Abstract
Given their safe use in humans and inherent adjuvanticity, Lactic Acid Bacteria may offer several advantages over other mucosal delivery strategies for cancer vaccines. The objective of this study is to evaluate the immune responses in mice after oral immunization with Lactobacillus (L) plantarum WCFS1 expressing a cell-wall anchored tumor antigen NY-ESO-1. And to investigate the immunostimulatory potency of this new candidate vaccine on human dendritic cells (DCs). L. plantarum displaying NY-ESO-1 induced NY-ESO-1 specific antibodies and T-cell responses in mice. By contrast, L. plantarum displaying conserved proteins such as heat shock protein-27 and galectin-1, did not induce immunity, suggesting that immune tolerance to self-proteins cannot be broken by oral administration of L. plantarum. With respect to immunomodulation, immature DCs incubated with wild type or L. plantarum-NY-ESO-1 upregulated the expression of co-stimulatory molecules and secreted a large amount of interleukin (IL)-12, TNF-α, but not IL-4. Moreover, they upregulated the expression of immunosuppressive factors such as IL-10 and indoleamine 2,3-dioxygenase. Although L. plantarum-matured DCs expressed inhibitory molecules, they stimulated allogeneic T cells in-vitro. Collectively, the data indicate that L. plantarum-NY-ESO-1 can evoke antigen-specific immunity upon oral administration and induce DC maturation, raising the potential of its use in cancer immunotherapies.
Abbreviations
| APC | = | antigen presenting cells |
| DC | = | dendritic cells |
| HPV | = | human papilloma virus |
| HSP | = | heat shock protein |
| iDC | = | immature DCs |
| IDO | = | indoleamine 2,3-dioxygenase |
| Ig | = | immunoglobulin |
| IL | = | interleukin |
| LAB | = | lactic acid bacteria |
| L | = | lactobacillus |
| mDC | = | mature DC |
| PD | = | programmed cell death |
| Th | = | T helper |
Introduction
Mucosal vaccines offer great potential since they can be administered via oral or intranasal delivery.Citation1,2 To induce immune responses, however, a delivery system is needed that protects antigen from degradation, facilitates antigen uptake in the gastrointestinal tract, and triggers immunity rather than tolerance often seen in oral vaccination with soluble antigens.Citation3 Given their food grade status and history of safe use in humans, Lactic Acid Bacteria (LAB) are considered more attractive than other live vaccine vectors such as Salmonella.Citation1,4 Moreover, several members of LAB such as Lactobacillus (L.) casei, L. plantarum, L. acidophilus, and Lactococcus lactis, exhibited self-adjuvant properties and found to be weakly immunogenic themselves [reviewed in 1]. With respect to the effects of antigen location (cytoplasmic, secreted, or anchored to the cell wall) on immunogenicity, there is no clear consensus on which cloning strategy is preferable.Citation5,6 This is likely due to several factors such as the expression level of the antigen and the nature of the bacterial strain. Conflicting results are also reported regarding the comparison between intragastric and intranasal routes of administration.Citation7,8 Regardless of the applied strategy, in most studies mucosal immunization resulted in induction of antigen-specific immunity.
Because mucosal immunization induces not only mucosal IgA, but also systemic T-cell and antibody responses in peripheral lymphoid tissues, this route of vaccination has been used to induce immunity against infections and certain cancer types.Citation9–16 Indeed, a number of studies of oral vaccines generated from genetically engineered pathogenic or commensal bacteria have been reported. For example, administration of L. casei expressing a cell-wall anchored E7 antigen from human papilloma virus (HPV) 16, which is the main etiological agent of cervical cancer, induced cellular immunity and protected mice from tumor development.Citation15 Intranasal administration of recombinant lactococci displaying E7 antigen and secreting biologically active IL-12 induced E7-specific immune responses and showed therapeutic effects against HPV-16-induced tumors.Citation16 Interestingly, E7-expressing L. casei vaccine induced E7-specific immunity and clinical response in patients with cervical intraepithelial neoplasia grade 3,Citation17 confirming the clinical feasibility of developed vaccines against HPV-related cervical cancer in humans.
Notably, cancer vaccines hold great promise in the treatment of certain cancers such as melanoma, and have been the focus of extensive pre-clinical and clinical testing in recent years.Citation18 Among cancer associated antigens, NY-ESO-1 has emerged as one of the most promising targets in vaccination.Citation19 The frequent finding of humoral and cellular immune responses against this antigen in cancer patients with NY-ESO-1-expressing tumors makes it one of the most immunogenic human tumor antigens.Citation19 Many strategies for development of NY-ESO-1-based vaccines have been reported, including recombinant live vector vaccines and protein or peptide vaccines.Citation20,21 In contrast to the currently used vectors, LAB are generally regarded as safe microorganisms, and some of them are able to stimulate the immune system of the host as adjuvants due to their probiotic and anti-inflammatory properties.Citation1,22 Except for cervical cancer, little is known about the use of LAB in cancer vaccines. We previously reported that the 37 kDa immunogenic oncofetal antigen (OFA) expressed on the cell surface of L. plantarum can induce antibody response.Citation23 Encouraged by this finding, in this study we investigated whether L. plantarum expressing NY-ESO-1 can induce specific immunity in mice. As controls for immunogenicity, we included 2 conserved proteins between human and mouse, HSP-27 and galectin-1 (Gal-1). We furthermore evaluated the immunomodulatory properties of recombinant and wild type L. plantarum on human monocytes, immature (i) DCs, and mature (m) DCs derived from the same donors. Immunization analysis demonstrated that surface-displayed NY-ESO-1 induced immune responses and exhibited an adjuvant activity on iDCs, providing a new strategy for the development of cancer vaccines.
Results
Expression of the recombinant proteins
The cancer/testis protein NY-ESO-1 is a suitable model antigen to explore systemic and mucosal immune responses. Full-length NY-ESO-1 protein and control proteins (HSP-27, Gal-1) were expressed on the cell wall of L. plantarum (). To evaluate gene expression, equal amounts of whole cell protein extracts of L. plantarum carrying the empty vector (pEV), expressing Gal-1, NY-ESO-1, or HSP-27 protein were analyzed by Western blotting using specific monoclonal antibodies (). Immune-reactive fusion proteins with the appropriate sizes were detected. In all experiments Gal-1 seems to migrate as a double band. This is more likely due to a specific degradation because the same results were obtained with 2 different antibodies. It should be noted that in human cells Gal-1 was detected as a single isoform.Citation24 Using flow cytometry, we further confirmed the display of the expressed proteins on the surface of L. plantarum (). Indeed, recombinant L. plantarum stained positively with the corresponding antibodies, whereas none of the used monoclonal antibodies stained the cells harboring the pEV vector ( last panel). These results validate our surface expression system to display tumor antigens for mucosal vaccines and/or other therapies.
Figure 1. Characterization of recombinant Lactobacillus. (A) Schematic overview of the expression cassette for C-terminal anchoring of the 3 target proteins, NY-ESO-1, HSP-27 and Gal-1. The expression cassette is translationally fused to the inducible PsppA promoter. The target genes are fused through a SalI linker to a signal peptide (SP) derived from the L. plantarum. The C-terminal LPxTG anchor sequence derived from the L. plantarum Lp_2578 is translationally fused to the target genes through a MluI linker. Three variants of this linker have been developed [(named, cwa1, cwa2 and cwa3; (21)] which differ in terms of how large a part of the lactobacillal protein preceding the LPxTG motif is included; see Materials & Methods for details. Previously published plasmids that were used as starting points for these constructions contain the same restriction sites. (B) Western blotting. Whole-cell protein extracts prepared from L. plantarum expressing Gal-1, NY-ESO-1, or HSP-27 were prepared, analyzed by 10% SDS-PAGE gels, transferred to nitrocellulose and incubated with protrein-specific antibody as indicated. L. plantarum harboring pEV vector was used as a control. The three immunoblots are triplicates. (C) Flow cytometry analysis of L. plantarum expressing Gal-1 (L.p-Gal-1), NY-ESO-1 (L.p-NY-ESO-1), or HSP-27 (L.p-HSP-27). After induction of protein expression, the live cells were washed with PBS, stained with the corresponding biotin-conjugated monoclonal antibodies (grew histograms) followed by PE-conjugated streptavidin and then analyzed by flow cytometry. Black histograms correspond to cells stained with only PE-conjugated streptavidin (negative control). None of the used biotin conjugated monoclonal antibodies against NY-ESO-1, Gal-1, or HSP-27 stained L. plantarum harboring the pEV vector (L.p-pEV, last panel).
![Figure 1. Characterization of recombinant Lactobacillus. (A) Schematic overview of the expression cassette for C-terminal anchoring of the 3 target proteins, NY-ESO-1, HSP-27 and Gal-1. The expression cassette is translationally fused to the inducible PsppA promoter. The target genes are fused through a SalI linker to a signal peptide (SP) derived from the L. plantarum. The C-terminal LPxTG anchor sequence derived from the L. plantarum Lp_2578 is translationally fused to the target genes through a MluI linker. Three variants of this linker have been developed [(named, cwa1, cwa2 and cwa3; (21)] which differ in terms of how large a part of the lactobacillal protein preceding the LPxTG motif is included; see Materials & Methods for details. Previously published plasmids that were used as starting points for these constructions contain the same restriction sites. (B) Western blotting. Whole-cell protein extracts prepared from L. plantarum expressing Gal-1, NY-ESO-1, or HSP-27 were prepared, analyzed by 10% SDS-PAGE gels, transferred to nitrocellulose and incubated with protrein-specific antibody as indicated. L. plantarum harboring pEV vector was used as a control. The three immunoblots are triplicates. (C) Flow cytometry analysis of L. plantarum expressing Gal-1 (L.p-Gal-1), NY-ESO-1 (L.p-NY-ESO-1), or HSP-27 (L.p-HSP-27). After induction of protein expression, the live cells were washed with PBS, stained with the corresponding biotin-conjugated monoclonal antibodies (grew histograms) followed by PE-conjugated streptavidin and then analyzed by flow cytometry. Black histograms correspond to cells stained with only PE-conjugated streptavidin (negative control). None of the used biotin conjugated monoclonal antibodies against NY-ESO-1, Gal-1, or HSP-27 stained L. plantarum harboring the pEV vector (L.p-pEV, last panel).](/cms/asset/14df6af3-4d47-4273-bca5-8dc960051647/khvi_a_1056952_f0001_oc.gif)
Antibody response to oral administration of recombinant L. plantarum
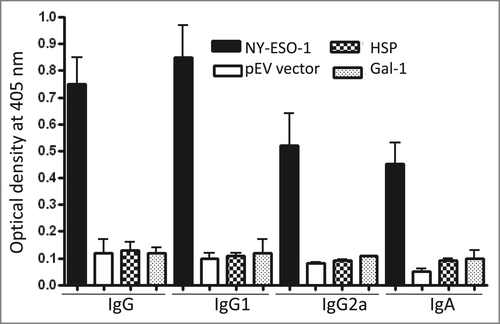
To evaluate the ability of the recombinant L. plantarum to induce an antigen-specific immune response, first BALB/c mice were orally immunized with L. plantarum expressing NY-ESO-1 or harboring the pEV vector. NY-ESO-1 specific serum IgG, IgG1, IgG2a, and mucosal IgA levels in intestinal lavages, were determined by ELISA. Mice vaccinated with L. plantarum expressing NY-ESO-1 developed IgG, IgG1 and IgG2a against NY-ESO-1 protein when compared to mice immunized with L. plantarum harboring the pEV vector (). Furthermore, mice developed IgA antibodies in intestinal lavages. Thus, both systemic (serum IgG) and mucosal (intestinal IgA) NY-ESO-1- specific antibodies were induced by oral administration. By contrast, mice immunized with L. plantarum expressing Gal-1 or HSP-27 did not develop antibody response (). These results may be expected because both proteins are conserved between human and mouse.
Figure 2. Antibody responses to L. plantarum expressing NY-ESO-1. BALB/c mice immunized with either L. plantarum expressing NY-ESO-1 (A) or harboring the pEV vector (B) were analyzed for the presence of NY-ESO-1-specific IgG, IgG1, IgG2a, and IgA by ELISA. Bars represent the median value of each experimental group (n = 6 ).
Oral immunization induces antigen-specific cellular immune response in spleen
In mice the IgG2a B-cell response is associated with a Th1-like immune response, whereas IgG1 with a Th2-like response. The induction of both responses requires T-cell help. Therefore, we have tested the ability of L. plantarum expressing NY-ESO-1 to induce T-cell response. After vaccination, splenocytes were prepared on day 40 from control and immunized mice and T-cell proliferation in response to recombinant NY-ESO-1 was assayed by thymidine incorporation (). Spleenic T cells isolated from L. plantarum-NY-ESO-1 immunized mice proliferated upon in-vitro stimulation with recombinant NY-ESO-1 (p < 0.001). Mice orally immunized with the L. plantarum harboring pEV vector showed a moderate response that was similar to that seen in mice injected with PBS buffer (control). In accordance with the antibody response, spleenic T cells from mice immunized with L. plantarum expressing HSP-27 or Gal-1 protein failed to proliferate in response to recombinant proteins (data not shown).
Figure 3. T-cell response to NY-ESO-1. Spleenic cells from mice immunized with either L. plantarum expressing NY-ESO-1 or L. plantarum harboring the vector (pEV) were prepared and stimulated for 6 d with NY-ESO-1 protein (10 μg/ml). Thereafter, T-cell proliferation was determined by incorporation of [3H]-thymidine following overnight pulsing. The results are expressed as the mean values of stimulation index ±SD for triplicate determination. Stimulation index is calculated by dividing the number of cpm for NY-ESO-1-stimulated cells by the number of cpm in unstimulated cells. Control = mice injected with PBS buffer. The data are representative for 5 separate experiments. ***p < 0.001.
![Figure 3. T-cell response to NY-ESO-1. Spleenic cells from mice immunized with either L. plantarum expressing NY-ESO-1 or L. plantarum harboring the vector (pEV) were prepared and stimulated for 6 d with NY-ESO-1 protein (10 μg/ml). Thereafter, T-cell proliferation was determined by incorporation of [3H]-thymidine following overnight pulsing. The results are expressed as the mean values of stimulation index ±SD for triplicate determination. Stimulation index is calculated by dividing the number of cpm for NY-ESO-1-stimulated cells by the number of cpm in unstimulated cells. Control = mice injected with PBS buffer. The data are representative for 5 separate experiments. ***p < 0.001.](/cms/asset/f647bc96-e14b-4a2a-a2d9-ab4367a28817/khvi_a_1056952_f0003_b.gif)
Dendritic cell response to recombinant and wild type L. plantarum
Given the potential use of L. plantarum-based cancer vaccines in humans,Citation1 we investigated the responses of iDC and mDC to wild type L. plantarum expressing NY-ESO-1. In these experiments, iDC and TNF-α-matured DC (mDC) derived from the same donors were co-cultured with live wild type or L. plantarum expressing NY-ESO-1 for 18 h, culture supernatants were collected and the amount of pro-inflammatory cytokines TNF-α, IL-12, and anti-inflammatory cytokine IL-10 was quantified by ELISA. Control samples were un-stimulated cells. L. plantarum expressing NY-ESO-1 or harboring the pEV vector induced high levels of IL-12 and TNF-α (). Notably, the level of IL-10 was significantly lower than that of IL-12 (900 ± 150 vs 3100 ± 500 pg/ml). In contrast to iDC, mDC did not respond to L. plantarum, indicating that the signaling pathways activated in iDC are switched off in mDC. Comparable cytokine induction profiles were obtained with UV-killed L. plantarum (data not shown). We also quantified the Th-2 cytokine IL-4. Both recombinant and wild type L. plantarum did not induce IL-4 production (). Similar to iDCs, monocytes prepared from the same donors responded to L. plantarum by producing IL-12, TNF-α and IL-10 (data not shown).
Table 1. Cytokine production by L. plantarum-treated DCs
iDC-matured with L. plantarum upregulated the expression of co-stimulatory molecules
To investigate whether L. plantarum-treated iDCs upregulate the expression of co-stimulatory molecules, cells were stimulated for 48 h with either wild type, recombinant L. plantarum or TNF−α and then they were stained with marker-specific antibodies. Data were acquired on a flow cytometer and expressed as mean fluorescence (). Both recombinant and wild type L. plantarum increased the expression all markers when compared to un-stimulated iDCs. Moreover, the expression of CD80, CD86 and CCR7 was significantly increased as compared to TNFα-matured DCs (p < 0.01). In general, TNF-α-matured DCs showed less pronounced phenotype as the wild type or recombinant L. plantarum-matured DCs.
Table 2. Surface expression of the activation markers on DCs
L. plantarum- matured DCs upregulated the expression of immunosuppressive factors
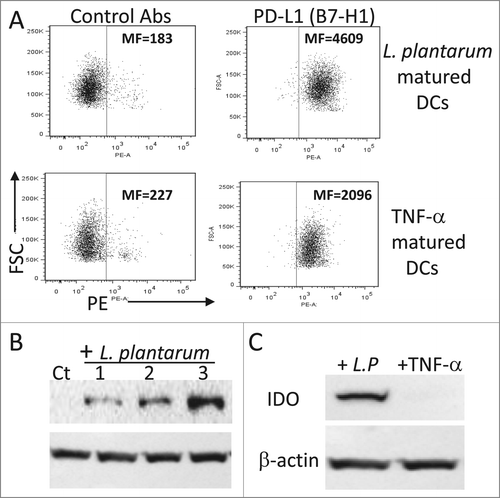
Since the programmed death (PD, B7-H1)-ligand-1 and IDO play a major role in immune regulation,Citation25 their expression in DCs exposed to L. plantarum was analyzed. The expression level of PD-L1 was significantly increased (p < 0.01) in L. plantarum-treated iDCs as compared to that of TNFα-treated cells (). Moreover, L. plantarum-treated iDCs upregulated the expression of IDO, an immunosuppressive enzyme (). In contrast, TNF-α treated iDCs did not express IDO (). Similar to the wild type, L. plantarum expressing NY-ESO-1 induced IDO and PD-L1 gene expression (data not shown).
Figure 4. Expression of PD-L1 and IDO by iDC in response to L. plantarum. (A) PD-L1 expression. iDCs were stimulated with TNF-α or live L. plantarum for 48 h and then the expression of PD-L1 was analyzed by flow cytometry using a specific monoclonal antibody. (B) Expression of IDO in response to various L. plantarum concentrations. iDC were stimulated for 18 h and then protein extracts were prepared and analyzed by Western blotting. Lanes 1, 2, and 3 correspond to cells stimulated with 105, 106 and 107 CFU/ml, respectively. (C) As in B, iDCs were stimulated with L. plantarum (107 CFU/ml) or TNF-α (50 ng/ml) and then the expression of IDO was analyzed by Western blotting. Ct = unstimulated cells. MF = mean fluorescence intensity. The data are representative of 4 separate experiments.
L. plantarum-matured DCs induced T-cell proliferation
Having shown that L. plantarum treated iDCs express co-stimulatory molecules and secreted IL-12, next we investigated whether the increased expression of inhibitory factors (e.g., IL-10, IDO, PD-L1) by L. plantarum affects T-cell response. In these experiments, we used purified CD4+ T cells from one donor as responder and DCs from a second donor as stimulator in mixed lymphocyte reaction (MLR) assays (). iDCs induced only weak proliferative responses in CD4+ T cells. As expected, TNF-α matured DCs induced a strong allogeneic response as compared to iDCs. The potency of L. plantarum-matured DCs to induce T-cell proliferation at DC/T cell ratios of 1:40 and 1:20 was comparable to that of TNF-α matured DCs. However, at DC/T ratio of 1:10, L. plantarum-matured DCs were less T-cell stimulator than TNF-α matured DCs (p < 0.01).
Figure 5. Potency of various DC preparations to stimulate allogeneic T cell proliferation. DCs were co-cultured with allogeneic CD4+ T cells at a T cell/DC ratio of 1:40, 1:20 or 1:10. Cells were co-cultured for 5 d at 37°C and T-cell proliferation was determined by incorporation of [3H]-thymidine following overnight pulsing. The results are presented as mean values ±SD for triplicate determination and are representative for 3 separate experiments. p < 0.01.
![Figure 5. Potency of various DC preparations to stimulate allogeneic T cell proliferation. DCs were co-cultured with allogeneic CD4+ T cells at a T cell/DC ratio of 1:40, 1:20 or 1:10. Cells were co-cultured for 5 d at 37°C and T-cell proliferation was determined by incorporation of [3H]-thymidine following overnight pulsing. The results are presented as mean values ±SD for triplicate determination and are representative for 3 separate experiments. p < 0.01.](/cms/asset/a9a6b74b-c816-4a0b-a16c-57fbcd33e03d/khvi_a_1056952_f0005_b.gif)
Discussion
Oral vaccines, depending on their composition and nature, can activate every effector arm of the immune system.Citation1 The present study demonstrated the expression of NY-ESO-1 antigen at the surface of L. plantarum and confirmed that oral immunization with this recombinant bacterium induced both humoral and T-cell responses in mice. Although L. plantarum matured iDC expressed IL-10, IDO, and PD-L1 as potential inhibitory molecules, they activated T cells more likely via the overexpression of IL-12 and co-stimulatory molecules. IL-12 is a critical factor in switching naïve or memory T cells to Th-1 response against infections and other disease such as cancers.Citation26 It is generally acknowledged that excessive activation of immune effector cells may result in host detrimental conditions, such as in chronic inflammation and autoimmunity. To ensure the proper regulation of effector mechanisms, the production of proinflammatory molecules (e.g., IL-12, TNF-α) is always associated with the upregulation of secreted and membrane-bound inhibitory factors such as IL-10 and PD-L1.Citation25,27 The ability of L. plantarum WCFS1 strain to induce counter-regulatory cytokines should provide an important regulatory mechanism and may explain their anti-inflammatory and protective effects Citation22,28
Most studies in the literature have focused on mucosal vaccination against infections.Citation1 With respect to cancer immunotherapy, recently Kawana and colleagues demonstrated that oral vaccination with L. casei expressing modified E7 protein can induce clinical response in patients with cervical intraepithelial neoplasia.Citation17 NY-ESO-1 tumor antigen that is expressed in a variety of human malignancies, but not in normal tissues except for the testis and placenta.Citation19 Therefore, a vaccine that can induce both mucosal and systemic immunity might be more effective for eradicating NY-ESO-1-expressing tumors than parenteral vaccines. Our strategy of expressing NY-ESO-1 in L. plantarum induced both systemic and mucosal immunity after oral administration. The present designed self-adjuvanted subunit vaccine does not require additional adjuvants to induce immune responses in mice. Consistent with our data, Meng and colleagues showed that oral vaccination with attenuated Salmonella enteric strains encoding T- cell epitopes from NY-ESO-1 can induce specific cytotoxic T-lymphocyte responses in mice.Citation20 The authors have used the Tafi display system to express NY-ESO-1 T-cell epitopes.
Notably, innate immunity plays a key role in the development of protective adaptive immune responses during vaccination. Several adjuvants are used in vaccines to activate innate immune cells, including DCs. Such activation of innate cells by a vaccine is critical to subsequent activation and differentiation of effector T cells. Since DCs are the most potent APCs,Citation18 several studies have investigated their capacity to response to LAB. For example, monocyte-derived DC stimulated with L. Johnsonii, L. reuteri, or L. gasseri produced IL-12, but not IL-10.Citation29 Bone marrow-derived murine DCs stimulated with L. casei produced a significant amount of TNF-α, IL-6, and IL-12, whereas those stimulated with L. reuteri produced low level of IL-12.Citation30 Likewise, murine DCs stimulated with L. acidophilus produced IL-10, IL-12 and TNF-α.Citation31 Human monocyte-derived iDCs stimulated with L. plantarum expressing OspA lipoprotein from B. burgdogferi produced TNF-α and IL-10, but not IL-12. By contrast, iDCs stimulated with wild type L. plantarum did not produce significant levels of TNF-α and IL-10.Citation32 Although the induction levels of the investigated cytokines varied between donors, monocytes and monocyte-derived iDCs prepared from more than 20 healthy individuals responded to L. plantarum WCFS1 strain by secreting TNF-α, IL-12, and IL-10 (). The variation in strain immune profiles suggest that there could be some underlying strain-dependent genetic differences from the cytokine secretion. Indeed, Meijerink et al. identified gene loci in L. plantarum WCFS1 that modulate the immune response of DCs.Citation33 The authors used 42 different L. plantarum strains isolated from humans and different food resources. The strains differed considerably in their ability to induce cytokine expression in monocyte-derived iDCs. Consistent with the present study, the levels of IL-12, IL-10, and TNF-α induced by the wild type L. plantarum WCFS1 were around 3500, 250, and 2400 pg/ml, respectively. We found that in contrast to monocytes and iDCs, TNF-α matured DCs are unresponsive to wild type and recombinant L. plantarum WCFS1. Although further work is needed, this observation indicates that the maturation stage of DCs may influence their response to bacterial cell components.
Among the regulatory factors, IDO has emerged as one of the most used inhibitory molecule by the immune system.Citation34 IDO-competent DCs exert regulatory effects on T cells that are mediated by tryptophan depletion and by the production of metabolic byproducts collectively known as kynurenines.Citation34 As shown in this study, L. plantarum activated iDCs upregulated the expression of IDO. This observation is interesting given the anti-inflammatory properties of certain probiotic bacteria. In response to L. plantarum, DCs also overexpressed PD-L1. The engagement of PD1/PDL-1 induces negative signals to receptor signaling pathways, leading to inhibition of T-cell proliferation.Citation27 Despite the expression of these 2 inhibitory molecules, L. plantarum-matured DCs activated T cell proliferation in-vitro. However, at high DC number some inhibition effect was evident as compared to TNF-α matured DCs. Thus, depending on the number of DCs within inflammatory sites and/or lymph nodes, IL-12 might override IDO, IL10, and PD-L1-driven blockade of T-cell proliferation.
In conclusion, the present study demonstrates that genetically engineered L. plantarum expressing NY-ESO-1 can induce humoral and T-cell responses and should therefore represent a promising strategy for oral immunization against tumor expressing NY-ESO-1. Moreover, L. plantarum induction of DC maturation along with the secretion of IL-12, a major cytokine that triggers Th-1 response, should support the use of L. plantarum WCFS1 strain as a delivery vector.
Materials and Methods
Reagents
Recombinant NY-ESO-1, Gal-1 and HSP-27 proteins were purchased from ProSpec-Tany TechnologyGene Ltd (East Brunswick, New Jersey), Nordic BioSite (Täby, Sweden), and R& D systems (Minneapolis, Minnesota), respectively. Recombinant proteins were detected using the following monoclonal antibodies: NY-ESO-1 (Santa Cruz Biotechnology, Dallas, Texas), biotin-conjugated Gal-1 (R&D systems), and anti-HSP-27 (R&D systems). Antibodies against NY-ESO-1 and HSP-27 were biotin-conjugated using Sulfo-NHS-biotin (Santa Cruz Biotechnology) as described by the manufacturer's instructions. Anti-mouse IgG and IgA were purchased from Sigma (St. Louis, Missouri) and goat anti-mouse IgG1 and IgG2a were purchased from AbD Serotec (Kidlington, UK). Other antibodies were purchased from Dako (Glostrup, Denmark) or BD Biosciences (San Jose, California).
Animals
Female BALB/c and C57BL/6 mice (5 weeks old) were purchased from Harlan (Boxmeer, The Netherlands) and housed under specific pathogen-free conditions. Mice were acclimated to the new environment for at least one week after arrival before being used for the experiments. They were on average 5 to 10 weeks of age at the onset of use. Animal studies were carried out in agreement with the protocols approved by the Animal Care Committee at the Norwegian Radium Hospital, Institute for Cancer Research.
Cloning
All plasmids used in the present study are derivatives of pSIP401, a vector constructed for inducible gene expression in L. plantarum.Citation35 The parental vectors used for cloning were pSIP401 derivatives that had previously been modified for C-terminal cell wall anchoring of the heterologously expressed protein of interest.Citation23 The NY-ESO-1 sequence previously optimized for secretionCitation36 was codon-optimized for expression in L. plantarum WCFS1 and ordered from Genscript (Piscataway, NJ, USA) in a pUC57-vector (pUC57-NY-ESO). The NY-ESO-1 gene fragment was amplified using pUC57-NY-ESO as template with the primer pair NYESOF (TGCTTCATCAGTCGACCAAGCGGAAGGCCGA) and NYESOcwa3R (CTGGTTGGCT ACGCGTTCGCCGTTGAC) (restriction sites in italics). The resulting fragment was In-Fusion cloned (In-Fusion HD cloning kit, Clontech Laboratories, Mountain View, CA, USA) into the SalI and MluI restriction sites of pLp_0373sOFAcwa3 (4), yielding the plasmid pLp_0373sESOcwa3. The expression vector, pLp_0373ESOcwa3, was directly transformed into electrocompetent L. plantarum WCFS1,Citation37 following the protocol of Holo and colleagues.Citation38 The code “0373” indicates that secretion of NY-ESO-1 is driven by an N-terminal signal peptide (SP) derived from the L. plantarum protein Lp_0373.Citation39 The cell wall anchor referred to as cwa3 originates from L. plantarum protein Lp_2578 and comprises 128 residues (see ref. Twenty-three for more details). The Gal-1 sequence (NP_002296.1) was codon-optimized (Genscript) and cloned into a pUC57-vector pUC57-Gal-1). To allow for cloning directly into the pSIP-secretion vectors, the synthesized gene deviated for the wild-type gene as follows, the start codon in gal-1 was removed and 2 6 bp sequences corresponding to SalI and EcoRI restriction sites were introduced at sites corresponding to the extreme N-terminus and C-terminus of the amino acid sequence, respectively. The pUC57-Gal-1 vector was digested with SalI and EcoRI and ligated into the SalI/EcoRI-digested pLp_3050sNucA,Citation40 yielding pLp_3050sGal-1. The Gal-1 anchoring construct was made by amplifying the 3050sGal-1 fragment (Gal-1 fused to the Lp_3050 signal peptide) from pLp_3050sGal-1 using the primer pair 3050F (GGAGTATGATTCATATGAAAAAATTTAACT-TTAAAACCATGTT) and Galcwa2R (GTTCAGTGACAC-GCGTATCAAACGCCACACACTTAATC) and subsequently in-fusion cloned into the NdeI/MluI digested pLp_0373sOFAcwa2 plasmid,Citation23 yielding pLp_3050sGal-1cwa2. The cell wall anchor referred to as cwa2 is similar to cwa3, described above, but the anchor sequence consist of 194 residues. The hsp-27 sequence (accession no. NP_001531.1) was amplified from cDNA with the primer pair HspF (GTCGACACCGAGCGC-CGC) and HspR (GAATTCTTACTTGGCGGCAGTCTCATCGGAT) and directly sub-cloned into the PCR-Blunt II TOPO vector (Invitrogen, Carlsbad, CA). The hsp-27 gene was then re-amplified from the TOPO vector using the primer pair 3050sHsp27F (GGCCTCCAAGGTCGACACCGAGC GCC-GC) and Hspcwa2R (GTTCAGTGACACGCGTCTTGGCG-GCAGTCTCATCGGA) and In-Fusion cloned into the Sal/MluI-digested pLp_3050sGal-1cwa2, yielding pLp_3050sHsp-27cwa2. Both pLp_3050sGal-1cwa2 and pLp_3050sHsp-27cwa2 were transformed into E. coli TOP10 (Invitrogen) for generation of plasmid DNA subsequently used for electroporation into L. plantarum WCFS1. All PCR generated inserts were verified by DNA sequencing.
Culture conditions
Frozen stocks of recombinant L. plantarum strains were used to inoculate Man-Rogosa-Sharpe (MRS) agar and broth medium supplemented with erythromycin (5μg/ml) at 30°C in an anaerobic cabinet. Bacteria are usually collected during the log phase corresponding to approximately 109 CFU/ml. To induce gene expression, the cells were grown overnight at 30°C in MRS medium containing erythromycin (5 μg/ml). The overnight cultures were diluted to 1/100 in MRS medium and cultured for 2 h at 30°C. Gene expression of the recombinant proteins was then induced by adding SppIP peptide pheromone to a final concentration of 25 ng/ml. Bacteria were subsequently collected when their OD600 corresponded to 5 × 108 cell/ml (log phase). Cells were harvested by centrifugation at 12000 g for 10 min at 4°C, washed twice in sterile phosphate-buffered saline (PBS) and re-suspended in PBS at a concentration of approximately 109 CFU/ml. Gene expression was always verified by Western blotting.
Analysis of gene expression by immunoblotting and flow cytometry
To analyze the expression of the cloned proteins, subsequent to gene induction, L. plantarum cultures were collected by centrifugation at 12000 g for 10 min. The pellets were washed twice with PBS buffer and total proteins were prepared with boiling SDS-extraction buffer (phosphate buffered saline, 5% SDS). Proteins were separated on 10% SDS-PAGE, electro-blotted onto nitrocellulose membranes, blocked for 60 min at room temperature in 5% non-fat milk Tris-buffer-saline (TBS) and then incubated overnight at 4°C with mouse monoclonal antibodies against human NY-ESO-1, HSP-27, or Gal-1. After washing, the membranes were incubated with HRP-conjugated anti-mouse IgG antibodies (Dako, Glostrup, Denmark) and reactive bands were visualized by an ECL kit. For flow cytometry, recombinant L. plantarum (5 × 105 cells/well/200 μl staining buffer) were incubated for 30 min at 4°C with biotin-conjugated monoclonal antibodies against Gal-1, NY-ESO-1 or HSP-27. Thereafter, the cells were incubated with PE-streptavidin for 30 min, washed and then analyzed with FACSCanto II flow cytometer (BD Biosciences, San Jose, CA, USA). Data were analyzed with FlowJo software.
Immunization
After gene induction, recombinant L. plantarum cells were harvested, washed twice with PBS and resuspended in PBS and used for immunization. Administration was performed intragastrically using a ball-tipped syringe for oral gavage inoculation (6 BALB/c mice/group). Each mouse received approximately 5 × 108 CFU recombinant L. plantarum. The control group received L. plantarum harboring the pEV, a control vector without target gene. Mice received 3 to 4 immunizations. One week after the last immunization, mice were bled and sera were analyzed by ELISA for the presence of antibodies against the recombinant proteins. At the end of the experiments, mice were killed and blood, intestinal lavages and spleens were collected for further analysis. With respect to T-cell response, prepared splenocytes were incubated with recombinant proteins for 5 days, pulsed with [3H]-thymidine and then DNA-associated reactivity was quantified by using a gamma counter. Some C75BL/6 mice were also immunized with L. plantarum expressing NY-ESO-1, HSP-27, or Gal-1 protein.
ELISA
Sera and intestinal lavages from orally inoculated mice were tested by indirect ELISA for the presence of total IgG, IgG1, IgG2a or IgA to recombinant NY-ESO-1, HSP-27, or Gal-1 protein. Purified recombinant proteins were coated at 0.2 μg/well/100 μl coating buffer, blocked and ELISA was performed using serum 1/100 and intestinal lavage (1/20). Subsequent to incubation, plates were washed and further incubated with alkaline phosphatase-conjugated goat-anti mouse IgG or IgA. For the detection of IgG1 and IgG2a antibodies, goat anti-mouse IgG1 or IgG2a antibodies conjugated to alkaline phosphatase were used. Absorbance values were read at 405 nm on an ELISA plate reader.
Preparation of monocytes-derived DCs
Peripheral blood mononuclear cells (PBMCs) were isolated from blood donor buffy coats by density-gradient centrifugation with Lymphoprep (Nycomed, Oslo, Norway). Monocytes were isolated by plastic adherence. Immature monocyte-derived dendritic cells (iDCs) were generated by culturing monocytes in complete RPMI 1640 medium supplemented with granulocyte macrophage-colony-stimulating factor (GM-CSF, 25 ng/ml; R&D systems) and interleukin 4 (IL-4, 50 ng/ml; R&D systems) for 5 d To generate mature (m) monocyte-derived DCs, iDCs were stimulated with tumor necrosis factor α (TNF-α 50 ng/ml, R&D systems) for additional 2 d In parallel, iDCs were stimulated with live L. plantarum (106 or 107 CFU/ml) for 2 d in order to investigate the adjuvant activity of the bacteria.
Cytokine induction
Monocytes, iDCs or mDCs derived from the same donors were resuspended in RPMI medium supplemented with 10% FCS and antibiotics and then seeded in 12-well tissue culture plates at 106 cells/ml. Cells were stimulated with live wild type or L. plantarum expressing NY-ESO-1 (107 CFU/ml) for 18 h. Negative control cultures contained unstimulated cells. In some experiments, the cells were also stimulated with UV-killed L. plantarum. Thereafter, culture supernatants were collected and TNF-α, IL-12, IL-10, and IL-4 were quantified by ELISA (Quantikine, R&D Systems). For phenotypic analysis, iDCs were stimulated with the test molecules for 48 h. All cultures were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Phenotypic analysis
The phenotype of DCs was analyzed by direct immunofluorescence staining of cell surface antigens using fluorochrome-conjugate mAbs for CD40, CD80, CD83, CD86, CCR7, B7-H1 (PD-L-1), and appropriate control Ab isotypes. After staining, the cells were analyzed on FACSCantoII flow cytometer. The data were processed using FlowJo software and expressed as mean fluorescence intensity.
Allogeneic stimulation of CD4+ T cells with monocyte-derived DCs
Allogeneic CD4+ T cells were isolated from PBMCs by direct magnetic labeling with anti-CD4+ Dynabeads according to the manufacturer's protocol (Dynal Biotechn, Oslo, Norway). Thereafter, T cells (105/250 μl medium) were cocultured with allogeneic iDCs, TNF-α-matured DCs or L. plantarum-matured mDCs at DC/T cell ratios 1:10, 1:20, and 1:40. Co-cultures were incubated for 5 d and then pulsed with 1 μCi/well of [3H]-thymidine for 18 h before harvesting onto glass filters.
Statistical analysis
All data are presented as means ± standard deviation. Statistical analysis was performed using GraphPad Prism, version 5.00 for Windows.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported in part by grants from the Gene Therapy Program at the Oslo University Hospital to M. Sioud.
References
- Pasetti MF, Simon JK, Sztein MB: Levine MM. Immunology of gut mucosal vaccines. Immunol Rev 2011; 239:125-148; PMID:21198669; http://dx.doi.org/10.1111/j.1600-065X.2010.00970.x
- Bermúdez-Humarán LG, Aubry C, Motta JP, Deraison C, Steidler L, Vergnolle N, Chatel JM, Langella P. Curr Opin Microbiol 2013; 16:278-283; http://dx.doi.org/10.1016/j.mib.2013.06.002
- Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Förster R, Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. JEM 2006; 203:519-527; http://dx.doi.org/10.1084/jem.20052016
- Abd El, Ghany M, Jansen A, Clare S, Hall I, Pickard D, Kingsley RA, Dougan G. Candidate live, attenuated Salmonella enteric serotype Typhimurium vaccines with reduced fecal shedding are immunogenic and effective oral vaccines. Infect Immun 2007; 75:1835-1842; PMID:17296764; http://dx.doi.org/10.1128/IAI.01655-06
- Reveneau N, Geoffroy MC, Locht C, Chagnaud P, Mercenier A. Comparison of the immune responses induced by local immunizations with recombinant Lactobacillus plantarum producing tetanus toxin fragment C in different cellular locations. Vaccine 2002; 20:1769-1777; PMID:11906764; http://dx.doi.org/10.1016/S0264-410X(02)00027-0
- Yigang XU, Yijing LI. Construction of recombinant Lactobacillus casei efficiently surface displayed and secreted porcine parvovirus VP2 protein and comparison of the immune responses induced by oral immunization. Immunology. 2008; 124:68-75; PMID:18034821; http://dx.doi.org/10.1111/j.1365-2567.2007.02738.x
- Ramasamy R, Yasawardena S, Zomer A, Venema G, Kok J, Leenhouts K. Immunogenicity of a malaria parasite antigen displayed by Lactococcus lactis in oral immunisations. Vaccine 2006; 24:3900-3908; PMID:16545511; http://dx.doi.org/10.1016/j.vaccine.2006.02.040
- Cortes-Perez NG, Lefèvre F, Corthier G, Adel-Patient K, Langella P, Bermúdez-Humarán LG. Influence of the route of immunization and the nature of the bacterial vector on immunogenicity of mucosal vaccines based on lactic acid bacteria. Vaccine 2007; 25:6581-6588; PMID:17675182; http://dx.doi.org/10.1016/j.vaccine.2007.06.062
- Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 2006; 314:1157-1160; PMID:17110582; http://dx.doi.org/10.1126/science.1132742
- Bermúdez-Humarán LG, Cortes-Perez NG, Le Loir Y, Alcocer-González JM, Tamez-Guerra RS, de Oca-Luna RM, Langella P. An inducible surface presentation system improves cellular immunity against human papillomavirus type 16 E7 antigen in mice after nasal administration with recombiunant lactococci. J Med Microbiol 2004; 53:427-433; http://dx.doi.org/10.1099/jmm.0.05472-0
- Bahey-El-Din M, Casey PG, Griffin BT, Gahan CG. Lactococcus lactis-expressing listeriolysin O (LLO) provides protection and specific CD8(+) T cells against Listeria monocytogenes in the murine infection model. Vaccine 2008; 26:5304-5314; PMID:18691625; http://dx.doi.org/10.1016/j.vaccine.2008.07.047
- Campos IB, Darrieux M, Ferreira DM, Miyaji EN, Silva DA, Arêas AP, Aires KA, Leite LC, Ho PL, Oliveira ML. Nasal immunization of mice with Lactobacillus casei expressing the pneumococcal surface protein A: induction of antibodies, complement deposition and partial protection against Streptococcus pneumonia challenge. Microbes Infect 2008; 10:481-488; PMID:18403234; http://dx.doi.org/10.1016/j.micinf.2008.01.007
- Ribelles P, Benbouziane B, Langella P, Suárez JE, Bermúdez-Humarán, Riasi A. Protection against human papillomavirus type 16-induced tumors in mice using non-genetically modified lactic acid bacteria displaying E7 antigen at its surface. Appl Microbial Biotechnol 2013; 97:1231-1239; http://dx.doi.org/10.1007/s00253-012-4575-1
- del Rio B, Dattwyler RJ, Aroso M, Neves V, Meirelles L, Seegers JF, Gomes-Solecki M. Oral immunization with recombinant Lactobacillus planatarum induces a protective immune response in mice with Lyme disease. Clin Vaccine Immunol 2008; 15:1429-1435; PMID:18632920; http://dx.doi.org/10.1128/CVI.00169-08
- Adachi K, Kawana K, Yokoyama T, Fujii T, Tomio A, Miura S, Tomio K, Kojima S, Oda K, Sewaki T, et al. Oral immunization with a Lactobacillus casei vaccine expressing human papillomavirus (HPV) type 16 E7 is an effective strategy to induce mucosal cytotoxic lymphocytes against HPV 16 E7. Vaccine 2010; 28:2810-2817; PMID:20170766; http://dx.doi.org/10.1016/j.vaccine.2010.02.005
- Bermúdez-Humarán LG, Cortes-Perez NG, Lefèvre F, Guimarães V, Rabot S, Alcocer-Gonzalez JM, Gratadoux JJ, Rodriguez-Padilla C, Tamez-Guerra RS, Corthier G, et al. A novel mucosal vaccine based on live Lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16-induced tumors. J Immunol 2005; 175:7297-7302; http://dx.doi.org/10.4049/jimmunol.175.11.7297
- Kawana K, Adachi K, Kojima S, Taguchi A, Tomio K, Yamashita A, Nishida H, Nagasaka K, Arimoto T, Yokoyama T, et al. Oral vaccination against HPV E7 for treatment of cervical intraepithelial neoplasia grade 3 (CIN3) elicits E7-specific mucosal immunity in the cervix of CIN3 patients. Vaccine 2014; 32:6233-6239; PMID:25258102; http://dx.doi.org/10.1016/j.vaccine.2014.09.020
- Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity 2010; 33:464-478; PMID:21029958; http://dx.doi.org/10.1016/j.immuni.2010.10.007
- Jager E, Chen YT, Drijfhout JW, Karbach J, Ringhoffer M, Jäger D, Arand M, Wada H, Noguchi Y, Stockert E, et al. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility antigen (HLA)-A2-binding peptide epitopes. J Exp Med 1998; 187:265-270; PMID:9432985; http://dx.doi.org/10.1084/jem.187.2.265
- Meng JZ, Dong YJ, Huang H, Li S, Zhong Y, Liu SL, Wang YD. Oral vaccination with attenuated Salmonella enteric Strains Encoding T-cell epitopes from tumor antigen NY-ESO-1 induces specific cytotoxic T-lymphocyte Resaponses. Clin Vaccine Immunol 2010; 17:889-894; PMID:20375244; http://dx.doi.org/10.1128/CVI.00044-10
- Maraskovsky E, Sjölander S, Drane DP, Schnurr M, Le TT, Mateo L, Luft T, Masterman KA, Tai TY, Chen Q, et al. NY-ESO-1 protein formulated in ISCOMATRIX adjuvant is a potent anticancer vaccine inducing both humoral and CD8+ T cell-mediated immunity and protection against NY-ESO-1 tumors. Clin Cancer Res 2004; 10:2879-2890; PMID:15102697; http://dx.doi.org/10.1158/1078-0432.CCR-03-0245
- Von Schillde MA, Hörmannsperger G, Weiher M, Alpert CA, Hahne H, Bäuerl C, van Huynegem K, Steidler L, Hrncir T, Pérez-Martínez G, Kuster B, Haller D. Lactoceptin Secreted by Lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines. Cell Host Microbe 2012; 11:387-396; PMID:22520466; http://dx.doi.org/10.1016/j.chom.2012.02.006
- Fredriksen L, Mathiesen G, Sioud M, Eijsink VGH. Cell wall anchoring of the 37-kilodalton oncofetal antigen by Lactobacillus plantarum for mucosal cancer vaccine delivery. Appl Environm Microbiol 2010; 76:7359-7362; http://dx.doi.org/10.1128/AEM.01031-10
- Mobergslien A, Sioud M. Galectin-1 and -3 gene silencing in immature and mature dendritic cells enhances T cell activation and interferon-γ production. J. Leukoc. Biol. 2012; 91:461-46; PMID:22167721
- Sioud M. Engineering better immunotherapies via RNA interference. Hum Vaccin Immunother. 2014; 10:3165-3174; PMID:25483669; http://dx.doi.org/10.4161/hv.29754
- Zygmunt B, Veldhoen M. T helper cell differentiation more than just cytokines. Adv Immunol. 2011; 109:159-196; PMID:21569915
- Honjo T, Okazaki T. The PD-1-PD-L pathways in immunological tolerance. Trends Immunol 2006; 27:195-201; PMID:16500147; http://dx.doi.org/10.1016/j.it.2006.02.001
- Fernandez EM, Valenti V, Rockel C, Hermann C, Pot B, Boneca IG, Grangette C. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidogycan-derived muropeptide. Gut 2011; 60:1050-1059; PMID:21471573; http://dx.doi.org/10.1136/gut.2010.232918
- Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfiled KL, Bavari S, Klaenhammer TR. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci 2005; 102:2880-2885; PMID:15710900; http://dx.doi.org/10.1073/pnas.0500098102
- Christensen HR, Frokiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine DCs. J Immunol 2002; 168:171-178; PMID:11751960; http://dx.doi.org/10.4049/jimmunol.168.1.171
- Weis G, Rasmussen S, Zeuthen LH, Nielsen BN, Jarmer H, Jespersen L, Frøkiær H. Lactobacillus acidophilus induces virus immune defense genes in murine dendritic cells by a Toll-like receptor-2-dependent mechanism. Immunology 2010; 131:268-281; PMID:20545783; http://dx.doi.org/10.1111/j.1365-2567.2010.03301.x
- Del Rio B, Seegers JFML, Gomes-Solecki M. Immune response to Lactobacillus plantarum expressing Borrelia burgdorferi OspA is modulated by the lipid modification of the antigen. Plos One 2010; 5:e11199; PMID:20585451; http://dx.doi.org/10.1371/journal.pone.0011199
- Meijerink M, van Hemert S, Taverne N, Wels M, de Vos P, Bron PA, Savelkoul HF, van Bilsen J, Kleerebezem M, Wells JM. Identification of Genetic Loci in Lactobacillus plantarum that modulate the immune response of dendritic cells using comparative genome hybridization. PloS One 2010; 5:e10632; PMID:20498715; http://dx.doi.org/10.1371/journal.pone.0010632
- Platten M, Wick W, Van den Eynde B. Tryptophan Catabolism in Cancer: Beyond IDO and tryptophan depletion. Cancer Res 2012; 72:5435-5440; PMID:23090118; http://dx.doi.org/10.1158/0008-5472.CAN-12-0569
- Sørvig E, Grönqvist S, Naterstad K, Mathiesen G, Eijsink VG, Axelsson L. Construction of vectors for inducible gene expression in Lactobacillus sakei and L plantarum. FEMS Microbiol Lett 2003; 229:119-126; PMID:14659551; http://dx.doi.org/10.1016/S0378-1097(03)00798-5
- Piatesi A, Howland SW, Rakestraw JA, Renner C, Robson N, Cebon J, Maraskovsky E, Ritter G, Old L, Wittrup KD. Directed evolution for improved secretion of cancer-testisantigen NY-ESO-1 from yeast. Protein Expr Purif 2006; 48:232-242; PMID:16563796; http://dx.doi.org/10.1016/j.pep.2006.01.026
- Kleerebezem M, Boekhorst J, van Kranenburg R, Molenaar D, Kuipers OP, Leer R, Tarchini R, Peters SA, Sandbrink HM, Fiers MW, et al. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci. 2003; 100:1990-1995; http://dx.doi.org/10.1073/pnas.0337704100
- Holo H, Nes IF. High-Frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol 1989; 55:3119-3123; PMID:16348073
- Mathiesen G, Sveen A, Piard JC, Axelsson L, Eijsink VG. Heterologous protein secretion by Lactobacillus plantarum using homologous signal peptides. J Appl Microbiol 2008; 105:215-226; PMID:18298538; http://dx.doi.org/10.1111/j.1365-2672.2008.03734.x
- Mathiesen G, Sveen A, Brurberg MB, Fredriksen L, Axelsson L, Eijsink VG: Genome-wide analysis of signal peptide functionality in Lactobacillus plantarum WCFS1. BMC Genomics 2009; 10:425-430; PMID:19744343; http://dx.doi.org/10.1186/1471-2164-10-425
