Abstract
Streptococcus pneumoniae is an important pathogen causing invasive diseases such as sepsis, meningitis, and pneumonia. Vaccines have become the most effective way to prevent pneumococcal infections. This phase III trial was designed to evaluate the immunogenicity and safety of a 23-valent pneumococcal polysaccharide vaccine in Chinese healthy population aged >2 years. We conducted a randomized, double-blinded, active-controlled, multicenter trial in which 1660 healthy population (>2 years of age) were randomly assigned in a 1 : 1 ratio to receive 2 intramuscular doses of either the treatment vaccine or the active control vaccine, PNEUMOVAX 23. The surveillance period was 30 days. The primary end point was the 2-fold increase rate of anti-pneumococcal antibody for all 23 included serotypes in each group. In the intention-to-treat cohort, the 2-fold increase rate of anti-pneumococcal antibody for 23 included serotypes varied from 62.47% to 97.01% in the treatment group, and from 51.49% to 95.77% in the control group. According to −10% non-inferiority margin and 95% confidence intervals of rate difference, almost all included serotypes of the treatment group reached non-inferiority to control group except for serotype 6B, the lower limit of rate difference of which was −10.00%, equal to the non-inferiority margin. The 2-fold increase rates of anti-pneumococcal antibody were significantly higher in the treatment group for serotype 2, 3, 4, 10A, 11A and 20. Furthermore, for all 23 serotypes, IgG geometric mean concentrations (GMCs) at day 30 were significantly higher in treatment group for serotype 2, 3, 4, 9V, 10A, 11A, 15B, 18C, 19A, 22F and 33F. Higher geometric mean fold increase (GMFI) were also observed in the treatment group correspondingly. Serious adverse events occurred in 3 of 830 participants in the treatment group (0.36%) and 2 of 830 participants in the control group (0.24%). No death occurred during the trial. The frequencies of both solicited and unsolicited adverse events (AEs) were small lower in the treatment group (34.34% vs 35.66% for solicited AEs, 4.34% vs 5.42% for unsolicited AEs). Both vaccines were well tolerated and most AEs were mild or moderate in intensity. The newly vaccine was well tolerated and immunologically non-inferior to the active control vaccine PNEUMOVAX 23 for all 23 vaccine serotypes in the Chinese population (>2 years of age).
Introduction
Streptococcus pneumoniae is a common cause of invasive disease and respiratory-tract infections in more and less developed countries. Risk groups for diseases caused by pneumococci, such as meningitis, sepsis, and pneumonia, include young children, elderly people, and patients with immunodeficiencies.Citation1 Despite the use of antibiotics and intensive care for over 50 years, the case fatality rate of pneumococcal bacteremia has remained at 15% to 20% in children and young adults and 30 to 40% in the elderly. Moreover, the rates of antimicrobial resistance in pneumococci have steadily increased.Citation2-4 Vaccines have become the most effective way to prevent pneumococcal infections.Citation1
The serotype distribution varied with age, year, specimen and geographic location, which played an important role in the development and formulation of the vaccines.Citation5,6 The first national investigation of serotype distribution of S. pneumoniae in hinterland of China was organized by WHO which involved 27 hospitals and institutes in 18 provinces and cities in 1981. The study showed that the 8 common serotypes were serotypes 5, 6, 1, 19, 2, 14, 23 and 3, which together accounted for 63.3% of all isolates, the most common serotype causing lung infection in infants were 1 and 14 while those isolated from older children and adults with lung infections were 1 and 5.Citation7,8 As a result of the serious abuse of antibiotics in China, the resistance of S. pneumoniae to various antibiotics is increasing. A series of studies reported after 2000 from several locations in China suggested that the distribution of common serotypes of S. pneumoniae isolated from healthy children or adults was different significantly. Serotypes 19A, 19F, 23F, 6B, 15B and 14 became common in most of the studies.Citation9-15
The current 7-valent pneumococcal conjugate vaccine (PCV7) covering serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, PCV10 (PCV7 plus serotypes 1, 5, and 7F), and PCV13 (PCV10 plus serotypes 3, 6A, and 19A) have been shown to be effective in preventing invasive diseases caused by the vaccine-type strains.Citation16,17 However, with the increasing rates of antimicrobial resistance in pneumococci and the shifting serotypes distribution, the protective coverage of the conjugate vaccines may be lower, and replacement by non-vaccine serotypes resulting in disease is a serious threat for the near future.Citation18-23 The effective vaccines with more extensive coverage of serotype distribution of S.pneumoniae, included wider geographical areas and in different epidemiological/clinical settings including rural localities are most suitable for China. 23-valent PPV (PPV23), expanding serotype coverage to more than 85% of the organisms that caused invasive pneumococcal disease (IPD), and has been shown to be highly cost-effective when provided as a routine immunization.Citation24-32 23-valent PPV is currently recommended for persons >65 years of age and for high-risk individuals 2–64 y of age in many developed countries.Citation33,34
In the present large scale, double-blinded, randomized, active-controlled, phase III study, we assessed the safety and immunogenicity of a newly developed 23-valent pneumococcal polysaccharide vaccine in Chinese population, and according to the age strata of the participants.
Results
Study population
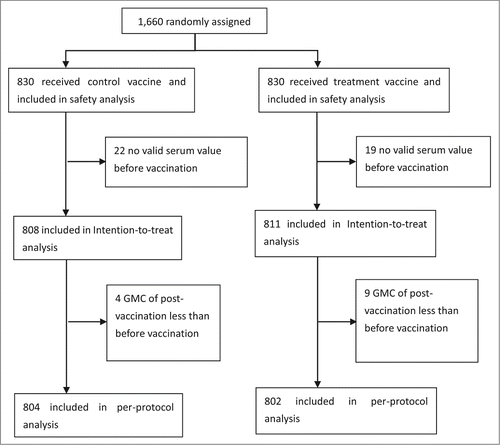
A total of 1,660 participants (>2 years of age) were enrolled and received the treatment or control vaccine. Of these, 19 participants in the treatment group and 22 in the control group were excluded from immunogenicity analysis for they did not have valid serum value before vaccination. As a result, 811(97.71%) participants in the treatment group and 808(97.35%) in the control group were included in intention-to-treat analysis ().
Moreover, 9 participants in the treatment group and 4 in the control group were excluded from the per-protocol analysis due to the GMCs of post-vaccination less than pre-vaccination, which was not logical. Finally, 802(96.63%) of the treatment group and 804(96.87%) of the control group were included in the per-protocol analysis ().The demographic characteristics of the participants were well balanced in the two groups ().
Table 1. Baseline characteristics in safety analysis set
Efficacy end points and immunogenicity
In the treatment group, the 2-fold increase rate of anti-pneumococcal antibody varied from 62.47% to 97.01% for all 23 serotypes, serotype 2, 8, 9N, 18C and 33F increase exceeded 90%. In the control group, the rate varied from 51.49% to 95.77% for all 23 serotypes, serotype 8, 9N, 18C and 33F increase exceeded 90%.
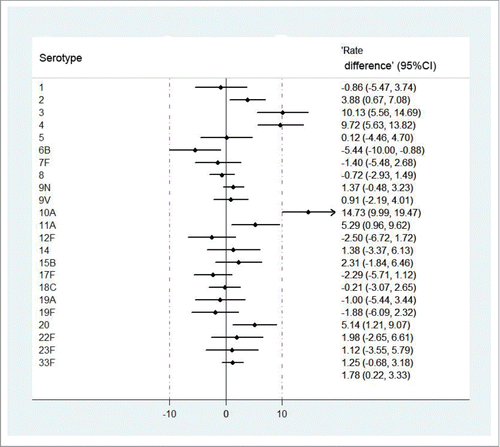
According to −10% non-inferiority margin and 2-sided 95% confidence intervals (CIs) of rate difference, the treatment group was non-inferior to the control group except for serotype 6B. For serotype 2, 3, 4, 10A, 11A and 20, rate of 2-fold increase treatment were significantly higher in the treatment group compared to the control group (90.02% in treatment group vs. 85.82% in control group for serotype 2; 72.19% in treatment group vs. 61.69% in control group for serotype 3; 81.67% in treatment group vs. 71.89% in control group for serotype 4; 66.58% in treatment group vs. 51.49% in control group for serotype 10A; 75.69% in treatment group vs. 70.40% in control group for serotype 11A; 82.42% in treatment group vs. 77.24% in control group for serotype 20).While for serotype 6B, the lower limit of rate difference between the two groups was −10.00%, equal to the non-inferiority margin ( ; Table S1).
For IgG geometric mean concentrations (GMCs) of participants, pre-immunization antibody levels were balanced in both groups. At day 30, the levels of the treatment group were statistically significantly higher by 2–3 folds for serotype 2, 3, 4, 9V, 10A, 11A, 15B, 18C, 19A, 22F and 33F, compared with the control recipients, with the exception of serotype 6B, which was lower in the treatment group (; Fig. S1). Similarly, geometric mean fold increase (GMFI) in participants vaccinated with the treatment vaccine were higher than those in participants vaccinated with the control vaccine for all serotypes except 6B and 19F, which were lower in the treatment group ().
Table 2. Immune response to pneumococcal vaccine in the intention-to-treat population
Subgroup analysis
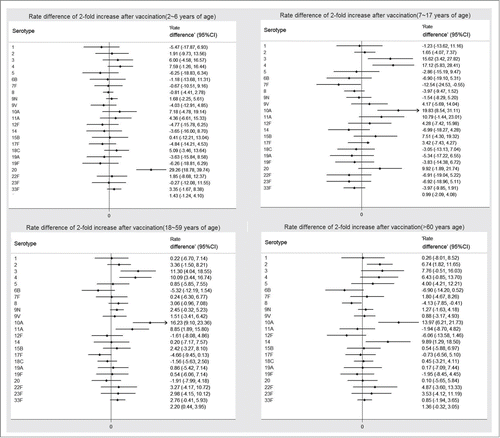
According to the age strata of the participants in the study design and randomization, we analyzed the primary endpoint for the 2 subgroups (2∼6 y and >60 years) respectively. The similar trend of antibody responses were observed. In the subgroup of 2∼6 y of age, the 2-fold increase rate of all included anti-pneumococcal antibody serotype were no statistical difference except 20 (ITT: 88.43% vs. 59.17% at day30, P < 0.0001), the rate difference (95%CI) was 29.26(95%CI, 18.78 to 39.74). In the subgroup of >60 years of age, the 2-fold increase rate of serotype 2, 8, 10A and 14 showed statistical difference (p = 0.0076, 0.0306, 0.0005 and 0.025 respectively), the rate differences (95% CIs) were 6.74(95%CI, 1.82 to 11.65), −4.13(95%CI, −7.85 to −0.41), 13.97(95%CI, 6.21 to 21.73) and 9.89(95%CI, 1.29 to 18.50) respectively. The 2-fold increase rates of the other included serotypes were no statistical difference. (; Tables S2–5).
Adverse reactions
During the entire study period, 5 serious adverse events were reported and considered to be unrelated to vaccination: 3 in the treatment group (1 case of thyrophyma, 1 case of renal calculus, and 1 case of bronchitis) and 2 in the control group (1 case of cervical spondylopathy, and 1 case of cerebral infarction). No death occurred during the trial.
The incidence of all adverse reactions was 32.53% and 34.10% in the treatment and control groups (p = 0.5321). Within each subgroup, there was also no significant difference in the occurrence of adverse reactions between the two groups. The frequencies of both solicited and unsolicited adverse events were similar in the 2 study groups (). The most common systemic adverse reactions were fever, headache, fatigue, and nausea, the most common injection-site adverse reactions were pain, redness and swelling. A total of 20 participants in the treatment group (2.41%) and 19 participants in the control group (2.29%) had grade 3 adverse events within 7 d after an injection.
Table 3. Adverse events in the safety analysis population
Discussion
In this large scale, double blinded, randomized phase III study, the efficacy, immunogenicity and safety of a newly 23-valent pneumococcal polysaccharide vaccine developed by a Chinese local biotech manufacture have been extensively studied. The study clearly showed that both vaccines were highly immunogenic. The newly PPV23 vaccine have similar efficacy and safety with the established control vaccine PNEUMOVAX 23 (Merck & Co., Inc.) in all included serotypes.
A series of studies reported serotype 19A, 19F, 23F, 6B and 14 were common among children <5 years of age in China.Citation8-15 The treatment vaccine showed similar efficacy and safety with the control group, all reached the pre-defined non-inferiority margin for the serotype 19A, 19F, 23F and 14. For serotype 6B, lower efficacy was observed in the treatment group, the primary end point was equaled to the non-inferiority margin. While in the subgroup analysis of 2∼6 y of age, all efficacy endpoints for serotype 6B have no statistical difference between the two groups. A number of studies suggested that serotype 6B is just common in infants and younger children, but not in older children and adults,Citation8,14,19 which means their natural exposed chance is low, and resulted in the magnitude of antibody response to vaccination is not as high and sensitive as in younger children's. In addition, the sample size of younger children was just a small part of our all participants. As a consequence, the antibody response to serotype 6B in in all age groups was attenuated, while still remained highly in the subgroup.
Pneumococcal disease also remains a major cause of morbidity and mortality among elderly persons. PPV23 is currently recommended for use in elderly persons and in persons at high risk of infection. In the United States, the Advisory Committee on Immunization Practices (ACIP) recommends PPV23 vaccination for all adults aged >65 years and for individuals aged >2 years with risk factors for pneumococcal disease.Citation33,34 It provides approximately 50–80% protection against invasive disease in the general elderly population. The vaccine significantly reduced mortality, stay duration, and intensive care admissions among adults hospitalized with community-acquired pneumonia.Citation19,36,37 In our treatment vaccine, the recent studies reported prevalent serotypes (serotype 19A, 19F, 14, 3 and 23F) in older adults were observed as efficacious as the control vaccine, and totally reached the pre-defined non-inferiority margin. Furthermore, the results of all endpoints have no statistical difference between the two groups in subgroup analysis.
The immunogenicity of the treatment vaccine in our study was small superior to that of the control for 2 of 23 serotypes studied (3, and 10A). Referred to the results of several previous studiesCitation38-40 critically, small different immune responses are accep via diverse prevalence and pre-exposure in different region or population. In addition, double-blinding principle across the whole trail ensured the same storage and administration were conducted in both groups.
With respect to the duration of PPV23 neutralizing antibody titers, vaccination with polysaccharide antigens does not establish T-cell mediated immune memory, although it may induce memory plasma cells. The duration of the immune response of PPV23 is likely limited, indicating a need for periodic revaccination. Musher et al. recently reported both primary vaccination and revaccination with PPV23 induce antibody responses that persist during 5 y of observation in middle-aged and older adults, consistent with the findings of previous studies, antibody levels 30 d after revaccination were generally modestly lower than those observed after primary vaccination. Induction of relatively long-lived memory suppressor T regulatory cells may be responsible for the observed suppression and may be intrinsic to the immune response to polysaccharide antigens.Citation39 The utility of routine pneumococcal revaccination in long-lived populations, to avoid further decline in anti-pneumococcal antibodies to levels like those among unvaccinated adults. Instead, revaccination seems to sustain antibody levels. Routine revaccination could reduce susceptibility to vaccine serotypes, to the extent that antibody levels are associated with protection. Both primary vaccination and revaccination with PPV23 in elderly persons result in significant and sustained increases in total IgG and functional antibody activity levels without evidence of significant hyporesponsiveness with revaccination.Citation40-43
Pneumococcal polysaccharides are T-cell independent antigens that are poorly immunogenic for important pneumococcal serotypes in infancy. The conjugation of pneumococcal capsular polysaccharide to carrier proteins results in a T-cell dependent immune response, characterized by increased antibody concentrations in infants, induction of memory cells, and a booster response upon subsequent antigenic exposures. Schedules combining PCV with 23-valent pneumococcal polysaccharide vaccine (PPV23) have been studied and proposed as a means to expand disease protection against serotypes not included in the PCVs. Several studies in children, using a variety of conjugate formulations and schedules in different countries, have shown that PPV23 as a booster dose following a priming series of PCV induces higher concentrations of antibodies than a PCV booster. The same results still observed in studies with PCV/PPV23 combined regimens have been done in patient populations for whom PPV23 is routinely recommended.Citation44-48
In conclusion, this newly vaccine is immunologically non-inferior and as well tolerated as the established vaccine PNEUMOVAX 23. Long-term antibody persistence and efficacy evaluations should be preferred in further studies.
Methods
Study design
This double-blinded, randomized, active-controlled non-inferiority clinical trial was designed by the Center for Disease Control and Prevention of the Guangxi Zhuang Autonomous Region (Guangxi CDC); Walvax Biotech (the study sponsor and manufacture of the vaccine) and the Department of Health Statistics of Fourth Military Medical University (FMMU). Data were collected by Guangxi CDC and monitored by Simon Record, a Contract Research Organization (CRO).This trial was performed from September 2012 to May 2013 at 2 centers (Jinchengjiang and Liucheng Counties) comprising 25 sites in Guangxi, China. The trial protocol and the informed-consent form were approved by the ethics committee of the Guangxi CDC. Before enrollment, written informed consent was obtained from each participant or legal guardian. The trial was conducted in accordance with the principles of the Declaration of Helsinki, the standards of Good Clinical Practice and Chinese regulatory requirements. The trial was registered at Clinicaltrials.gov (NCT02285036).
Each dose of treatment and control vaccine was assigned a code from a randomization list byage stratified block randomization (block size 10). The randomization list was generated by an external statistician with SAS (version 9.1). All packages of treatment and control vaccine were identical in appearance, with the code as the only identifier. Each participant was assigned a sequential number according to their sequence of enrolment and received the treatment or the control labeled with the same numbers. Thus, participants were randomly assigned to receive the treatment or the control in a 1:1 ratio. Investigators involved in randomization and masking did not participate in any other part of the trial. Allocation was masked from all participants, their guardians, and investigators.
Participants
Healthy participants (>2 years of age), with axillary temperature less than Celsius 37 degrees, not yet having received pneumococcal vaccine or other prevention biological products within 7 d were eligible for enrollment in this study. The exclusion criteria included (1) any known primary or secondary immunodeficiency, (2) allergy, (3) severe cardiovascular disease bleeding disorders, (4) receipt of immunoglobulin or blood products within one month and so on. All eligible participants were stratified by age (2∼6,7∼17, 18∼59 and >60 years), were randomly assigned in a 1:1 ratio to receive the treatment vaccine or the control. The same dose of either the treatment vaccine or the control vaccine were administered intramuscularly, with a 30 d surveillance period after an injection.
Vaccine
The treatment pneumococcal vaccine was developed by Yunnan Walvax Biotech Co. Ltd (Walvax) China, is a sterile, liquid vaccine for intramuscular or subcutaneous injection. The vaccine contained a mixture of highly purified capsular polysaccharides from the 23 most prevalent or invasive serotypes of Streptococcus pneumonia(serotype 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F). The dose of vaccine is contained 23 kinds of pneumococcal polysaccharide serotypes each 25μg in a 0.5ml dose per individual, and without any preservatives and stored at 2–8°C.
The active control, PNEUMOVAX 23, is an established US-licensed vaccine and manufactured by Merck. Each 0.5 ml dose of vaccine contains 25μg of each polysaccharide type in isotonic saline solution with 0.25% phenol as a preservative and stored at 2–8°C.
Both the treatment and control vaccines were prepared in a facility that was compliant with Good Manufacturing Practices and were tested by the National Institutes for Food and Drug Control before the start of the study.
Efficacy end points and Immunogenicity
The primary endpoint was the 2-fold increase rate of anti-pneumococcal antibody for all included 23 serotypes in each group.
The secondary endpoints included geometric mean fold increase (GMFI) and geometric mean concentration (GMCs) of IgG after vaccination in each group.
Blood samples were collected at baseline and day 30 after the injection for immunogenicity evaluation. All serum samples were assessed for anti-pneumococcal neutralizing antibody by means of enzyme-linked immunospot assay (ELISA) at the Chinese National Institute for Food and Drug Control in blindness.
Safety assessment
All participants or guardians who received the treatment or control vaccines were asked to fill out diary cards that listed injection-site adverse reactions (e.g., pain, redness, and swelling) and systemic adverse reactions (e.g., fever, headache, and diarrhea). Safety data were collected on solicited adverse events that occurred within 7 d after an injection and unsolicited adverse events (those reported spontaneously by a participant or guardian) that occurred within 30 d after an injection. Data on serious adverse events were collected throughout the trial. The grade and the relationship of the adverse event or serious adverse event with receipt of an injection were decided by investigators before unblinding.
Statistical analysis
On the assumption of the 2-fold increase rate of 92% and the non-inferiority margin of −10%, 830 participants per group were required to achieve at least the overall power of 80% with the one-sided significance level of 2.5%. The Bonferroni method was employed for the primary endpoint to control the overall type II error.
All analysis was performed on the intention-to-treat (ITT) population and per-protocol (PP) population. For the primary endpoint, the null hypothesis is rejected unless each serotype of the treatment group is non-inferior to the control group. The 95% confidence interval (CI) of the 2-fold increase rate difference for all serotypes between the two groups were calculated and the non-inferiority was considered if the lower bound of 95% CI was larger than −10%, which was determined by all investigators and recorded in the protocol before enrollment. The Student's t-test was used for the analysis of log-transformed GMI and GMC and the Chi-square test or Fisher's exact test (when data were sparse) was used for the analysis of dichotomous outcomes. All data were analyzed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA), hypothesis testing was 2-sided with an α value of 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
We deeply appreciate reviewers' helpful comments.
Supplemental_Materials.zip
Download Zip (6 MB)Funding
This work was partially supported by Research Grants from the National Natural Science Foundation of China (Program No. 81473069, 81273176, 81302509) and the Natural Science Basic Research Plan in Shaanxi Province of China (Program No. 2015JM8440).
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. Hib and pneumococcal global burden of disease study team; Hib and pneumococcal global burden of disease study team. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009; 374(9693):893-902; PMID:19748398; http://dx.doi.org/10.1016/S0140-6736(09)61204-6
- Schrag SJ, Beall B, Dowell SF. Limiting the spread of resistant pneumococci: biological and epidemiologic evidence for the effectiveness of alternative interventions. Clin Microbiol Rev 2000; 13:588-601; PMID:11023959; http://dx.doi.org/10.1128/CMR.13.4.588-601.2000
- Whitney CG, Farley MM, Hadler J, Harrison LH, Lexau C, Reingold A, Lefkowitz L, Cieslak PR, Cetron M, Zell ER, et al. Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med 2000; 343:1917-24; PMID:11136262; http://dx.doi.org/10.1056/NEJM200012283432603
- McCormick AW, Whitney CG, Farley MM, Lynfield R, Harrison LH, Bennett NM, Schaffner W, Reingold A, Hadler J, Cieslak P, et al. Geographic diversity and temporal trends of antimicrobial resistance in Streptococcus pneumoniae in the United States. Nat Med 2003; 9:424-30; PMID:12627227; http://dx.doi.org/10.1038/nm839
- Bogaert D, de Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 2004; 4:144-54; PMID:14998500; http://dx.doi.org/10.1016/S1473-3099(04)00938-7
- Hausdorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis 2005; 5:83-93; PMID:15680778; http://dx.doi.org/10.1016/S1473-3099(05)01280-6
- Type Study Group for Pneumococcus. Serotype study for Streptococcus pneumonia in 18 provinces, China. Chin J Epidemiol 1989; 10(3):133-7. [in Chinese]; PMID:2673527
- Yao KH, Yang YH. Streptococcus pneumoniae diseases in Chinese children: past, present and future. Vaccine 2008; 26:4425-33; PMID:18602435; http://dx.doi.org/10.1016/j.vaccine.2008.06.052
- Zhao GM, Black S, Shinefield H, Wang CQ, Zhang YH, Lin YZ, Lu JL, Guo YF, Jiang QW. Serotype distribution and antimicrobial resistance patterns in Streptococcus pneumoniae isolates from hospitalized pediatric patients with respiratory infections in Shanghai, China [J]. Pediatr Infect Dis J 2003; 22:739-42; PMID:12913778; http://dx.doi.org/10.1097/01.inf.0000078373.54515.40
- Liu Y, Wang H, Chen M, Sun Z, Zhao R, Zhang L, Wang H, Zhang H, Wang L, Chu Y, et al. Serotype distribution and antimicrobial resistance patterns of Streptococcus pneumoniae isolated from children in China younger than 5 years. Diagn Microbiol Infect Dis 2008; 61:256-63; PMID:18358662; http://dx.doi.org/10.1016/j.diagmicrobio.2008.02.004
- Xue L, Yao K, Xie G, Zheng Y, Wang C, Shang Y, Wang H, Wan L, Liu L, Li C, et al. Serotype distribution and antimicrobial resistance of Streptococcus pneumonia isolates that cause invasive disease among Chinese children. Clinical Infect Dis 2010; 50:741-4; http://dx.doi.org/10.1086/650534
- Zhou L, Ma X, Gao W, Yao KH, Shen AD, Yu SJ, Yang YH. Molecular characteristics of erythromycin-resistant Streptococcus pneumoniae from pediatric patients younger than five years in Beijing, 2010. BMC Microbiol 2012; 12:228; PMID:23043378; http://dx.doi.org/10.1186/1471-2180-12-228
- Ma X, Zhao R, Ma Z, Yao K, Yu S, Zheng Y, Yang Y. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates causing invasive diseases from Shenzhen Children's Hospital. PLoS One 2013; 8(6):e67507; PMID:23840728; http://dx.doi.org/10.1371/journal.pone.0067507
- Zhao C, Zhang F, Chu Y, Liu Y, Cao B, Chen M, Yu Y, Liao K, Zhang L, Sun Z, et al. Phenotypic and genotypic characteristic of invasive Pneumococcal isolates from both children and adult patients from a multicenter surveillance in China 2005–2011. PLoS One 2013; 8(12):e82361; PMID:24349263; http://dx.doi.org/10.1371/journal.pone.0082361
- Zhou L, Yu SJ, Gao W, Yao KH, Shen AD, Yang YH. Serotype distribution and antibiotic resistance of 140 pneumococcal isolates from pediatric patients with upper respiratory infections in Beijing, 2010. Vaccine 2011; (29):7704-10; PMID:21839135; http://dx.doi.org/10.1016/j.vaccine.2011.07.137
- Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, et al. Active bacterial core surveillance of the emerging infections program network. Decline in invasive pneumococcal disease after the introduction of protein polysaccharide conjugate vaccine. N Engl J Med 2003; 348:1737-46; PMID:12724479; http://dx.doi.org/10.1056/NEJMoa022823
- Kyaw MH, Lynfield R, Schaffner W, Craig AS, Hadler J, Reingold A, Thomas AR, Harrison LH, Bennett NM, Farley MM, et al. Active bacterial core surveillance of the emerging infections program network. Effect of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med 2006; 354:1455-63; PMID:16598044; http://dx.doi.org/10.1056/NEJMoa051642
- Hanage WP, Huang SS, Lipsitch M, Bishop CJ, Godoy D, Pelton SI, Goldstein R, Huot H, Finkelstein JA. Diversity and antibiotic resistance among nonvaccine serotypes of Streptococcus pneumonia carriage isolates in the post-heptavalent conjugate vaccine era. J Infect Dis 2007; 195:347-52; PMID:17205472; http://dx.doi.org/10.1086/510249
- Lexau CA, Lynfield R, Danila R, Pilishvili T, Facklam R, Farley MM, Harrison LH, Schaffner W, Reingold A, Bennett NM, et al. Active bacterial core surveillance team. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 2005; 294:2043-51; PMID:16249418; http://dx.doi.org/10.1001/jama.294.16.2043
- Jansen AG, Rodenburg GD, van der Ende A, van Alphen L, Veenhoven RH, Spanjaard L, Sanders EA, Hak E. Invasive pneumococcal disease among adults: associations among serotypes, disease characteristics, and outcome. Clin Infect Dis 2009; 49(2):e23-9; PMID:19522653; http://dx.doi.org/10.1086/600045
- Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013; 369:155-63; PMID:23841730; http://dx.doi.org/10.1056/NEJMoa1209165
- Dagan R. Impact of pneumococcal conjugate vaccine on infections caused by antibiotic-resistant Streptococcus pneumoniae. Clin Microbiol Infect 2009; 15(Suppl 3):16-20; PMID:19366365; http://dx.doi.org/10.1111/j.1469-0691.2009.02726.x
- Burgos J, Falcó V, Borrego A, Sordé R, Larrosa MN, Martinez X, Planes AM, Sánchez A, Palomar M, Rello J, et al. Impact of the emergence of non-vaccine pneumococcal serotypes on the clinical presentation and outcome of adults with invasive pneumococcal pneumonia. Clin Microbiol Infect 2013; 1:385-91; PMID:22583156; http://dx.doi.org/10.1111/j.1469-0691.2012.03895.x
- Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, Adair RK, Clemens JD. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med 1991; 21:1453-60; PMID:1944423; http://dx.doi.org/10.1056/NEJM199111213252101
- Mangtani P, Cutts F, Hall AJ. Efficacy of polysaccharide pneumococcal vaccine in adults in more developed countries: the state of the evidence. Lancet Infect Dis 2003; 3:71-8; PMID:12560191; http://dx.doi.org/10.1016/S1473-3099(03)00514-0
- Vila-Corcoles A, Salsench E, Rodriguez-Blanco T, Ochoa-Gondar O, de Diego C, Valdivieso A, Hospital I, Gomez-Bertomeu F, Raga X. Clinical effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumonia in middle-aged and older adults: a matched case–control study. Vaccine 2009; 27:1504-10; PMID:19171174; http://dx.doi.org/10.1016/j.vaccine.2009.01.013
- Johnstone J, Eurich DT, Minhas JK, Marrie TJ, Majumdar SR. Impact of the pneumococcal vaccine on long-term morbidity and mortality of adults at high risk for pneumonia. Clin Infect Dis 2010; 51(1):15-22; PMID:20504233; http://dx.doi.org/10.1086/653114
- Conaty S, Watson L, Dinnes J, Waugh N. The effectiveness of pneumococcal polysaccharide vaccines in adults: a systematic review of observational studies and comparison with results from randomised controlled trials. Vaccine 2004; 22:3214-24; PMID:15297076; http://dx.doi.org/10.1016/j.vaccine.2003.08.050
- Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, Hanson CA, Mahoney LD, Shay DK, Thompson WW. Vaccine Safety Datalink. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med 2003; 348:1747-55; PMID:12724480; http://dx.doi.org/10.1056/NEJMoa022678
- Vila-Córcoles A, Ochoa-Gondar O, Hospital I, Ansa X, Vilanova A, Rodríguez T, Llor C. EVAN Study Group. Protective effects of the 23-Valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin Infect Dis 2006; 43:860-8; PMID:16941367; http://dx.doi.org/10.1086/507340
- de Roux A, Schmöle-Thoma B, Siber GR, Hackell JG, Kuhnke A, Ahlers N, Baker SA, Razmpour A, Emini EA, Fernsten PD, et al. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis 2008; 46:1015-23; PMID:18444818; http://dx.doi.org/10.1086/529142
- Wiemken TL, Carrico RM, Klein SL, Jonsson CB, Peyrani P, Kelley RR, Aliberti S, Blasi F, Fernandez-Gonzalez R, Lopardo G, et al. CAPO Investigators. The effectiveness of the polysaccharide pneumococcal vaccine for the prevention of hospitalizations due to Streptococcus pneumonia community-acquired pneumonia in the elderly differs between the sexes: Results from the Community-Acquired Pneumonia Organization (CAPO) international cohort study. Vaccine 2014; 32:2198-203; PMID:24613522; http://dx.doi.org/10.1016/j.vaccine.2014.02.048
- Centers for Disease Control and Prevention. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997; 46(RR-8):1-24; PMID:9132580
- Centers for Disease Control Prevention (CDC); Advisory Committee on Immunization Practices. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep 2010; 59:102-106; PMID:20134399
- Lexau CA, Lynfield R, Danila R, Pilishvili T, Facklam R, Farley MM, Harrison LH, Schaffner W, Reingold A, Bennett NM, et al. Active bacterial core surveillance team. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 2005; 294:2043-2051; PMID:16249418; http://dx.doi.org/10.1001/jama.294.16.2043
- Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2008; (1):CD000422; PMID:18253977
- Fisman DN, Abrutyn E, Spaude KA, Kim A, Kirchner C, Daley J. Prior pneumococcal vaccination is associated with reduced death, complications, and length of stay among hospitalized adults with community-acquired pneumonia. Clin Infect Dis 2006; 42(April 15):1093-101; PMID:16575726; http://dx.doi.org/10.1086/501354
- Macintyre CR, Ridda I, Gao Z, Moa AM, McIntyre PB, Sullivan JS, Jones TR, Hayen A, Lindley RI. A randomized clinical trial of the immunogenicity of 7-Valent pneumococcal conjugate vaccine compared to 23-Valent polysaccharide vaccine in frail, hospitalized elderly. PLoS One 2014 Apr 23; 9(4):e94578; PMID:24760002; http://dx.doi.org/10.1371/journal.pone.0094578
- Jackson LA, Gurtman A, van Cleeff M, Frenck RW, Treanor J, Jansen KU, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine 2013 Aug 2; 31(35):3594-602; PMID:23688525; http://dx.doi.org/10.1016/j.vaccine.2013.04.084
- Jackson LA, Gurtman A, van Cleeff M, Jansen KU, Jayawardene D, Devlin C, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine 2013 Aug 2; 31(35):3577-84; PMID:23688526; http://dx.doi.org/10.1016/j.vaccine.2013.04.085
- Musher DM, Manof SB, Liss C, McFetridge RD, Marchese RD, Bushnell B, Alvarez F, Painter C, Blum MD, Silber JL. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis 2010; 201(4):516-24; PMID:20092407; http://dx.doi.org/10.1086/649839
- Manoff SB, Liss C, Caulfield MJ, Marchese RD, Silber J, Boslego J, Romero-Steiner S, Rajam G, Glass NE, Whitney CG, et al. Revaccination with a 23-valent pneumococcal polysaccharide vaccine induces elevated and persistent functional antibody responses in adults aged >65 years. J Infect Dis 2010; 201(4):525-33; PMID:20088694; http://dx.doi.org/10.1086/651131
- Nichol KL. Pneumococcal vaccination and revaccination in the elderly population. J Infect Dis 2010; 201:659-61; PMID:20113178; http://dx.doi.org/10.1086/651376
- Musher DM, Rueda AM, Nahm MH, Graviss EA, Rodriguez-Barradas MC. Initial and subsequent response to pneumococcal polysaccharide and protein conjugate vaccines administered sequentially to adults who have recovered from pneumococcal pneumonia. J Infect Dis 2008; 198(October 1):1019-27; PMID:18710324; http://dx.doi.org/10.1086/591629
- Lazarus R, Clutterbuck E, Yu LM, Bowman J, Bateman EA, Diggle L, Angus B, Peto TE, Beverley PC, Mant D, et al. A randomized study comparing combined pneumococcal conjugate and polysaccharide vaccination schedules in adults. Clin Infect Dis 2011; 52(6):736-42; PMID:21367726; http://dx.doi.org/10.1093/cid/cir003
- Jackson LA, Neuzil KM, Nahm MH, Whitney CG, Yu O, Nelson JC, Starkovich PT, Dunstan M, Carste B, Shay DK, et al. Immunogenicity of varying dosages of 7-valent pneumococcal polysaccharide-protein conjugate vaccine in seniors previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 2007; 25(20):4029-37; PMID:17391816; http://dx.doi.org/10.1016/j.vaccine.2007.02.062
- Goldblatt D, Southern J, Andrews N, Ashton L, Burbidge P, Woodgate S, Pebody R, Miller E. The immunogenicity of 7-valent pneumococcal conjugate vaccine versus 23-valent polysaccharide vaccine in adults aged 50–80 years. Clin Infect Dis 2009; 49(9):1318-25; PMID:19814624; http://dx.doi.org/10.1086/606046
- O'Brien KL, Hochman M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect Dis 2007; 7(9):597-606; PMID:17714673; http://dx.doi.org/10.1016/S1473-3099(07)70210-4