.Citation97,98,100
Protein Vaccine Studies in Special Populations/Geographies
In 2012, Sanofi Pasteur commenced (with International Center for Diarrheal Research, Bangladesh) a phase I clinical trial of a vaccine containing three Spn proteins (PcpA, PhtD, PlyD1) in different dosage forms (NCT01764126). This was an observer-blind, randomized, vaccine controlled, vaccine trial to determine the safety and immunogenicity of the vaccine in an age down approach beginning with adults, then toddlers and infants, with data safety review at each step before stepping down to the next age group. In a separate phase I study conducted in Switzerland, promising safety profiles and immunogenicity of monovalent (PhtD or PcpA) and bivalent (PhtD and PcpA) protein vaccine candidates were demonstrated in an adult population.Citation138 In another clinical trial, GSK is working with PATH, the Medical Research Council of The Gambia, and London School of Tropical Medicine and Hygiene in a phase II clinical study in the Gambia of GSK's protein-plus-conjugate vaccine candidate Citation139,140 ().
Table 3. Pneumococcal protein vaccine candidate evaluated in clinical trials
There is empirical evidence to suggest that different populations in different geographical regions might exhibit differences in response to vaccinations. For instance, efficacy of BCG in the prevention of pulmonary TB ranges between 0 and 80%; nearly half of this variability can be explained by population/geographical differences among study sites. For example, clinical trials show good protection against adult pulmonary TB in the UK, but little protection in Malawi.Citation141-143 A recent study compared the ontogeny of TLR induced immune responses from study populations of different population/geographical regions. A significant divergence in innate immune response was noted.Citation68,70 Composition of the NP microflora is a key factor in the transition of Spn from commensal to pathogen, and a substantial difference in the microflora between different populations/geographical regions has been reported.Citation61 Outside first world countries, routine and overarching immunization programs against Flu A, Spn, and Haemophilus influenzae are not performed. This increased microbial burden in the NP along with a number of other crucial factors including malnutrition, poor environmental air quality, and genetics not only predisposes some populations to increased susceptibility for infections but may also lead to compromised vaccine efficacy or vaccine hyporesponsiveness (). Therefore, it is plausible that as explained above in the case of BCG, Spn protein vaccines might have different efficacies in diverse geographical regions having differential upper respiratory polymicrobial niche in response to routine vaccinations.
Table 4 Vulnerable populations, risk factors for Spn infections and immune system
Conclusions
The cost of Spn conjugate vaccination is a major global concern. PCV formulations are expensive to produce because the product is given as a single shot but consists of 7, 10 or 13 vaccines. A protein-based vaccination strategy likely will require less or no boosting because natural boosting should continue to occur since Spn will not be eliminated as a colonizing bacteria. However, it should be noted that a vaccine inducing a moderate immune response might be sufficient for invasive but not non-invasive infections.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Käyhty H, Nurkka A, Soininen A, Väkeväinen M. The immunological basis for immunization series, module 12: Pneumococcal vaccines. (Ed.ˆ(Eds) ( Department of Immunization, Vaccines and Biologicals, World Wealthy Orginiation, Publications of the World Health Organization 2009
- Denoel P, Philipp MT, Doyle L, Martin D, Carletti G, Poolman JT. A protein-based pneumococcal vaccine protects rhesus macaques from pneumonia after experimental infection with Streptococcus pneumoniae. Vaccine 2011; 29(33):5495-501; PMID:21624422; http://dx.doi.org/10.1016/j.vaccine.2011.05.051
- Moffitt KL, Malley R. Next generation pneumococcal vaccines. Curr Opinion Immunl 2011; 23(3):407-13; PMID:21514128; http://dx.doi.org/10.1016/j.coi.2011.04.002
- Feldman C, Anderson R. Review: Current and new generation pneumococcal vaccines. J Infect 2014; 69(4):309-25; PMID:24968238; http://dx.doi.org/10.1016/j.jinf.2014.06.006
- Darrieux M, Goulart C, Briles D, Leite LC. Current status and perspectives on protein-based pneumococcal vaccines. Crit Rev Microbiol 2013; 41:1-11
- WHO. Pneumococcal vaccines-WHO (World Healthy Orginization) position paper - 2012. In: 6 april 2012, 87th year / 6 avril 2012, 87e année, No. 14, 2012, 87, 129-144, http://www.who.int/wer. WHO (Ed.ˆ(Eds) (Geneva, Switzerland 2012)
- Jedrzejas MJ. Pneumococcal virulence factors: structure and function. Microbiol Mol Biol Rev 2001; 65(2):187-207; first page, table of contents; PMID:11381099; http://dx.doi.org/10.1128/MMBR.65.2.187-207.2001
- Calix JJ, Porambo RJ, Brady AM, Larson TR, Yother J, Abeygunwardana C, Nahm MH. Biochemical, genetic, and serological characterization of two capsule subtypes among Streptococcus pneumoniae Serotype 20 strains: discovery of a new pneumococcal serotype. J Biol Chem 2012; 287(33):27885-94; PMID:22736767; http://dx.doi.org/10.1074/jbc.M112.380451
- van der Linden M, Reinert RR, Kern WV, Imohl M. Epidemiology of serotype 19A isolates from invasive pneumococcal disease in German children. BMC Infect Dis 2013; 13:70; PMID:23384407; http://dx.doi.org/10.1186/1471-2334-13-70
- Calix JJ, Dagan R, Pelton SI, Porat N, Nahm MH. Differential occurrence of Streptococcus pneumoniae serotype 11E between asymptomatic carriage and invasive pneumococcal disease isolates reflects a unique model of pathogen microevolution. Clin Infect Dis 2012; 54(6):794-9; PMID:22267713; http://dx.doi.org/10.1093/cid/cir953
- Song JY, Nahm MH, Moseley MA. Clinical implications of pneumococcal serotypes: invasive disease potential, clinical presentations, and antibiotic resistance. J Korean Med Sci 2013; 28(1):4-15; PMID:23341706; http://dx.doi.org/10.3346/jkms.2013.28.1.4
- Khan MN, Pichichero ME. The host immune dynamics of pneumococcal colonization: implications for novel vaccine development. Hum Vaccines Immunother 2014; 10(12):3688-99; PMID:25668673; http://dx.doi.org/10.4161/21645515.2014.979631
- Lynch JP, 3rd, Zhanel GG. Streptococcus pneumoniae: epidemiology and risk factors, evolution of antimicrobial resistance, and impact of vaccines. Curr Opinion Pulmon Med 2010; 16(3):217-25; PMID:20375783
- Besser TE, McGuire TC, Gay CC, Pritchett LC. Transfer of functional immunoglobulin G (IgG) antibody into the gastrointestinal tract accounts for IgG clearance in calves. J Virol 1988; 62(7):2234-7; PMID:3373569
- Pichichero ME, Casey JR. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. Jama 2007; 298(15):1772-8; PMID:17940232; http://dx.doi.org/10.1001/jama.298.15.1772
- Kaplan SL, Barson WJ, Lin PL, Stovall SH, Bradley JS, Tan TQ, Hoffman JA, Givner LB, Mason EO Jr. Serotype 19A Is the most common serotype causing invasive pneumococcal infections in children. Pediatrics 2010; 125(3):429-36; PMID:20176669; http://dx.doi.org/10.1542/peds.2008-1702
- Jambo KC, Sepako E, Heyderman RS, Gordon SB. Potential role for mucosally active vaccines against pneumococcal pneumonia. Trend Microbiol 2010; 18(2):81-9; PMID:20031415; http://dx.doi.org/10.1016/j.tim.2009.12.001
- Mann B, Thornton J, Heath R, Wade KR, Tweten RK, Gao G, El Kasmi K, Jordan JB, Mitrea DM, Kriwacki R. Broadly protective protein-based pneumococcal vaccine composed of pneumolysin toxoid-CbpA peptide recombinant fusion protein. J Infect Dis 2014; 209(7):1116-25; PMID:24041791; http://dx.doi.org/10.1093/infdis/jit502
- Grabenstein JD, Klugman KP. A century of pneumococcal vaccination research in humans. Clin Microbiol Infect 2012; 18 Suppl 5:15-24; PMID:22882735; http://dx.doi.org/10.1111/j.1469-0691.2012.03943.x
- Otczyk D, Cripps A. Vaccination for the control of childhood bacterial pneumonia–Haemophilus influenzae type b and pneumococcal vaccines. Pneumonia 2013; (Feb 14): 2:2-15.
- Bosch AA, Biesbroek G, Trzcinski K, Sanders EA, Bogaert D. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog 2013; 9(1), e1003057; PMID:23326226; http://dx.doi.org/10.1371/journal.ppat.1003057
- Pettigrew MM, Gent JF, Pyles RB, Miller AL, Nokso-Koivisto J, Chonmaitree T. Viral-bacterial interactions and risk of acute otitis media complicating upper respiratory tract infection. J Clin Microbiol 2011; 49(11):3750-5; PMID:21900518; http://dx.doi.org/10.1128/JCM.01186-11
- McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 2006; 19(3):571-82; PMID:16847087; http://dx.doi.org/10.1128/CMR.00058-05
- Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 2004; 4(3):144-54; PMID:14998500; http://dx.doi.org/10.1016/S1473-3099(04)00938-7
- Vanderkooi OG, Church DL, MacDonald J, Zucol F, Kellner JD. Community-based outbreaks in vulnerable populations of invasive infections caused by Streptococcus pneumoniae serotypes 5 and 8 in Calgary, Canada. PloS one 2011; 6(12), e28547; PMID:22216100; http://dx.doi.org/10.1371/journal.pone.0028547
- Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J 2010; 29(4):304-9; PMID:19935445
- Friedel V, Zilora S, Bogaard D, Casey JR, Pichichero ME. Five-year prospective study of paediatric acute otitis media in Rochester, NY: modelling analysis of the risk of pneumococcal colonization in the nasopharynx and infection. Epidemiol Infect 2013; 1-9.
- Garcia-Rodriguez JA, Fresnadillo Martinez MJ. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J Antimicrob Chemother 2002; 50 Suppl S2:59-73; PMID:12556435; http://dx.doi.org/10.1093/jac/dkf506
- McCool TL, Cate TR, Moy G, Weiser JN. The immune response to pneumococcal proteins during experimental human carriage. J Exp Med 2002; 195(3):359-65; PMID:11828011; http://dx.doi.org/10.1084/jem.20011576
- Mureithi MW, Finn A, Ota MO, Zhang Q, Davenport V, Mitchell TJ, Williams NA, Adegbola RA, Heyderman RS. T cell memory response to pneumococcal protein antigens in an area of high pneumococcal carriage and disease. J Infect Dis 2009; 200(5):783-93; PMID:19642930; http://dx.doi.org/10.1086/605023
- Pichichero ME, Kaur R, Casey JR, Xu Q, Almudevar A, Ochs M. Antibody response to Streptococcus pneumoniae proteins PhtD, LytB, PcpA, PhtE and Ply after nasopharyngeal colonization and acute otitis media in children. Hum Vaccines Immunother 2012; 8(6):799-805; PMID:22495112; http://dx.doi.org/10.4161/hv.19820
- Wright AK, Bangert M, Gritzfeld JF, Ferreira DM, Jambo KC, Wright AD, Collins AM, Gordon SB. Experimental human pneumococcal carriage augments IL-17A-dependent T-cell defence of the lung. PLoS Pathog 2013; 9(3), e1003274; PMID:23555269; http://dx.doi.org/10.1371/journal.ppat.1003274
- Richards L, Ferreira DM, Miyaji EN, Andrew PW, Kadioglu A. The immunising effect of pneumococcal nasopharyngeal colonisation; protection against future colonisation and fatal invasive disease. Immunobiology 2010; 215(4):251-63; PMID:20071053; http://dx.doi.org/10.1016/j.imbio.2009.12.004
- Lee H, Choi EH, Lee HJ. Efficacy and effectiveness of extended-valency pneumococcal conjugate vaccines. Korean J Pediatr 2014; 57(2):55-66; PMID:24678328; http://dx.doi.org/10.3345/kjp.2014.57.2.55
- Oosterhuis-Kafeja F, Beutels P, Van Damme P. Immunogenicity, efficacy, safety and effectiveness of pneumococcal conjugate vaccines (1998–2006). Vaccine 2007; 25(12):2194-212; PMID:17267077; http://dx.doi.org/10.1016/j.vaccine.2006.11.032
- Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, Nyquist AC, Gershman KA, Vazquez M, Bennett NM. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 2006; 368(9546):1495-502; PMID:17071283; http://dx.doi.org/10.1016/S0140-6736(06)69637-2
- Xu Q, Almudervar A, Casey JR, Pichichero ME. Nasopharyngeal bacterial interactions in children. Emerg Infect Dis 2012; 18(11):1738-45; PMID:23092680; http://dx.doi.org/10.3201/eid1811.111904
- Xu Q, Pichichero ME. Co-colonization by Haemophilus influenzae with Streptococcus pneumoniae enhances pneumococcal-specific antibody response in young children. Vaccine 2014; 32(6):706-11; PMID:24355091; http://dx.doi.org/10.1016/j.vaccine.2013.11.096
- van Rossum AM, Lysenko ES, Weiser JN. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect Immun 2005; 73(11):7718-26; PMID:16239576; http://dx.doi.org/10.1128/IAI.73.11.7718-7726.2005
- Smith AM, Adler FR, Ribeiro RM, Gutenkunst RN, McAuley JL, McCullers JA, Perelson AS. Kinetics of coinfection with influenza A virus and Streptococcus pneumoniae. PLoS Pathog 2013; 9(3), e1003238; PMID:23555251; http://dx.doi.org/10.1371/journal.ppat.1003238
- Small CL, Shaler CR, McCormick S, Jeyanathan M, Damjanovic D, Brown EG, Arck P, Jordana M, Kaushic C, Ashkar AA, et al. Influenza infection leads to increased susceptibility to subsequent bacterial superinfection by impairing NK cell responses in the lung. J Immunol 2010; 184(4):2048-56; PMID:20083661; http://dx.doi.org/10.4049/jimmunol.0902772
- Wu Y, Tu W, Lam KT, Chow KH, Ho PL, Guan Y, Peiris JS, Lau YL. Lethal coinfection of influenza virus and Streptococcus pneumoniae lowers antibody response to influenza virus in lung and reduces numbers of germinal center B cells, T follicular helper cells, and plasma cells in mediastinal lymph Node. J Birol 2015; 89(4):2013-23; PMID:25428873; http://dx.doi.org/10.1128/JVI.02455-14
- Wolf AI, Strauman MC, Mozdzanowska K, Williams KL, Osborne LC, Shen H, Liu Q, Garlick D, Artis D, Hensley SE, et al. Pneumolysin expression by streptococcus pneumoniae protects colonized mice from influenza virus-induced disease. Virology 2014; 462–463:254-65; PMID:24999050
- Wolf AI, Strauman MC, Mozdzanowska K, Whittle JR, Williams KL, Sharpe AH, Weiser JN, Caton AJ, Hensley SE, Erikson J. Coinfection with Streptococcus pneumoniae modulates the B cell response to influenza virus. J Virol 2014; 88(20):11995-2005; PMID:25100838; http://dx.doi.org/10.1128/JVI.01833-14
- Ono A, Okada F, Takata S, Hiramatsu K, Ando Y, Nakayama T, Maeda T, Mori H. A comparative study of thin-section CT findings between seasonal influenza virus pneumonia and Streptococcus pneumoniae pneumonia. Br J Radiol 2014; 87(1039):20140051; PMID:24834476; http://dx.doi.org/10.1259/bjr.20140051
- Katsura H, Piao Z, Iwatsuki-Horimoto K, Akeda Y, Watanabe S, Horimoto T, Oishi K, Kawaoka Y. A bivalent vaccine based on a replication-incompetent influenza virus protects against Streptococcus pneumoniae and influenza virus infection. J Virol 2014; 88(22):13410-7; PMID:25210171; http://dx.doi.org/10.1128/JVI.01205-14
- Fukuda Y, Furuya Y, Nozaki Y, Takahata M, Nomura N, Mitsuyama J. Therapeutic effects of garenoxacin in murine experimental secondary pneumonia by Streptococcus pneumoniae after influenza virus infection. Diagn Microbiol Infect Dis 2014; 78(2):168-71; PMID:24321356; http://dx.doi.org/10.1016/j.diagmicrobio.2013.11.003
- Hament JM, Aerts PC, Fleer A, van Dijk H, Harmsen T, Kimpen JL, Wolfs TF. Direct binding of respiratory syncytial virus to pneumococci: a phenomenon that enhances both pneumococcal adherence to human epithelial cells and pneumococcal invasiveness in a murine model. Pediatr Res 2005; 58(6):1198-203; PMID:16306193; http://dx.doi.org/10.1203/01.pdr.0000188699.55279.1b
- Tong HH, Fisher LM, Kosunick GM, DeMaria TF. Effect of adenovirus type 1 and influenza A virus on Streptococcus pneumoniae nasopharyngeal colonization and otitis media in the chinchilla. Annal Otol, Rhinol Laryngol 2000; 109(11):1021-7; PMID:11089992; http://dx.doi.org/10.1177/000348940010901106
- Murrah KA, Turner RL, Pang B, Perez AC, Reimche JL, King LB, Wren J, Gandhi U, Swords WE, Ornelles DA. Replication of type 5 adenovirus promotes middle ear infection by Streptococcus pneumoniae in the chinchilla model of otitis media. Path Dis 2015; 73(2):1-8; PMID:25251686
- Ludewick HP, Aerts L, Hamelin ME, Boivin G. Long-term impairment of Streptococcus pneumoniae lung clearance is observed after initial infection with influenza A virus but not human metapneumovirus in mice. J Gen Virol 2011; 92(Pt 7):1662-5; PMID:21411678; http://dx.doi.org/10.1099/vir.0.030825-0
- Alymova IV, Portner A, Takimoto T, Boyd KL, Babu YS, McCullers JA. The novel parainfluenza virus hemagglutinin-neuraminidase inhibitor BCX 2798 prevents lethal synergism between a paramyxovirus and Streptococcus pneumoniae. Antimicrob Agents Chemother 2005; 49(1):398-405; PMID:15616320; http://dx.doi.org/10.1128/AAC.49.1.398-405.2005
- Short KR, Habets MN, Hermans PW, Diavatopoulos DA. Interactions between Streptococcus pneumoniae and influenza virus: a mutually beneficial relationship? Future Microbiol 2012; 7(5):609-24; PMID:22568716; http://dx.doi.org/10.2217/fmb.12.29
- McNamee LA, Harmsen AG. Both influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infection. Infect Immun 2006; 74(12):6707-21; PMID:16982840; http://dx.doi.org/10.1128/IAI.00789-06
- Avadhanula V, Rodriguez CA, Devincenzo JP, Wang Y, Webby RJ, Ulett GC, Adderson EE. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J Virol 2006; 80(4):1629-36; PMID:16439519; http://dx.doi.org/10.1128/JVI.80.4.1629-1636.2006
- van der Sluijs KF, van Elden LJ, Nijhuis M, Schuurman R, Pater JM, Florquin S, Goldman M, Jansen HM, Lutter R, van der Poll T. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J Immunol 2004; 172(12):7603-9; PMID:15187140; http://dx.doi.org/10.4049/jimmunol.172.12.7603
- Lijek RS, Weiser JN. Co-infection subverts mucosal immunity in the upper respiratory tract. Curr Opin Immunol 2012; 24(4):417-23; PMID:22658762; http://dx.doi.org/10.1016/j.coi.2012.05.005
- Brouwer MC, de Gans J, Heckenberg SG, Zwinderman AH, van der Poll T, van de Beek D. Host genetic susceptibility to pneumococcal and meningococcal disease: a systematic review and meta-analysis. Lancet Infect Dis 2009; 9(1):31-44; PMID:19036641; http://dx.doi.org/10.1016/S1473-3099(08)70261-5
- Kunzli N, Kaiser R, Medina S, Studnicka M, Chanel O, Filliger P, Herry M, Horak F Jr, Puybonnieux-Texier V, Quénel P, et al. Public-health impact of outdoor and traffic-related air pollution: a European assessment. Lancet 2000; 356(9232):795-801; PMID:11022926; http://dx.doi.org/10.1016/S0140-6736(00)02653-2
- Kanatani KT, Ito I, Al-Delaimy WK, Adachi Y, Mathews WC, Ramsdell JW, Toyama Asian Desert Dust and Asthma Study Team. Desert dust exposure is associated with increased risk of asthma hospitalization in children. Am J Respir Crit Care Med 2010; 182(12):1475-81; PMID:20656941; http://dx.doi.org/10.1164/rccm.201002-0296OC
- Cremers AJ, Zomer AL, Gritzfeld JF, Ferwerda G, van Hijum SA, Ferreira DM, Shak JR, Klugman KP, Boekhorst J, Timmerman HM, et al. The adult nasopharyngeal microbiome as a determinant of pneumococcal acquisition. Microbiome 2014; 2:44; PMID:25671106
- Regev-Yochay G, Trzcinski K, Thompson CM, Malley R, Lipsitch M. Interference between Streptococcus pneumoniae and Staphylococcus aureus: In vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae. J Bacteriol 2006; 188(13):4996-5001; PMID:16788209; http://dx.doi.org/10.1128/JB.00317-06
- Spijkerman J, Prevaes SM, van Gils EJ, Veenhoven RH, Bruin JP, Bogaert D, Wijmenga-Monsuur AJ, van den Dobbelsteen GP, Sanders EA. Long-term effects of pneumococcal conjugate vaccine on nasopharyngeal carriage of S. pneumoniae, S. aureus, H. influenzae and M. catarrhalis. PloS one 2012; 7(6), e39730; PMID:22761879; http://dx.doi.org/10.1371/journal.pone.0039730
- Thaler I, Manor D, Itskovitz J, Rottem S, Levit N, Timor-Tritsch I, Brandes JM. Changes in uterine blood flow during human pregnancy. Am J Obstet Gynecol 1990; 162(1):121-5; PMID:2301480; http://dx.doi.org/10.1016/0002-9378(90)90834-T
- Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol 2011; 12(6):509-17; PMID:21739679; http://dx.doi.org/10.1038/ni.2039
- Bogaert D, Weinberger D, Thompson C, Lipsitch M, Malley R. Impaired innate and adaptive immunity to Streptococcus pneumoniae and its effect on colonization in an infant mouse model. Infect Immun 2009; 77(4):1613-22; PMID:19168741; http://dx.doi.org/10.1128/IAI.00871-08
- Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity 2012; 37(5):771-83; PMID:23159225; http://dx.doi.org/10.1016/j.immuni.2012.10.014
- Smolen KK, Cai B, Gelinas L, Fortuno ES 3rd, Larsen M, Speert DP, Chamekh M, Cooper PJ, Esser M, Marchant A. Single-cell analysis of innate cytokine responses to pattern recognition receptor stimulation in children across four continents. J Immunol 2014; 193(6):3003-12; PMID:25135829; http://dx.doi.org/10.4049/jimmunol.1400895
- Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES 3rd, Hajjar AM, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol 2009; 183(11):7150-60; PMID:19917677; http://dx.doi.org/10.4049/jimmunol.0901481
- Smolen KK, Ruck CE, Fortuno ES, 3rd, Ho K, Dimitriu P, Mohn WW, Speert DP, Cooper PJ, Esser M, Goetghebuer T, et al. Pattern recognition receptor-mediated cytokine response in infants across 4 continents. J Allergy Clin Immunol 2014; 133(3):818-26 e814; PMID:24290283; http://dx.doi.org/10.1016/j.jaci.2013.09.038
- Pichichero ME. Protein carriers of conjugate vaccines: characteristics, development, and clinical trials. Hum Vaccines Immunother 2013; 9(12):2505-23; PMID:23955057; http://dx.doi.org/10.4161/hv.26109
- Pichichero ME. Booster vaccinations: can immunologic memory outpace disease pathogenesis? Pediatrics 2009; 124(6):1633-41; PMID:19933727; http://dx.doi.org/10.1542/peds.2008-3645
- Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci U S A 2005; 102(13):4848-53; PMID:15781870; http://dx.doi.org/10.1073/pnas.0501254102
- Musher DM, Manof SB, Liss C, McFetridge RD, Marchese RD, Bushnell B, Alvarez F, Painter C, Blum MD, Silber JL. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis 2010; 201(4):516-24; PMID:20092407; http://dx.doi.org/10.1086/649839
- Ekstrom N, Vakevainen M, Verho J, Kilpi T, Kayhty H. Functional antibodies elicited by two heptavalent pneumococcal conjugate vaccines in the Finnish Otitis Media Vaccine Trial. Infect Immun 2007; 75(4):1794-800; PMID:17261612; http://dx.doi.org/10.1128/IAI.01673-06
- Song JY, Moseley MA, Burton RL, Nahm MH. Pneumococcal vaccine and opsonic pneumococcal antibody. J Infect Chemother 2013; 19(3):412-25; PMID:23657429; http://dx.doi.org/10.1007/s10156-013-0601-1
- Marchant A, Kollmann TR. Understanding the ontogeny of the immune system to promote immune-mediated health for life. Front Immunol 2015; 6:77; PMID:25755655; http://dx.doi.org/10.3389/fimmu.2015.00077
- MacGillivray DM, Kollmann TR. The role of environmental factors in modulating immune responses in early life. Front Immunol 2014; 5:434; PMID:25309535; http://dx.doi.org/10.3389/fimmu.2014.00434
- Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol 2009; 9(2):91-105; PMID:19151746; http://dx.doi.org/10.1038/nri2487
- Zhang Q, Leong SC, McNamara PS, Mubarak A, Malley R, Finn A. Characterisation of regulatory T cells in nasal associated lymphoid tissue in children: relationships with pneumococcal colonization. PLoS Pathog 2011; 7(8), e1002175; PMID:21852948; http://dx.doi.org/10.1371/journal.ppat.1002175
- Debock I, Flamand V. Unbalanced Neonatal CD4(+) T-Cell Immunity. Front Immunol 2014; 5:393; PMID:25221551; http://dx.doi.org/10.3389/fimmu.2014.00393
- Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, Kolls JK, Srivastava A, Lundgren A, Forte S, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog 2009; 4(9), e1000159; PMID:18802458; http://dx.doi.org/10.1371/journal.ppat.1000159
- Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest 2009; 119(7):1899-909; PMID:19509469
- Gray C, Ahmed MS, Mubarak A, Kasbekar AV, Derbyshire S, McCormick MS, Mughal MK, McNamara PS, Mitchell T, Zhang Q. Activation of memory Th17 cells by domain 4 pneumolysin in human nasopharynx-associated lymphoid tissue and its association with pneumococcal carriage. Mucosal Immunol 2014 May; 7(3):705-17
- O'Connor W, Jr., Zenewicz LA, Flavell RA. The dual nature of T(H)17 cells: shifting the focus to function. Nat Immunol 2010; 11(6):471-6; PMID:20485275; http://dx.doi.org/10.1038/ni.1882
- Zenewicz LA, Flavell RA. IL-22 and inflammation: leukin' through a glass onion. Eur J Immunol 2008; 38(12):3265-8; PMID:19016525; http://dx.doi.org/10.1002/eji.200838655
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005; 6(11):1133-41; PMID:16200068; http://dx.doi.org/10.1038/ni1261
- McAleer JP, Kolls JK. Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol Rev 2014; 260(1):129-44; PMID:24942687; http://dx.doi.org/10.1111/imr.12183
- Lee FE, Georas SN, Beck LA. IL-17: important for host defense, autoimmunity, and allergy? J Invest Dermatol 2010; 130(11):2540-42; PMID:20944637; http://dx.doi.org/10.1038/jid.2010.295
- Angelone DF, Wessels MR, Coughlin M, Suter EE, Valentini P, Kalish LA, Levy O. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatr Res 2006; 60(2):205-9; PMID:16864705; http://dx.doi.org/10.1203/01.pdr.0000228319.10481.ea
- Vanden Eijnden S, Goriely S, De Wit D, Goldman M, Willems F. Preferential production of the IL-12(p40)/IL-23(p19) heterodimer by dendritic cells from human newborns. Eur J Immunol 2006; 36(1):21-6; PMID:16342235; http://dx.doi.org/10.1002/eji.200535467
- Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol 2007; 7(5):379-90; PMID:17457344; http://dx.doi.org/10.1038/nri2075
- Lundgren A, Bhuiyan TR, Novak D, Kaim J, Reske A, Lu YJ, Qadri F, Malley R. Characterization of Th17 responses to Streptococcus pneumoniae in humans: comparisons between adults and children in a developed and a developing country. Vaccine 2012; 30(26):3897-907; PMID:22504663; http://dx.doi.org/10.1016/j.vaccine.2012.03.082
- Kollmann TR. Variation between Populations in the Innate Immune Response to Vaccine Adjuvants. Front Immunol 2013; 4:81; PMID:23565115; http://dx.doi.org/10.3389/fimmu.2013.00081
- Nurhonen M, Auranen K. Optimal serotype compositions for Pneumococcal conjugate vaccination under serotype replacement. PLoS Comput Biol 2014; 10(2), e1003477; PMID:24550722; http://dx.doi.org/10.1371/journal.pcbi.1003477
- Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med 2008; 205(3):543-55; PMID:18332181; http://dx.doi.org/10.1084/jem.20071949
- Verhoeven D, Perry S, Pichichero ME. Contributions to protection from streptococcus pneumoniae Infection using monovalent recombinant protein vaccine candidates PcpA, PhtD and PlyD1 in an infant murine model during challenge. Clin Vaccine Immunol 2014; 21; PMID:24850621
- Verhoeven D, Xu Q, Pichichero ME. Vaccination with a Streptococcus pneumoniae trivalent recombinant PcpA, PhtD and PlyD1 protein vaccine candidate protects against lethal pneumonia in an infant murine model. Vaccine 2014; 32(26):3205-10; PMID:24731814; http://dx.doi.org/10.1016/j.vaccine.2014.04.004
- Pope C, Oliver EH, Ma J, Langton Hewer C, Mitchell TJ, Finn A. Genetic conjugation of components in two pneumococcal fusion protein vaccines enhances paediatric mucosal immune responses. Vaccine 2015; 33(14):1711-8; PMID:25698489; http://dx.doi.org/10.1016/j.vaccine.2015.02.012
- Xu Q, Surendran N, Verhoeven D, Klapa J, Ochs M, Pichichero ME. Trivalent pneumococcal protein recombinant vaccine protects against lethal Streptococcus pneumoniae pneumonia and correlates with phagocytosis by neutrophils during early pathogenesis. Vaccine 2015; 33(8):993-1000; PMID:25597944; http://dx.doi.org/10.1016/j.vaccine.2015.01.014
- Anish C, Upadhyay AK, Sehgal D, Panda AK. Influences of process and formulation parameters on powder flow properties and immunogenicity of spray dried polymer particles entrapping recombinant pneumococcal surface protein A. Int J Pharm 2014; 466(1–2):198-210; PMID:24631054; http://dx.doi.org/10.1016/j.ijpharm.2014.03.025
- Schachern PA, Tsuprun V, Ferrieri P, Briles DE, Goetz S, Cureoglu S, Paparella MM, Juhn S. Pneumococcal PspA and PspC proteins: potential vaccine candidates for experimental otitis media. Int J Pediatr Otorhinolaryngol 2014; 78(9):1517-21; PMID:25015773; http://dx.doi.org/10.1016/j.ijporl.2014.06.024
- Vivas M, Force E, Tubau F, El Haj C, Ariza J, Cabellos C. Effect of dexamethasone on the efficacy of daptomycin in the therapy of experimental pneumococcal meningitis. Int J Antimicrobial Agents 2015; 46; PMID:25813395
- Skinner JM, Indrawati L, Cannon J, Blue J, Winters M, Macnair J, Pujar N, Manger W, Zhang Y, Antonello J, et al. Pre-clinical evaluation of a 15-valent pneumococcal conjugate vaccine (PCV15-CRM197) in an infant-rhesus monkey immunogenicity model. Vaccine 2011; 29(48):8870-6; PMID:21964055; http://dx.doi.org/10.1016/j.vaccine.2011.09.078
- Carolan LA, Butler J, Rockman S, Guarnaccia T, Hurt AC, Reading P, Kelso A, Barr I, Laurie KL. TaqMan real time RT-PCR assays for detecting ferret innate and adaptive immune responses. J Virolog Methods 2014; 205C; 38-52; PMID:24797460; http://dx.doi.org/10.1016/j.jviromet.2014.04.014
- Huelves L, del Prado G, Rodriguez-Cerrato V, Gracia M, Cenjor C, Granizo JJ, Ponte C, Soriano F. Adherence of Streptococcus pneumoniae to polystyrene plates, effect of serum on adhesion, and virulence in the gerbil otitis media model. Microbial Pathogenesis 2007; 43(2–3):114-9; PMID:17583465; http://dx.doi.org/10.1016/j.micpath.2007.05.005
- Chiavolini D, Pozzi G, Ricci S. Animal models of Streptococcus pneumoniae disease. Clin Microbiol Rev 2008; 21(4):666-85; PMID:18854486; http://dx.doi.org/10.1128/CMR.00012-08
- Ross KS. Novel Strategies To Prevent And Treat Experimental Pneumococcal Disease. In: Department of Infection & Immunity. (Ed.ˆ(Eds) (University of Glasgow 2010).
- Bhutta MF. Mouse models of otitis media: strengths and limitations. Otolaryngol Head Neck Surg 2012; 147(4):611-14; PMID:22661304; http://dx.doi.org/10.1177/0194599812449986
- Giefing C, Meinke AL, Hanner M, Henics T, Bui MD, Gelbmann D, Lundberg U, Senn BM, Schunn M, Habel A, et al. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J Exp Med 2008; 205(1):117-31; PMID:18166586; http://dx.doi.org/10.1084/jem.20071168
- Rioux S, Neyt C, Di Paolo E, Turpin L, Charland N, Labbé S, Mortier MC, Mitchell TJ, Feron C, Martin D, et al.. Transcriptional regulation, occurrence and putative role of the Pht family of Streptococcus pneumoniae. Microbiology 2011; 157(Pt 2):336-48; PMID:20966093; http://dx.doi.org/10.1099/mic.0.042184-0
- Khan MN, Pichichero ME. Vaccine candidates PhtD and PhtE of Streptococcus pneumoniae are adhesins that elicit functional antibodies in humans. Vaccine 2012; 30(18):2900-7; PMID:22349524; http://dx.doi.org/10.1016/j.vaccine.2012.02.023
- Khan MN, Pichichero ME. CD4 T cell memory and antibody responses directed against the pneumococcal histidine triad proteins PhtD and PhtE following nasopharyngeal colonization and immunization and their role in protection against pneumococcal colonization in mice. Infection and immunity 2013; 81(10):3781-92; PMID:23897609; http://dx.doi.org/10.1128/IAI.00313-13
- Kaur R, Casey JR, Pichichero ME. Serum antibody response to five Streptococcus pneumoniae proteins during acute otitis media in otitis-prone and non-otitis-prone children. Pediatr Infect Dis J 2011; 30(8):645-50; PMID:21487325; http://dx.doi.org/10.1097/INF.0b013e31821c2d8b
- Godfroid F, Hermand P, Verlant V, Denoel P, Poolman JT. Preclinical evaluation of the Pht proteins as potential cross-protective pneumococcal vaccine antigens. Infection and immunity 2011; 79(1):238-45; PMID:20956575; http://dx.doi.org/10.1128/IAI.00378-10
- Verhoeven D, Xu Q, Pichichero ME. Vaccination with a Streptococcus pneumoniae trivalent recombinant PcpA, PhtD and PlyD1 protein vaccine candidate protects against lethal pneumonia in an infant murine model. Vaccine 2014; 32; PMID:24731814
- Glover DT, Hollingshead SK, Briles DE. Streptococcus pneumoniae surface protein PcpA elicits protection against lung infection and fatal sepsis. Infect Immun 2008; 76(6):2767-76; PMID:18391008; http://dx.doi.org/10.1128/IAI.01126-07
- Marriott HM, Mitchell TJ, Dockrell DH. Pneumolysin: a double-edged sword during the host-pathogen interaction. Curr Mol Med 2008; 8(6):497-509; PMID:18781957; http://dx.doi.org/10.2174/156652408785747924
- Salha D, Szeto J, Myers L, Claus C, Sheung A, Tang M, Ljutic B, Hanwell D, Ogilvie K, Ming M, et al. Neutralizing antibodies elicited by a novel detoxified pneumolysin derivative, PlyD1, provide protection against both pneumococcal infection and lung injury. Infect Immun 2012; 80(6):2212-20; PMID:22473606; http://dx.doi.org/10.1128/IAI.06348-11
- Leroux-Roels G, Maes C, De Boever F, Traskine M, Ruggeberg JU, Borys D. Safety, reactogenicity and immunogenicity of a novel pneumococcal protein-based vaccine in adults: a phase I/II randomized clinical study. Vaccine 2014; 32(50):6838-46; PMID:24607003; http://dx.doi.org/10.1016/j.vaccine.2014.02.052
- Khan MN, Coleman JR, Vernatter J, Varshney AK, Dufaud C, Pirofski LA. An Ahemolytic Pneumolysin of Streptococcus Pneumoniae Manipulates Human Innate and CD4+ T-Cell Responses and Reduces Resistance to Colonization in Mice in a Serotype-Independent Manner. J Infect Dis 2014; 210(10):1658-69; PMID:25001458; http://dx.doi.org/10.1093/infdis/jiu321
- Witzenrath M, Pache F, Lorenz D, Koppe U, Gutbier B, Tabeling C, Reppe K, Meixenberger K, Dorhoi A, Ma J, et al. The NLRP3 inflammasome is differentially activated by pneumolysin variants and contributes to host defense in pneumococcal pneumonia. J Immunol 2011; 187(1):434-40; PMID:21646297; http://dx.doi.org/10.4049/jimmunol.1003143
- Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, Kurt-Jones E, Paton JC, Wessels MR, Golenbock DT, et al. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci U S A 2003; 100(4):1966-71; PMID:12569171; http://dx.doi.org/10.1073/pnas.0435928100
- Paton JC, Lock RA, Hansman DJ. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect Immun 1983; 40(2):548-52; PMID:6840851
- Hirst RA, Kadioglu A, O'Callaghan C, Andrew PW. The role of pneumolysin in pneumococcal pneumonia and meningitis. Clin Exp Immunol 2004; 138(2):195-201; PMID:15498026; http://dx.doi.org/10.1111/j.1365-2249.2004.02611.x
- Ren D, Almudevar AL, Pichichero ME. Synchrony in serum antibody response to conserved proteins of Streptococcus pneumoniae in young children. Hum Vaccines Immunotherapeutics 2015; 11(2):489-97; PMID:25692218; http://dx.doi.org/10.4161/21645515.2014.990861
- Sharma SK, Roumanes D, Almudevar A, Mosmann TR, Pichichero ME. CD4+ T-cell responses among adults and young children in response to Streptococcus pneumoniae and Haemophilus influenzae vaccine candidate protein antigens. Vaccine 2013; 31(30):3090-7; PMID:23632305; http://dx.doi.org/10.1016/j.vaccine.2013.03.060
- Xu Q, Casey JR, Pichichero ME. Higher levels of mucosal antibody to pneumococcal vaccine candidate proteins are associated with reduced acute otitis media caused by Streptococcus pneumoniae in young children. Mucosal Immunol 2015; 2015 Feb 4. [Epub ahead of print] PMID:25648056; http://dx.doi.org/10.1038/mi.2015.1.
- Zhang Q, Bagrade L, Clarke E, Paton JC, Nunez DA, Finn A. Bacterial lipoproteins differentially regulate human primary and memory CD4+ T and B cell responses to pneumococcal protein antigens through Toll-like receptor 2. J Infect Dis 2010; 201(11):1753-63; PMID:20402597; http://dx.doi.org/10.1086/652495
- Holmlund E, Quiambao B, Ollgren J, Nohynek H, Kayhty H. Development of natural antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A and pneumolysin in Filipino pregnant women and their infants in relation to pneumococcal carriage. Vaccine 2006; 24(1):57-65; PMID:16115703; http://dx.doi.org/10.1016/j.vaccine.2005.07.055
- Simell B, Ahokas P, Lahdenkari M, Poolman J, Henckaerts I, Kilpi TM, Käyhty H. Pneumococcal carriage and acute otitis media induce serum antibodies to pneumococcal surface proteins CbpA and PhtD in children. Vaccine 27(34):4615-21; PMID:19524618; http://dx.doi.org/10.1016/j.vaccine.2009.05.071
- Prevaes SM, van Wamel WJ, de Vogel CP, Veenhoven RH, van Gils EJ, van Belkum A, Sanders EA, Bogaert D, et al. Nasopharyngeal colonization elicits antibody responses to staphylococcal and pneumococcal proteins that are not associated with a reduced risk of subsequent carriage. Infect Immun 2012; 80(6):2186-93; PMID:22451514; http://dx.doi.org/10.1128/IAI.00037-12
- Pichichero ME, Kaur R, Casey JR, Xu Q, Almudevar A, Ochs M. Antibody response to Streptococcus pneumoniae proteins PhtD, LytB, PcpA, PhtE and Ply after nasopharyngeal colonization and acute otitis media in children. Hum.Vaccin.Immunother., 2012; 8(6):799-805; PMID:22495112; http://dx.doi.org/10.4161/hv.19820
- Sato S, Kiyono H. The mucosal immune system of the respiratory tract. Curr Opin Virol 2012; 2(3):225-32; PMID:22542216; http://dx.doi.org/10.1016/j.coviro.2012.03.009
- Xu Q, Casey JR, Pichichero ME. Higher levels of mucosal antibody to pneumococcal vaccine candidate proteins are associated with reduced acute otitis media caused by Streptococcus pneumoniae in young children. Mucosal Immunol 2015; Feb 4. http://dx.doi.org/10.1038/mi.2015.1. [Epub ahead of print]
- Xu Q, Casey JR, Newman M, Pichichero ME. Protective Mucosal Antibody Response to Pneumococcal Protein Vaccine Candidates in Otitis-prone Children after Nasopharyngeal Colonization. Submission under review 2015.
- Kaur R, Surendran N, Ochs M, Pichichero ME. Human antibodies to PhtD, PcpA, and Ply reduce adherence to human lung epithelial cells and murine nasopharyngeal colonization by Streptococcus pneumoniae. Infect Immun 2014; 82(12):5069-75; PMID:25245804; http://dx.doi.org/10.1128/IAI.02124-14
- Bologa M, Kamtchoua T, Hopfer R, Sheng X, Hicks B, Bixler G, Hou V, Pehlic V, Yuan T, Gurunathan S, et al. Safety and immunogenicity of pneumococcal protein vaccine candidates: monovalent choline-binding protein A (PcpA) vaccine and bivalent PcpA-pneumococcal histidine triad protein D vaccine. Vaccine 2012; 30(52):7461-8; PMID:23123106; http://dx.doi.org/10.1016/j.vaccine.2012.10.076
- Leroux-Roels G, Maes C, De Boever F, Traskine M, Ruggeberg JU, Borys D. Safety, reactogenicity and immunogenicity of a novel pneumococcal protein-based vaccine in adults: A phase I/II randomized clinical study. Vaccine 2014; PMID:24607003
- Prymula R, Pazdiora P, Traskine M, Ruggeberg JU, Borys D. Safety and immunogenicity of an investigational vaccine containing two common pneumococcal proteins in toddlers: a phase II randomized clinical trial. Vaccine 2014; 32(25):3025-34; PMID:24699466; http://dx.doi.org/10.1016/j.vaccine.2014.03.066
- Brewer TF. Preventing tuberculosis with bacillus Calmette-Guerin vaccine: a meta-analysis of the literature. Clin Infect Dis 2000; 31 Suppl 3, S64-67; PMID:11010824; http://dx.doi.org/10.1086/314072
- Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 1995; 346(8986):1339-45; PMID:7475776; http://dx.doi.org/10.1016/S0140-6736(95)92348-9
- Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, Rodrigues LC, Smith PG, Lipman M, Whiting PF, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 2014; 58(4):470-80; PMID:24336911; http://dx.doi.org/10.1093/cid/cit790