Abstract
Clostridium difficile is the major cause of hospital-acquired infectious diarrhea and colitis in developed countries. The pathogenicity of C. difficile is mainly mediated by the release of 2 large potent exotoxins, toxin A (TcdA) and toxin B (TcdB), both of which require neutralization to prevent disease occurrence. We have generated a novel chimeric protein, designated mTcd138, comprised of the glucosyltransferase and cysteine proteinase domains of TcdB and the receptor binding domain of TcdA and expressed it in Bacillus megaterium. To ensure that mTcd138 is atoxic, 2 point mutations were introduced to the glucosyltransferase domain of TcdB, which essentially eliminates toxicity of mTcd138. Parenteral immunizations of mice and hamsters with mTcd138 induced protective antibodies to both toxins and provided protection against infection with the hyper-virulent C. difficile strain UK6.
Introduction
Clostridium difficile is the most common cause of antibiotic-associated diarrhea in hospitalized patients in the developed world. Since 2005, C. difficile infection (CDI) has been increasingly reported among young and healthy individuals. Today, CDI is a huge social and economic burden Citation1 causing an estimated $3.2 billion of health care cost to US hospitals alone.Citation2,3
Advanced age (≥65 years), antibiotic use, immunosuppression, exposure to health care system and long-time hospitalization are major risk factors for CDI.Citation4 C. difficile toxins A (TcdA) and B (TcdB) are the major virulence factors. Both toxins share similar domain structures, including the N-terminal glucosyltransferase domain (GT), the autocatalytic cysteine proteinase domain (CPD), the central translocation domain (TMD), and the C-terminal receptor binding domain (RBD).Citation5,6
Currently, standard treatment of severe CDI is the use of vancomycin, metronidazole or fidaxomicin.Citation7-9 While effective, these antibiotics may contribute to a very high recurrence rate ranging from 20–35%.Citation10,11 A recent computer simulation shows that vaccination could be the cost-effective approach in the prevention and treatment of CDI, especially the recurrent CDI.Citation12
It was initially reported that anti-TcdA antibodies were sufficient to protect the host against CDI.Citation13,14 However, recent studies demonstrated an even more important role of TcdB in the pathogenesis of CDI,Citation15-18 suggesting that an effective vaccine should target both toxins.
Vaccines targeting the C. difficile toxins include toxoids Citation19-23 and toxin fragments.Citation24-29 Formaldehyde-inactivated native C. difficile toxins have been reported to be well tolerated and able to induce protective immunity in CDI in humans.Citation22,30,31 However, chemical toxoiding requires establishing rigorous conditions to eliminate toxicity in the final drug product while minimizing any loss of immunogenicity. Genetic toxoiding has the advantage of avoiding chemical-treatment steps during vaccine bioprocess development. Therefore, recombinant polypeptides have been considered in several studies as potential vaccine candidates. In particular, RBDs of TcdA and TcdB have been evaluated for their ability to induce protective immunity.Citation25,32-34
Recent studies have indicated that the N-terminal GT domain of TcdB can serve as an excellent immunogen.Citation35,36 This notion was initially supported by our recent construction of a chimeric recombinant vaccine against TcdA and TcdB, i.e., cTxAB, in which the original RBD of a full-length TcdB was replaced with the corresponding portion of TcdA.Citation37 cTxAB is protective in animal models. However, the cTxAB protein has a very low yield in B. megaterium, possibly because of the large size of the construct.
We also found that the neutralizing epitopes in TcdB are located in the N terminusCitation37 in addition to other domains.Citation32 Moreover, the N terminus of TcdB is more conserved than its RBD.Citation38,39 In contrast, the RBD of TcdA has potent adjuvant activity, e.g., it has been reported that a TcdA receptor peptide fragment enhanced mucosal antibody responses in mice when co-administered with heterologous protective antigensCitation40. The RBD of TcdA has been used as an immunogen in several studies.Citation28,34,40
In this study, we have generated a much simplified new chimeric protein, mTcd138, containing the GTD and CPD domains of TcdB and RBD of TcdA. mTcd138 was evaluated in mice and hamsters for its immunogenicity, and protection efficacy against CDI. Our data show that mTcd138 fusion protein may represent an alternative vaccine candidate for the protection against CDI.
Results
Construction and cytotoxicity testing of mTcd138
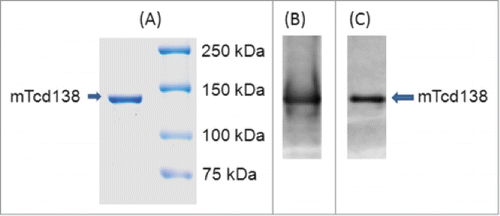
A construct encoding the mTcd138 fusion protein was generated that contains the GT and CPD domains of TcdB and the RBD of TcdA linked by a 4-aa sequence (Gly-Gly-Ser-Gly) (). Recombinant mTcd138 with a 6xHis-tag was expressed in B. megaterium and purified by Ni-affinity chromatography followed by ion exchange purification. The purification process yielded a highly pure product of about 138 kDa (). Western blot analysis using specific antibodies against TcdA and TcdB verified the presence of TcdA and TcdB fragments (). Approximately, 4–5 mg of mTcd138 was obtained from one liter of bacterial culture.
Figure 1. Domains of TcdA and TcdB and construction of mTcd138. (A) Both toxins share similar domains, including the glucosyltransferase domain (GT), the autocatalytic cysteine proteinase domain (CPD), the translocation domain (TMD) and the receptor binding domain (RBD). The DXD motif and a conserved tryptophan in the GT are involved in the enzymatic activity. (B) mTcd138 was constructed by fusing the GT and CPD of TcdB with the RBD of TcdA. Two point mutations were made in the GT of TcdB to eliminate the toxicity of mTcd138.
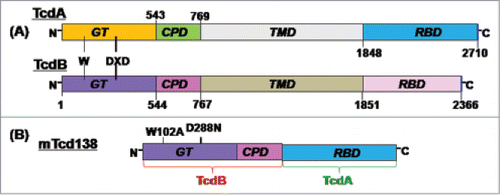
Figure 2. Expression and purification of mTcd138. Analysis of purified 138 kDa fusion protein by SDS-PAGE (A) and Western blot analysis with anti-TcdA antibody (B) and anti-TcdB antibody (C).
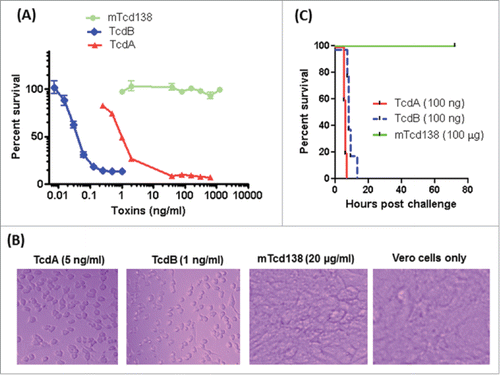
Residue toxic activity of fragments from receptor binding domain of TcdB has been reported at 100 µg/ml.Citation41 In addition, trans-membrane domain of TcdB has also been reported to contribute to toxicity.Citation42 To ensure that mTcd138 was atoxic, 2 amino acids, which have been reported to be the key residues involved in the GT activity,Citation43,44 were mutated in the GT domain of TcdB (). mTcd138 did not show detectable toxicity in in vitro (). mTcd138 at a dose of 20 µg/ml did not cause visible cell morphological changes in Vero cells, while TcdA at 5 ng/ml or TcdB at 1 ng/ml led to complete cell rounding after 72-hour incubation (). To further test in vivo toxicity of mTcd138, groups of mice were intraperitoneal (i.p.) challenged with TcdA, TcdB or mTcd138. All mice challenged i.p. with 100 ng of TcdA or TcdB died within 20 hours, while those challenged with 100 µg of mTcd138 survived () for 80 hours without any symptoms.
Figure 3. Toxicity of mTcd138. (A) Vero cells in a 96-well plate were exposed to TcdA, TcdB or mTcd138 at different concentrations for 72 h. MTT assays were performed, and cell viability was expressed as the percentage of surviving cells compared to cells without toxin exposure. (B) Vero cells were treated with TcdA, TcdB, or mTcd138 at the indicated doses for 72 hours, and representative cell images were taken. (C) C57/BL6 mice were intraperitoneally challenged with 100 ng of TcdA or TcdB or 100 μg of mTcd138. Mouse survival was monitored for 80 hours.
Immunization of mice with mTcd138 induces antibody and protects against both TcdA and TcdB
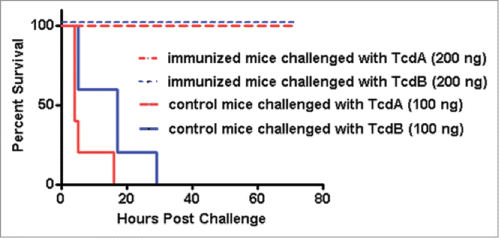
Immunization of mice with mTcd138 via i.p., intramuscular (i.m.) or intradermal (i.d.) routes induced potent but similar levels of IgG antibody responses against both TcdA and TcdB (). Significant anti-TcdA and anti-TcdB IgG responses were induced in the first and second immunizations. mTcd138 immunization induced potent neutralizing antibodies against both toxins (), though neutralizing titers against TcdA were much higher than those against TcdB. More importantly, immunization of mice with mTcd138 provided full protection against systemic challenge of lethal dose of TcdA / TcdB (). All mTcd138-immunized mice survived the intraperitoneal injection of 200 ng of either TcdA or TcdB, whereas all the placebo-immunized mice died from a i.p. injection with 100 ng of either toxin. Double mutant E. coli heat labile toxin (dmLT) was used in i.d. immunization as an adjuvant with an additional goal to stimulate mucosal response, however, no appreciable anti-TcdA or anti-TcdB IgA was detected in feces of mice immunized via the i.d. route (Data not shown).
Figure 4. mTcd138 immunization via intraperitoneal (i.p.), intramuscular (i.m.) or intradermal (i.d.) route induces similar levels of antibody response. Groups of C57 BL/6 mice were immunized 3 times at 14-day intervals via i.p., i.m. or i.d. route with 10 µg of mTcd138 in the presence of alum (i.m. and i.p.) or double mutant E.coli heat labile toxin (dmLT) (i.d.) as an adjuvant. Sera were collected, and anti-TcdA (A) or anti-TcdB (B) IgG titers measured by standard ELISA.
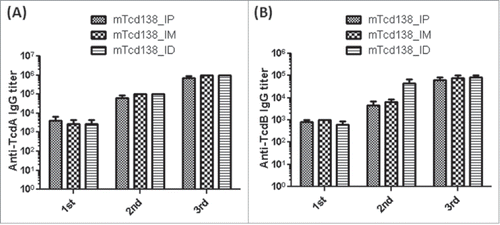
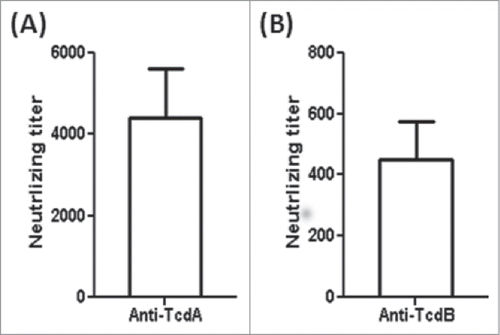
Figure 5. Serum anti-toxin neutralizing titers of the mTcd138-immunized mice (i.p.). Vero cells were used to determine in vitro neutralizing activities of sera. The neutralizing titer is expressed as the maximum dilution of the sera that inhibits cell rounding caused by toxin at a given concentration. This given concentration is the minimum toxin dose causing cell rounding after a 16 h of toxin exposure, i.e., 2.5 and 0.1 ng/ml for TcdA and TcdB, respectively.
mTc138 vaccination protects mice from infection with an epidemic C. difficile strain
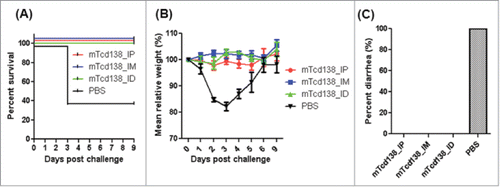
We further evaluated the protection efficacy of mTcd138 in a mouse model of CDI. After three immunizations via i.p., i.m. or i.d. routes, mice were challenged with 106 spores of C. difficile UK6 (BI/NAP1/027). All mice in all PBS-immunized mice developed diarrhea () and weight loss (). Approximately 60% mice died or became moribund and were euthanized by day 4 post-infection (). In contrast, mTcd138-immunized mice showed no appreciable signs of disease ().
Figure 7. mTcd138 immunization of mice via i.p, i.m. or i.d. routes provides full protection against infection with a hypervirulent C. difficile strain. Mice were challenged with C. difficile UK6 spores (106 /mouse) 14 days after the third immunization with mTcd138 or PBS. Kaplan-Meier survival plots (P = 0.0384 between PBS-immunized and the 3 mTcd138-immunized groups) (A), mean relative weight of all surviving mice (up to the day of death) (B) of different groups, and frequency of diarrhea (C) are illustrated. Data were presented as mean relative weight ± standard error.
Protective efficacy of mTcd138 in the hamster model of C. difficile infection
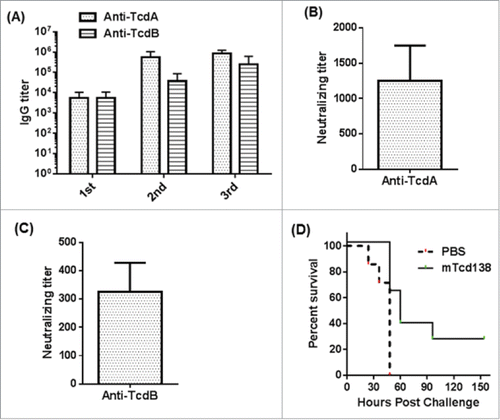
Hamsters are extremely sensitive to C. difficile infection, developing clinical signs of CDI rapidly, and die within 2 to 3 days of infection even at a very low dose of 100 spores. Therefore, hamster is an ideal animal model to test the strength of vaccines against CDI. To test the immunogenicity and protective response of mTcd138, 2 groups of hamsters (n = 8) were i.p. immunized for 3 times at 14-day intervals with 10 µg of mTcd138 or the same volume of PBS with alum as an adjuvant. Immunization of mTcd138 induced rapid antibody responses against both toxins after first immunization (), and induced high-levels of anti-toxin antibodies similar to those in mice (). Importantly, sera from immunized hamsters were able to neutralize TcdA and TcdB (), although neutralizing titers against both toxins are lower than those of sera from mTcd138 immunized mice (). To test the strength of mTcd138, 14 days after the third immunization, hamsters were i.p. injected with 30 mg/kg clindamycin followed by challenge at 2 × 105 C. difficile UK6 spores (lethal challenge dose is 100–1000 spores) 5 days later. All PBS-immunized hamsters developed diarrhea and died within 24–48 hours (). All mTcd138-immunized hamsters also developed diarrhea, however, they survived significantly longer (P = 0.0075) and 2 hamsters survived 7-day of monitoring period ().
Figure 8. Protective response of mTcd138 vaccination in hamsters. (A) Serum anti-TcdA/TcdB IgG titers after each immunization with mTcd138 (10 µg, IP); (B) Serum (from 3rd immunization) anti-TcdA neutralizing titers; (C) Serum (from 3rd immunization) anti-TcdB neutralizing titers; (D) Kaplan-Meier survival curves of mTcd138-immunized or PBS-immunized hamsters challenged with 2 × 105 C. difficile UK6 spores (P = 0.0075).
Discussion
Both TcdA and TcdB are major C. difficile virulence factors. Vaccination targeting both toxins is necessary to provide the host full protection against CDI.
It was reported that TcdA is relatively well conserved, while TcdB has much variability,Citation38,39 especially the RBD region.Citation38 The N terminus encompassing the GTD and CPD domains is more conserved between historical and epidemic strains. In our previous study Citation37 and consistent with others,Citation35,36 we indicated that the N-terminus of TcdB was able to elicit a protective antibody response. In contrast, the RBD of TcdA has potent adjuvant activity Citation40, and has been used as vaccine candidates in several studies.Citation28,34,40 Therefore, we hypothesized that a fusion protein containing the N-terminus of TcdB and RBD of TcdA would be immunogenic and sufficient to induce protection against both toxins.
Previously, we described the development of a chimeric recombinant protein vaccine (cTxAB) targeting both toxins.Citation37 Here, we reported the construction of a much simpler immunogen targeting both TcdA and TcdB by fusing the glucosyltransferase and cysteine proteinase domains of TcdB with the receptor binding domain of TcdA. This new construct is stable, and is easy to express and purify in large quantities. It is non-toxic and expressed in B. megaterium, a nonpathogenic and endotoxin-free production system. Immunization with mTcd138 fully protected mice against systemic challenge with lethal doses of toxins, and against infection with the hypervirulent strain C. difficile UK6. mTcd138 immunization also induced significant protective responses in hamsters. However, the protection in hamsters is not as impressive as in mice, which may partially due to the very high challenge C. difficile dose (2 × 105) used in hamsters. Further evaluation of immunization does, routes and challenge dose of C. difficile spores will be pursued in both mice and hamsters in the near future.
Recently, Leuzzi et al. reported that the GT of TcdB, when expressed separately, was immunogenic and was able to induce neutralizing antibodies to TcdB. When expressed as a fusion with part of RBD of TcdA, however, the fusion protein could not induce anti-TcdB neutralizing antibodies.Citation36 The fusion protein constructed by this group does not include the CPD domain of TcdB. The different outcomes of our study and that of Leuzzi et al., indicate that CPD is immunogenic and/or plays important roles in maintaining the native structure or epitope conformation of GTD. In fact, the importance of the CPD in eliciting anti-TcdB neutralizing antibodies was also documented by a recent report, in which a TcdB fragment (TxB4) containing RBD and TMD only induced half the amount of anti-TcdB neutralizing antibodies when compared to another TcdB fragment (TxB5) comprised of the CPD in addition to the TMD and RBD.Citation45
Materials and Methods
Preparation of C. difficile spores
C. difficile UK6, an epidemic strain (kindly provided by Dale Gerding and Abraham L. Sonenshein)Citation46 was isolated in the United Kingdom. Sporulation of the C. difficile UK6 strain was induced in Clospore medium as described previously.Citation47 Briefly, an overnight 20 ml of C. difficile culture in Columbia Broth was inoculated into 500 ml of Clospore medium, and incubated for 1–2 weeks at 37°C in an anaerobic incubator. The spore suspension was centrifuged at 1000g for 10 min, and the pellet was washed 5 times with sterile water and suspended in 10 ml of ddH2O. The spore suspension was heated at 60°C for 20 min to kill vegetative cells and stored at 4°C. The spore concentration was determined by serial dilution on TCCFA or BHI plates.Citation48
Expression of recombinant fusion protein mTcd138 in Bacillus megaterium
Genes encoding TcdA and TcdB from C. difficile VPI10463 were previously cloned in an E.coli-B. megaterium shuttle vector pHis 1525 and expressed in B. megaterium.Citation49 Recombinant TcdA and TcdB were expressed in B. megaterium and purified as described previously.Citation49
To generate mTcd138, the DNA sequences from C. difficile VPI 10463, encoding the glucosyltransferase (GTD 1–543 aa) with 2 amino acid mutations (W102A and D288N) and cysteine proteinase (CPD, 543–767) domains of TcdB and receptor binding domain (RBD) of TcdA were bridged with a linker (ggt ggc tct ggt) sequence, synthesized by Geneart (Germany) and cloned between the BsrGI and EagI sites of the vector pHis1525. mTcd138 was expressed in B. megeatarium and purified as described previously.Citation49
Cytotoxicity of mTcd138 in cells
Cytotoxicity of the toxins was assayed as described previously.Citation49 Briefly, vero cells in 96-well plates were exposed to TcdA/TcdB or mTcd138 at different concentrations. MTT [3-(4,5-dimethylthiazol-2-yl)2 2,5-diphenyl tetrazolium bromide] assays were used to determine the toxin or mTcd138 cytotoxicity. After 72 h of treatment, 10 μl of MTT (5 mg/ml) was added to each well, and were incubated for additional 4 h at 37°C, followed by dissolving the formed formazan 0.4 N HCl in absolute isopropanol. The absorbance was recorded at 570 nm. Cell viability was defined as the percentage of survived cells in comparison with the non-treated cells.
Cytotoxicity of mTcd138 in mice
Female C57 BL/6 mice (n = 10) were i.p. challenged with 100 ng TcdA/ TcdB or 100 µg mTcd138 per mouse. Mouse survival was monitored and analyzed by Kaplan-Meier survival analysis.
Mouse immunization and mouse model of C. difficile infection
Female C57/BL6 mice were housed under the same conditions. All studies followed the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Tufts University Institutional Animal Care and Use Committee under the protocol #G2012-70. Mice (n = 10) were immunized 3 times at 14-day intervals via i.p., intramuscular (i.m.) or intradernal (i.d.) routes with 10 µg of mTcd138 in PBS along with alum (i.m. and i.p.) or double mutant E. coli heat labile toxin (dmLT) at 5 µg/mouse (i.d.) as adjuvants. dmLT, an adjuvant for mucosal immunization, was used in i.d. immunization with an additional goal to stimulate mucosal response. Lyophilized dmLT was manufactured at Walter Reed Army Institute for Research (Silver Spring, MD, BPR-928-00, Lot No, 1575).Citation50 Control mice received PBS with alum. Sera were collected. Immunized or control mice were pretreated with an antibiotic cocktail followed by C. difficile spore infection via oral gavage as described previously.Citation51,52 Fourteen days after the third immunization, mice were challenged with 106 C. difficile UK6 spores.
Hamster immunization and challenge with C. difficile
Golden Syrian female hamsters used in the experiments were housed in cages individually under the same conditions. All studies followed the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Tufts University Institutional Animal Care and Use Committee under the protocol #G2014-90.
Hamsters were i.p. immunized with 10 µg of mTcd138 in 100 µl of PBS with alum as an adjuvant for 3 times at 14-days intervals. Control hamsters were immunized with same volume of PBS plus alum. Sera were collected, and anti-TcdA/TcdB IgG titers were determined by ELISA. Two weeks after third immunization, hamsters were i.p. administered with one dose of clindamycin at 30 mg/kg followed by oral challenge with 2 × 105 C. difficile UK6 spores 5 days later. The hamsters were monitored for 7 days for diarrhea and other disease symptoms.
Antibody titers and neutralizing assays
Antibody titers were measured using a standard ELISA against purified recombinant wild-type TcdA or TcdB. Vero cells were used to assess neutralizing activities of serum samples. The neutralizing titer is defined as the maximum dilution of the samples that blocks cell rounding caused by toxin at a given concentration. This given concentration is the minimum dose of the toxin that causes all cells to round after a 16-h exposure to the toxin, i.e., ca 2.5 and 0.1 ng/ml for TcdA and TcdB, respectively.
Statistical analysis
Data were analyzed by Kaplan-Meier survival analysis using Prism statistical software. Results are expressed as means ± standard errors of means.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
X. Sun conceived and designed the experiments. Y. Wang, H.B. Kim, X. Ju, S. Zhao, K. Zhang and X. Sun performed the experiments. Y. Wang, Y. Yan and X. Sun analyzed the data. X. Sun, Y. Wang, Y. Yan and S. Tzipori contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We thank Hanping Feng (University of Maryland) for providing B. megaterium strains expressing wild-type and mutant TcdA/TcdB. We thank Abraham L. Sonenshein, Giovanni F. Widmer, Diane Schmidt (Tufts University) and Gary Ostroff (University of Massachusetts Medical School) for critical comments of the manuscript. We also thank Weijia Nie for technical assistance.
Funding
This work was supported by NIH grant K01DK092352 (X. Sun), Tufts Collaborates 2013_V330421 (X. Sun), Tufts Collaborates 2014_V330423 (X. Sun), Tufts Innovation Institute Award 2014_V330427 (X. Sun) and partially by NIH grant AI088748 (S. Tzipori/H. Feng).
References
- Rebeaud F, MF Bachmann. Immunization strategies for Clostridium difficile infections. Expert Rev Vaccines. 11(4): p. 469-79; PMID:22551032; http://dx.doi.org/10.1586/erv.12.18
- O'Brien JA, Lahue BJ, Caro JJ, Davidson DM. The emerging infectious challenge of clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol, 2007. 28(11): p. 1219-27; http://dx.doi.org/10.1086/522676
- Dubberke ER, AI Wertheimer. Review of current literature on the economic burden of Clostridium difficile infection. Infect Control Hosp Epidemiol, 2009. 30(1): p. 57-66; http://dx.doi.org/10.1086/592981
- Bauer MP, Notermans DW, van Benthem BH, Brazier JS, Wilcox MH, Rupnik M, Monnet DL, van Dissel JT, Kuijper EJ; ECDIS Study Group. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 377(9759): p. 63-73; PMID:21084111; http://dx.doi.org/10.1016/S0140-6736(10)61266-4
- Jank T, K Aktories. Structure and mode of action of clostridial glucosylating toxins: the ABCD model. Trends Microbiol, 2008. 16(5): p. 222-9; http://dx.doi.org/10.1016/j.tim.2008.01.011
- Pruitt RN, DB Lacy. Toward a structural understanding of Clostridium difficile toxins A and B. Front Cell Infect Microbiol. 2: p. 28; PMID:22919620
- McDonald LC, Coignard B, Dubberke E, Song X, Horan T, Kutty PK; Ad Hoc Clostridium difficile Surveillance Working Group. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol, 2007. 28(2): p. 140-5; http://dx.doi.org/10.1086/511798
- Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America, Clinical practice guidelines for Clostridium difficile infection in adults: update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010; 31(5): p. 431-55; PMID:20307191; http://dx.doi.org/10.1086/651706
- Cornely OA, Crook DW, Esposito R, Poirier A, Somero MS, Weiss K, Sears P, Gorbach S; OPT-80-004 Clinical Study Group. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 12(4): p. 281-9; PMID:22321770
- Tonna I, PD Welsby. Pathogenesis and treatment of Clostridium difficile infection. Postgrad Med J, 2005. 81(956): p. 367-9; http://dx.doi.org/10.1136/pgmj.2004.028480
- Barbut F, Richard A, Hamadi K, Chomette V, Burghoffer B, Petit JC. Epidemiology of recurrences or reinfections of Clostridium difficile-associated diarrhea. J Clin Microbiol, 2000. 38(6): p. 2386-8.
- Lee BY, Popovich MJ, Tian Y, Bailey RR, Ufberg PJ, Wiringa AE, Muder RR. The potential value of Clostridium difficile vaccine: an economic computer simulation model. Vaccine, 2010. 28(32): p. 5245-53; http://dx.doi.org/10.1016/j.vaccine.2010.05.062
- Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med, 2000. 342(6): p. 390-7; http://dx.doi.org/10.1056/NEJM200002103420604
- Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet, 2001. 357(9251): p. 189-93; http://dx.doi.org/10.1016/S0140-6736(00)03592-3
- Johnson S, Kent SA, O'Leary KJ, Merrigan MM, Sambol SP, Peterson LR, Gerding DN. Fatal pseudomembranous colitis associated with a variant clostridium difficile strain not detected by toxin A immunoassay. Ann Intern Med, 2001. 135(6): p. 434-8; http://dx.doi.org/10.7326/0003-4819-135-6-200109180-00012
- Drudy D, S Fanning, L Kyne. Toxin A-negative, toxin B-positive Clostridium difficile. Int J Infect Dis, 2007. 11(1): p. 5-10; http://dx.doi.org/10.1016/j.ijid.2006.04.003
- Kuijper EJ, de Weerdt J, Kato H, Kato N, van Dam AP, van der Vorm ER, Weel J, van Rheenen C, Dankert J. Nosocomial outbreak of Clostridium difficile-associated diarrhoea due to a clindamycin-resistant enterotoxin A-negative strain. Eur J Clin Microbiol Infect Dis, 2001. 20(8): p. 528-34; http://dx.doi.org/10.1007/s100960100550
- van den Berg RJ, Claas EC, Oyib DH, Klaassen CH, Dijkshoorn L, Brazier JS, Kuijper EJ. Characterization of toxin A-negative, toxin B-positive Clostridium difficile isolates from outbreaks in different countries by amplified fragment length polymorphism and PCR ribotyping. J Clin Microbiol, 2004. 42(3): p. 1035-41; http://dx.doi.org/10.1128/JCM.42.3.1035-1041.2004
- Aboudola S, Kotloff KL, Kyne L, Warny M, Kelly EC, Sougioultzis S, Giannasca PJ, Monath TP, Kelly CP. Clostridium difficile vaccine and serum immunoglobulin G antibody response to toxin A. Infect Immun, 2003. 71(3): p. 1608-10; http://dx.doi.org/10.1128/IAI.71.3.1608-1610.2003
- Giannasca PJ, Zhang ZX, Lei WD, Boden JA, Giel MA, Monath TP, Thomas WD Jr. Serum antitoxin antibodies mediate systemic and mucosal protection from Clostridium difficile disease in hamsters. Infect Immun, 1999. 67(2): p. 527-38
- Siddiqui F, O'Connor JR, Nagaro K, Cheknis A, Sambol SP, Vedantam G, Gerding DN, Johnson S. Vaccination with parenteral toxoid B protects hamsters against lethal challenge with toxin A-negative, toxin B-positive clostridium difficile but does not prevent colonization. J Infect Dis. 205(1): p. 128-33; PMID:22124129; http://dx.doi.org/10.1093/infdis/jir688
- Sougioultzis S, Kyne L, Drudy D, Keates S, Maroo S, Pothoulakis C, Giannasca PJ, Lee CK, Warny M, Monath TP, et al. Clostridium difficile toxoid vaccine in recurrent C. difficile-associated diarrhea. Gastroenterology, 2005. 128(3): p. 764-70; http://dx.doi.org/10.1053/j.gastro.2004.11.004
- Anosova NG, Brown AM, Li L, Liu N, Cole LE, Zhang J, Mehta H, Kleanthous H. Systemic antibody responses induced by a two-component Clostridium difficile toxoid vaccine protect against C. difficile-associated disease in hamsters. J Med Microbiol. 62(Pt 9): p. 1394-404; PMID:23518659
- Belyi IF, NA Varfolomeeva. Construction of a fusion protein carrying antigenic determinants of enteric clostridial toxins. FEMS Microbiol Lett, 2003. 225(2): p. 325-9; http://dx.doi.org/10.1016/S0378-1097(03)00560-3
- Permpoonpattana P, Hong HA, Phetcharaburanin J, Huang JM, Cook J, Fairweather NF, Cutting SM. Immunization with Bacillus spores expressing toxin A peptide repeats protects against infection with Clostridium difficile strains producing toxins A and B. Infect Immun. 79(6): p. 2295-302; PMID:21482682; http://dx.doi.org/10.1128/IAI.00130-11
- Ryan ET, Butterton JR, Smith RN, Carroll PA, Crean TI, Calderwood SB. Protective immunity against Clostridium difficile toxin A induced by oral immunization with a live, attenuated Vibrio cholerae vector strain. Infect Immun, 1997. 65(7): p. 2941-9
- Seregin SS, Aldhamen YA, Rastall DP, Godbehere S, Amalfitano A. Adenovirus-based vaccination against Clostridium difficile toxin A allows for rapid humoral immunity and complete protection from toxin A lethal challenge in mice. Vaccine. 30(8): p. 1492-501; PMID:22200503; http://dx.doi.org/10.1016/j.vaccine.2011.12.064
- Ward SJ, Douce G, Dougan G, Wren BW. Local and systemic neutralizing antibody responses induced by intranasal immunization with the nontoxic binding domain of toxin A from Clostridium difficile. Infect Immun, 1999. 67(10): p. 5124-32
- Ward SJ, Douce G, Figueiredo D, Dougan G, Wren BW. Immunogenicity of a Salmonella typhimurium aroA aroD vaccine expressing a nontoxic domain of Clostridium difficile toxin A. Infect Immun, 1999. 67(5): p. 2145-52
- Greenberg RN, Marbury TC, Foglia G, Warny M. Phase I dose finding studies of an adjuvanted Clostridium difficile toxoid vaccine. Vaccine. 30(13): p. 2245-9; PMID:22306375; http://dx.doi.org/10.1016/j.vaccine.2012.01.065
- Kotloff KL, Wasserman SS, Losonsky GA, Thomas W Jr, Nichols R, Edelman R, Bridwell M, Monath TP. Safety and immunogenicity of increasing doses of a Clostridium difficile toxoid vaccine administered to healthy adults. Infect Immun, 2001. 69(2): p. 988-95; http://dx.doi.org/10.1128/IAI.69.2.988-995.2001
- Tian JH, Fuhrmann SR, Kluepfel-Stahl S, Carman RJ, Ellingsworth L, Flyer DC. A novel fusion protein containing the receptor binding domains of C. difficile toxin A and toxin B elicits protective immunity against lethal toxin and spore challenge in preclinical efficacy models. Vaccine. 30(28): p. 4249-58; PMID:22537987; http://dx.doi.org/10.1016/j.vaccine.2012.04.045
- Ghose C, Verhagen JM, Chen X, Yu J, Huang Y, Chenesseau O, Kelly CP, Ho DD. Toll-like receptor 5-dependent immunogenicity and protective efficacy of a recombinant fusion protein vaccine containing the nontoxic domains of Clostridium difficile toxins A and B and Salmonella enterica serovar typhimurium flagellin in a mouse model of Clostridium difficile disease. Infect Immun. 81(6): p. 2190-6; PMID:23545305; http://dx.doi.org/10.1128/IAI.01074-12
- Gardiner DF, Rosenberg T, Zaharatos J, Franco D, Ho DD. A DNA vaccine targeting the receptor-binding domain of Clostridium difficile toxin A. Vaccine, 2009. 27(27): p. 3598-604; http://dx.doi.org/10.1016/j.vaccine.2009.03.058
- Jin K, Wang S, Zhang C, Xiao Y, Lu S, Huang Z. Protective antibody responses against Clostridium difficile elicited by a DNA vaccine expressing the enzymatic domain of toxin B. Hum Vaccin Immunother. 9(1): p. 63-73; PMID:23143772; http://dx.doi.org/10.4161/hv.22434
- Leuzzi R, Spencer J, Buckley A, Brettoni C, Martinelli M, Tulli L, Marchi S, Luzzi E, Irvine J, Candlish D, et al. Protective efficacy induced by recombinant Clostridium difficile toxin fragments. Infect Immun. 81(8): p. 2851-60; PMID:23716610; http://dx.doi.org/10.1128/IAI.01341-12
- Wang H, Sun X, Zhang Y, Li S, Chen K, Shi L, Nie W, Kumar R, Tzipori S, Wang J, et al. A chimeric toxin vaccine protects against primary and recurrent Clostridium difficile infection. Infect Immun. 80(8): p. 2678-88; PMID:22615245; http://dx.doi.org/10.1128/IAI.00215-12
- Lanis JM, S Barua, JD Ballard. Variations in TcdB activity and the hypervirulence of emerging strains of Clostridium difficile. PLoS Pathog. 6(8): p. e1001061; PMID:20808849; http://dx.doi.org/10.1371/journal.ppat.1001061
- Stabler RA, He M, Dawson L, Martin M, Valiente E, Corton C, Lawley TD, Sebaihia M, Quail MA, Rose G, et al. Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome Biol, 2009. 10(9): p. R102; http://dx.doi.org/10.1186/gb-2009-10-9-r102
- Brun P, Scarpa M, Grillo A, Palù G, Mengoli C, Zecconi A, Spigaglia P, Mastrantonio P, Castagliuolo I. Clostridium difficile TxAC314 and SLP-36kDa enhance the immune response toward a co-administered antigen. J Med Microbiol, 2008. 57(Pt 6): p. 725-31; http://dx.doi.org/10.1099/jmm.0.47736-0
- Zemljic M, Rupnik M, Scarpa M, Anderluh G, Palù G, Castagliuolo I. Repetitive domain of Clostridium difficile toxin B exhibits cytotoxic effects on human intestinal epithelial cells and decreases epithelial barrier function. Anaerobe, 2010. 16(5): p. 527-32; http://dx.doi.org/10.1016/j.anaerobe.2010.06.010
- Donald RG, Flint M, Kalyan N, Johnson E, Witko SE, Kotash C, Zhao P, Megati S, Yurgelonis I, Lee PK, et al. A novel approach to generate a recombinant toxoid vaccine against Clostridium difficile. Microbiology, 2013. 159(Pt 7): p. 1254-66; http://dx.doi.org/10.1099/mic.0.066712-0
- Teichert M, Tatge H, Schoentaube J, Just I, Gerhard R. Application of mutated Clostridium difficile toxin A for determination of glucosyltransferase-dependent effects. Infect Immun, 2006. 74(10): p. 6006-10; http://dx.doi.org/10.1128/IAI.00545-06
- Busch C, Hofmann F, Selzer J, Munro S, Jeckel D, Aktories K. A common motif of eukaryotic glycosyltransferases is essential for the enzyme activity of large clostridial cytotoxins. J Biol Chem, 1998. 273(31): p. 19566-72; http://dx.doi.org/10.1074/jbc.273.31.19566
- Maynard-Smith M, Ahern H, McGlashan J, Nugent P, Ling R, Denton H, Coxon R, Landon J, Roberts A, Shone C. Recombinant antigens based on toxins A and B of Clostridium difficile that evoke a potent toxin-neutralising immune response. Vaccine. 32(6): p. 700-5; PMID:24342251; http://dx.doi.org/10.1016/j.vaccine.2013.11.099
- Killgore G, Thompson A, Johnson S, Brazier J, Kuijper E, Pepin J, Frost EH, Savelkoul P, Nicholson B, van den Berg RJ, et al. Comparison of seven techniques for typing international epidemic strains of Clostridium difficile: restriction endonuclease analysis, pulsed-field gel electrophoresis, PCR-ribotyping, multilocus sequence typing, multilocus variable-number tandem-repeat analysis, amplified fragment length polymorphism, and surface layer protein A gene sequence typing. J Clin Microbiol, 2008. 46(2): p. 431-7; http://dx.doi.org/10.1128/JCM.01484-07
- Perez J, VS Springthorpe, SA Sattar. Clospore: a liquid medium for producing high titers of semi-purified spores of Clostridium difficile. J AOAC Int, 2011. 94(2): p. 618-26.
- Sorg JA, AL Sonenshein. Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. J Bacteriol, 2010. 192(19): p. 4983-90; http://dx.doi.org/10.1128/JB.00610-10
- Yang G, Zhou B, Wang J, He X, Sun X, Nie W, Tzipori S, Feng H. Expression of recombinant Clostridium difficile toxin A and B in Bacillus megaterium. BMC Microbiol, 2008. 8: p. 192; http://dx.doi.org/10.1186/1471-2180-8-192
- Lee S, WL Picking, S Tzipori. The immune response of two microbial antigens delivered intradermally, sublingually, or the combination thereof. Microbes Infect 2014; 16(9):796-803; PMID:25111827; http://dx.doi.org/10.1016/j.micinf.2014.07.013
- Chen X, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding DN, Kelly CP. A mouse model of Clostridium difficile-associated disease. Gastroenterology, 2008. 135(6): p. 1984-92; http://dx.doi.org/10.1053/j.gastro.2008.09.002
- Sun X, Wang H, Zhang Y, Chen K, Davis B, Feng H. Mouse relapse model of Clostridium difficile infection. Infect Immun, 2011. 79(7): p. 2856-64; http://dx.doi.org/10.1128/IAI.01336-10