Abstract
Both Tim-3 and Tim-4 belong to the T-cell immunoglobulin and mucin domain (Tim) gene family, which plays a critical role in immunoregulation. Tim-3 has been suggested as a negative regulator of anti-tumor immunity due to its function on inducing T cells exhaustion in cancer. In addition to its expression on exhausted T cells, Tim-3 also has been reported to up-regulate on nature killer (NK) cells and promote NK cells functionally exhausted in cancer. While Tim-3 selectively expression on most types of leukemia stem cells, it promotes the progression of acute myeloid leukemia. Recently, data from experimental models of tumor discovered that Tim-3 and Tim-4 up-regulation on tumor associated dendritic cells and macrophages attenuated the anti-tumor effects of cancer vaccines and chemotherapy. Moreover, co-blockage of Tim-3 and PD-1, Tim-3 and CD137, Tim-3 and carcinoembryonic antigen cell adhesion molecule 1 (CEACAM1) could enhance cell-mediated immunity in advanced tumor, and combined treatment with anti-Tim-3 and anti-Tim-4 mAbs further increase the efficacy of cancer vaccines. The therapeutic manipulation of TIM-3 and TIM-4 may provide a novel strategy to improve the clinical efficacy of cancer immunotherapy.
Abbreviations
| APC | = | antigen-presenting cells |
| BTLA | = | B and T lymphocyte attenuator |
| CEACAM1 | = | carcinoembryonic antigen cell adhesion molecule 1 |
| CTL | = | cytotoxicity T lymphocyte |
| CTLA-4 | = | ytotoxic T lymphocyte antigen-4 |
| DAMPs | = | danger associated pattern molecules |
| DCs | = | dendritic cells |
| HCC | = | hepatocellular carcinoma |
| HMGB1 | = | high mobility group protein B1 |
| LAG-3 | = | lymphocyte activation gene-3 |
| mAbs | = | monoclonal antibodies |
| MDSC | = | myeloid-derived suppressor cells |
| NK | = | natural killer cells |
| NKT | = | natural killer T cells |
| NSCLC | = | non-small cell lung cancer |
| PD-1 | = | programmed death-1 |
| PS | = | phosphatidylserine |
| RCC | = | renal cell cancer |
| TADC | = | tumor associated dendritic cells |
| TAM | = | tumor associated macrophages |
| Th1-T TGF-β | = | transforming growth factor-β |
| helper type | = | 1 cells |
| Tim | = | T-cell immunoglobulin and mucin domain |
| VEGF | = | vascular endothelial growth factor |
Introduction
The T-cell immunoglobulin and mucin domain (Tim) gene family was discovered in 2001, which plays a critical role in immunoregulation.Citation1 The Tim gene family comprises of 8 members (TIM-1–8) on mouse chromosome 11B1.1 and 3 members (TIM-1, TIM-3, and TIM-4) on human chromosome 5q33.2.Citation2 Tim-3 is expressed on many types of immune cells, including T cells, dendritic cells (DCs), macrophages, nature killer cells (NK), cancer stem cells, and so on.Citation3 TIM-3 is previously known as a receptor for galectin-9 and phosphatidylserine (PS), it may induce the apoptosis of T cells, enhance the secretion of proinflammatory cytokines such as TNF by DCs and NK cells, and promote the phagocytosis of apoptotic cells by monocytes and macrophages through interaction with its ligands.Citation4,5 Recently, carcinoembryonic antigen cell adhesion molecule 1 (CEACAM1), another well-known molecule expressed on activated T cells and involved in T-cell inhibition, was discovered as a heterophilic ligand for TIM-3, and their interaction had a crucial role in regulating autoimmunity.Citation6 Unlike Tim-3, Tim-4 is exclusively expressed on antigen-presenting cells (APCs),Citation7 and serves as a critical sensor for controlling the functions of naive and activated T cells and phagocytosis of apoptotic cells by APCs through interaction with PS.Citation8,9
Indeed, Tim-3 has been reported as a negative regulator of anti-tumor immunity. The expression of Tim-3 on T cells in cancer may induce T cell exhaustion, and promote the expansion of immunosuppressive CD4+FoxP3+ regulatory T (Treg) cells and CD11b+Gr-1+ myeloid suppressor cells (MDSC).Citation10 Recent reports also showed that TIM-3, as a surface molecule, is selectively expressed on leukemia stem cells (LSCs) in acute myeloid leukemia (AML), and as such it can be a good target in eradicating AML stem cells, leaving normal hematopietic stem cells intact.Citation11-13 In addition, Tim-3 has also been reported to up-regulate on tumor associated dendritic cells (TADC) and macrophages (TAM), and attenuate the antitumor effects of cancer vaccinesCitation4 (). The expression of Tim-4 on TAM could mediate degradation of dying tumor cells by autophagy, reduce antigen presentation and impaired cytotoxicity T lymphocyte (CTL) responses, and suppress the antitumor effects of chemotherapy.Citation14 Co-blockage of Tim-3 with other negative checkpoint regulators expressed on T cells, such as programmed death-1 (PD-1) and CD137, would enhance cell-mediated immunity in advanced tumor, and combined treatment with anti-Tim-3 and anti-Tim-4 mAbs further increase the efficacy of cancer vaccines.
Figure 1. Tim-3 negatively regulates cell-mediated antitumor immunity. Tim-3 expression on T cells in cancer may induce T cell exhaustion, promote the expansion of immunosuppressive CD4+FoxP3+ regulatory T (Treg) cells and CD11b+Gr-1+ myeloid suppressor cells (MDSC). TIM-3 as a surface molecule is selectively expressed on leukemia stem cells (LSC), especially in acute myeloid leukemia (AML), and as such promotes the progression of AML. In addition, Tim-3 also up-regulates on tumor associated dendritic cells (TADC) and macrophages (TAM), and attenuates the antitumor effects of cancer vaccines.
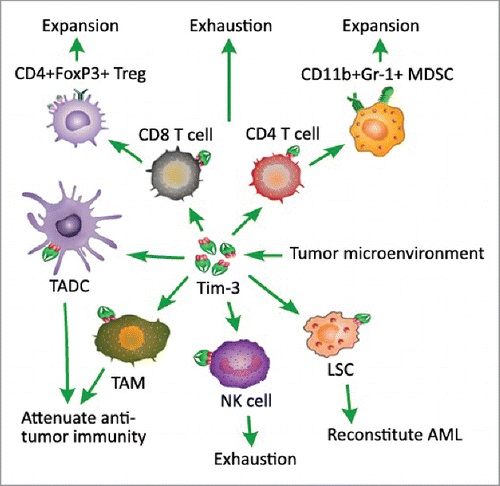
Tim-3 on T cell subsets in tumor
Tim-3 was discovered nearly 10 y ago as a molecule expressed on IFN-γ-producing CD4+ T helper type 1 (Th1) and CD8+ T cytotoxic type 1 (Tc1) cells, and induced T cell apoptosis through interaction with its ligand galectin-9. And then, several studies have identified TIM-3 as an important immune regulator in the tumor microenvironment due to its negative regulation on various T-cell subsets. Within the tumor microenvironment, a cross-talk between the infiltrating cells may occur conditioning the characteristic of the in situ immune response. CD4+ or CD8+ Tumor-Induced Senescent T cells may promote production of pro-inflammatory cytokines (TNF, IL-1β and IL-6) and angiogenic factors (MMP-9, VEGF-A and IL-8) by CD14+ monocytes/macrophages (Mo/Ma), and Tim-3 and CD40 are involved in this modulation.Citation15
In addition, a synergistic action between negative checkpoint regulators expressed on T cells may also exist. The expression of negative checkpoint regulator PD-1 and TIM-3 impairing cell-mediated immunity was observed in advanced melanoma and colorectal cancer.Citation16,17 Co-blockage of Tim-3 and PD-1 could enhance the expansion and function of tumor antigen–specific CD8+ T cells in vitro and in vivo, induce tumor rejection in experimental models and enhance the vaccine effect in patients with advanced melanoma.Citation18,19 New treatment targeting TIM-3 and PD-1 on CD4+ and CD8+ T cells may provide a breakthrough treatment to cancer patients. In a murine model of ovarian cancer, co-blockage of Tim-3 and CD137 was able to prevent the tumor progression in advanced established tumor by increasing the number of CD4+ and CD8+ cells and decreasing immunosuppressive CD4+FoxP3+ regulatory T (Treg) cells and CD11b+Gr-1+ MDSC.Citation20 Also, co-blockade of CEACAM1 and TIM-3 leads to enhancement of anti-tumor immune responses with improved elimination of tumors in mouse colorectal cancer models.Citation6 Other negative checkpoint regulators expression on tumor-infiltrating lymphocytes, such as cytotoxic T lymphocyte antigen-4 (CTLA-4), lymphocyte activation gene-3 (LAG-3) and BTLA (B and T lymphocyte attenuator) also negatively regulate antitumor immunity in tumor microenvironments, the effects of co-blockage of them with Tim-3 still need further investigation.Citation21-23
Tim-3 and TIM-4 on TADC and TAM
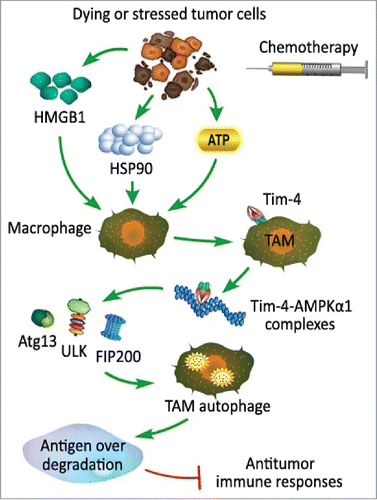
Despite Tim-3 and Tim-4 expressed by DCs and macrophages promote the phagocytosis of apoptotic cells through interaction with PS, little is known about what regulates Tim-3 and Tim-4 expression. Tumor microenvironments play a determinant role in tumor survival, suppressing responsiveness to anticancer drugs and accelerating subsequent tumor growth, thus may be responsible for the expression of Tim-3 and Tim-4 on tumor infiltrating cells. Chiba et al pointed out that in tumor microenvironments, tumor-derived immunoregulatory factors such as IL-10 and vascular endothelial growth factor (VEGF) promoted TIM-3 expression on DCs alone or together, in a dose-dependent manner, and the mechanisms used by tumor cells to induce their expression of TIM-3 distinct from those used by DCs.Citation4 When upregulation on TADC, TIM-3 directly interacted with high mobility group protein B1 (HMGB1) and suppressed nucleic acid-mediated activation of an effective antitumor immune response. Recently, Yan et al showed that, Tim-3 expression on TAM in hepatocellular carcinoma (HCC) is induced by tumor-derived signals, including transforming growth factor-β (TGF-β). In turn, TAM promotes the growth of HCC by secretion of soluble factors such as interleukin-6 (IL-6). Additionally, Tim-3 inhibits the activation of tumor-specific CD8+ T cells. Inhibition of Tim-3 could target HCC from 2 angles, first by blocking growth promotion mediated by TAM and second by releasing CD8+ T cell cytotoxicity.Citation24,25 While Baghdadi et al also found that danger associated pattern molecules (DAMPs) such as HMGB1, HSP90 (heat shock protein 90), MSU (monosodium urate), S100A8, and ATP (apyrase) released from dying or stressed tumor cells treated with chemotherapeutic drugs could promote TIM-4 expression on macrophages and DCs.Citation14
Autophagy is an important homeostatic cellular recycling mechanism responsible for degrading unnecessary or dysfunctional cellular organelles and proteins in all living cells,Citation26 and has an important role in cancer-cell resistance to anticancer therapies such as radiation, chemotherapy, and some other targeted therapies.Citation27-29 In the study of Baghdadi et al, they pointed out the mechanism of cancer-cell resistance to chemotherapy. After recognition of DAMPs from dying or stressed tumor cells by chemotherapy, TIM-4 up-regulated on TAM and directly interacted with AMPKa1, thus promoted phosphorylation of ULK1 at Ser555, recruited Atg13/FIP200 and initiated autophagic vesicle formation and activated autophagy mediated degradation of ingested tumors, leading to reduced antigen presentation and impaired cytotoxicity T lymphocyte (CTL) responses (). Consistently, blockage of the TIM-4-AMPKa1-autophagy pathway augmented the antitumor effect of chemotherapeutics by enhancing tumor-specific CTL responses. So, targeting of the TIM-4-AMPKa1 interaction may constitute a unique strategy for augmenting antitumor immunity and improving cancer chemotherapy.Citation14
Figure 2. Tim-4 expression on tumor associated macrophages mediates degradation of dying tumor cells by autophagy. Danger associated pattern molecules (DAMPs) released from dying or stressed tumor cells treated with chemotherapeutic drugs such as high mobility group protein B1 (HMGB1), heat shock protein 90 (HSP90) and apyrase (ATP) promote TIM-4 expression on macrophage. After recognition of DAMPs released from dying or stressed tumor cells with chemotherapy, TIM-4 expression on TAM directly interacts with AMPKa1, thus promotes phosphorylation of ULK1 at Ser555, which is critical to recruit Atg13/FIP200 and initiate autophagic vesicle formation and activates autophagy mediated degradation of ingested tumors, leading to reduced antigen presentation and impaired antitumor immune responses.
Both TIM-3 and TIM-4 attenuate the antitumor effect of cancer vaccines, a combined treatment with anti-TIM-3 and anti-TIM-4 mAbs may further increase the efficacy of cancer vaccines and chemotherapy. In established B16 melanoma, combining anti-TIM-3 and anti-TIM-4 mAbs markedly increased vaccine-induced antitumor responses by increasing the numbers and effector functions of both NK cells and CD8+ T cells.Citation30 The therapeutic manipulation of TIM-3 and TIM-4 may provide a novel strategy to improve the clinical efficacy of cancer immunotherapy. Taken together, these findings identify TIM3 and Tim-4 as potential targets for inducing antitumor immunity in conjunction with DNA vaccines and/or immunogenic chemotherapy in clinical settings.
Other roles of Tim-3 in tumor
Tim-3 also functions as a human NK-cell co-receptor to enhance IFN-γ production, which has important implications for the control of infectious disease and cancer.Citation31 Meanwhile, NK-cell responses may be negatively regulated when NK cells encounter target cells expressing cognate ligands of Tim-3.Citation32 For patients with metastatic melanoma, expression of Tim-3 on NK cells induce functionally impaired/exhausted NK cells, and Tim-3 blockage reversed this exhausted phenotype, thus Tim-3–targeted therapies may be an effective method to restore antitumor immunity.Citation33
In the studies of Kikushige et al, Tim-3 is selectively expressed on LSCs in most types of AML, with the exception of acute promyelocytic leukemia, not on normal haematopoietic stem cells (HSCs). Although it is not surprising in mouse models reconstituted with human AML LSCs or human haematopoietic stem cells, a human TIM-3 mouse IgG2a antibody with complement-dependent and antibody-dependent cellular cytotoxic activities eradicates AML LSCs in vivo without affecting normal human hematopoiesis.Citation11-13 Thus, TIM-3 may serve as one of the promising targets to eradicate AML LSCs. Jajosky et al also pointed out that, RepSox (a reprogramming tool and inhibitor of transforming growth factor-β receptor 1) accelerated loss of Tim-3 from the surface of AML cells by inhibiting TGF-β signaling.Citation34
Tim-3 as human cancer prognostic factor
The expression of TIM-3 on T cells was poor clinic pathological parameters such as nodal metastasis and advanced cancer stages in gastric cancer,Citation35 lung cancer,Citation36 cervical cancerCitation37 and ovarian cancer.Citation38 The ectopic expression of TIM-3 in tumor cells has been suggested as a potential, independent prognostic factor for patients with lung cancer,Citation36 cervical cancerCitation37 and prostate cancer.Citation39 The Tim-3/galectin-9 signaling pathway mediates T-cell senescence was also involved in patients with hepatitis B virus (HBV)-associated HCC, thus it has been a potential immunotherapeutic target.Citation40
In addition, polymorphisms in TIM-3 gene were showed to be associated with various cancers. Subjects carrying +4259TG genotype had a significantly higher risk of NSCLC, pancreatic cancer and renal cell carcinoma compared to the wide-type genotype.Citation41-43 TIM3–1516 genotypes GT or TT may differentially and interactively predispose cirrhosis and HCC in chronic HBV infection.Citation44 The relationship between TIM3–1516 polymorphic genotype and the distant metastasis of gastric cancer was also found.Citation45 Overall, the results suggest that TIM-3 may play important roles in regulating the prognosis of these diseases.
Conclusion and future perspectives
Despite the fact that Tim-3 expression on various T cell subsets could negatively regulates the antitumor immunity in patients with cancer, the expression of Tim-3 on NK cells and TADC, and the expression of Tim-4 on TAM also attenuates the cell-mediated antitumor effects in established tumors. In addition, the expression of Tim-3 on LSCs also promotes the progression of AML. New treatment targeting TIM-3 and PD-1, Tim-3 and CD137, Tim-3 and CEACAM1, Tim-3 and other negative checkpoint regulators on T cells, Tim-3 on NK cells and LSCs, and combined chemotherapy or cancer vaccines with mAb to Tim-3 or Tim-4 may provide a breakthrough in the treatment for the patients with advanced cancer. Since polymorphisms in TIM-3 may play important roles in regulating the prognosis of various cancers, the mechanisms behind this still need further investigation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
LC carried out collection and assembly of data, and manuscript writing. ZHR conceived of the review and helped to draft the manuscript, and gave final approval of manuscript. All authors read and approved the final manuscript.
References
- McIntire JJ, Umetsu SE, Akbari O, Potter M, Kuchroo VK, Barsh GS, Freeman GJ, Umetsu DT, DeKruyff RH. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nature immunology 2001; 2:1109-16; PMID:11725301; http://dx.doi.org/10.1038/ni739
- Li Z, Ju Z, Frieri M. The T-cell immunoglobulin and mucin domain (Tim) gene family in asthma, allergy, and autoimmunity. Allergy and asthma proceedings : the official journal of regional and state allergy societies 2013; 34:e21-6; PMID:23406933; http://dx.doi.org/10.2500/aap.2013.34.3646
- Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunological reviews 2010; 235:172-89; PMID:20536563
- Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan JD, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nature immunology 2012; 13:832-42; PMID:22842346; http://dx.doi.org/10.1038/ni.2376
- Tang D, Lotze MT. Tumor immunity times out: TIM-3 and HMGB1. Nature immunology 2012; 13:808-10; PMID:22910384; http://dx.doi.org/10.1038/ni.2396
- Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 2015; 517:386-90; PMID:25363763; http://dx.doi.org/10.1038/nature13848
- Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity 2007; 27:927-40; PMID:18082433; http://dx.doi.org/10.1016/j.immuni.2007.11.011
- Rodriguez-Manzanet R, Sanjuan MA, Wu HY, Quintana FJ, Xiao S, Anderson AC, Weiner HL, Green DR, Kuchroo VK. T and B cell hyperactivity and autoimmunity associated with niche-specific defects in apoptotic body clearance in TIM-4-deficient mice. Proceedings of the National Academy of Sciences of the United States of America 2010; 107:8706-11; PMID:20368430; http://dx.doi.org/10.1073/pnas.0910359107
- Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature 2007; 450:435-9; PMID:17960135; http://dx.doi.org/10.1038/nature06307
- Anderson AC. Tim-3, a negative regulator of anti-tumor immunity. Current opinion in immunology 2012; 24:213-6; PMID:22226204; http://dx.doi.org/10.1016/j.coi.2011.12.005
- Kikushige Y, Shima T, Takayanagi S, Urata S, Miyamoto T, Iwasaki H, Takenaka K, Teshima T, Tanaka T, Inagaki Y, et al. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell stem cell 2010; 7:708-17; PMID:21112565; http://dx.doi.org/10.1016/j.stem.2010.11.014
- Kikushige Y, Akashi K. TIM-3 as a therapeutic target for malignant stem cells in acute myelogenous leukemia. Annals of the New York Academy of Sciences 2012; 1266:118-23; PMID:22901263; http://dx.doi.org/10.1111/j.1749-6632.2012.06550.x
- Kikushige Y, Miyamoto T. TIM-3 as a novel therapeutic target for eradicating acute myelogenous leukemia stem cells. International journal of hematology 2013; 98:627-33; PMID:24046178; http://dx.doi.org/10.1007/s12185-013-1433-6
- Baghdadi M, Yoneda A, Yamashina T, Nagao H, Komohara Y, Nagai S, Akiba H, Foretz M, Yoshiyama H, Kinoshita I, et al. TIM-4 glycoprotein-mediated degradation of dying tumor cells by autophagy leads to reduced antigen presentation and increased immune tolerance. Immunity 2013; 39:1070-81; PMID:24315994; http://dx.doi.org/10.1016/j.immuni.2013.09.014
- Ramello MC, Boari JT, Canale FP, Mena HA, Negrotto S, Gastman B, Gruppi A, Rodriguez EV, Montes CL. Tumor-induced senescent T cells promote the secretion of pro-inflammatory cytokines and angiogenic factors by human monocytes/macrophages through a mechanism that involves Tim-3 and CD40L. Cell death & disease 2014; 5:e1507; PMID:25375372; http://dx.doi.org/10.1038/cddis.2014.451
- Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. The Journal of experimental medicine 2010; 207:2175-86; PMID:20819923; http://dx.doi.org/10.1084/jem.20100637
- Arai Y, Saito H, Ikeguchi M. Upregulation of TIM-3 and PD-1 on CD4+ and CD8+ T Cells Associated with Dysfunction of Cell-Mediated Immunity after Colorectal Cancer Operation. Yonago acta medica 2012; 55:1-9; PMID:24031134
- Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. The Journal of experimental medicine 2010; 207:2187-94; PMID:20819927; http://dx.doi.org/10.1084/jem.20100643
- Fourcade J, Sun Z, Pagliano O, Chauvin JM, Sander C, Janjic B, Tarhini AA, Tawbi HA, Kirkwood JM, Moschos S, et al. PD-1 and Tim-3 regulate the expansion of tumor antigen-specific CD8(+) T cells induced by melanoma vaccines. Cancer research 2014; 74:1045-55; PMID:24343228; http://dx.doi.org/10.1158/0008-5472.CAN-13-2908
- Guo Z, Cheng D, Xia Z, Luan M, Wu L, Wang G, Zhang S. Combined TIM-3 blockade and CD137 activation affords the long-term protection in a murine model of ovarian cancer. Journal of translational medicine 2013; 11:215; PMID:24044888; http://dx.doi.org/10.1186/1479-5876-11-215
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer 2012; 12:252-64; PMID:22437870; http://dx.doi.org/10.1038/nrc3239
- Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer research 2012; 72:917-27; PMID:22186141; http://dx.doi.org/10.1158/0008-5472.CAN-11-1620
- Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Olive D, Kuchroo V, Zarour HM. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer research 2012; 72:887-96; PMID:22205715; http://dx.doi.org/10.1158/0008-5472.CAN-11-2637
- Yan W, Liu X, Ma H, Zhang H, Song X, Gao L, Liang X, Ma C. Tim-3 fosters HCC development by enhancing TGF-beta-mediated alternative activation of macrophages. Gut 2015
- Flecken T, Sarobe P. Tim-3 expression in tumour-associated macrophages: a new player in HCC progression. Gut 2015; PMID:25694141
- Janku F, McConkey DJ, Hong DS, Kurzrock R. Autophagy as a target for anticancer therapy. Nature reviews Clinical oncology 2011; 8:528-39; PMID:21587219; http://dx.doi.org/10.1038/nrclinonc.2011.71
- Qadir MA, Kwok B, Dragowska WH, To KH, Le D, Bally MB, Gorski SM. Macroautophagy inhibition sensitizes tamoxifen-resistant breast cancer cells and enhances mitochondrial depolarization. Breast cancer research and treatment 2008; 112:389-403; PMID:18172760; http://dx.doi.org/10.1007/s10549-007-9873-4
- Carew JS, Medina EC, Esquivel JA, 2nd, Mahalingam D, Swords R, Kelly K, Zhang H, Huang P, Mita AC, Mita MM, et al. Autophagy inhibition enhances vorinostat-induced apoptosis via ubiquitinated protein accumulation. Journal of cellular and molecular medicine 2010; 14:2448-59; PMID:19583815; http://dx.doi.org/10.1111/j.1582-4934.2009.00832.x
- Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer research 2008; 68:1485-94; PMID:18316613; http://dx.doi.org/10.1158/0008-5472.CAN-07-0562
- Baghdadi M, Nagao H, Yoshiyama H, Akiba H, Yagita H, Dosaka-Akita H, Jinushi M. Combined blockade of TIM-3 and TIM-4 augments cancer vaccine efficacy against established melanomas. Cancer immunology, immunotherapy : CII 2013; 62:629-37; PMID:23143694; http://dx.doi.org/10.1007/s00262-012-1371-9
- Gleason MK, Lenvik TR, McCullar V, Felices M, O'Brien MS, Cooley SA, Verneris MR, Cichocki F, Holman CJ, Panoskaltsis-Mortari A, et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood 2012; 119:3064-72; PMID:22323453; http://dx.doi.org/10.1182/blood-2011-06-360321
- Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF, Lanier LL. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012; 119:3734-43; PMID:22383801; http://dx.doi.org/10.1182/blood-2011-11-392951
- da Silva IP, Gallois A, Jimenez-Baranda S, Khan S, Anderson AC, Kuchroo VK, Osman I, Bhardwaj N. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer immunology research 2014; 2:410-22; PMID:24795354; http://dx.doi.org/10.1158/2326-6066.CIR-13-0171
- Jajosky AN, Coad JE, Vos JA, Martin KH, Senft JR, Wenger SL, Gibson LF. RepSox slows decay of CD34+ acute myeloid leukemia cells and decreases T cell immunoglobulin mucin-3 expression. Stem cells translational medicine 2014; 3:836-48; PMID:24855276; http://dx.doi.org/10.5966/sctm.2013-0193
- Jiang J, Jin MS, Kong F, Cao D, Ma HX, Jia Z, Wang YP, Suo J, Cao X. Decreased galectin-9 and increased Tim-3 expression are related to poor prognosis in gastric cancer. PloS one 2013; 8:e81799; PMID:24339967; http://dx.doi.org/10.1371/journal.pone.0081799
- Zhuang X, Zhang X, Xia X, Zhang C, Liang X, Gao L, Zhang X, Ma C. Ectopic expression of TIM-3 in lung cancers: a potential independent prognostic factor for patients with NSCLC. American journal of clinical pathology 2012; 137:978-85; PMID:22586058; http://dx.doi.org/10.1309/AJCP9Q6OVLVSHTMY
- Cao Y, Zhou X, Huang X, Li Q, Gao L, Jiang L, Huang M, Zhou J. Tim-3 expression in cervical cancer promotes tumor metastasis. PloS one 2013; 8:e53834; PMID:23335978; http://dx.doi.org/10.1371/journal.pone.0053834
- Wu J, Liu C, Qian S, Hou H. The expression of Tim-3 in peripheral blood of ovarian cancer. DNA and cell biology 2013; 32:648-53; PMID:24007284; http://dx.doi.org/10.1089/dna.2013.2116
- Piao YR, Piao LZ, Zhu LH, Jin ZH, Dong XZ. Prognostic value of T cell immunoglobulin mucin-3 in prostate cancer. Asian Pacific journal of cancer prevention : APJCP 2013; 14:3897-901; PMID:23886204; http://dx.doi.org/10.7314/APJCP.2013.14.6.3897
- Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, Liu J, Shi L, Liu C, Wang G, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology 2012; 56:1342-51; PMID:22505239; http://dx.doi.org/10.1002/hep.25777
- Bai J, Li X, Tong D, Shi W, Song H, Li Q. T-cell immunoglobulin- and mucin-domain-containing molecule 3 gene polymorphisms and prognosis of non-small-cell lung cancer. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2013; 34:805-9; PMID:23359271; http://dx.doi.org/10.1007/s13277-012-0610-1
- Tong D, Zhou Y, Chen W, Deng Y, Li L, Jia Z, Qi D. T cell immunoglobulin- and mucin-domain-containing molecule 3 gene polymorphisms and susceptibility to pancreatic cancer. Molecular biology reports 2012; 39:9941-6; PMID:22733499; http://dx.doi.org/10.1007/s11033-012-1862-y
- Cai C, Wang L, Wu Z, Li M, Chen W, Sun Y. T-cell immunoglobulin- and mucin-domain-containing molecule 3 gene polymorphisms and renal cell carcinoma. DNA and cell biology 2012; 31:1285-9; PMID:22472081; http://dx.doi.org/10.1089/dna.2012.1625
- Li Z, Li N, Zhu Q, Zhang G, Han Q, Zhang P, Xun M, Wang Y, Zeng X, Yang C, et al. Genetic variations of PD1 and TIM3 are differentially and interactively associated with the development of cirrhosis and HCC in patients with chronic HBV infection. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases 2013; 14:240-6; PMID:23291409; http://dx.doi.org/10.1016/j.meegid.2012.12.008
- Cao B, Zhu L, Zhu S, Li D, Zhang C, Xu C, Zhang S. Genetic variations and haplotypes in TIM-3 gene and the risk of gastric cancer. Cancer immunology, immunotherapy : CII 2010; 59:1851-7; PMID:20811886; http://dx.doi.org/10.1007/s00262-010-0910-5
