Abstract
There is still no suitable routine hepatitis B immunization strategy for adults in China. To establish an optimal vaccination schedule for healthy adults, we investigated various schedules in healthy adults. In this randomized 5143 healthy adults received 10 μg hepatitis B vaccine at 0, 1 and 3 months(group A), 0, 1 and 6 months(group B), or 0, 1 and 12 months(group C). Blood samples were collected after 1 month and 12 months after the third dose. The geometric mean titer (GMT), seroconversion rate (levels of anti-HBs ≥10 mIU/mL) and high response rate (levels of anti-HBs ≥100 mIU/mL) were assayed. In our study, 2438 healthy adults finished the full vaccination program and follow-up. The seroconversion/sero-protective rate of groups A–C at one and 12 month after administration of the third vaccine dose was 100%, 99.9% and 97.9% verse 64.9%, 75.7% and 79.0%, respectively. GMT for anti-HBs tested in group A to C within 1 or 12 month after the third vaccination was 213.16, 432.58 and 451.47 mIU/ml verse 22.07, 46.70 and 56.18 mIU/ml, respectively. There were significant differences of seroconversion/sero-protective rate and GMT among the 3 groups (p < 0.01). Given the high anti-HBs seroconversion rate and GMT in all 3 groups, a flexible schedule for Hepatitis B vaccine should be recommended to adults, but 0-1-12 schedule is a better choice.
Introduction
It is well-known that hepatitis B virus (HBV) immunization strategy is the most effective measure for prevention of HBV transmission. Although hepatitis B vaccine has been available since 1982, HBV infection remains a serious global public health problem. According to WHO, there are currently >2 billion people infected with HBV, with ˜360 million chronically infected worldwide.Citation1 HBV-related liver disease or hepatocellular carcinoma results in 600,000 deaths annually.Citation2 In China, the 2006 national serosurvey showed 7.18% of hepatitis B surface antigen (HBsAg) prevalence among a general population, with 26.36% dropped according to 1992 national serosurvey (HBsAg prevalence: 9.75%).Citation2 Although the prevalence of HBsAg in the whole population has fallen, HBsAg prevalence in adults will not decrease dramatically due to the life-long HBsAg carrier status and the increased life expectancy. According to a study in Jiangsu province, China, hepatitis B vaccination in adults might decrease the rate of HBsAg positivity and routine immunization of adults is recommended.Citation3 In addition, it was estimated that there were 221 million migratory populations at the end of 2010 in China, mostly of young adults.Citation4 They left home to find better employment opportunities and higher income in cities and would come back home without fixed time, but usually at the end of year. The nature of perennial motility increased their danger of HBV infection. This special national situation of our nation decides the necessity for adult immunization investigation. Therefore, the National Health and Family Planning Commission of the People's Republic of China intends to expand vaccination based on routine vaccination of infants, encouraging adults to be vaccinated, especially in high-risk populations. To date, there have been no informed types and dosage of hepatitis B vaccine available for Chinese adults, the recommendation for adults on hepatitis B vaccination put forward by Chinese National CDC follows the conventional immunization programs available for infants. Though there have been several studies in terms of adult vaccination against hepatitis B in developed countries, there are no standard immunization programs for adults in China, needing more studies to explore optimal immunization programs.
In the present study, we investigated the immunogenicity and persistence of hepatitis B surface antibody (anti-HBs) titers after 2 different dosages (10 and 20μg) of the common vaccines in Chinese market, and compared the immunogenicity and persistence of anti-HBs titers after 3 different schedules of 10 μg common vaccines in Chinese market in healthy adults.
Results
Study population
A total of 5143 individuals were randomized into this study between March and June 2010 and received the full vaccination schedule. Blood samples were collected at 1 month after the third dose. There were a total of 986participants who are excluded from the efficacy analysis: 826 showed positive for anti-HBc, 150 were aged <16 years, and 10 had incomplete age information. The loss to follow-up at 12 months after full vaccination was surprising, at nearly half of the individuals recruited (41.4 %). The final number included was 2438 (). As shown in , the sex ratio was 1:1.56 (male: female), there were no significant differences in baseline demographics among groups A–C, accepting the hypothesis of homogeneity. The mean age of the subjects was 33.07 ± 7.87 years old.
Figure 1. Participant disposition (Group A: 0-1-3 schedule, group B: 0-1-6 schedule, group C: 0-1-12 schedule; *: To analysis the immunogenicity effect of hepatitis B vaccine, subjects with positive anti-HBc antibody who also got full vaccination was deleted in the process of analysis.).
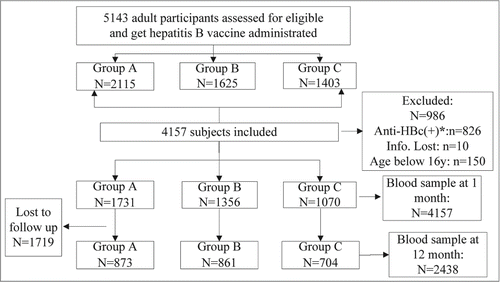
Table 1. Gender and age distribution of participants after 12 months follow-up
Antibody response among different vaccination programs
100%, 99.9% [95% confidence interval (CI): 99.7%,100%] and 97.9% (95% CI:96.8%,98.9%) of groups A–C developed protective levels of anti-HBs ≥10 IU/L within 1 month after administration of the third vaccine dose, respectively (). The sero-protective rate at 12 months after the third dose of vaccine was 65.0% (95% CI: 61.8%, 68.1%), 75.7% (95% CI: 72.9%, 78.6%) and 79.0% (95% CI: 76.0%, 82.0%), respectively. The seroconversion rates from each blood sample had significant differences among different vaccination programs (p <0.001).
Table 2. Summary of seroconversion rate and GMT by different variables
Among the volunteers who presented with seroconversion rates of 100%, 99.9% and 97.9% within 1 month after administration of the full vaccination, 64.4%, 74.6% and 76.8% of those administered the 0-1-3, 0-1-6 or 0-1-12 schedule, respectively, were considered to be high responders (anti-HBs ≥100 mIU/ml). Similarly, the sero-protective rate of high responders about 12 months after administration of the third vaccine dose was 22.0%, 39.7% and 44.2%, respectively (). Significant differences were observed among different vaccination programs (p < 0.001).
Table 3. High-response rate among groups A–C
GMT for anti-HBs tested within 1 month after the third vaccination was 213.16 mIU/ml (95% CI: 189.93, 239.23) in group A, compared with 432.58 mIU/ml (95% CI: 382.91, 488.69) in group B, and 451.47 mIU/ml (95% CI: 383.92, 530.92) in group C. Statistical analysis of log-transformed data demonstrated significantly higher GMT of anti-HBs for the 3 group at 1 month (). GMT for anti-HBs tested within 12 months after the third vaccination was 22.07 mIU/ml (95% CI: 19.19, 25.37), 46.70 mIU/ml (95% CI: 39.69, 54.94) and 56.18 mIU/ml (95% CI: 45.68, 69.11) in the 0-1-3, 0-1-6 and 0-1-12 vaccination schedules, respectively.
According to , in group A, the sero-protective rate at 12 months had dropped 35.0% compared with 1 month. Similar changes occurred in groups B and C with 24.2% and 19.3% decreases, respectively. There were significant differences in all the 3 groups between the 2 time points. In addition, GMT for anti-HBs in each schedule decreased significantly from 1 month after the full vaccination to 12 months.
Immunogenicity-related factors
When stratified by demographic characteristics, the 1 month GMT was significantly higher in females than in males, but there were no significant differences between men and women neither with seroconversion/sero-protective nor GMT at 12 month (). As to age stratification, a significant difference for sero-protective rate was shown at 12 month after the third dose other than one month. All four age groups had a seroconversion rate of ˜99.0% at 1 month after the third dose; and sero-protective rates of 80.1%, 7.3%, 69.1% and 69.0% at 12 months after the third dose in the 4 years age group, respectively. GMTs for anti-HBs levels in each age group at 1 and 12 months were 402.08 (330.13, 489.70) mIU/ml, 362.43 (321.99, 407.95) mIU/ml, 306.30 (268.50, 349.42) mIU/ml, 258.96 (200.00, 335.29) mIU/ml versus 58.48 (47.64, 71.78) mIU/ml, 40.27 (34.61, 46.85) mIU/ml, 30.15 (25.33, 35.88) mIU/ml and 25.09 (16.88, 37.29) mIU/ml, respectively. Apart from the seroconversion rate at 1 month (p > 0.05), significant differences were identified of the sero-protective rate at 12 months and GMT for anti-HBs tested at 2 time points (p = 0.0003, 0.014 and 0.0007).
As shows, the trend in terms of GMT for anti-HBs tested within 1 month and 12 months after the third vaccination resembled the trend in sero-protective rates at 12 months when samples were collected among the age groups. The overall correlation between GMTs and age groups was negative at 1 month and 12 months (r = −0.072, P = 0.0004; r = −0.105, P = 0.00092).
Table 4. Correlation between GMTs and the age groups using bivariate correlations
Discussion
Vaccinating adults against hepatitis B is important because adults can be carriers of HBV, especially who have not received hepatitis B immunization from the childhood. At the present time, there are a large number of adults without hepatitis B inoculation. As we all know, the hepatitis B immunization coverage rate in China has increased each year since 1992. Infant vaccination coverage with both the timely birth dose and the complete vaccine series was substantially higher among children born during 2003 than among those born during 1997; timely birth-dose coverage increased from 29.1% to 75.8%.Citation5 If we calculated the age from 1997, it is 18 year old till now. Based on the above data, there are nearly 70.9% adults without hepatitis B immunization except the population who are born before 1997. And with the economic increasing, most of them left home for good job and would return home at different time, especially at the end of year. Therefore, routine 0-1-6 schedule may not be suitable for them. We have to search for better immunization program and optimal dosage among healthy adults to prevent the horizontal transmission. Up to now, there were few large-scale studies regarding the hepatitis B vaccine on immunogenicity in the healthy adults. According to a recent report from most developed countries, adult vaccines are mostly recommended for specific high-risk groups of adults rather than recommended to all adults. The situation in China is similar. And as a result, it is of great necessity to explore optimal hepatitis B immunization program for all healthy adults, especially in China.
According to previous studies, conventional hepatitis B vaccination programs (0-1-6 schedule) in healthy adults induce seroconversion rates >90%.Citation6,7 In our study, where hepatitis B vaccines were administered intramuscularly among 7 counties, excellent immunogenicity was demonstrated for all 3 schedules. 99.3% of vaccine recipients were converted to sero-protective at 1 month after different full vaccination schedules were administered. The reason may be that the subjects were comparative young with over 90% below 45 years old. Although the seroconversion rate remained similar at 1 month among participants after full immunization, there was significant higher sero-protective rate in the 0-1-12 schedule than the other 2 groups at 12 months after dose 3. Pitifully, we did not find similar investigation among adults. However, we surprisingly found that our results were similar to other studies conducted in children or infants, in which the concentrations of anti-HBs after the third injection were dependent on the interval between the second and third dose. Children who received the third vaccine dose relatively late obtained higher antibody titers than those received the third dose on schedule.Citation8–10 However, regarding this evidence, Agladioglu et al.Citation11 did not observe any positive impact on antibody levels of the interval between the second and third doses for those infants vaccinated at months 0, 2 and 9 or 2, 4 and 9. As there were no similar study conducted in adults, so we dare to infer the reason may be that the impact on antibody levels of the interval between the second and third doses would be affected by age, and needs further investigation.
According to the American Academy of Immunization Practices standardsCitation12 (anti-HBs titers 10–99 mIU/mL as hypo-responders,100 −999 mIU/mL as good-responders, ≥1000 mIU/mL as hyper-responders),an anti-HBs level ≥10 mIU/ml is correlated with vaccine-elicited protection against HBV infection,Citation12,13 and an anti-HBs level ≥100 mIU/ml is considered as good reponse.Citation14 The authentic persistence of immunity after HB vaccination is currently unknown. According to several medical professions, peak antibody levels after vaccination mainly determine the long-term efficacy of hepatitis B vaccine. It is generally assumed that anti-HBs titers decline rapidly within the first year and then more slowly after primary immunization, and the higher the antibody levels, the longer the period of protection.Citation8,9,15,16 The total anti-HBs GMT observed at 1 month and 12 months after the third dose was 375.43 and 40.60 mIU/ml, respectively. There were significant differences among the schedules that showed a significant increase from the 0-1-3 to 0-1-12 schedule at both time points. Therefore, it could be convinced that the hepatitis B immunization of 0,1 and 12 month might be the appropriate schedule that provides longer protection than that vaccinated at 0, 1 and 3 or 6 month, which needs further investigation.
It is recognized that the immunogenicity of hepatitis B vaccines in healthy individuals differs. The well known historical factors include male sex, advanced age, smoking, obesity, alcohol intake, injection site and storage conditions, cirrhosis, immune suppression, diabetes mellitus, chronic renal failure and genetically determined resistance,Citation17-23 apart from the dosage and immunization schedules. In our investigation, the 1 month GMT was significantly higher in females than in males without significant difference of seroconversion, which was consistent with other research.Citation24 Furthermore, advanced age was the main demographic variable with a significant impact on the seroconversion rates and GMT, regardless of the dose and number of doses administered, which is in agreement with other trials.Citation17,19 Given that younger adults had good immunization, hepatitis B vaccine is recommended for younger individuals. In terms of the vaccination schedule, all 3 of the 3-dose schedules induced high seroconversion rates, while the 0-1-12 schedule was the first choice due to its higher sero-protective rate at 12 months and GMT at both time points.
However, there were several limitations to the present study. A large number of participants were lost to follow-up. There are 2 reasons. First, the study was approved by the Institutional Ethics Committee of the Zhejiang Center for Disease Control and Prevention, China. Once the subjects want to quit, we comply their wishes. Second, migrants are features of China. Younger adults migrate to urban areas seeking work. How to getting these individuals immunized? Combined with our present study, we think that flexible schedules (0, 1, 3˜12 month) might be a better way and 0-1-12 month was recommended, as some individuals may return home at irregular time while most of them would be certainly back home at the end of year. We also failed to obtain data on body mass index and history of smoking. Therefore, the demographic distribution was perhaps unbalanced and the impact of these factors on seroconversion rates and GMT was unclear. We should set more time points after the first and second doses in order to analyze the trend in seroconversion rate among the 3 vaccination schedules and their differences. We should continue to observe the cohort in order to determine the time point at which anti-HBs became negative. Only when we establish the key time point can we perform booster vaccination for better prevention of HBV infection among adults.
In conclusion, the present study shows a highly satisfactory immunogenicity profile of hepatitis B vaccines with all 3 schedules in healthy adults, the second dose should be given 1 month after the first dose, and the interval between the third and the first dose could vary from 3 to 12 months, but 0-1-12 schedule is a better choice. In addition, hepatitis B vaccine should be administrated among younger adults.
Methods
Study participants
We conducted an investigation in 7 counties of Zhejiang Province (Deqing, Changxing, Anji, Nanxun, Wuxing, Shaoxing and Tongxiang). All the counties had a similar economic level. Each participant gave signed informed consent prior to any study procedure. Participants were randomly classified into 3 groups from A to C according to the vaccination schedule administrated. All participants were screened serologically before the first dose of vaccine and vaccinated after the blood collected. The study was approved by the Institutional Ethics Committee of the Zhejiang Center for Disease Control and Prevention, China. Inclusion criteria were: (a) age 16–59 years; (b) willing to participate in the study; (c) signed informed consent; (d) willing to participate in the follow-up study. Exclusion criteria were: (a) reluctant to participate in the study; (b) HBsAg positive and/or anti-HBs positive; (c) history of allergies or severe reaction to vaccination; (d) history of hepatitis B vaccination; (e) history of any type of vaccination within the previous 4 weeks; (f) high risk of becoming immunologically compromised; (g) previous immunosuppressive therapy (intravenous or oral cortisone or chemotherapy); (h) previous immunostimulatory therapy; (i) any observational or experimental drugs during the past 4 weeks; (j) acute illness within the past 7 days; (k) infection that required antibacterial or antiviral therapy within the past 7 days; (l) fever within the past 3 days (subaxillary temperature ≥38°C); (m) known or anticipated immune dysfunction.
Vaccines
According to our previous studies, the commonly available vaccines of 10μg dose on the Chinese market from 4 different companies have similar immunization effects in healthy adults.Citation25 So we still choose the 10μg vaccines as follows: recombinant hepatitis B vaccine: lot No. 20090521, Shenzhen Kangtai Biological Products Co. Ltd., China; lot Nos. 2009030906 and 2010010106, the Dalian High-Tech Biopharmaceutical Co., Ltd., China; lot No. XHBVB554AA, the GlaxoSmithKline Company, UK; Chinese Hamster Ovary (CHO): lot No.200904A3101, the North China Pharmaceutical Company, GeneTech Biotechnology Pharmaceutical Co., Ltd, China. According to the vaccination schedule, the hepatitis B vaccines were randomly categorized into groups A–C. Group A referred to the participants who received injection in the deltoid muscle at 0, 1 and 3 months; group B received the vaccine at 0, 1 and 6 months; and group C received the vaccine at 0, 1 and 12 months.
Laboratory testing
Sample collection and processing
Each 3-ml blood sample was collected before vaccination, and 1 month and 12 months after the third vaccination. The samples from each participant were preserved at −20°C for later analysis. At the same time, all participants were monitored for 30 min after each injection for immediate reactions. Frozen separated serum samples were sent to ADICON Clinical Laboratories (Hangzhou, China) for quantification of HBsAg, anti-HBs and anti-HBc by chemiluminescence immunoassay (CLIA).
Apparatus and reagents
We used an Architect-i2000 (Abbot, Chicago, IL, USA) to perform the CLIA. The reagent lot number for HBsAg tests was 70318HN00, with the criterion that a signal-to-noise ratio (S/N) ≥0.05 was considered to be positive. The reagent lot number for the anti-HBs test was 75684M100, with the criterion that an antibody level ≥10 mIU/ml was considered to be positive. The main outcome was the seroprotective levels of HBsAb. Anti-HBs antibody level (≥10 mIU/ml) was considered as seroconversion and defined as having protective effects against HBV infection, and values ≥100 mIU/ml were taken as good response. The reagent lot number for the anti-HBc test was 72448M100, with the criterion that an anti-HBc antibody level ≥1 mIU/ml was defined as positive.
Data collation and analysis
We established a database using EpiData3.2 and statistical analysis was performed using SPSS 18.0 and Excel 2007. We were unable to detect anti-HBs antibody titer levels <0.01 mIU/ml, therefore, we assigned a value of 0.005 mIU/ml to these subjects when calculating geometric mean titer (GMT) of anti-HBs. A χ2 test or Fisher's exact test was used for enumeration data. GMTs of anti-HBs obtained for each group, which were quantified at 1 month or 12 months after the full vaccination schedule, were compared by analysis of variance, using the F test to determine the equality of means. The relationship between GMT and age group was compared by bivariable correlation test. A two-tailed probability in statistical tests with α of 0.05 was considered to be significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the Deqing, Changxing, Anji, Nanxun, Wuxing, Shaoxing and Tongxiang Center for Disease Control and Prevention (CDC) and their relevant personnel for their contribution to this study.
Funding
This project was supported by the National Scientific and Technological Major Project of China (No. 2011ZX10004-901,2013ZX10004-904, No. 2014ZX10004008).
References
- World Health Organization. Available from: URL: http://www.who.int/biologicals/vaccines/Hepatitis_B/en/.
- Li X, Zheng Y, Liau A, Cai B, Ye D, Huang F, Sheng X, Ge F, Xuan L, Li S, et al. Hepatitis B virus infections and risk factors among the general population in Anhui Province, China: an epidemiological study. BMC Public Health 2012; 12:272; PMID:22475135; http://dx.doi.org/10.1186/1471-2458-12-272
- Zhu L, Zhai X, Zhu Y, Xu W, Bao C, Peng H, Bian Q, Yang H, Wang H, Hu Z. Evaluation of the Impact of Hepatitis B Vaccination in Adults in Jiangsu Province, China. PLoS One 2014; 9(6):e101501; PMID:24979048; http://dx.doi.org/10.1371/journal.pone.0101501
- Ministry of Health of the People's Republic of China. 2010 Health statistical yearbook of China. Beijing: Ministry of Health, China; 2011
- Centers for Disease Control and Prevention (CDC). Progress in hepatitis B prevention through universal infant vaccination—China, 1997–2006. MMWR Morb Mortal Wkly Rep 2007; 56:441-5; PMID:17495790
- Maynard JE, Kane MA, Alter MJ, et al. Control of hepatitis B by immunization: global perspectives. In: Viral hepatitis and liver disease. Vyas GN, Dienstag JL, Hoofnagle JH, ed. New York: Grune & Stratton; 1988:967-9.
- André FE. Overview of a 5-year clinical experience with a yeast derived hepatitis B vaccine. Vaccine 1990; 8(Suppl):S74-8; PMID:2139288; http://dx.doi.org/10.1016/0264-410X(90)90222-8
- Hadler SC, de Monzon MA, Lugo DR, Perez M. Effect of timing of hepatitis B vaccine doses on response to vaccine in Yucpa Indians. Vaccine 1989; 7:106-10; PMID:2526419; http://dx.doi.org/10.1016/0264-410X(89)90046-7
- Jilg W, Schmidt M, Deinhardt F. Vaccination against hepatitis B: comparison of three different vaccination schedules. J Infect Dis 1989; 160:766-9; PMID:2530289; http://dx.doi.org/10.1093/infdis/160.5.766
- Girisha KM, Kamat JR, Nataraj G. Immunological response to two hepatitis B vaccines administered in two different schedules. Indian J Pediatr 2006; 73:489-91; PMID:16816509; http://dx.doi.org/10.1007/BF02759892
- Agladioglu S, Beyazova U, Camurdan AD, Sahin F, Atak A. Immunogenicity of recombinant hepatitis B vaccine: comparison of two different vaccination schedules. Infection 2010; 38(4):269-73; PMID:20512395; http://dx.doi.org/10.1007/s15010-010-0031-2
- Mast EE, Weinbaum CM, Fiore AE, Alter MJ, Bell BP, Finelli L, Rodewald LE, Douglas JM Jr, Janssen RS, Ward JW; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC).. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006; 55:1-33; PMID:17159833
- Szmuness W, Stevens CE, Zang EA, Harley EJ, Kellner A. A controlled clinical trial of the efficacy of the hepatitis B vaccine (Heptavax B): a final report. Hepatology 1981;1:377-85; PMID:7030902; http://dx.doi.org/10.1002/hep.1840010502
- Hadler SC, de Monzon MA, Lugo DR, Perez M. Effect of timing of hepatitis B vaccine doses on response to vaccine in Yucpa Indians. Vaccine 1989 Apr; 7(2):106-10; PMID:2526419; http://dx.doi.org/10.1016/0264-410X(89)90046-7
- McMahon BJ, Bruden DL, Petersen KM, Bulkow LR, Parkinson AJ, Nainan O, Khristova M, Zanis C, Peters H, Margolis HS. Antibody levels and protection after hepatitis B vaccination: results of a 15-year follow-up. Ann Intern Med 2005; 142:333-41; PMID:15738452; http://dx.doi.org/10.7326/0003-4819-142-5-200503-010-00008
- McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, Bulkow L, Fiore AE, Bell BP, Hennessy TW. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis 2009; 200:1390-6; PMID:19785526; http://dx.doi.org/10.1086/606119
- Fisman DN, Agrawal D, Leder K. The Effect of Age on Immunologic Response to Recombinant Hepatitis B Vaccine: A Meta-analysis. Clin Infect Dis 2002; 35:1368-75; PMID:12439800; http://dx.doi.org/10.1086/344271
- Weber DJ, Rutala WA, Samsa GP, Santimaw JE, Lemon SM. Obesity as a predictor of poor antibody response to hepatitis B plasma vaccine. JAMA1985; 254:3187-489; PMID:2933532
- Shaw FE Jr, Guess HA, Roets JM, Mohr FE, Coleman PJ, Mandel EJ, Roehm RR Jr, Talley WS, Hadler SC. Effect of anatomic injection site, age and smoking on the immune response to hepatitis B vaccination. Vaccine 1989; 7:425-30; PMID:2530717; http://dx.doi.org/10.1016/0264-410X(89)90157-6
- Averhoff F, Mahoney F, Coleman P, Schatz G, Hurwitz E, Margolis H. Immunogenicity of hepatitis B vaccines. Implications for persons at occupational risk of hepatitis B virus infection. Am J Prev Med 1998; 15:1-8; PMID:9651632; http://dx.doi.org/10.1016/S0749-3797(98)00003-8
- Hennig BJ, Fielding K, Broxholme J, Diatta M, Mendy M, Moore C, Pollard AJ, Rayco-Solon P, Sirugo G, van der Sande MA, et al. Host Genetic Factors and Vaccine-Induced Immunity to Hepatitis B Virus Infection. PLoS One 2008; 3:1-10; PMID:18365030
- DeRave S, Heijtink RA, Bakker-Bendik M, Boot J, Schalm SW. Immunogenicity of standard and low dose vaccination using yeast-derived recombinant hepatitis B surface antigen in elderly volunteers. Vaccine 1994; 12:532-4; PMID:8036828; http://dx.doi.org/10.1016/0264-410X(94)90313-1
- Estévez ZC, Betancourt AA, González VM, Baile NF, Silva CV, Bernal FH, Arias EP, Delhanty Fernández A, Olazábal NM, del Río Martín A, et al. Immunogenicity and safety assessment of the Cuban recombinant hepatitis B vaccine in healthy adults. Biologicals 2007; 35:115-22; PMID:17056272; http://dx.doi.org/10.1016/j.biologicals.2006.06.001
- Yuan YB, Wang ZQ, Ma XP, Zhang GH. Comparison of the immunogenicity of different dosages of recombinant yeast-derived hepatitis B vaccines in adults. J Prev Med Chin PLA 2003; 21(3):176-9. [In Chinese]
- Ren JJ, Dai XW, Jiang ZG, Shen LZ, Chen YD, Li Q, Ren W, Liu Y, Yao J, Li LJ. Immunological effects of a 10-μg dose of domestic hepatitis B vaccine in adults. J Zhejiang Univ Sci B 2012; 13(11):948-54; PMID:23125088; http://dx.doi.org/10.1631/jzus.B1200179
