Abstract
BR-TD-1001 was developed as a booster for the immunity maintenance of diphtheria and tetanus. The aim of this study was to evaluate the safety and immunogenicity of BR-TD-1001 (test vaccine) in comparison with placebo and an active comparator in healthy Korean adults. A randomized, double-blind, placebo-controlled, active comparator, phase I clinical trial was conducted. Fifty subjects were randomly assigned to one of 3 treatment groups in a ratio of 2:2:1, and were administered a single intramuscular dose of test vaccine, active comparator, or placebo, respectively. All subjects were monitored for 4 weeks after injection. The antibody titers of the patients 2 and 4 weeks after vaccination were compared with the baseline. The frequencies of all adverse events including adverse drug reactions in the test group were not statistically different from those of the other treatment groups (P = 0.4974, 0.3061). No serious adverse event occurred, and no subject was withdrawn from the study for safety. The seroprotection rates against both tetanus and diphtheria at 4 weeks after vaccination were over 0.95. For anti-tetanus antibody, the geometric mean titer in the test group was significantly higher than those of the other groups (P = 0.0364, 0.0033). The geometric mean titer of anti-diphtheria antibody in the test group was significantly higher than the value of the placebo (P = 0.0347) while it was not for the value of the active comparator (P = 0.8484). In conclusion, BR-TD-1001 was safe, well-tolerated, and showed sufficient immunogenicity as a booster for diphtheria and tetanus.
Introduction
Diphtheria and tetanus are highly contagious and potentially life-threatening infectious diseases. Corynebacterium diphtheriae is the pathogen for diphtheria, which spreads through physical contact or aerosol and induces inflammation of the upper respiratory tract and skin via its exotoxin. The late effects of diphtheria are sometimes fatal when it involves the myocardium, which leads to myocarditis, as well as the cranial and peripheral nerves, which results in the motor and/or sensory palsies.Citation1 Tetanus is caused by the neurotoxin (tetanospasmin) of Clostridium tetani, which penetrates through damaged skin tissue. The tetanus toxin migrates to the central nervous system by retrograde transport along the nerve, binds to receptors at the nerve termini, and then blocks the release of inhibitory neurotransmitters such as glycine and gamma-aminobutyric acid.Citation2
Immunity against both diphtheria and tetanus is not naturally acquired, but is obtained only by vaccination.Citation3 However, the antibody titers against diphtheria and tetanus toxin gradually decrease in adults over time even if they have received the Diphtheria, Tetanus, and Pertussis (DTP) vaccination in their childhood.Citation4,5 This implies that the maintenance of immunity against tetanus and diphtheria is essential.Citation6,7 In this context, the US Center for Disease Control recommended that all adults be given a booster dose of Tetanus and Diphtheria toxoid (Td) vaccine every 10 years from the age of 11–12 years.Citation8,9 Likewise, in Korea, where the DTP vaccination was already essential for most children, the Korean School Health Law amendment in 2005 imposed an obligatory Td booster vaccination for adolescents.
Accordingly, many pharmaceutical companies in Korea began production of the Td vaccine. BR-TD-1001 is a newly developed Td vaccine that targets booster immunization. BR-Td-1001 is manufactured using the techniques of advanced fermentation and toxin production, followed by inactivation and purification which enables the production of relatively homogenous antibodies. As an initial evaluation for the biological activities of BR-TD-1001 in humans, this study aimed to explore the safety and immunogenicity of the vaccine in healthy Korean adults in comparison to placebo and an active comparator (a licensed Td vaccine).
Results
Subjects
A total of 130 Korean male adult subjects were screened and 50 were enrolled. The reasons for screening failures were as follows; the eligibility criteria were not met for 75 subjects and consent withdrawal for 5 subjects. All of the subjects completed the study according to the protocol. At a significance level of 0.05, the demographic data, including age, height, weight, and lifestyle habits, showed no significant differences among the treatment groups ().
Table 1. Subject demographics
Safety
Over the entire study period, 63 AEs (local or systemic) were reported in 30 subjects. For the test group, 27 AEs occurred in 13 subjects, while 32 AEs in 13 subjects occurred in the reference group, and 4 AEs in 4 subjects occurred in the placebo group. Among the AEs, 22 from the test group, 28 from the reference group, and 2 from the placebo group were considered as ADRs. Neither the incidence of AEs (P = 0.4974) nor that of ADRs (P = 0. 3061) was significantly different among the treatment groups. No SAE was observed. The occurrence of AEs is summarized in .
Table 2. Summary of adverse events
Most of the AEs were mild (56 out of 63), and the remainder of the AEs, which occurred in 2 subjects (one from the reference group and the other from the test group), were moderate. Approximately 80% of the AEs (51 out of 63) were solicited (local/systemic AEs). All AEs needed no further medical intervention and spontaneously resolved. The number and the severity of the AEs are summarized by treatment groups in .
Table 3. Local and systemic AEs reported (solicited and unsolicited
All of the safety test results remained within the normal clinical ranges and there were no meaningful differences in the measurements among the treatment groups.
Immmunogenicity
The seroprotection rates against both diphtheria and tetanus at Visit 7 were over 0.95 in the actively-treated groups. The difference between treatment groups was not statistically significant for anti-diphtheria antibody (P = 1.0000, due to high seroprotection rate of placebo group at baseline) while it was significant for anti-tetanus antibody (P < 0.0001). In the subjects with baseline titer less than 0.1 IU/mL, the seroprotection rates were over 0.75 for anti-diphtheria antibody (P = 0.4167) and were 1.0 for anti-tetanus antibody (P = 0.0055) in the actively-treated groups (the rate of placebo group remained 0).
The anti-diphtheria GMT of the test group was significantly higher than the value of the placebo group (P = 0.0347) while it was not meaningfully different from the value of the reference group (P = 0.8484). For anti-tetanus antibody, the GMT of the test group showed statistical differences against both the reference and placebo group (P = 0.0364 and 0.0033, respectively). The time effects for GMTs were statistically significant in actively-treated groups (P ≤ 0.0001 for all cases) which mean that the antibody titers were clearly increased from the baseline values over time. shows the anti-diphtheria and anti-tetanus antibody GMTs of the each group throughout the study period.
Figure 1. Anti-diphtheria and anti-tetanus antibody titers at visit 6 and 7 in BR-TD-1001, active comparator or placebo group. Boxes represent geometric mean and bars show geometric standard deviations.
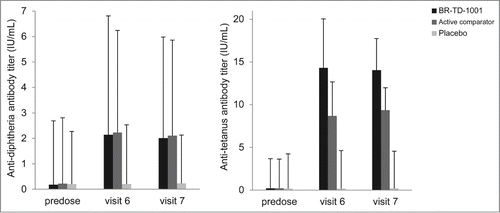
Accordingly, at Visits 7, the anti-diphtheria GMRs of the test group and the reference group were 11.12 and 9.81, respectively, and the similar outcomes could be found for the anti-tetanus antibody titers. Meanwhile, the extents of antibody titer increase were highly variable in the test and reference group. The anti-diphtheria antibody titer increased 0.86–288.89-fold in the test group and 1.75–115.26-fold in the reference group. The corresponding values of anti-tetanus antibody in the test group were 4.58–689.02, and 6.52–700 in the reference group. All the immunogenicity outcomes are summarized in .
Table 4. Summary of immunogenicity outcomes
Discussion
In this study, we tried to evaluate the safety and immunogenicity of a newly developed Td vaccine, BR-TD-1001, in Korean male adults who received a single vaccination, in comparison with participants who received an active comparator or placebo.
The incidence and number of AEs and ADRs showed no statistical differences among the treatment groups. Most of the AEs were mild and no SAE was reported or observed. Other assessment profiles for the safety evaluation such as physical exam, vital sign check-up, laboratory tests, and 12-lead ECG also showed no clinically significant results. Based on these observations, we suggest that BR-TD-1001 is safe and tolerable compared to the licensed Td vaccine.
The Td vaccine is known to have a lower incidence of local and systemic AEs compared to DPT vaccine,Citation10 especially for fever. In addition, most of the reported AEs were mild and subsided within 2–3 day.Citation11 Viella et al. Citation12 evaluated the safety and reactogenicity of the Td vaccine for over 3000 subjects and reported that the most common local AE was pain. In this study, we were able to verify these known characteristics of Td vaccine. The incidence of AEs following a Td vaccination (BR-TD-1001 and active comparator) was 65% (26/40), less than the 73.3% rate (22/30) reported by a study performed at another clinical trial center in Korea on a newly developed Td vaccine produced by different company.Citation13
In terms of efficacy, BR-TD-1001 showed equivalent immunogenicity as a booster for diphtheria and tetanus as compared to the licensed Td vaccine. The seroprotection rate after 4 weeks of BR-TD-1001 vaccination was similar to that of the active comparator for both diphtheria and tetanus. The high seroprotection rate of the placebo group (0.9 in diphtheria and 0.6 in tetanus) could be explained by the eligibility criterion which enabled the enrollment of subjects with antibody titers over 0.1 IU/mL. (Prior to vaccination, the GMTs of anti-diphtheria antibody were 0.18 IU/mL, 0.22 IU/mL, and 0.20 IU/mL in the test, reference, and placebo groups, respectively. The GMTs of anti-tetanus antibody at baseline were 0.20 IU/mL in the test group, 0.23 IU/mL in the reference group, and 0.17 IU/mL in the placebo group.) However, this was inevitable because of the difficulty of subject recruitment. We tried to conduct sub-group analysis of the seroprotection rate in the seronegative (antibody titer < 0.1 IU/mL) subjects, but the number of seronegative subjects was too small to achieve statistical and clinical significance. Since the seroprotection rate of the placebo group showed no interval change after injection, we could show the clinical importance of vaccination. The GMT and GMR values 2 and 4 weeks after BR-TD-1001 vaccination also showed more changes in the antibody titers as compared to the active comparator.
The geometric mean titers of anti-tetanus antibody at 2 and 4 weeks after active comparator vaccination were higher than those of GC1107 even though it was the same active comparator.Citation13 The GMT of the anti-tetanus antibody of BR-TD-1001 at 2 and 4 weeks was higher than that of the active comparator. However, the extent of the increase in anti-tetanus antibody titer after BR-TD-1001 vaccination compared with active comparator was lower than the case of GC1007.Citation13 This is due to the small scale of the phase I study. An additional clinical study with a large number of subjects is required.
In addition, even though this study was a phase I study that sought to explore safety and immunogenicity, we should also recognize that diphtheria and tetanus could pose serious health problems if the booster is not properly performed. Despite their passive immunization history, 20% and 32% of the enrolled subjects had insufficient immunity against diphtheria and tetanus, respectively, at the time of screening. At the end of the study, we found that most of those subjects gained seroprotection after being administered active vaccines. Subjects in the placebo group with low antibody titers were told to receive a booster after all of the study-related procedures were terminated.
In conclusion, BR-TD-1001 showed satisfactory tolerability and immunogenicity which may enable the product to proceed to the next step of development. Adult booster Td vaccines including BR-TD-1001 are expected to contribute to further decreases in the incidence of tetanus and diphtheria infection in Korea.
Materials and Methods
Study subjects and design
Healthy male Korean volunteers were enrolled at the screening visit (Visit 1). The subject screening procedure involved laboratory tests on hematology, blood chemistry, and urine analysis, physical examination, vital sign check-up, medical history with previous vaccination history, chest X-ray, and 12-lead electrocardiography (ECG). Subjects were enrolled if they met the following eligibility criteria: age ≥20 years, weight within 20% of the ideal body weight, and no clinically significant abnormalities. Subjects were excluded if they had any history or evidence of disease in the major organ systems, or any other acute or chronic disease including history of diphtheria, tetanus, or pertussis infection. Subjects who were vaccinated against diphtheria, tetanus, or pertussis within the last 5 years, experienced complications after DTP vaccination, had a history of tetanus immunoglobulin administration, or had antibody levels ≥1.0 U/mL against either diphtheria or tetanus were also excluded. Participants abstained from drugs, foods, or any other lifestyle factors which might alter the antibody titer throughout their participation.
The study was designed as a randomized, double-blind, placebo and active-controlled parallel trial. All of the study-related procedures were conducted in Seoul St. Mary's Hospital. The subjects were assigned to one of 3 treatment groups (BR-TD-1001, active comparator, and placebo) at a ratio of 2:2:1 according to the randomization table constructed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). To maintain the double-blind nature of the study, an un-blinded pharmacist, nurse, and monitor were selected who did not participate in any endpoint assessment or data analysis. The principal investigator kept one set of the randomization codes in a sealed envelope for emergencies when un-blinding was required.
A single dose of study vaccines (BR-TD-1001 for the ‘test group’, active comparator for the ‘reference group’) or placebo (‘placebo group’) was administered into the deltoid muscle of the non-dominant arm using pre-filled syringes with a 25-gauge needle (2.54 cm) by the un-blinded nurse at Day 0 (Visit 2). Identical syringes with opaquely covered exteriors were used to prevent subjects from identifying the injectant. The administration was performed in a separated space where the other research staffs were not allowed to approach.
Subjects visited the study site for 3 subsequent days after injection (Visits 3–5), and then on Day 14 (Visit 6) and Day 28 (Visit 7) for safety and immunogenicity evaluation. For Visits 6 and 7, time windows of 3 days and 5 days, respectively, were allowed. The last safety data was collected through telephone calls at Day 42 (Visit 8).
The study was conducted in accordance with the Korean Good Clinical Practice guidelines and with the Declaration of Helsinki. An independent Institutional Review Board (Seoul St. Mary's Hospital) approved the protocol before the execution of the trial, and all participants gave written informed consent. A clinical trial registration procedure was not performed.
Study vaccine
BR-TD-1001 was manufactured by Boryung Biopharma Co., Ltd., Seoul, Korea. Diphtheria and tetanus toxins were produced by growing C. diphtheriae and C. tetani in a semisynthetic, casein-based medium in a fermenter. Diphtheria toxin (or tetanus toxoid) underwent ammonium sulfate fractionation and a chromatography purification process. The toxin was detoxified into a toxoid by chemical modification. The toxoid was purified into a high quality vaccine antigen with a purity level greater than 2500 Lf/mg PN. The purified toxoid solution was then adsorbed onto aluminum hydroxide and diluted using normal saline.
The complete BR-TD-1001 product contained ≥2 IU (≥1.5 Lf) of diphtheria and ≥20 IU (≥5 Lf)of tetanus toxoid within a volume of 0.5 mL, and also contained 4.25 mg of sodium chloride as an isotonic agent and 0.5 mg of aluminum hydroxide as an adsorbent. The amount of toxoids in the active comparator (SK Td-pur®, SK chemicals, Seongnam, Korea) was identical to that of BR-TD-1001. Normal saline 0.5 mL was used as the placebo.
Safety measures
For the monitoring of immediate adverse events (AEs) by the administration of investigational products (IPs), the subjects were monitored for 30 minutes after the injection on Visit 2 and discharged if they did not show any acute AEs, including anaphylaxis. The safety monitoring was maintained and included history taking, physical examination, vital sign check-ups, laboratory tests, and ECG at each scheduled visit over the entire study period.
Frequently occurring AEs, including reactions that were local (pain, tenderness, erythema/redness, induration/swelling) and systemic (fever, nausea/vomiting, diarrhea, headache, fatigue, myalgia), were classified as solicited AEs. These were extensively monitored using diary cards which were distributed to the subjects at Visit 2. All other AEs found by the investigators or reported by the subjects were recorded as unsolicited AEs. All AEs were evaluated for their relationships to the biological activities of the IPs. When the causal effects of the IPs could not be excluded, the AEs were categorized as adverse drug reactions (ADRs). An AE was considered to be a serious adverse event (SAE) when the AE resulted in death, was life-threatening, required hospitalization or prolongation of current hospitalization, caused persistent or significant disability, was a congenital anomaly/birth defect, or required intervention to prevent permanent impairment or damage.
Immunoassay
Peripheral venous blood was sampled into heparinized tubes (Vacutainer, BD, Franklin Lakes, NJ, USA) just before the injection and on Visits 6 and 7. Plasma was immediately centrifuged at 3000 g for 20 min at 4°C, and then stored at −70°C until it was assayed. Two different kinds of enzyme-linked immunosorbent assay (ELISA) kits (RE56191 and RE56901; IBL International GmbH, Hamburg, Germany) were used to determine the serum antibody levels of diphtheria and tetanus. The assay procedure was performed following each kits' instructions.Citation14,15
Antibody levels ≥0.1 IU/mL were considered indicative of seroprotection against their corresponding pathogens.Citation6,7 The lower limits of quantification (LLOQ) of the antibodies were 0.004 IU/mL and 0.0172 IU/mL against diphtheria and tetanus, respectively. The precision and accuracy were satisfactory.
Endpoints and statistical analysis
The primary safety endpoint was the incidence of both solicited and unsolicited AEs during the overall study period. The changes in the results from the safety monitoring tests at Visit 7 from baseline (Visit 2) were also assessed as secondary safety endpoints. The primary efficacy (immunogenicity) endpoint was the seroprotection rate for both diphtheria and tetanus at Visit 7. The geometric mean titers (GMT) of the antibodies at Visits 6 and 7, as well as their ratio (Geometric Mean Ratio, GMR) to the baseline values were also taken into consideration as secondary endpoints. The seroprotection rate was defined as the proportion of subjects who achieved seroprotection.
The statistical analysis was originally planned to be performed using different subsets of the total data accordingly (e.g. intention-to-treat data for safety and per-protocol data for immunogenicity). However, since all enrolled subjects completed the study, the total data was used for all statistical analyses. To evaluate the similarities in the baseline characteristics of the treatment groups, the demographic data were summarized using descriptive statistics. The Kruskal-Wallis test or Fisher's exact test was performed when applicable.
For the safety assessment, Fisher's exact test was used to compare the incidence of AEs among the treatment groups and the 95% confidence interval (CI) was estimated. Since the incidence was basically a binomial parameter, the normal-theory method was attempted.Citation16 However, when the method could not be applied in cases of small sample size,Citation16 the Wilson score interval was used. The statistical significance of the changes in the safety measures and from baseline was tested using the paired t-test and the Kruskal-Wallis test was applied to analyze the differences among the treatment groups.
For the immunogenicity assessment, the seroprotection rate of each group was compared using the Fisher's exact test. Repeated measure analysis of variance (RM-ANOVA) was used to assess the changes in GMT values for each antibody by group and time. GMR values were summarized using descriptive statistics.
All of the statistical procedures were performed with SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Disclosure of Potential Conflicts of Interest
The authors Yong-Ju Chung and Tae-Yeon Kim were employees of Boryung Biopharma Co., Ltd. at the time the study was conducted.
Funding
This study was sponsored by Boryung Biopharma Co., Ltd., Seoul, Korea.
References
- Scheifele DW, Ochnio JJ. The immunological basis for immunization series. Module 2: diphtheria. World Health Organization. 2009. Available from: http://whqlibdoc.who.int/publications/2009/9789241597869_eng.pdf
- Borrow R, Balmer P, Roper MH. The immunological basis for immunization series. Module 3: Tetanus. World Health Organization. 2006. Available from: http://whqlibdoc.who.int/publications/2007/9789241595551_eng.pdf
- Williams WW, Hikson MA, Kane MA, Kendal AP, Spika JS, Hinman AR. Immunization policies and vaccine coverage among adults; the missed opportunities. Ann Intern Med 1988; 108: 616-25; PMID:2964806; http://dx.doi.org/10.7326/0003-4819-108-4-616
- Kang JH, Hur JK, Kim JH, Lee KI, Park SE, Ma SH, Lee MS, Baek SY, Hong SH, Min HK. Age related seroepidemiological study of diphtheria among Koreans. Korean J Infect Dis 2000; 32:1-7.
- Kang JH, Hur JK, Kim JH, Lee KI, Park SE, Ma SH, Lee MS, Ban SJ, Hong SH, Cho DH, et al. Age related serosurvey of immunity to tetanus in Korean populations. Korean J Infect Dis. 2001; 33:104-11.
- Vitek CR, Wharton M. Diphtheria toxoid In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 5th edition. Philadelphia: WB Saunders Co; 2008. p. 139-56.
- Wassilak SG, Roper MH, Kretsinger K, Orenstein WA. Tetanus toxoid In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 5th edition. Philadelphia: WB Saunders Co; 2008. p. 805-40.
- Centers for Disease Control and Prevention. Tetanus, diphtheria (Td) or tetanus, diphtheria, pertussis (Tdap) vaccine; 2008. Available from: http://www.immunize.org/vis/td_tdap.pdf
- Broder KR, Cortese MM, Iskander JK, Kretsinger K, Slade BA, Brown KH, Mijalski CM, Tiwari T, Weston EJ, Cohn AC, et al. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55(RR-3):1-34
- Kang JH. The need of Td vaccination according to the changes of tetanus and diphtheria immunity. J Korean Med Assoc 2008; 51:127-36; http://dx.doi.org/10.5124/jkma.2008.51.2.127
- Scheifele DW, Dobson S, Kallos A, Bjornson G, Ochnio JJ. Comparative safety of a tetanus-diphtheria toxoids booster immunization in students in Grade 6 and 9. Pediatr Infect Dis J 1998; 17:1121-6; PMID:9877359; http://dx.doi.org/10.1097/00006454-199812000-00004
- Vilella A, Dal-Ré R, Simó D, García-Corbeira P, Diego P, Bayas JM. Reactogenicity profile of tetanus-diphtheria (adulttype) vaccine: results of a naturalistic study performed at an adult vaccination center. J Clin Pharmacol 2000; 40:1267-73; PMID:11075312
- Lee S, Park WB, Shin KH, Ahn DH, Yoon SH, Cho JY, Shin SG, Jang IJ, Yu KS. Immunogenicity and safety of a single intramuscular dose of a diphtheria-tetanus (Td) vaccine (GC1107) in Korean adults. Vaccine 2011; 29:7638-43; PMID:21864613; http://dx.doi.org/10.1016/j.vaccine.2011.08.003
- Instruction for use, Diphtheria IgG ELlSA, IBL international GmbH, Hambrug, Germany. Available from: http://www.ibl-international.com/media/catalog/product/R/E/RE56191_IFU_en_Diphtheria_IgG_ELISA_V2012_09_sym3.pdf
- Instruction for use, Tetanus IgG ELlSA, IBL international GmbH, Hambrug, Germany. Available from: http://www.ibl-international.com/media/catalog/product/R/E/RE56901_IFU_en_Tetanus_IgG_ELISA_V2013_01_sym3.pdf
- Rosner B. Estimation. In: Taylor M, Seibert D, editors. Fundamentals of biostatistics. 7th ed. Boston: Brooks/Cole 2006. p. 184-189.
